Use of Time-Resolved Fluorescence to Monitor Bioactive Compounds in Plant Based Foodstuffs
Abstract
:1. Introduction
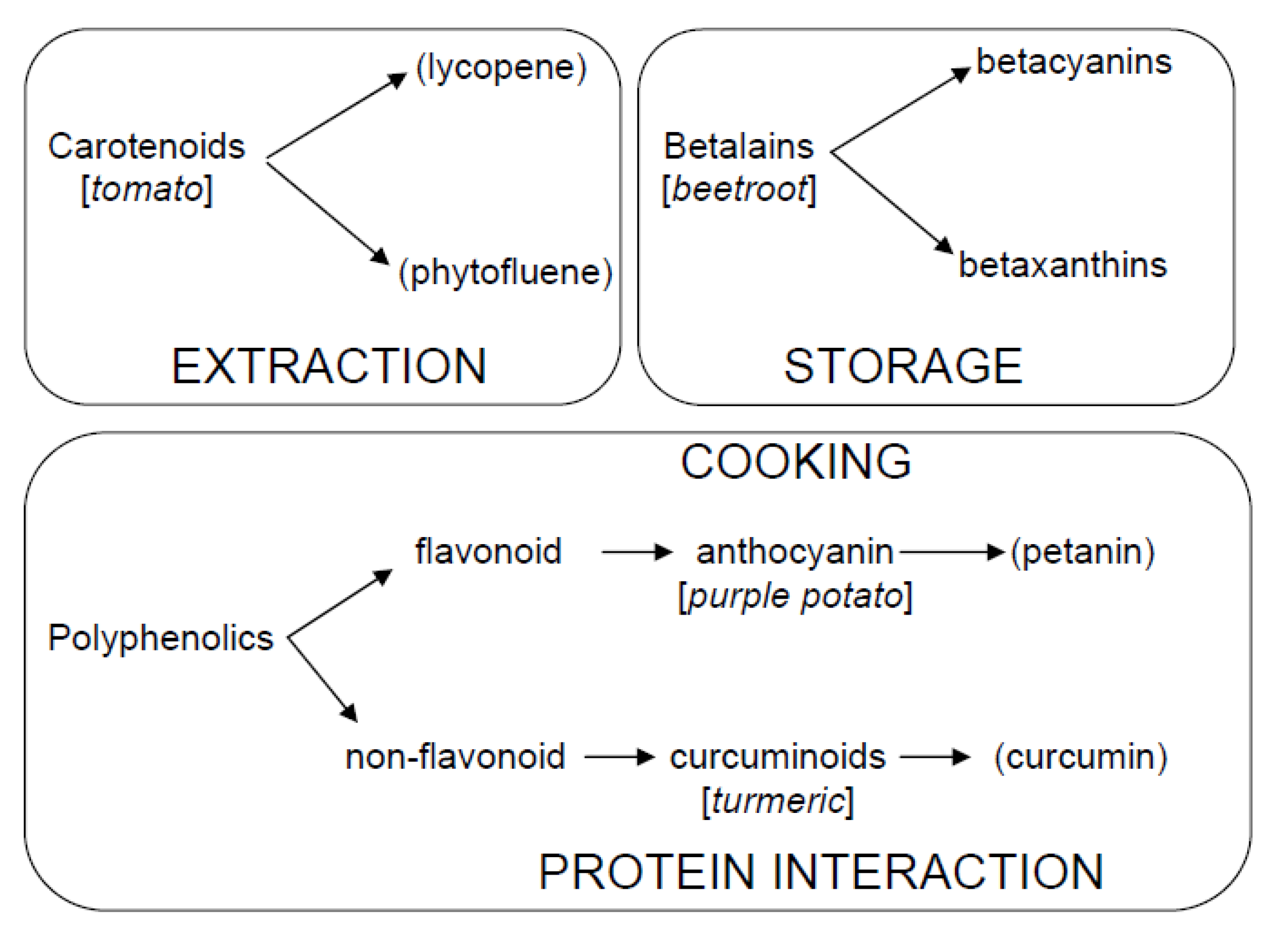
1.1. Interest in Studying Bioactive Compounds
1.2. Application of Time-Resolved Fluorescence
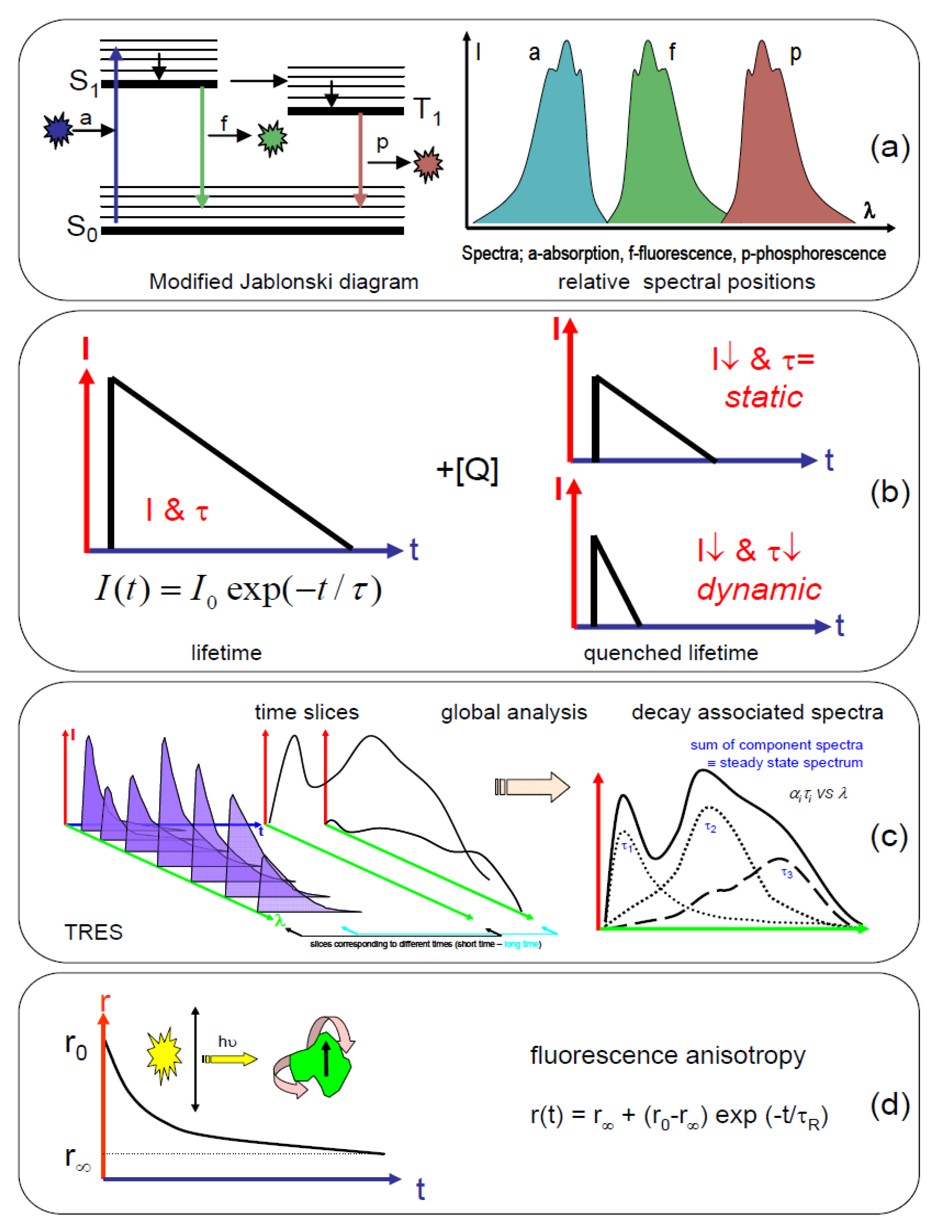
- changes in the nanoenvironment
