Detection of Superoxide Alterations Induced by 5-Fluorouracil on HeLa Cells with a Cell-Based Biosensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture Conditions
2.2. MTT Cell Proliferation Assay
2.3. Measurement of Mitochondrial Superoxide Production
2.4. Measurement of Caspase Activity
2.5. HeLa Cell Total Protein Extraction
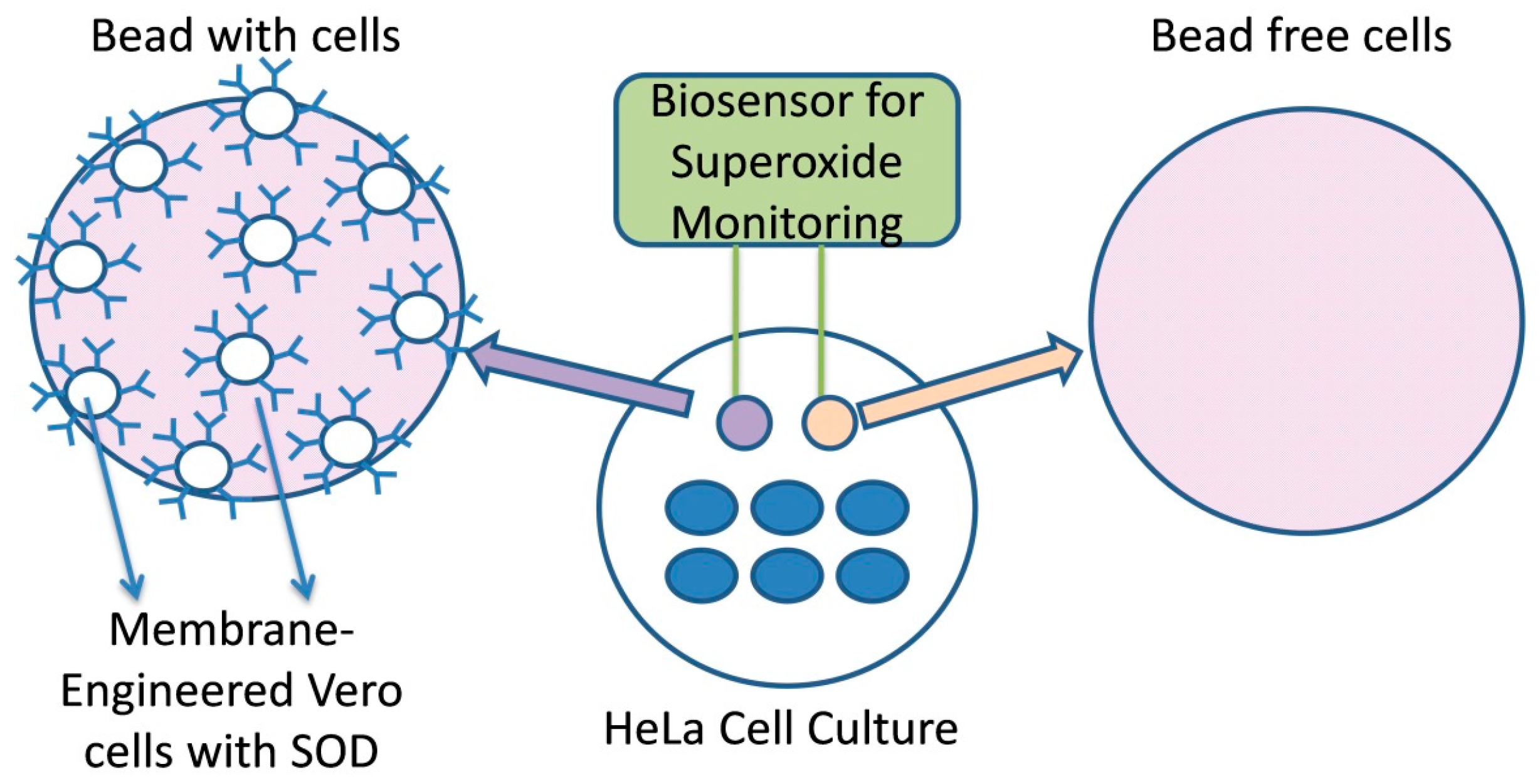
2.6. Creation of Membrane-Engineered Cells and Bioensor Fabrication (Vero-SOD)
2.7. Biosensor Setup for Recording Superoxide Concentration and Data Processing
2.8. Statistical Analysis
3. Results
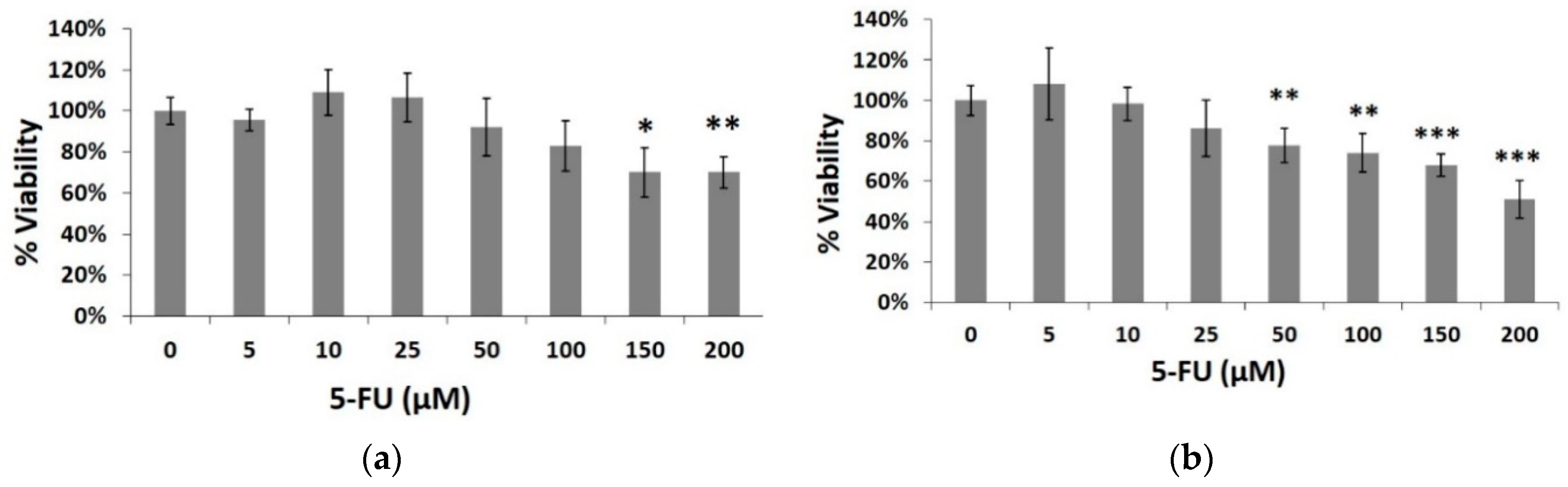
3.1. Assessment of the Effects of the Chemotherapeutic Agent 5-Fluorouracil (5-FU) on HeLa Cell Viability
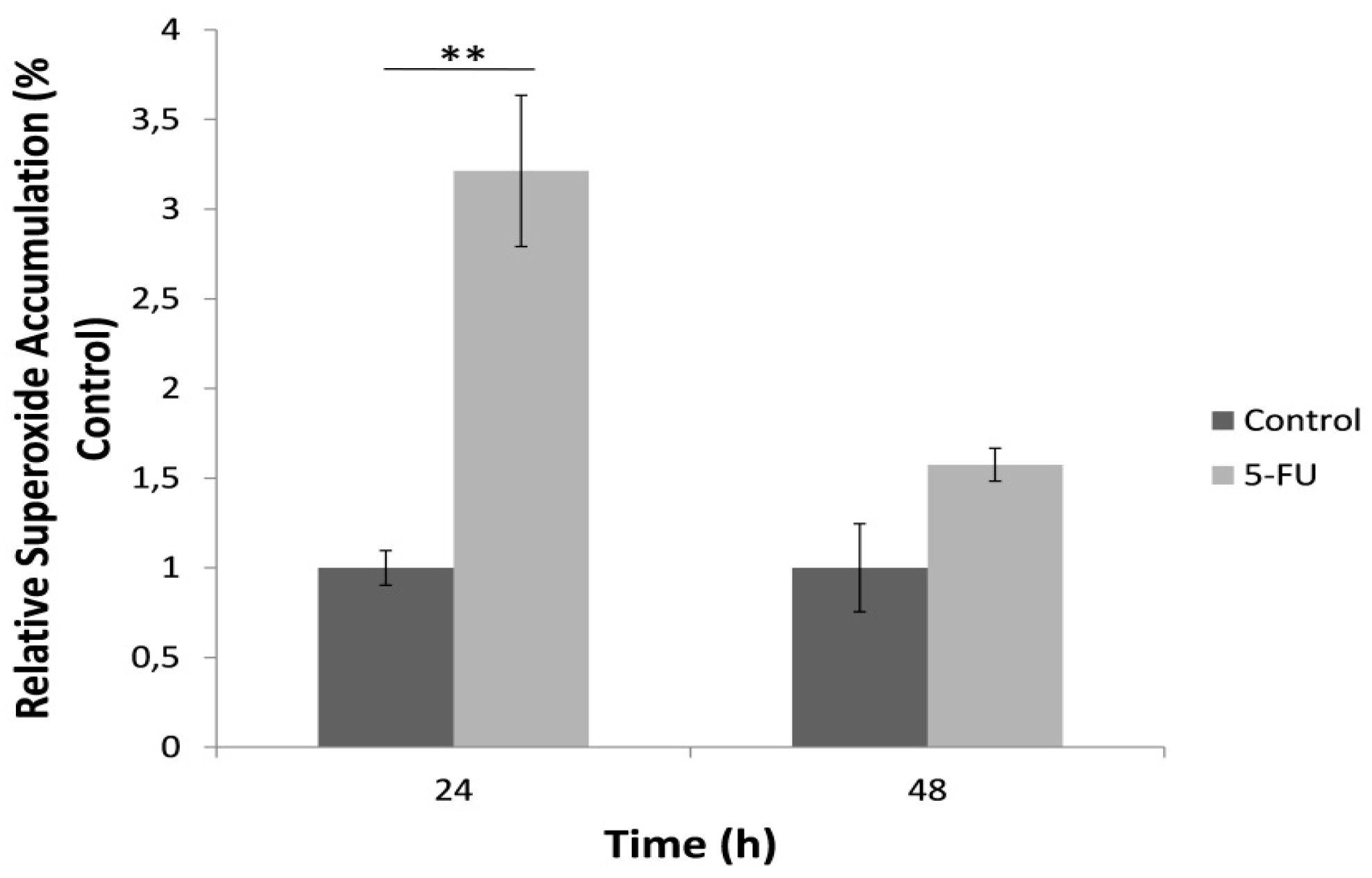
3.2. Increased Mitochondrial Superoxide Production in HeLa Cells is Observed after 24 h Treatment with 5-FU
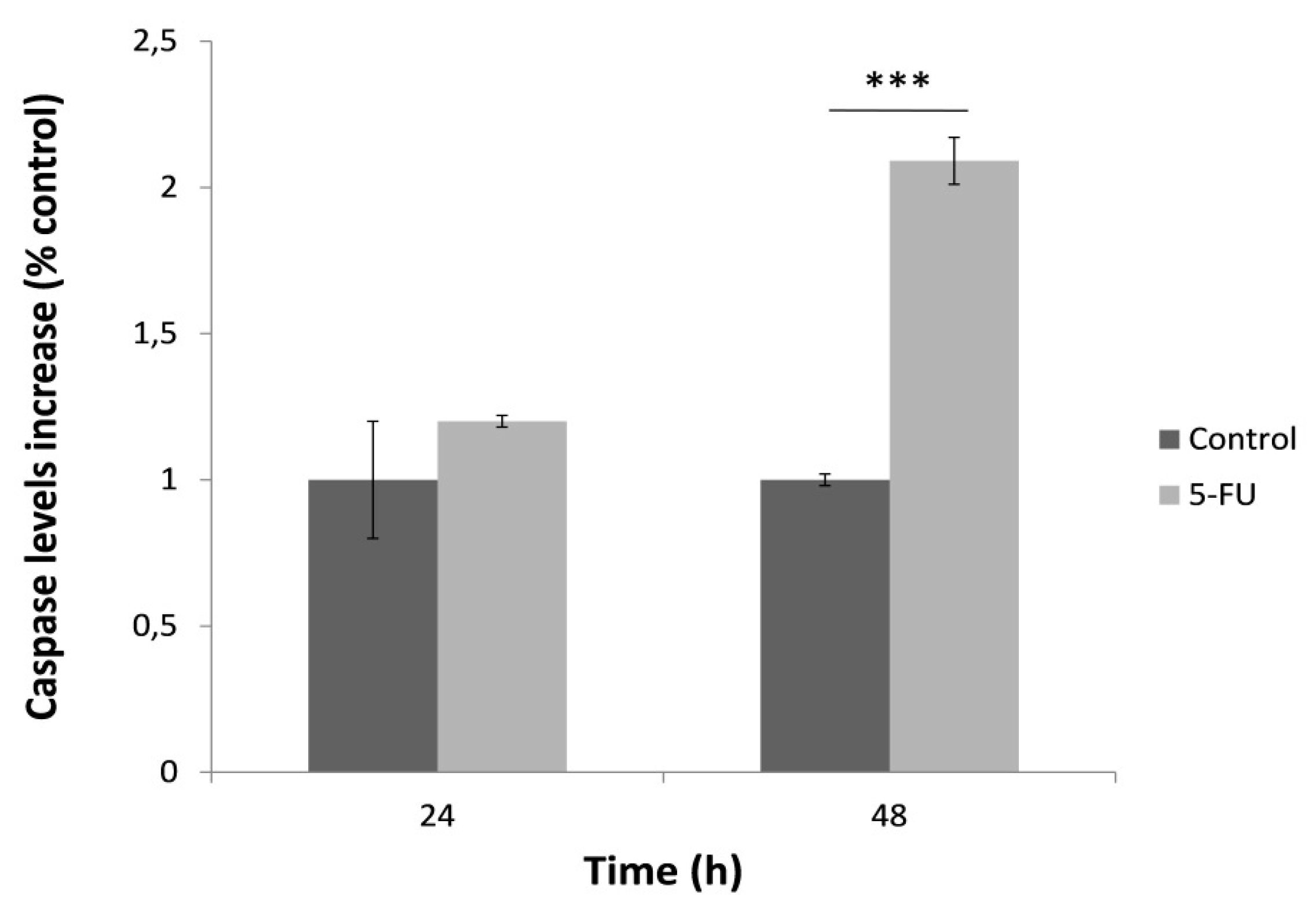
3.3. Caspase-3 Activation after 48 h Treatment with 5-FU
3.4. Superoxide Accumulation in Cell Culture Determined by the Bioelectric Cell-Based Biosensor
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Melville, A.; Eastwood, A.; Kleijnen, J.; Kitchener, H.; Martin-Hirsch, P.; Nelson, L. Management of gynaecological cancers. Qual. Health Care 1999, 8, 270–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grem, J.L. Mechanisms of Action and Modulation of Fluorouracil. Semin. Radiat. Oncol. 1997, 7, 249–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Talmon, G.; Wang, J. MicroRNA-587 antagonizes 5-FU-induced apoptosis and confers drug resistance by regulating PPP2R1B expression in colorectal cancer. Cell Death Dis. 2015, 6, e1845. [Google Scholar] [CrossRef]
- Noordhuis, P.; Holwerda, U.; van der Wilt, C.L.; Groeningen, C.; Smid, K.; Meijer, S.; Pinedo, H.; Peters, G. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef]
- Hwang, P.M.; Bunz, F.; Yu, J.; Rago, C.; Chan, T.A.; Murphy, M.P.; Kelso, G.F.; Smith, R.A.; Kinzler, K.W.; Vogelstein, B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 2001, 7, 1111–1117. [Google Scholar] [CrossRef]
