Ex Vivo Raman Spectrochemical Analysis Using a Handheld Probe Demonstrates High Predictive Capability of Brain Tumour Status
Abstract
1. Introduction
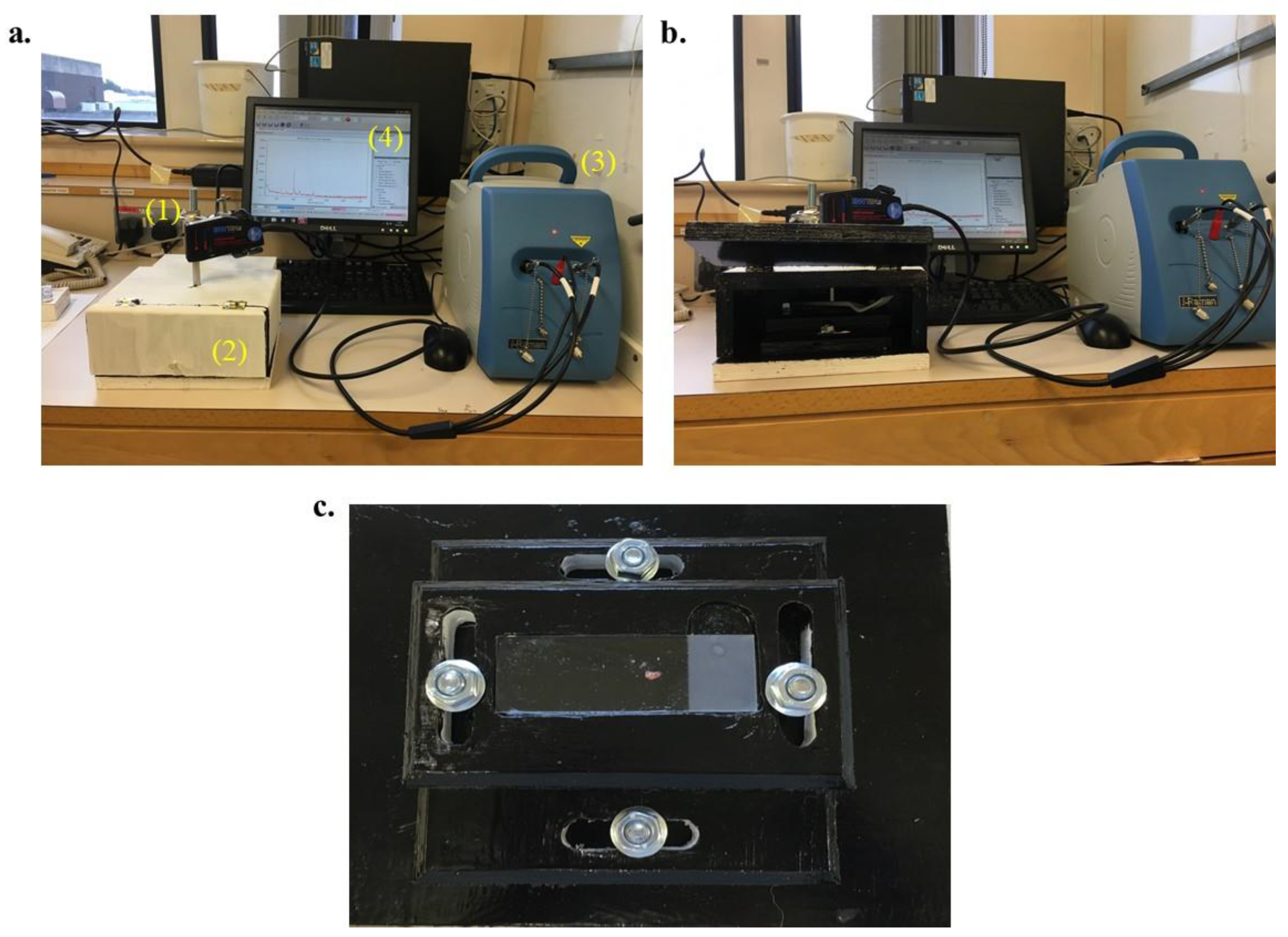
2. Materials and Methods