- ○
- (viscosity, pH, polarity, salvation)
- size and shape of molecules
- molecular interactions
- inter- and intramolecular distances
- kinetic and dynamic rates
- resolution of molecular mixtures
1.3. Scope of This Work
2. Experimental Section
2.1. Steady State Measurements
2.2. Time-Resolved Measurements
2.3. Time-Resolved Fluorescence Microscopy
2.4. Determination of Bioactive Components and Antioxidant Activity
3. Results and Discussion
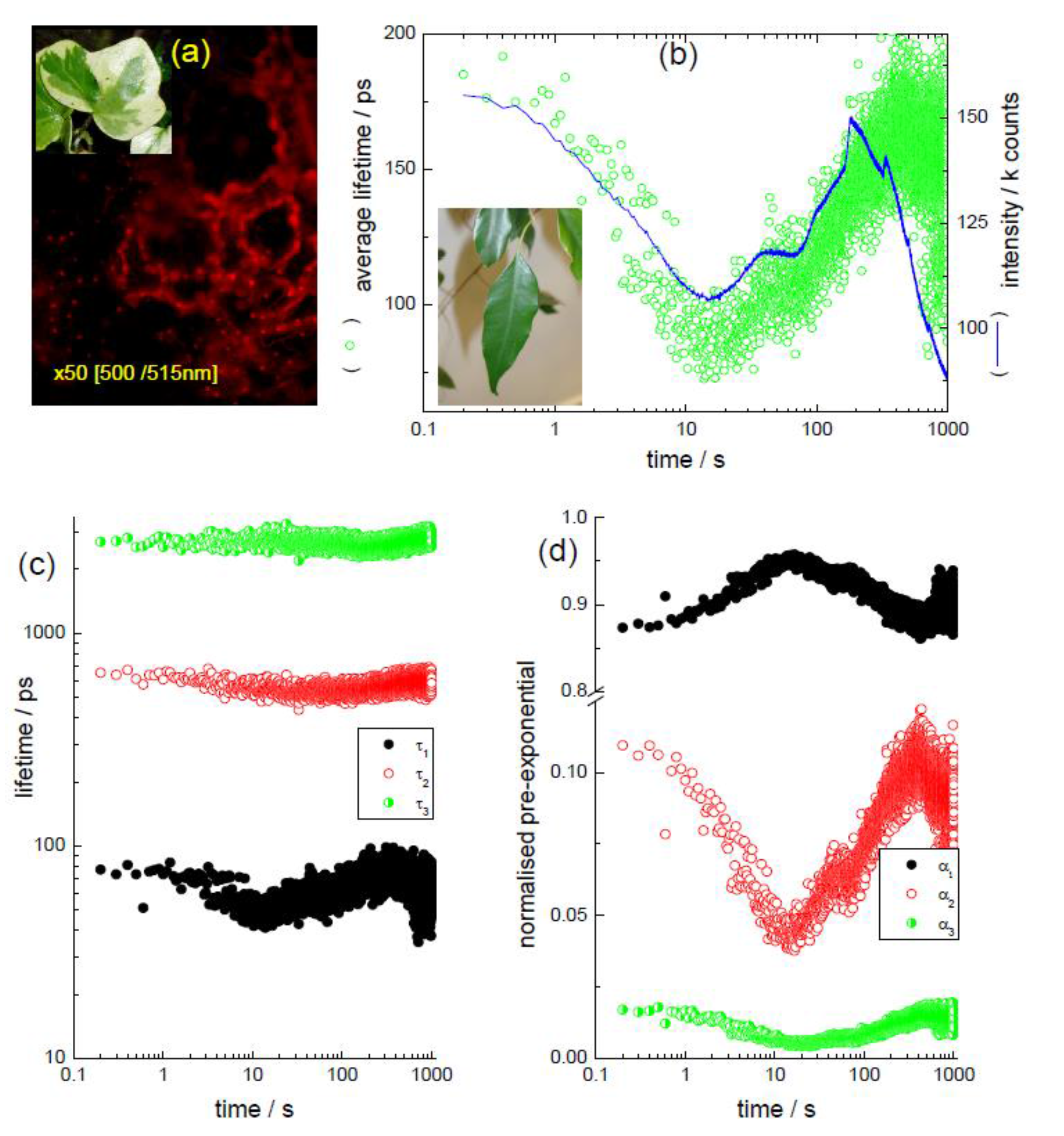
3.1. In the “Leaf” (Kinetic TCSPC and TRES to Monitor Chlorophyll)
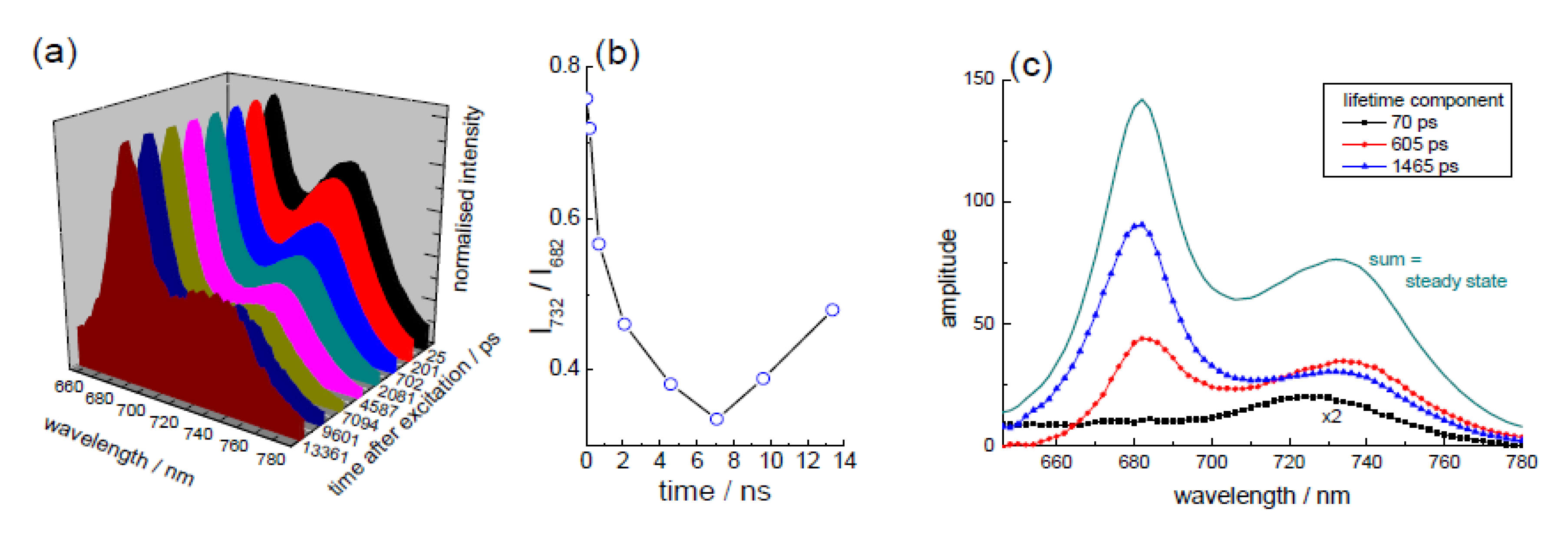