- Fan, C.; Chen, J.; Wang, Y.; Wong, Y.S.; Zhang, Y.; Zheng, W.; Cao, W.; Chen, T. Selenocystine potentiates cancer cell apoptosis induced by 5-fluorouracil by triggering reactive oxygen species-mediated DNA damage and inactivation of the ERK pathway. Free Radic. Biol. Med. 2013, 65, 305–316. [Google Scholar] [CrossRef]
- Liu, M.P.; Liao, M.; Dai, C.; Chen, J.F.; Yang, C.J.; Liu, M.; Chen, Z.G.; Yao, M.C. Sanguisorba officinalis L. synergistically enhanced 5-fluorouracil cytotoxicity in colorectal cancer cells by promoting a reactive oxygen species-mediated, mitochondria-caspase-dependent apoptotic pathway. Sci. Rep. 2016, 27, 34245. [Google Scholar] [CrossRef]
- Ohtani, T.; Hatori, M.; Ito, H.; Takizawa, K.; Kamijo, R.; Nagumo, M. Involvement of caspases in 5-FU induced apoptosis in an oral cancer cell line. Anticancer Res. 2000, 20, 3117–3121. [Google Scholar]
- Ricci, M.S.; Zong, W.X. Chemotherapeutic approaches for targeting cell death pathways. Oncologist 2006, 11, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Bouklihacene, M.; Thorne, R.F. 5-Fluorouracil-induced apoptosis in colorectal cancer cells is caspase-9-dependent and mediated by activation of protein kinase C-δ. Oncol. Lett. 2014, 8, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Poot, M.; Hu, D.; Oda, D. 5-Fluorouracil-induced apoptosis in cultured oral cancer cells. Oral Oncol. 2000, 36, 236–241. [Google Scholar] [CrossRef]
- Ponce-Cusi, R.; Calaf, G. Apoptotic activity of 5-fluorouracil in breast cancer cells transformed by low doses of ionizing α-particle radiation. Int. J. Oncol. 2016, 48, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Shigeishi, H.; Biddle, A.; Gammon, L.; Rodini, C.O.; Yamasaki, M.; Seino, S.; Sugiyama, M.; Takechi, M.; Mackenzie, I.C. Elevation in 5-FU-induced apoptosis in head and neck cancer stem cells by a combination of CDHP and GSK3β inhibitors. J. Oral Pathol. Med. 2015, 44, 201–207. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, J.; Pang, X.; Zhao, L.; Li, Q.; Cao, B. Effect of apatinib combined with 5-fluorouracil (5-FU) on proliferation, apoptosis and invasiveness of gastric cancer cells. Ann. Oncol. 2017, 28. [Google Scholar] [CrossRef]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Combination of phenylpropanoids with 5-fluorouracil as anti-cancer agents against human cervical cancer (HeLa) cell line. Phytomedicine 2012, 20, 151–158. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Gao, M.; He, M.; Yue, F.; Zhao, Y.; Sun, M.; He, K.; Chen, L. 5-FU preferably induces apoptosis in BRAF V600E colorectal cancer cells via downregulation of Bcl-xL. Mol. Cell. Biochem. 2019, 461, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhou, Q.; Zhu, S.; Liu, W. Anti-tumor mechanism of IL-21 used alone and in combination with 5-fluorouracil in vitro on human gastric cancer cells. J. Biol. Regul. Homeost. Agents 2018, 32, 619–625. [Google Scholar] [PubMed]
- Ghadban, T.; Dibbern, J.L.; Reeh, M.; Miro, J.T.; Tsui, T.Y.; Wellner, U.; Izbicki, J.R.; Güngör, C.; Vashist, Y.K. HSP90 is a promising target in gemcitabine and 5-fluorouracil resistant pancreatic cancer. Apoptosis 2017, 22, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Leung, G.P.H.; Zhang, Z.J.; Xiao, J.B.; Lao, L.X.; Feng, F.; Zhang, K.Y.B. Proanthocyanidins from Uncaria rhynchophylla induced apoptosis in MDA-MB-231 breast cancer cells while enhancing cytotoxic effects of 5-fluorouracil. Food Chem. Toxicol. 2017, 107, 248–260. [Google Scholar] [CrossRef]
- Deveci, H.A.; Nazıroğlu, M.; Nur, G. 5-Fluorouracil-induced mitochondrial oxidative cytotoxicity and apoptosis are increased in MCF-7 human breast cancer cells by TRPV1 channel activation but not Hypericum perforatum treatment. Mol. Cell. Biochem. 2018, 439, 189–198. [Google Scholar] [CrossRef]
