Nanoparticle-Based Biosensing of Tuberculosis, an Affordable and Practical Alternative to Current Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Clinical Samples
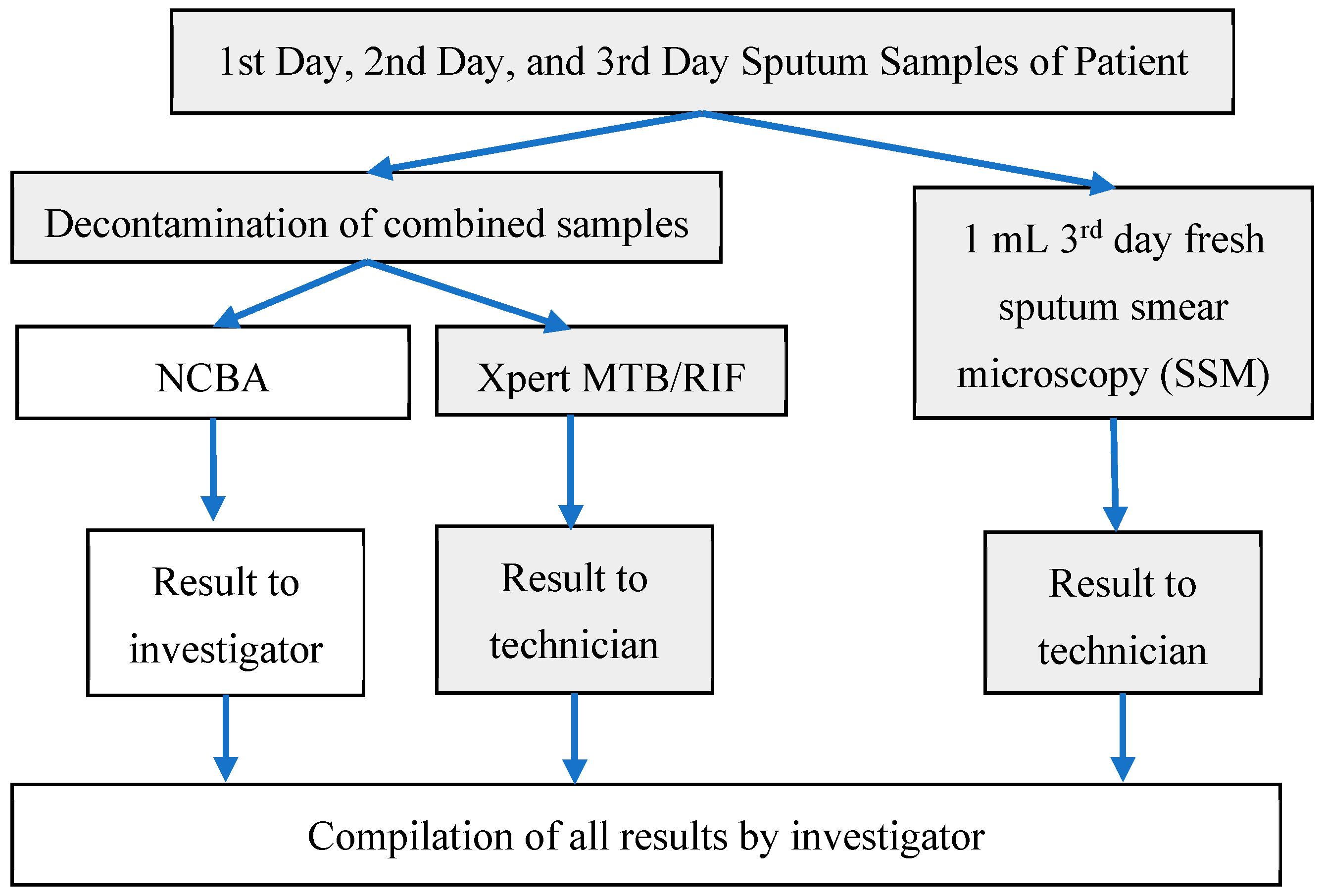
2.4. Sputum Sample Processing
- Sputum Smear Microscopy (SSM): A smear was prepared by the Ziehl–Neelsen (ZN) method of the third (fresh) sputum sample following the standard protocol by the International Union Against Tuberculosis and Lung Disease [19] within 2 h after receipt at the hospital. Briefly, about 20 µL of fresh sputum sample was placed on a glass slide and heat fixed by a passing flame from a Bunsen burner. The slide was placed on a staining rack and 0.3% carbol fuchsin was poured over the smear. The underside of the slide was gently heated by passing a flame under the rack until fumes appeared. After cooling for about 5 min, the smear was rinsed with distilled water until no color appeared in the effluent. The smear was washed with 25% sulphuric acid several times until the smear appeared light pink in color. The smear was washed with distilled water and then the counter stain (0.3% methylene blue) was added to cover the smear. Distilled water was used to wash off the counter stain and then the smear was air-dried. Once ready, the smear was examined under a light microscope using 100× oil immersion objective to observe the presence of red-colored AFB.
- Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA): Decontamination reagent from the kit was added to the combined sputum sample in a 2:1 (reagent to sample) ratio in a 15-mL falcon tube, which was then manually agitated twice (or quick vortex) during a 15-min incubation period at room temperature. Subsequently, 2 mL of the decontaminated sputum sample were transferred to the test cartridge using a sterile disposable pipette (provided with the kit). The cartridge was loaded into the Xpert MTB/RIF PCR machine and operated for 1 hour and 50 min. At the end of the real-time PCR run, the result was generated [20].
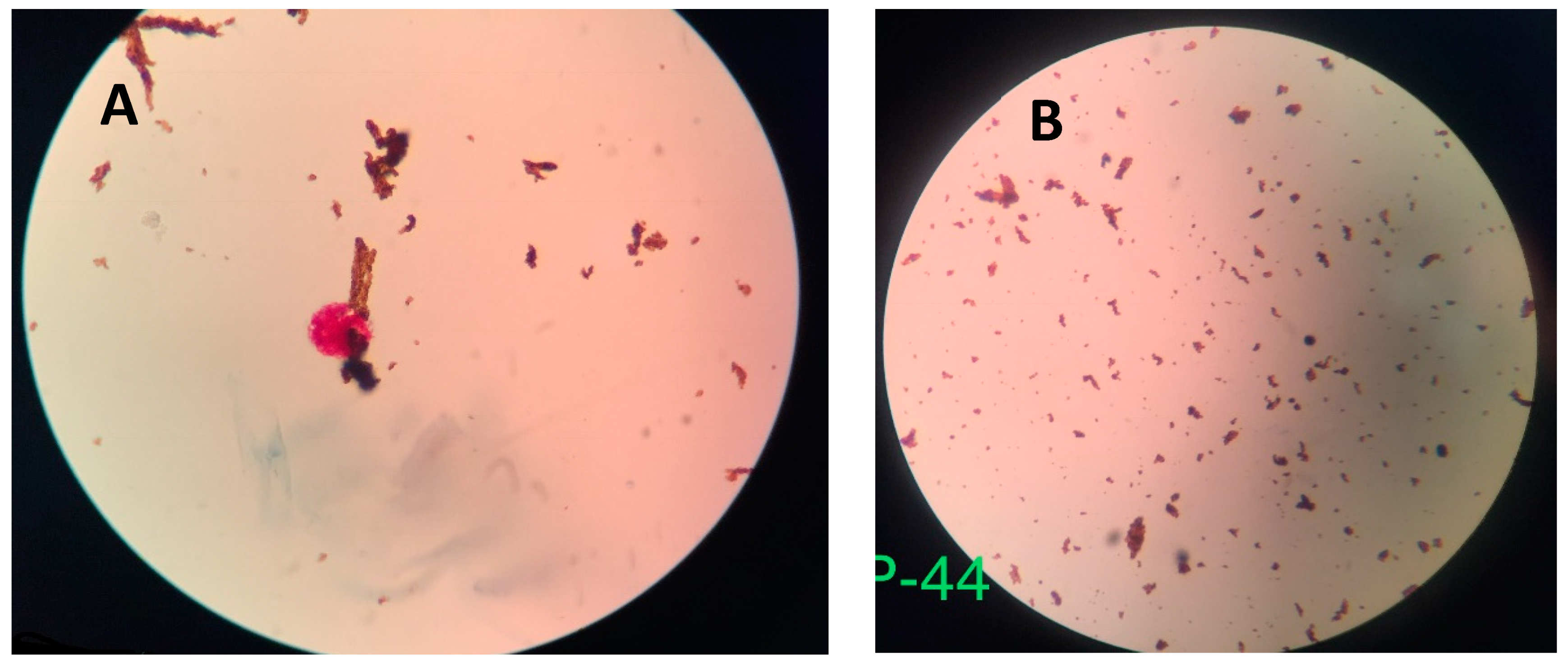
- Nanoparticle-based Colorimetric Biosensing Assay (NCBA): About 1 mL of the decontaminated sputum sample was added into a 1.5 mL tube containing 100 µL of the nanoparticles. The GMNP and sputum were mixed and allowed to incubate for 5 min at room temperature. The tube was then placed in a magnetic rack to separate the magnetic GMNP-AFB complex and the supernatant was discarded. A smear was prepared by adding 20 µL of the concentrated GMNP-AFB complex on a glass slide following the SSM procedure, as described in Section 1 above.