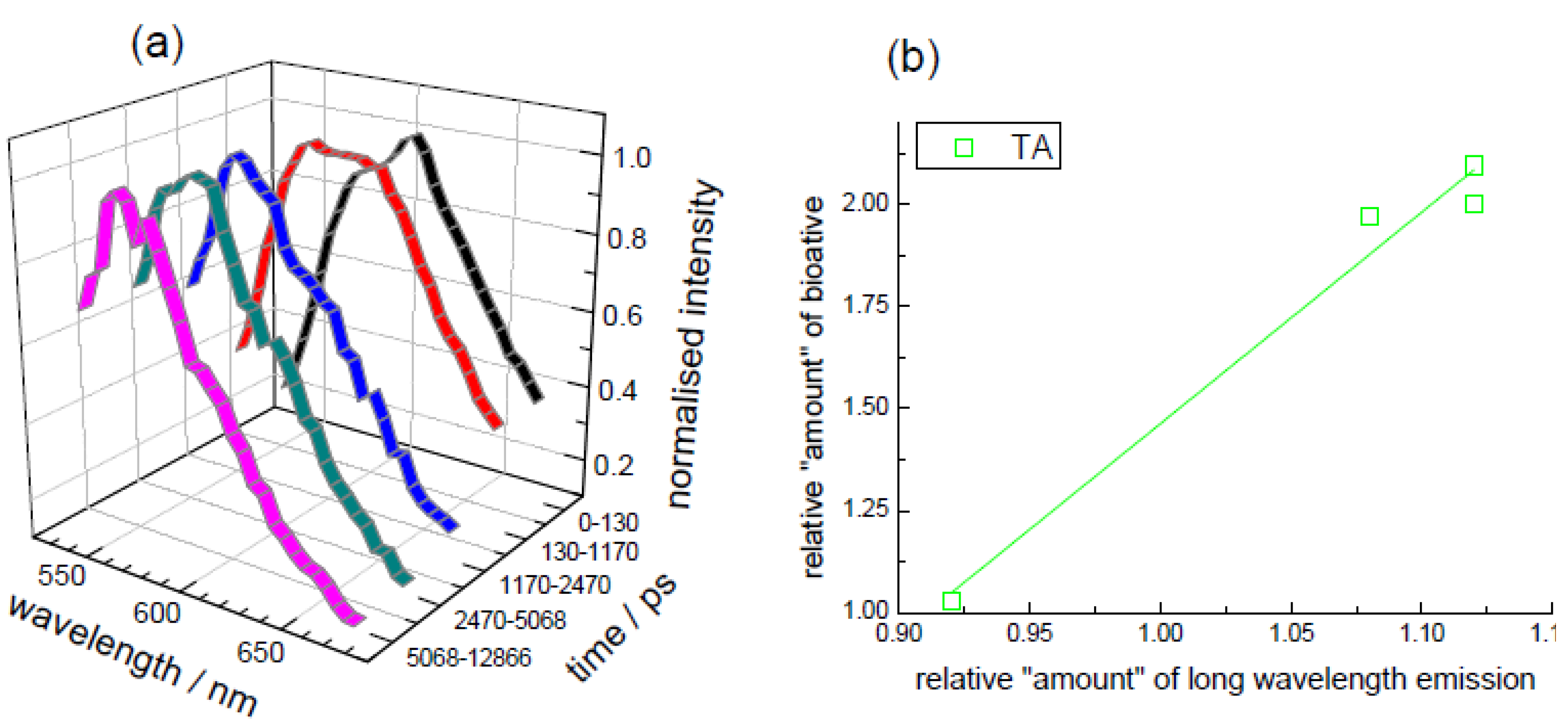
3.2. Extraction of Lycopene from Tomato Pulp Using Ultrasound (Decay Associated Spectra and Lifetime Determination)
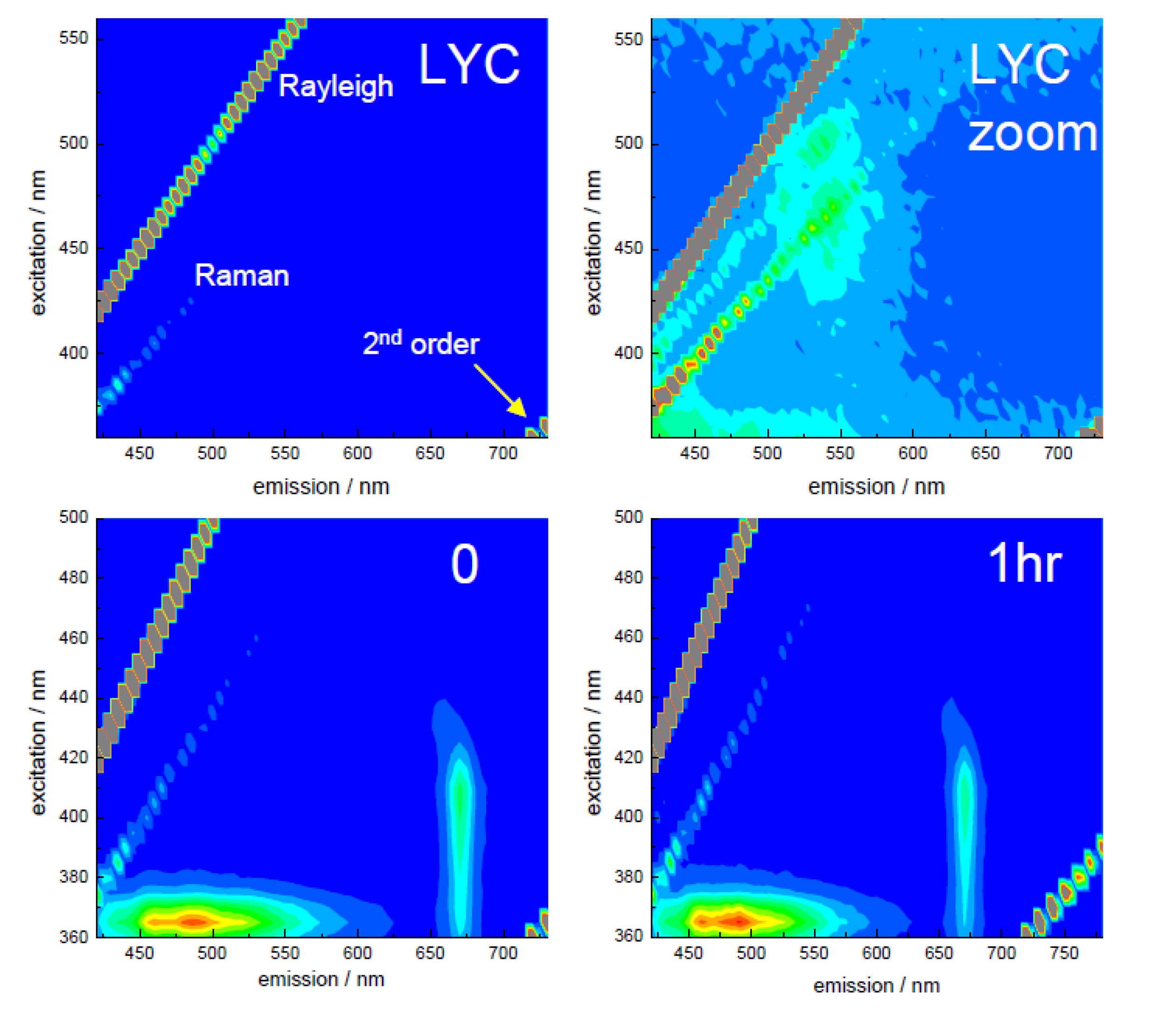
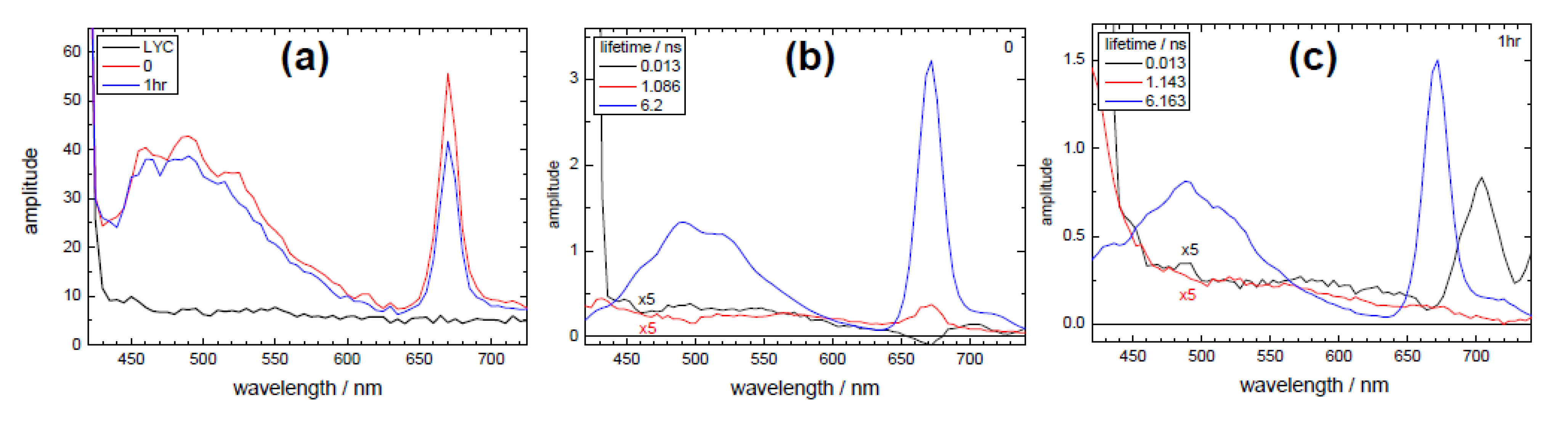

| Sample | Lifetime/ps | Fractional/% | ||||
|---|---|---|---|---|---|---|