- Lima, H.R.S.; da Silva, J.S.; de Oliveira Farias, E.A.; Teixeira, P.R.S.; Eiras, C.; Nunes, L.C.C. Electrochemical sensors and biosensors for the analysis of antineoplastic drugs. Biosens. Bioelectron. 2018, 108, 27–37. [Google Scholar] [CrossRef]
- Meneghello, A.; Tartaggia, S.; Alvau, M.D.; Polo, F.; Toffoli, G. Biosensing technologies for therapeutic drug monitoring. Curr. Med. Chem. 2018, 25, 4354–4377. [Google Scholar] [CrossRef]
- Shibayama-Imazu, T.; Sonoda, I.; Sakairi, S.; Aiuchi, T.; Ann, W.W.; Nakajo, S.; Itabe, H.; Nakaya, K. Production of superoxide and dissipation of mitochondrial transmembrane potential by vitamin K2 trigger apoptosis in human ovarian cancer TYK-nu cells. Apoptosis 2006, 11, 1535–1543. [Google Scholar] [CrossRef]
- Dhar, S.K.; St Clair, D.K. Manganese superoxide dismutase regulation and cancer. Free Radic. Biol. Med. 2012, 52, 2209–2222. [Google Scholar] [CrossRef]
- Coso, S.; Harrison, I.; Harrison, C.B.; Vinh, A.; Sobey, C.G.; Drummond, G.R.; Williams, E.D.; Selemidis, S. NADPH oxidases as regulators of tumor angiogenesis: Current and emerging concepts. Antioxid. Redox Signal. 2012, 16, 1229–1247. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G.; Derdak, Z.; Robson, S.C. Mitochondrial recoupling: A novel therapeutic strategy for cancer? Br. J. Cancer 2011, 105, 469–474. [Google Scholar] [CrossRef]
- Montero, A.J.; Jassem, J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs 2011, 71, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.P.; Chin-Sinex, H.; Nie, B.; Mendonca, M.S.; Wang, M. Targeting superoxide dismutase 1 to overcome cisplatin resistance in human ovarian cancer. Cancer Chemother. Pharmacol. 2009, 63, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Radenkovic, S.; Milosevic, Z.; Konjevic, G.; Karadzic, K.; Rovcanin, B.; Buta, M.; Gopcevic, K.; Jurisic, V. Lactate dehydrogenase, catalase, and superoxide dismutase in tumor tissue of breast cancer patients in respect to mammographic findings. Cell Biochem. Biophys. 2013, 66, 287–295. [Google Scholar] [CrossRef]