2.5. Statistical Analysis
3. Results
4. Discussion
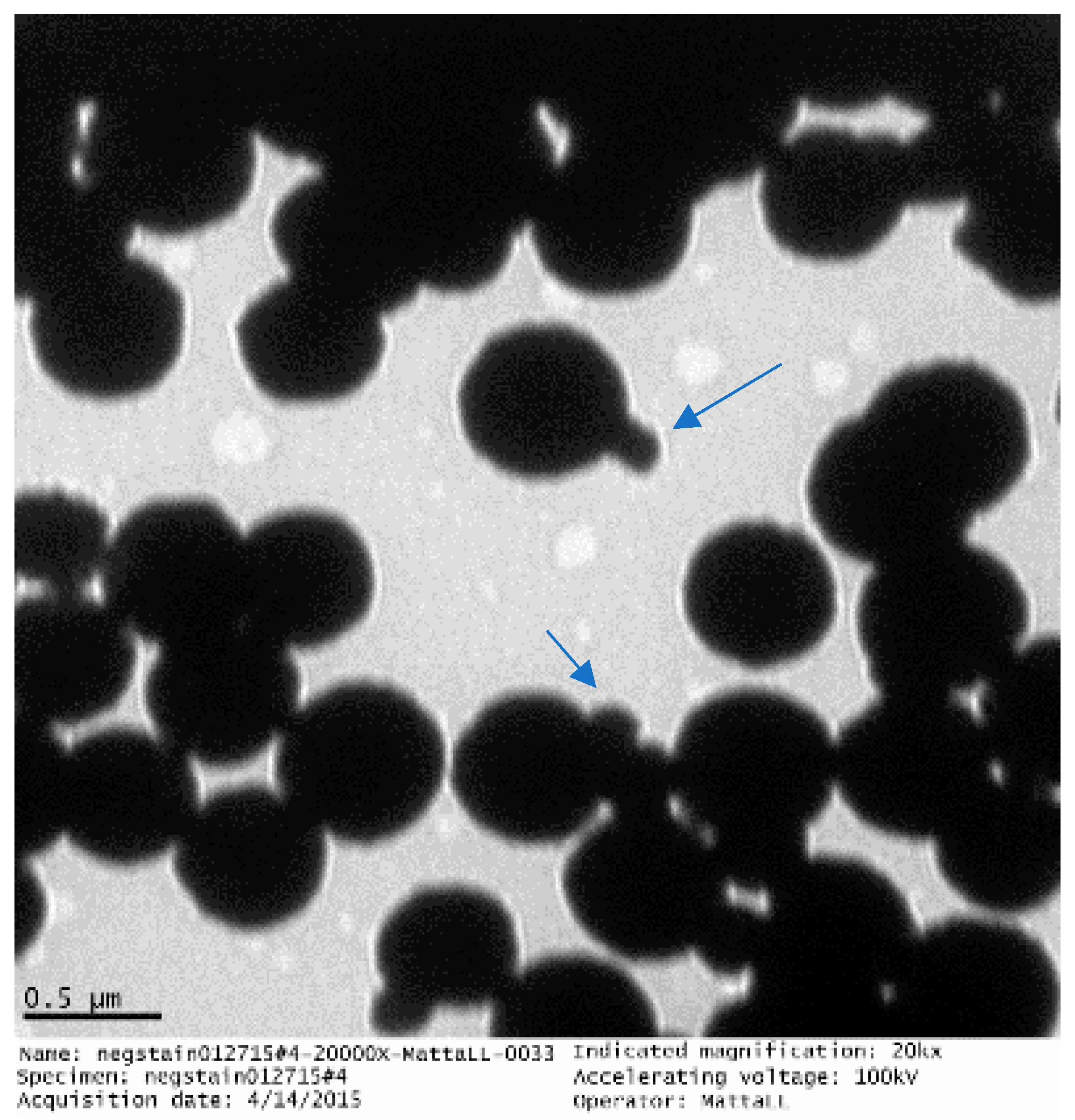
4.1. GMNP-AFB Complex
4.2. Novel Colorimetric Biosensing Mechanism
4.3. SSM vs. Xpert MTB/RIF
4.4. SSM vs. NCBA
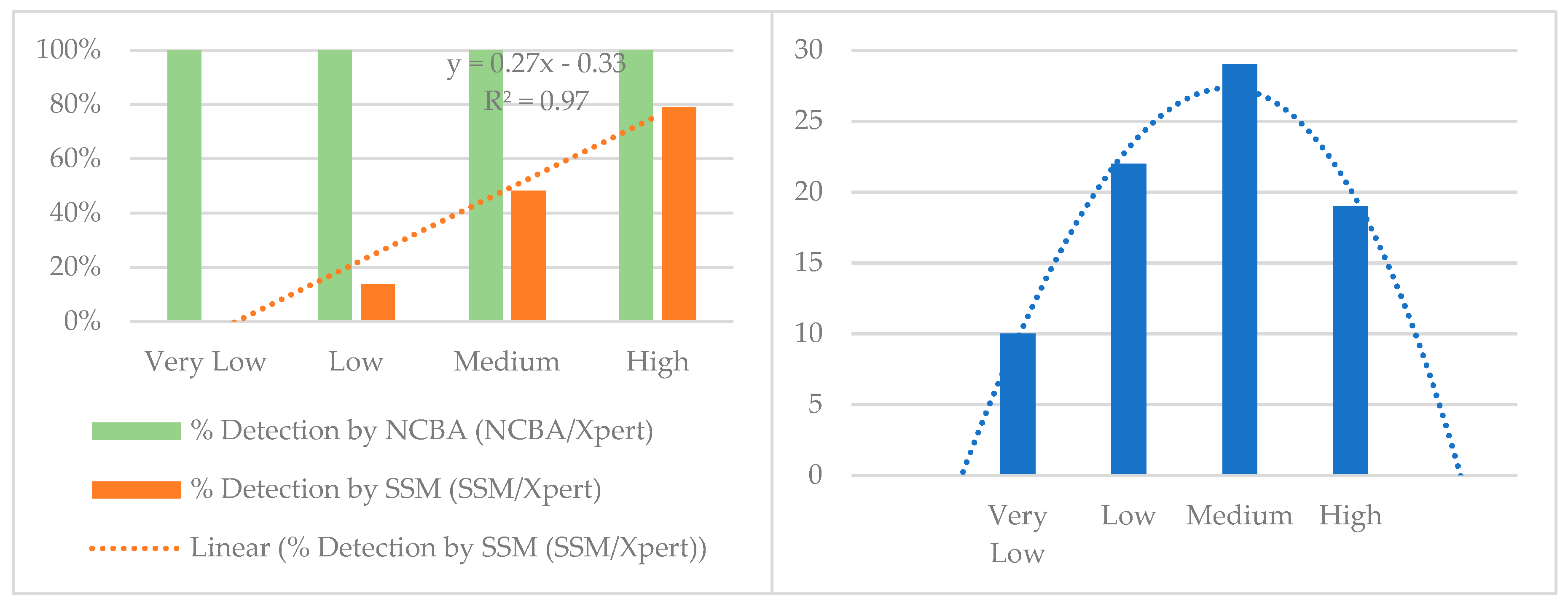
4.5. NCBA vs. Xpert MTB/RIF
5. Conclusions
6. Ethics Approval
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Tuberculosis (TB). Available online: http://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 9 May 2018).