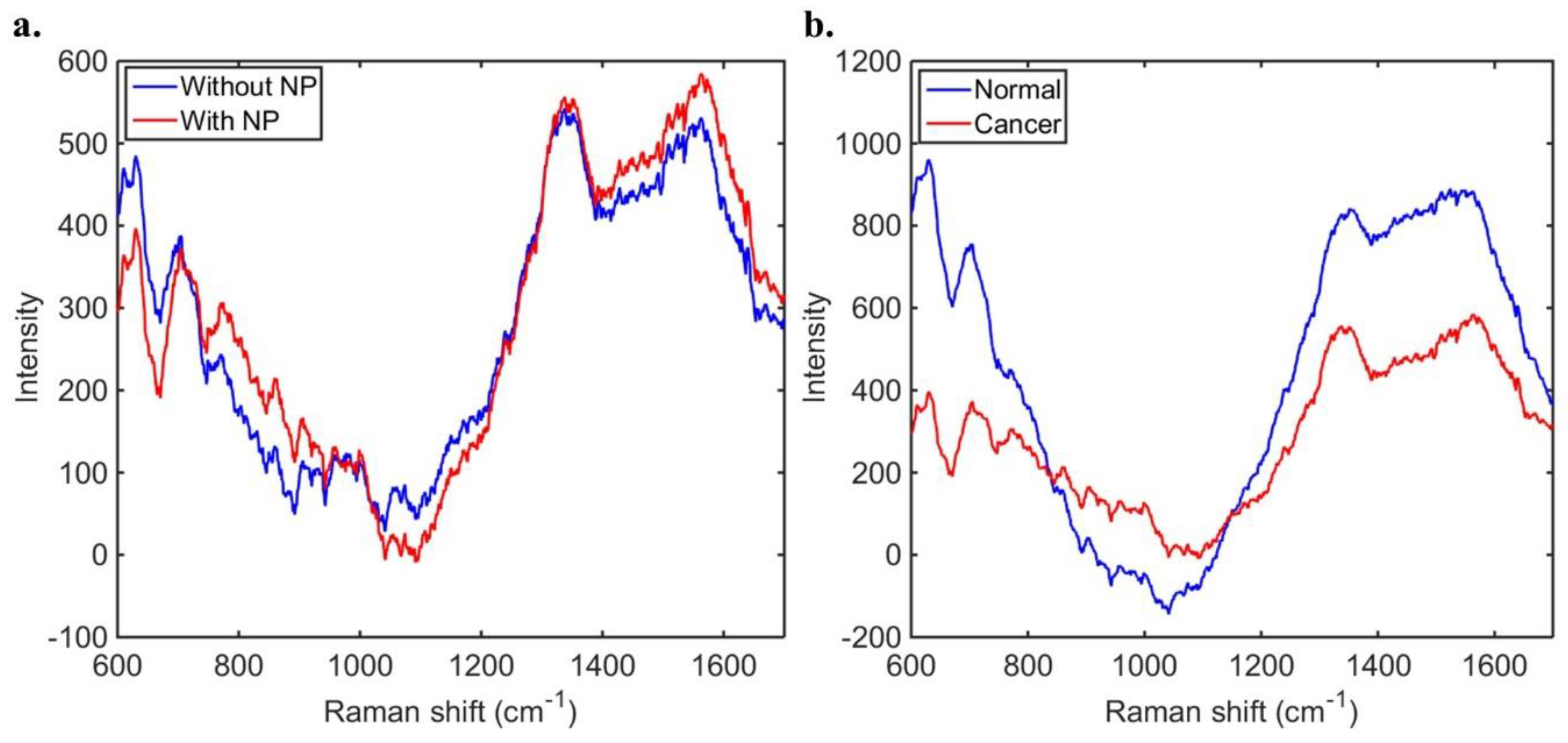
3. Results
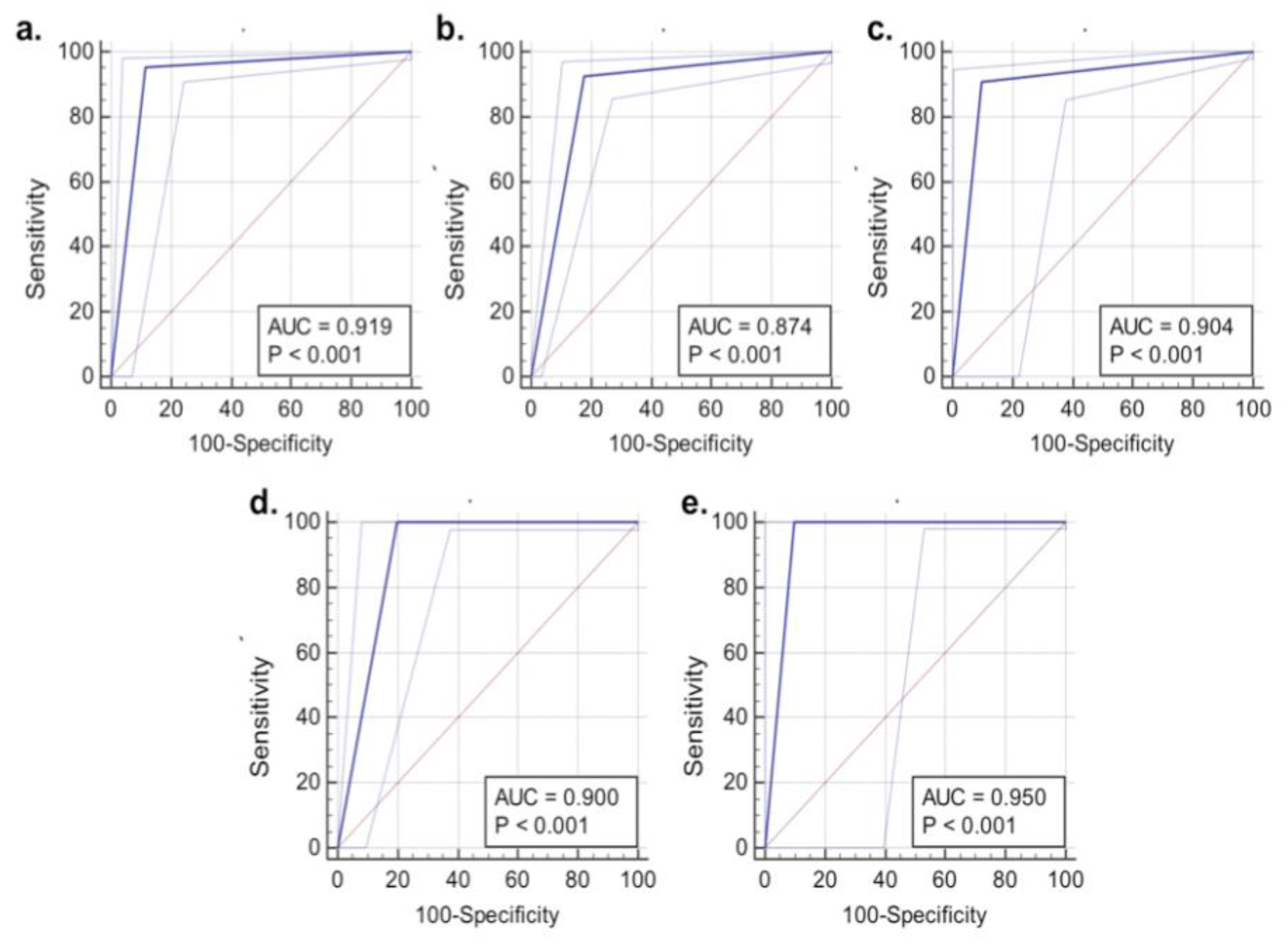
3.1. SERS Results Compared to Intraoperative Smear Preparation
3.2. SERS Results Compared to FFPE Tissue Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brain, Other CNS and Intracranial Tumours Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/brain-other-cns-and-intracranial-tumours#heading-Zero (accessed on 18 February 2018).