- Shacter, E.; Williams, J.A.; Hinson, R.M.; Senturker, S.; Lee, Y.-J. Oxidative stress interferes with cancer chemotherapy: Inhibition of lymphoma cell apoptosis and phagocytosis. Blood 2000, 96, 307–313. [Google Scholar] [CrossRef]
- Hu, Y.; Rosen, D.G.; Zhou, Y.; Feng, L.; Yang, G.; Liu, J.; Huang, P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: Role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005, 280, 39485–39492. [Google Scholar] [CrossRef]
- Tamaki, R.; Kanai-Mori, A.; Morishige, Y.; Koike, A.; Yanagihara, K.; Amano, F. Effects of 5-fluorouracil, adriamycin and irinotecan on HSC-39, a human scirrhous gastric cancer cell line. Oncol. Rep. 2017, 37, 2366–2374. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Kintzios, S. Non-invasive Superoxide Monitoring of In Vitro Neuronal Differentiation Using a Cell-Based Biosensor. J. Sens. 2015, 2015, 768352. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ueno, M.; Kakinuma, Y.; Yuhki, K.; Murakoshi, N.; Iemitsu, M.; Miyauchi, T.; Yamaguchi, I. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J. Pharmacol. Sci. 2006, 101, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Konorev, E.A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin Induces Apoptosis in Normal and Tumor Cells via Distinctly Different Mechanisms. J. Biol. Chem. 2004, 279, 25535–25543. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, G.; Valero, T.; Kintzios, S. Superoxide determination using membrane-engineered cells: An example of a novel concept for the construction of cell sensors with customized target recognition properties. Sens. Actuators B Chem. 2012, 175, 78–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Senthil, K.; Aranganathan, S.; Nalini, N. Evidence of oxidative stress in the circulation of ovarian cancer patients. Clin. Chim. Acta 2004, 339, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Nanda, R.; Sahoo, S.; Mohapatra, E. Biosensors in Health Care: The Milestones Achieved in Their Development towards Lab-on-Chip-Analysis. Biochem. Res. Int. 2016, 2016, 3130469. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as antioxidants and anticancer drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef]