- WHO. Global Tuberculosis Report 2017. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 10 May 2018).
- Google Docs. Annual_Report_2073-74.pdf. Available online: https://drive.google.com/file/d/125sRiD-5_2yBB67oNCZWAuOzk5wzU5_r/view?usp=drive_web&usp=embed_facebook (accessed on 10 May 2018).
- Riley, R.L.; Wells, W.F.; Mills, C.C.; Nyka, W.; Mclean, R.L. Air hygiene in tuberculosis: Quantitative studies of infectivity and control in a pilot ward. Am. Rev. Tuberc. 1957, 75, 420–431. [Google Scholar] [PubMed]
- Hobby, G.L.; Holman, A.P.; Iseman, M.D.; Jones, J.M. Enumeration of Tubercle Bacilli in Sputum of Patients with Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 1973, 4, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opota, O.; Senn, L.; Prod’hom, G.; Mazza-Stalder, J.; Tissot, F.; Greub, G.; Jaton, K. Added value of molecular assay Xpert MTB/RIF compared to sputum smear microscopy to assess the risk of tuberculosis transmission in a low-prevalence country. Clin. Microbiol. Infect. 2016, 22, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, G. The infectiousness of human tuberculosis; an epidemiological investigation. Acta Tuberc. Scand. Suppl. 1957, 38, 1–146. [Google Scholar] [PubMed]
- Yeager, H.; Lacy, J.; Smith, L.R.; LeMaistre, C.A. Quantitative studies of mycobacterial populations in sputum and saliva. Am. Rev. Respir. Dis. 1967, 95, 998–1004. [Google Scholar] [PubMed]
- Desikan, P. Sputum smear microscopy in tuberculosis: Is it still relevant? Indian J. Med. Res. 2013, 137, 442–444. [Google Scholar] [PubMed]
- Steingart, K.R.; Ng, V.; Henry, M.; Hopewell, P.C.; Ramsay, A.; Cunningham, J.; Urbanczik, R.; Perkins, M.D.; Aziz, M.A.; Pai, M. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A systematic review. Lancet Infect. Dis. 2006, 6, 664–674. [Google Scholar] [CrossRef]
- Lessem, E. The Tuberculosis Diagnostics Pipeline. Pipeline Report. Available online: http://www.pipelinereport.org/2017/tbdx (accessed on 19 May 2018).
- Singhal, R.; Myneedu, V.P. Microscopy as a diagnostic tool in pulmonary tuberculosis. Int. J. Mycobacteriol. 2015, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.J. Diagnosis of Pulmonary Tuberculosis: Recent Advances and Diagnostic Algorithms. Tuberc. Respir. Dis. 2015, 78, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Cudahy, P.; Shenoi, S. Diagnostics for pulmonary tuberculosis. Postgrad. Med. J. 2016, 92, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.J.; Starke, J.R.; Revell, P.A. Laboratory Diagnosis of Mycobacterium tuberculosis Infection and Disease in Children. J. Clin. Microbiol. 2016, 54, 1434–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyendak, M.R.; Lewinsohn, D.A.; Lewinsohn, D.M. New Diagnostic Methods for Tuberculosis. Curr. Opin. Infect. Dis. 2009, 22, 174–182. [Google Scholar] [CrossRef] [PubMed]
- García-Basteiro, A.L.; DiNardo, A.; Saavedra, B.; Silva, D.R.; Palmero, D.; Gegia, M.; Migliori, G.B.; Duarte, R.; Mambuque, E.; Centis, R.; et al. Point of care diagnostics for tuberculosis. Pulmonology 2018, 24, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- International Union Against Tuberculosis and Lung Disease (IUATLD). Activity Report, 1 July 2002–31 December 2003. Available online: https://www.theunion.org/official-documents/ar2003_en.pdf (accessed on 22 December 2018).