| τ1 | τ2 | τ3 | f1 | f2 | f3 | |
| LYC | 5.1 ± 0.8 | 807 ± 20 | 84 | 16 | ||
| 0 | 4.4 ± 0.4 | 313 ± 69 | 2014 ± 183 | 62 | 8 | 30 |
| 1hr | 5.1 ± 1.1 | 277 ± 57 | 1758 ± 123 | 62 | 7 | 31 |
3.3. Effect of Storage on Betalains in Raw Vacuum Beetroot (Lifetime Determination of Betalains)

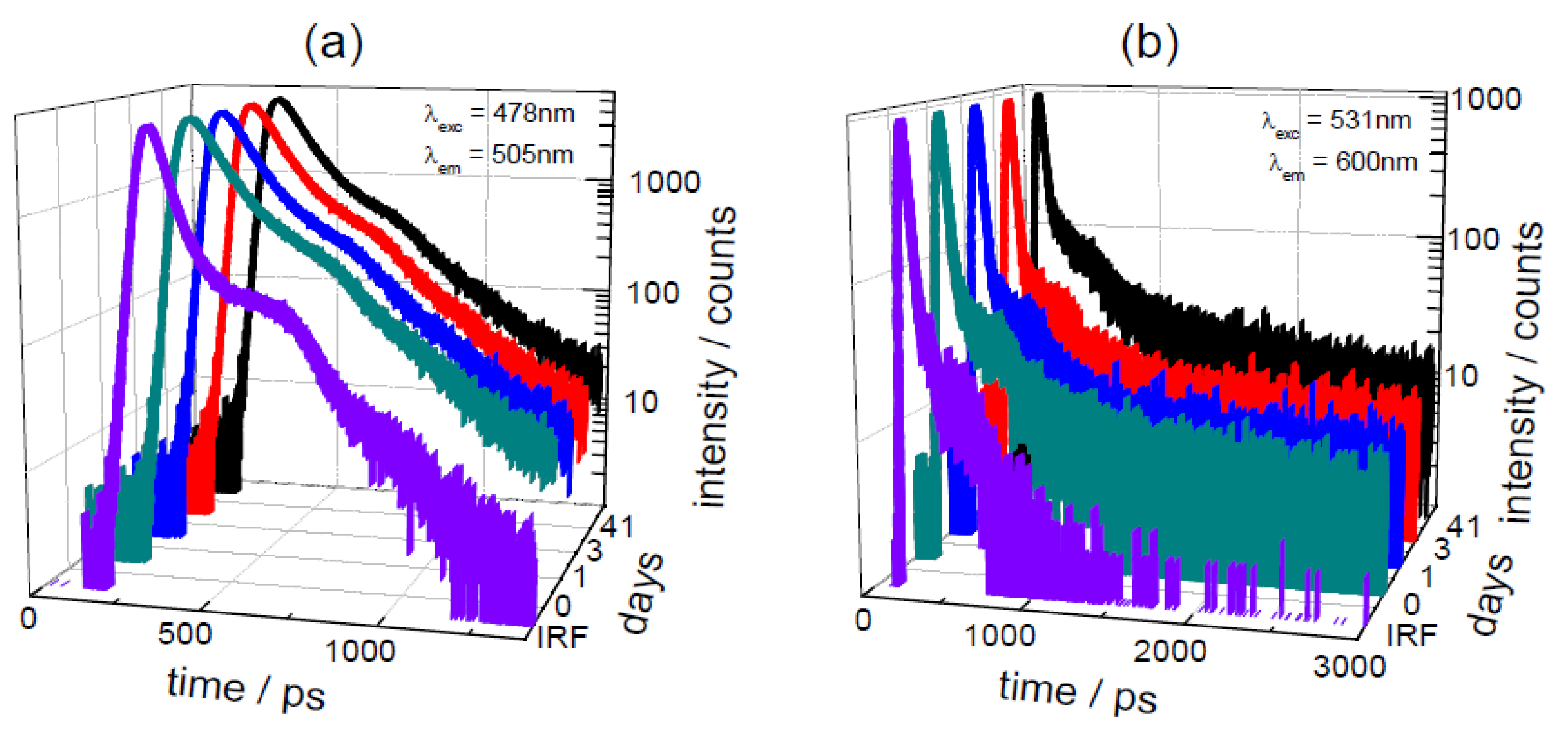