- Nunes, S.C.; Serpa, J. Glutathione in ovarian cancer: A double-edged sword. Int. J. Mol. Sci. 2018, 19, 1882. [Google Scholar] [CrossRef]
- Akpinar, B.; Bracht, E.V.; Reijnders, D.; Safarikova, B.; Jelinkova, I.; Grandien, A.; Vaculova, A.H.; Zhivotovsky, B.; Olsson, M. 5-Fluorouracil-induced RNA stress engages a TRAIL-DISC-dependent apoptosis axis facilitated by p53. Oncotarget 2015, 6, 43679–43697. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Kintzios, S. Application of “membrane-engineering” to bioelectric recognition cell sensors for the detection of picomole concentrations of superoxide radical: A novel biosensor principle. Anal. Chim. Acta 2006, 573–574, 90–96. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Vitsa, K.; Bem, F.; Vassilakos, N.; Perdikaris, A.; Blouhos, P.; Yialouris, C.; Frossiniotis, D.; Anthopoulos, I.; Maggana, O.; et al. Engineering of the membrane of fibroblast cells with virus-specific antibodies: A novel biosensor tool for virus detection. Biosens. Bioelectron. 2008, 24, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Perdikaris, A.; Alexandropoulos, N.; Kintzios, S. Development of a Novel, Ultra-rapid Biosensor for the Qualitative Detection of Hepatitis B Virus-associated Antigens and Anti-HBV, Based on “Membrane-engineered” Fibroblast Cells with Virus-Specific Antibodies and Antigens. Sensors 2009, 9, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Perdikaris, A.; Vassilakos, N.; Yiakoumettis, I.; Kektsidou, O.; Kintzios, S. Development of a portable, high throughput biosensor system for rapid plant virus detection. J. Virol. Methods 2011, 177, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gramberg, B.; Kintzios, S.; Schmidt, U.; Mewis, I.; Ulrichs, C. A basic approach towards the development of bioelectric bacterial biosensors for the detection of plant viruses. J. Phytopathol. 2012, 160, 106–111. [Google Scholar] [CrossRef]