- Hollon, T.; Lewis, S.; Freudiger, C.W.; Xie, S.; Orringer, D.A. Improving the accuracy of brain tumour surgery via Raman-based technology. Neurosurg. Focus 2016, 40, E9–E25. [Google Scholar] [CrossRef]
- Broadbent, B.; Tseng, J.; Kast, R.; Noh, T.; Brusatori, M.; Kalkanis, S.N.; Auner, G.W. Shining light on neurosurgery diagnostics using Raman spectroscopy. J. Neurooncol. 2016, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galli, R.; Uckermann, O.; Temme, A.; Leipnitz, E.; Meinhardt, M.; Koch, E.; Schackert, G.; Steiner, G.; Kirsch, M. Assessing the efficiency of coherent anti-Stokes Raman scattering microscopy for the detection of infiltrating glioblastoma in fresh brain tissue. J. Biophotonics 2017, 10, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.L.; Gajjar, K.; Trevisan, J.; Fogarty, S.W.; Taylor, S.E.; Da Gama-Rose, B.; Martin-Hirsch, P.L.; Martin, F.L. Vibrational biospectroscopy coupled with multivariate analysis extracts potentially diagnostic features in blood plasma/serum of ovarian cancer patients. J. Biophotonics 2014, 7, 200–209. [Google Scholar] [CrossRef] [PubMed]
- UK Hospital to trial Raman Probe for Brain Tumours. Available online: www.optics.org/news/6/1/18 (accessed on 22 February 2018).
- O’Faolain, E.; Hunter, M.; Byrne, J.; Kellehan, P.; McNamara, M.; Byrne, H.; Lyng, F. A Study Examining the Effects of Tissue Processing on Human Tissue Sections using Vibrational Spectroscopy. Vib. Spectrosc. 2005, 38, 121–127. [Google Scholar] [CrossRef]
- Huang, Z.; McWilliams, A.; Lam, S.; English, J.; McLean, D.I.; Lui, H.; Zeng, H. Effect of formalin fixation on the near-infrared Raman Spectroscopy of normal and cancerous human bronchial tissues. Int. J. Oncol. 2003, 23, 649–655. [Google Scholar] [CrossRef]
- Butler, H.J.; Fogarty, S.W.; Kerns, J.G.; Martin-Hirsch, P.L.; Fullwood, N.J.; Martin, F.L. Gold nanoparticles as a substrate in bio-analytical near-infrared surface-enhanced Raman spectroscopy. Analyst 2015, 140, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Velicka, M.; Pucetaite, M.; Urboniene, V.; Ceponkus, J.; Jankevicius, F.; Sablinkskas, V. Detection of cancerous kidney tissue by means of SERS spectroscopy of extracellular fluid. J. Raman Spectrosc. 2017, 48, 1744–1754. [Google Scholar] [CrossRef]
- Bury, D.; Martin-Hirsch, P.L.; Martin, F.L.; Dawson, T.P. Are new technologies translatable to point-of-care testing? Lancet 2017, 390, 2765–2766. [Google Scholar] [CrossRef]
- Stables, R.; Clemens, G.; Butler, H.J.; Ashton, K.M.; Brodbelt, A.; Dawson, T.P.; Fullwood, L.M.; Jenkinson, M.D.; Baker, M.J. Feature driven classification of Raman spectra for real-time spectral brain tumour diagnosis using sound. Analyst 2016, 142, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Desroches, J.; Jerymn, M.; Mok, K.; Lemieux-Leduc, C.; Mercier, J.; St-Arnaud, K.; Urmey, K.; Guiot, M.-C.; Marple, E.; Petrecca, K.; et al. Characterization of a Raman spectroscopy probe system for intraoperative brain tissue classification. Biomed. Opt. Exp. 2015, 6, 2380–2397. [Google Scholar] [CrossRef] [PubMed]
- Desroches, J.; Jerymn, M.; Pinto, M.; Picot, F.; Tremblay, M.-A.; Obaid, S.; Urmey, K.; Trudel, D.; Soulez, G.; Guiot, M.-C.; et al. A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy. Sci. Rep. 2018, 8, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Mu, X.; Ma, A.; Guo, S. Raman tags: Novel optical probes for intracellular sensing and imaging. Biotechnol. Adv. 2017, 35, 168–177. [Google Scholar] [CrossRef]