- Meawed, T.E.; Shaker, A. Assessment of diagnostic accuracy of Gene Xpert MTB/RIF in diagnosis of suspected retreatment pulmonary tuberculosis patients. Egypt. J. Chest Dis. Tuberc. 2016, 65, 637–641. [Google Scholar] [CrossRef]
- Varki, A.; Etzler, M.E.; Cummings, R.D.; Esko, J.D. Discovery and Classification of Glycan-Binding Proteins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Broeker, N.K.; Andres, D.; Kang, Y.; Gohlke, U.; Schmidt, A.; Kunstmann, S.; Santer, M.; Barbirza, S. Complex carbohydrate recognition by proteins: Fundamental insights from bacteriophage cell adhesion systems. Perspect. Sci. 2017, 11, 45–52. [Google Scholar] [CrossRef]
- Park, S.; Kim, G.-H.; Park, S.-H.; Pai, J.; Rathwell, D.; Park, J.-Y.; Kang, Y.-S.; Shin, I. Probing Cell-Surface Carbohydrate Binding Proteins with Dual-Modal Glycan-Conjugated Nanoparticles. J. Am. Chem. Soc. 2015, 137, 5961–5968. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Kremer, L. Acid-Fast Positive and Acid-Fast Negative Mycobacterium tuberculosis: The Koch Paradox. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Laserson, K.F.; Yen, N.T.N.; Thornton, C.G.; Mai, V.T.C.; Jones, W.; An, D.Q.; Phuoc, N.H.; Trinh, N.A.; Nhung, D.T.C.; Lien, T.X.; et al. Improved Sensitivity of Sputum Smear Microscopy after Processing Specimens with C18-Carboxypropylbetaine To Detect Acid-Fast Bacilli: A Study of United States-Bound Immigrants from Vietnam. J. Clin. Microbiol. 2005, 43, 3460–3462. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R.; Story, E.; Helb, D.; Kop, J.; Banada, P.; Owens, M.R.; Chakravorty, S.; Jones, M.; Alland, D. Evaluation of the Analytical Performance of the Xpert MTB/RIF Assay. J. Clin. Microbiol. 2010, 48, 2495–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
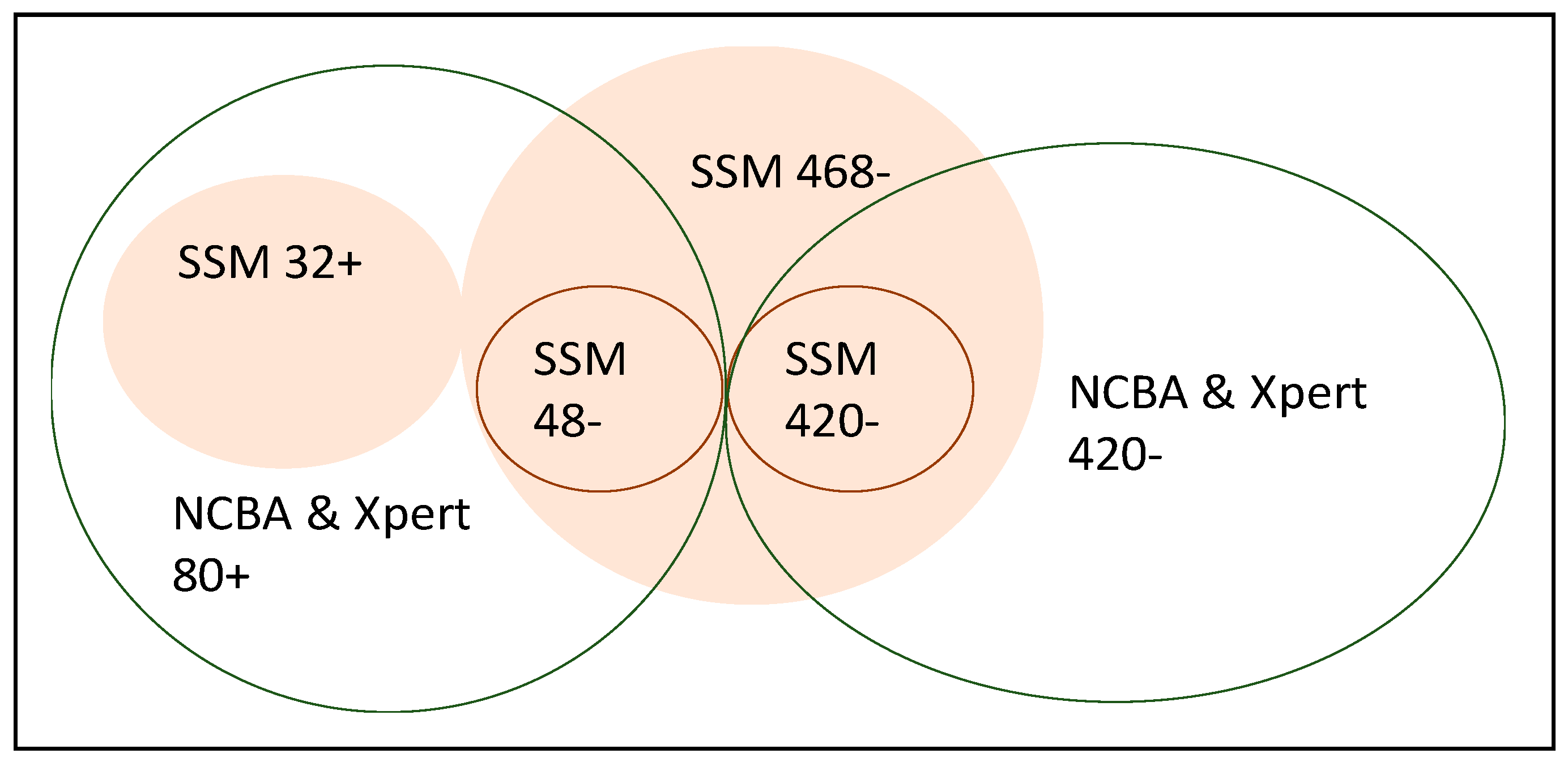