- Kokla, A.; Blouchos, P.; Livaniou, E.; Zikos, C.; Kakabakos, S.E.; Petrou, P.S.; Kintzios, S. Visualization of the membrane-engineering concept: Evidence for the specific orientation of electroinserted antibodies and selective binding of target analytes. J. Mol. Recognit. 2013, 26, 627–632. [Google Scholar] [CrossRef]
- Nin, V.; Hernandez, J.A.; Chiffle, S. Hyperpolarization of the plasma membrane potential provokes reorganization of the actin cytoskeleton and increases the stability of adherens junctions in bovine corneal endothelial cells in culture. Cell Motil. Cytoskelet. 2009, 66, 1087–1099. [Google Scholar] [CrossRef]
- Kintzios, S.; Bem, F.; Mangana, O.; Nomikou, K.; Markoulatos, P.; Alexandropoulos, N.; Fasseas, C.; Arakelyan, V.; Petrou, A.-L.; Soukouli, K.; et al. Study on the mechanism of Bioelectric Recognition Assay: Evidence for immobilized cell membrane interactions with viral fragments. Biosens. Bioelectron. 2004, 20, 907–916. [Google Scholar] [CrossRef]
- Mavrikou, S.; Flampouri, E.; Moschopoulou, G.; Mangana, O.; Michaelides, A.; Kintzios, S. Assessment of organophosphate and carbamate pesticide residues in cigarette tobacco with a novel cell biosensor. Sensors 2008, 8, 2818–2832. [Google Scholar] [CrossRef]
- Flampouri, E.; Mavrikou, S.; Kintzios, S.; Miliaids, G.; Aplada-Sarli, P. Development and Validation of a Cellular Biosensor Detecting Pesticide Residues in Tomatoes. Talanta 2010, 80, 1799–1804. [Google Scholar] [CrossRef]
- Crowe, S.M.; Kintzios, S.; Kaltsas, G.; Palmer, C.S. A Bioelectronic System to Measure the Glycolytic Metabolism of Activated CD4+ T Cells. Biosensors 2019, 9, 10. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Papanastasiou, I.; Makri, O.; Lambrou, N.; Economou, G.; Soukouli, K.; Kintzios, S. Cellular redox-status is associated with regulation of frond division in Spirodela polyrrhiza. Plant Cell Rep. 2007, 26, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. Methods Mol. Biol. 2012, 810, 183–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, H.; Kuppusamy, P.; Zweier, J.L.; Trush, M.A. Mitochondrial Electron Transport Chain-Derived Superoxide Exits Macrophages: Implications for Mononuclear Cell-Mediated Pathophysiological Processes. React. Oxyg. Species 2016, 1, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Solomides, C.; Parekh, H.; Simpkins, F.; Simpkins, H. Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer Chemother. Pharmacol. 2015, 75, 1217–1227. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, H.; Huang, C.; Lei, Y. Cancer drug resistance: Redox resetting renders a way. Oncotarget 2016, 7, 42740–42761. [Google Scholar] [CrossRef]
- Luo, M.; Wicha, M.S. Targeting cancer stem cell redox metabolism to enhance therapy responses. Semin. Radiat. Oncol. 2019, 29, 42–54. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrikou, S.; Tsekouras, V.; Karageorgou, M.-A.; Moschopoulou, G.; Kintzios, S. Detection of Superoxide Alterations Induced by 5-Fluorouracil on HeLa Cells with a Cell-Based Biosensor. Biosensors 2019, 9, 126. https://doi.org/10.3390/bios9040126
Mavrikou S, Tsekouras V, Karageorgou M-A, Moschopoulou G, Kintzios S. Detection of Superoxide Alterations Induced by 5-Fluorouracil on HeLa Cells with a Cell-Based Biosensor. Biosensors. 2019; 9(4):126. https://doi.org/10.3390/bios9040126
Chicago/Turabian StyleMavrikou, Sophia, Vasileios Tsekouras, Maria-Argyro Karageorgou, Georgia Moschopoulou, and Spyridon Kintzios. 2019. "Detection of Superoxide Alterations Induced by 5-Fluorouracil on HeLa Cells with a Cell-Based Biosensor" Biosensors 9, no. 4: 126. https://doi.org/10.3390/bios9040126
APA StyleMavrikou, S., Tsekouras, V., Karageorgou, M.-A., Moschopoulou, G., & Kintzios, S. (2019). Detection of Superoxide Alterations Induced by 5-Fluorouracil on HeLa Cells with a Cell-Based Biosensor. Biosensors, 9(4), 126. https://doi.org/10.3390/bios9040126