- Horsnell, J.; Stonelake, P.; Christie-Brown, J.; Shetty, G.; Hutchings, J.; Kendall, C.; Stone, N. Raman spectroscopy—A new method for the intra-operative assessment of axillary lymph nodes. Analyst 2010, 135, 3042–3047. [Google Scholar] [CrossRef]
- Horsnell, J.D.; Smith, J.A.; Sattlecker, M.; Sammon, A.; Chrisite-Brown, J.; Kendall, C.; Stone, N. Raman spectroscopy—A potential new method for the intra-operative assessment of axillary lymph nodes. Surgeon 2012, 10, 123–127. [Google Scholar] [CrossRef]
- Haka, A.S.; Volynskaya, Z.; Gardecki, J.A.; Nezemi, J.; Shenk, R.; Wang, N.; Dasari, R.R.; Fitzmaurice, M.; Feld, M.S. Diagnosing breast cancer using Raman spectroscopy: prospective analysis. J. Biomed. Opt. 2009, 14, 054023. [Google Scholar] [CrossRef]
- Haka, A.S.; Volynskaya, Z.; Gardecki, J.A.; Nazemi, J.; Lyons, J.; Hicks, D.; Fitzmaurice, M.; Dasari, R.R.; Crowe, J.P.; Feld, M.S. In vivo Margin Assessment during Partial Mastectomy Breast Surgery Using Raman Spectroscopy. Cancer Res. 2006, 66, 3317–3322. [Google Scholar] [CrossRef]
- Ellison, D.; Love, S.; Chimelli, L.; Harding, B.N.; Lowe, J.S.; Vinters, H.V.; Brandner, S.; Yong, W.H. Neuropathology: A reference text of CNS Pathology, 3rd ed.; Elsevier Mosby: St. Louis, MO, USA, 2013. [Google Scholar]
- Cui, L.; Butler, H.J.; Martin-Hirsch, P.L.; Martin, F.L. Aluminium foil as a potential substrate for ATR-FTIR, transflection FTIR or Raman spectrochemical analysis of biological samples. Anal. Meth. 2016, 8, 481–487. [Google Scholar] [CrossRef]
- Trevisan, J.; Angelov, P.P.; Scott, A.D.; Carmichael, P.L.; Martin, F.L. IRootLab: A free and open-source MATLAB toolbox for vibrational biospectroscopy data analysis. Bioinformatics 2013, 29, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.D.; Morais, C.L.M.; Nascimento, Y.M.; Araujo, J.M.G.; Lima, K.M.G. Spectroscopy with computational analysis in virological studies: A decafe (2006–2016). Trends Anal. Chem. 2017, 97, 244–256. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological material. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, K.; Heppenstall, L.D.; Pang, W.; Ashton, K.M.; Trevisan, J.; Patel, I.I.; Llabjani, V.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Dawson, T.; et al. Diagnostic segregation of human brain tumours using Fourier-transform infrared and/or Raman spectroscopy coupled with discriminant analysis. Anal. Meth. 2012, 5, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.I.; Trevisan, J.; Singh, P.B.; Nicholson, C.M.; Gopala Krishnan, R.K.; Matanhelia, S.S.; Martin, F.L. Segregation of human prostate tissues classified high-risk (UK) versus low risk (India) for adenocarcinoma using Fourier-transform infrared or Raman microspectroscopy coupled with discriminant analysis. Anal. Bioanal. Chem. 2011, 401, 969–982. [Google Scholar] [CrossRef]
- Bury, D.; Morais, C.L.M.; Paraskevaidi, M.; Ashton, K.M.; Dawson, T.P.; Martin, F.L. Spectral classification for diagnosis involving numerous pathologies in a complex clinical setting: A neuro-oncology example. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, K.; Aparna, B.; Anuradha, K.; Brijesh, T.; Sanjay, K.; Neetika, S. Intraoperative Squash Cytology of Central Nervous System and Spinal Cord Lesions with Histological Correlation. Arch. Pathol Lab. Med. 2016, 3, 61–72. [Google Scholar]
- Carmustine Implants and Temozolomide for the Treatment of Newly Diagnosed High-Grade Glioma. Technology appraisal guidance. Available online: https://www.nice.org.uk/Guidance/TA121 (accessed on 21 February 2018).