| SSM Test | True TB Cases | Non-TB Cases | NCBA Test | True TB Cases | Non-TB Cases |
|---|---|---|---|---|---|
| Positive test | 32 | 0 | Positive test | 80 | 0 |
| Negative test | 48 | 420 | Negative test | 0 | 420 |
| Technique | Xpert MTB/RIF as the Gold Standard, % (95% CI) | ||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| SSM Test | 40 (29–52) | 100 (99–100) | 100 | 90 (88–91) | 90 (87–93) |
| NCBA Test | 100 (95–100) | 100 (99–100) | 100 | 100 | 100 (99–100) |
| Xpert MTB/RIF Categories | Very Low | Low | Medium | High | Total |
|---|---|---|---|---|---|
| Xpert MTB/RIF | 10 | 22 | 29 | 19 | 80 |
| NCBA | 10 | 22 | 29 | 19 | 80 |
| SSM | 0 | 3 | 14 | 15 | 32 |
| % Detection (NCBA/Xpert) | 100% | 100% | 100% | 100% | |
| % Detection (SSM/Xpert) | 0% | 14% | 48% | 79% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhusal, N.; Shrestha, S.; Pote, N.; Alocilja, E.C. Nanoparticle-Based Biosensing of Tuberculosis, an Affordable and Practical Alternative to Current Methods. Biosensors 2019, 9, 1. https://doi.org/10.3390/bios9010001
Bhusal N, Shrestha S, Pote N, Alocilja EC. Nanoparticle-Based Biosensing of Tuberculosis, an Affordable and Practical Alternative to Current Methods. Biosensors. 2019; 9(1):1. https://doi.org/10.3390/bios9010001
Chicago/Turabian StyleBhusal, Nirajan, Sunaina Shrestha, Nisha Pote, and Evangelyn C. Alocilja. 2019. "Nanoparticle-Based Biosensing of Tuberculosis, an Affordable and Practical Alternative to Current Methods" Biosensors 9, no. 1: 1. https://doi.org/10.3390/bios9010001
APA StyleBhusal, N., Shrestha, S., Pote, N., & Alocilja, E. C. (2019). Nanoparticle-Based Biosensing of Tuberculosis, an Affordable and Practical Alternative to Current Methods. Biosensors, 9(1), 1. https://doi.org/10.3390/bios9010001