- Krafft, C.; Shapoval, L.; Sobottka, S.B.; Geiger, K.D.; Schackert, G.; Salzer, R. Identification of primary brain metastasis by SIMCA classification of IR spectroscopic images. Biochim. Biophys. Acta 2006, 1758, 883–891. [Google Scholar] [CrossRef]

| Case Number | Smear Result | Paraffin Result |
|---|---|---|
| 1 | Low-grade glioma | Glioblastoma |
| 2 | Meningioma | Meningioma |
| 3 | Metastasis | Ovarian serous carcinoma |
| 4 | High-grade glioma | Glioblastoma |
| 5 | High-grade glioma | Glioblastoma |
| 6 | Meningioma | Meningioma |
| 7 | Metastasis | Adenocarcinoma |
| 8 | High-grade glioma | Glioblastoma |
| 9 | High-grade glioma | Glioblastoma |
| 10 | Metastasis | Renal cell carcinoma |
| 11 | Metastasis | Lung adenocarcinoma |
| 12 | no tumour | Glioblastoma |
| 13 | Low-grade glioma | Astrocytoma Grade 2 |
| 14 | Inflammation | Astrocytoma Grade 2 |
| 15 | Inflammation | Astrocytoma Grade 2 |
| 16 | Metastasis | Ovarian serous carcinoma |
| 17 | High-grade glioma | Glioblastoma |
| 18 | High-grade glioma | Glioblastoma |
| 19 | High-grade glioma | Glioblastoma |
| 20 | High-grade glioma | Glioblastoma |
| 21 | High-grade glioma | Glioblastoma |
| 22 | reactive Low-grade glioma | Low grade glioma |
| 23 | Intermediate-grade glioma | Glioblastoma |
| 24 | Low-grade glioma | Astrocytoma Grade 3 |
| 25 | Lymphoma | High grade B cell lymphoma |
| 26 | Glioma | Astrocytoma Grade 2 |
| 27 | No definite tumour | Astrocytoma Grade 2 |
| 28 | Low- to intermediate-grade glioma | Astrocytoma Grade 2 |
| 29 | High-grade glioma | Glioblastoma |
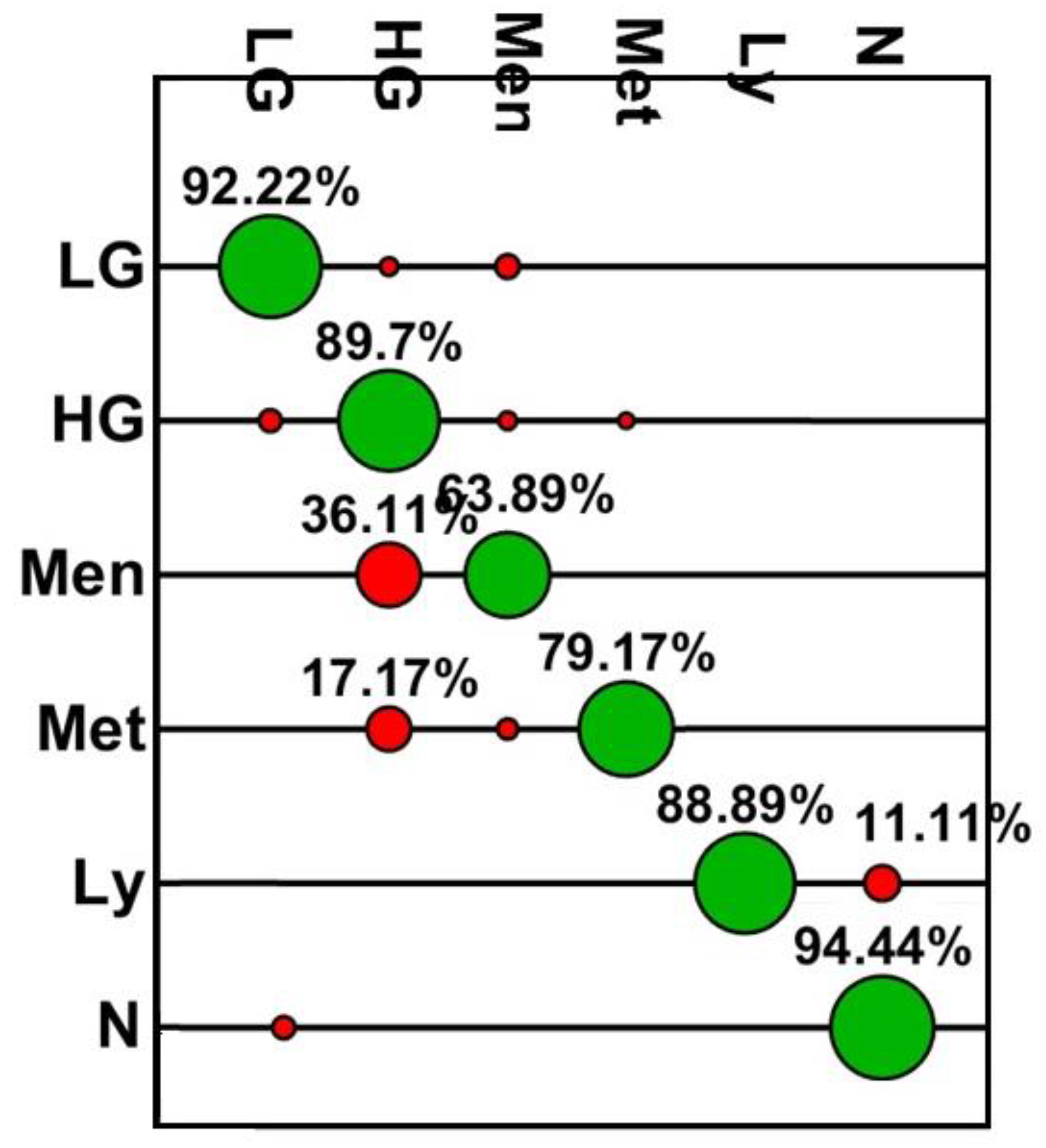
| Class | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| N | 98.6 | 94.4 | 99.5 | 97.7 | 98.8 |
| LG | 96.1 | 92.2 | 97.0 | 88.7 | 98.0 |
| HG | 90.3 | 89.7 | 90.6 | 83.5 | 94.4 |
| Men | 94.8 | 63.9 | 97.1 | 62.1 | 97.3 |
| Met | 95.4 | 79.2 | 98.8 | 93.3 | 95.8 |
| Lv | 99.6 | 88.9 | 100 | 100 | 99.6 |
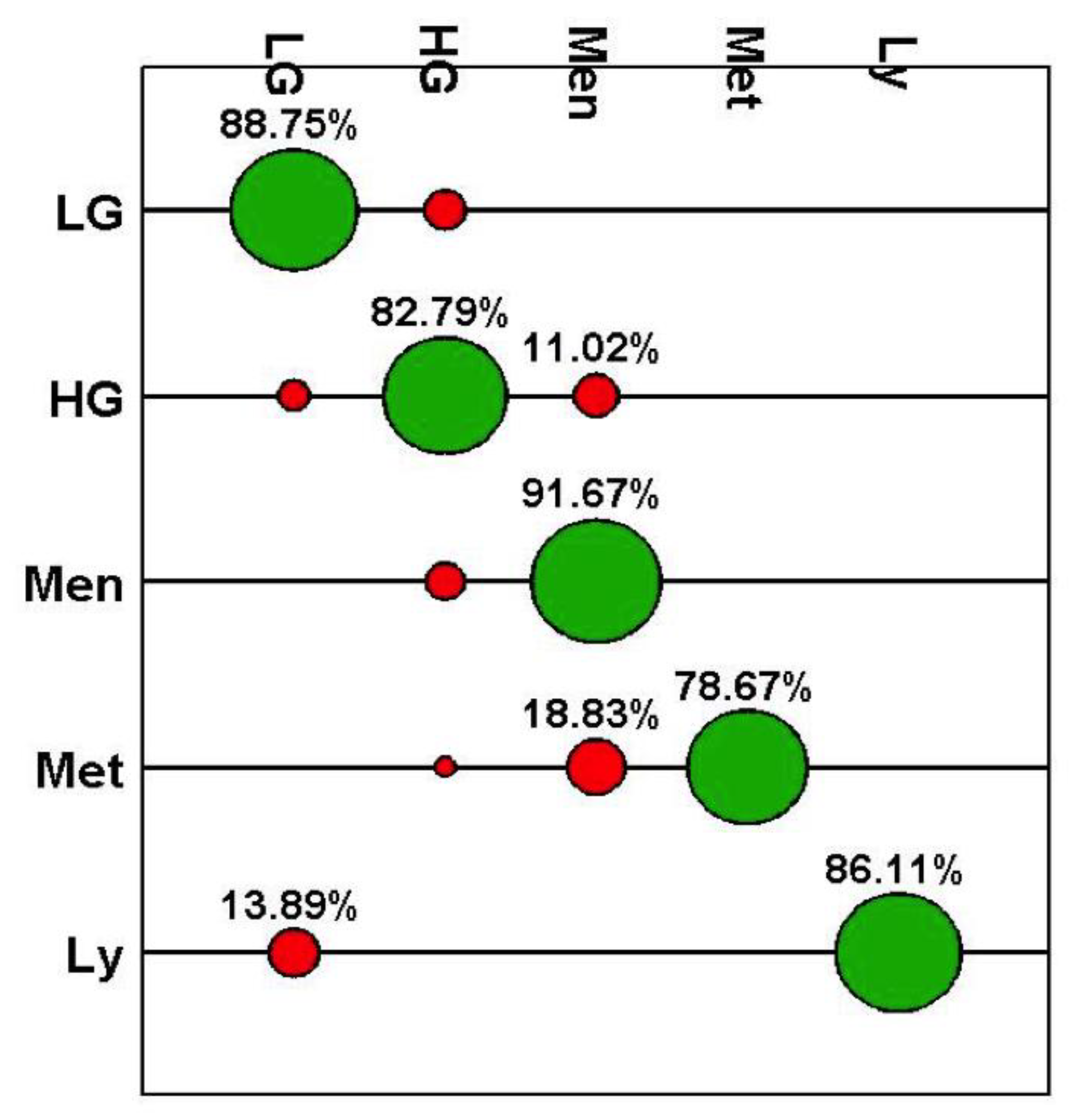
| Class | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| LG | 93.8 | 88.7 | 95.4 | 85.8 | 96.4 |
| HG | 88.0 | 82.8 | 92.8 | 91.6 | 85.1 |
| Men | 90.8 | 91.7 | 90.8 | 42.4 | 99.3 |
| Met | 96.3 | 78.7 | 100 | 100 | 95.7 |
| Lv | 99.5 | 86.1 | 100 | 100 | 99.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bury, D.; Morais, C.L.M.; Ashton, K.M.; Dawson, T.P.; Martin, F.L. Ex Vivo Raman Spectrochemical Analysis Using a Handheld Probe Demonstrates High Predictive Capability of Brain Tumour Status. Biosensors 2019, 9, 49. https://doi.org/10.3390/bios9020049
Bury D, Morais CLM, Ashton KM, Dawson TP, Martin FL. Ex Vivo Raman Spectrochemical Analysis Using a Handheld Probe Demonstrates High Predictive Capability of Brain Tumour Status. Biosensors. 2019; 9(2):49. https://doi.org/10.3390/bios9020049
Chicago/Turabian StyleBury, Danielle, Camilo L. M. Morais, Katherine M. Ashton, Timothy P. Dawson, and Francis L. Martin. 2019. "Ex Vivo Raman Spectrochemical Analysis Using a Handheld Probe Demonstrates High Predictive Capability of Brain Tumour Status" Biosensors 9, no. 2: 49. https://doi.org/10.3390/bios9020049
APA StyleBury, D., Morais, C. L. M., Ashton, K. M., Dawson, T. P., & Martin, F. L. (2019). Ex Vivo Raman Spectrochemical Analysis Using a Handheld Probe Demonstrates High Predictive Capability of Brain Tumour Status. Biosensors, 9(2), 49. https://doi.org/10.3390/bios9020049