| days | λexc | Lifetime/ps | Pre-Exponential αi [f i/%] | |||||
|---|---|---|---|---|---|---|---|---|
| stored | /nm | τ1 | τ2 | τ3 | <τ> | α1 [f1] | α2 [f2] | α3 [f3] |
| 0 | 478 | 15 ± 2 | 72 ± 2 | 264 ± 8 | 34 | 0.70 [31] | 0.28 [59] | 0.02 [10] |
| 1 | 478 | 16 ± 2 | 69 ± 2 | 265 ± 8 | 34 | 0.70 [31] | 0.29 [59] | 0.01 [10] |
| 3 | 478 | 14 ± 2 | 70 ± 2 | 259 ± 7 | 33 | 0.71 [31] | 0.28 [59] | 0.01 [10] |
| 41 | 478 | 14 ± 2 | 73 ± 2 | 300 ± 7 | 33 | 0.72 [31] | 0.26 [57] | 0.02 [12] |
| 0 | 531 | 8.6 ± 0.6 | 549 ± 37 | 10.0 | 1.00 [85] | 0.00 [15] | ||
| 1 | 531 | 8.4 ± 0.6 | 468 ± 39 | 9.6 | 1.00 [87] | 0.00 [13] | ||
| 3 | 531 | 8.0 ± 0.7 | 501 ± 45 | 9.2 | 1.00 [86] | 0.00 [14] | ||
| 41 | 531 | 7.7 ± 0.9 | 195 ± 15 | 1141 ± 67 | 14.1 | 0.98 [54] | 0.01 [19] | 0.01 [27] |
3.4. Effect of Cooking on Anthocyanin and Antioxidant Activity (Decay Associated Spectra)
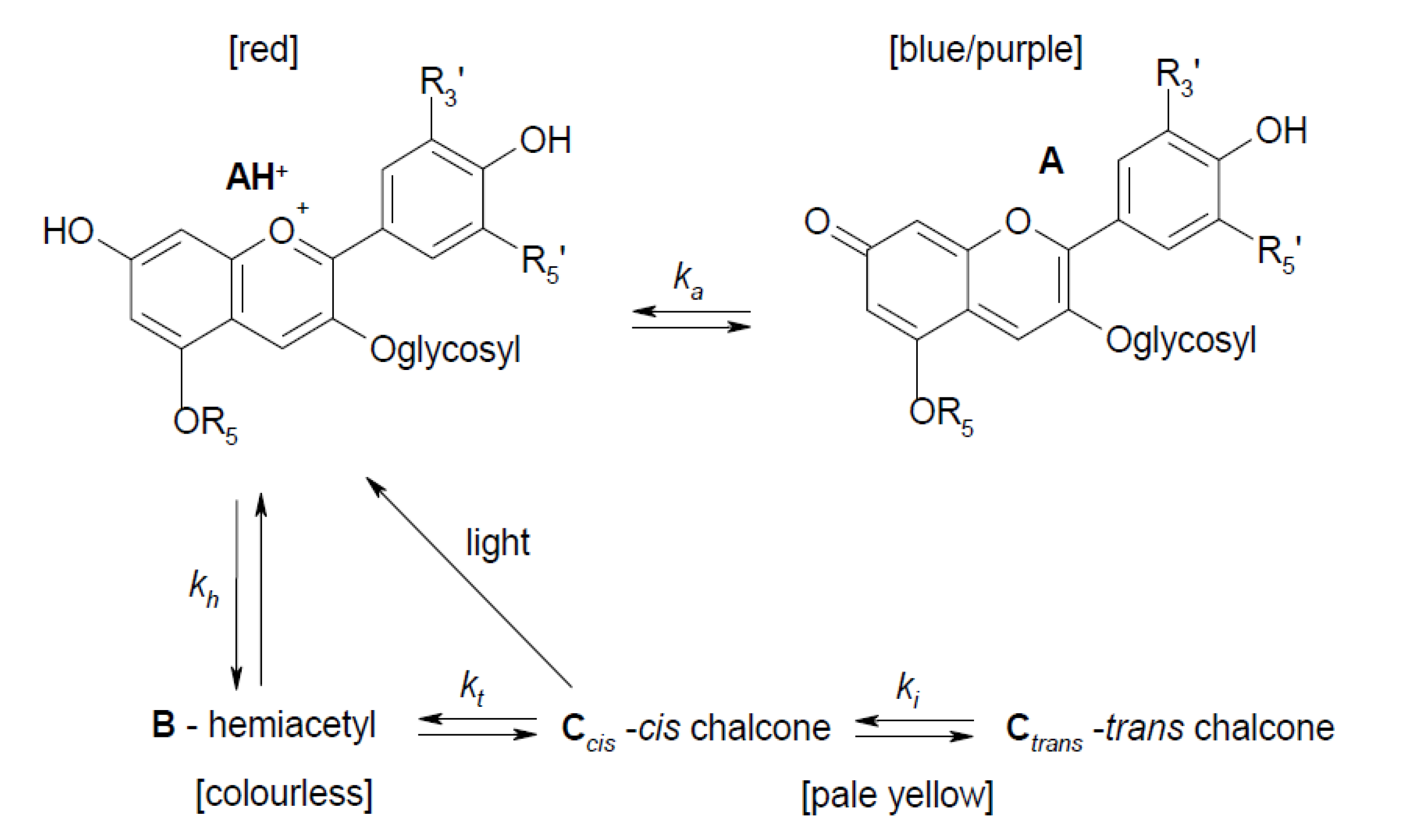
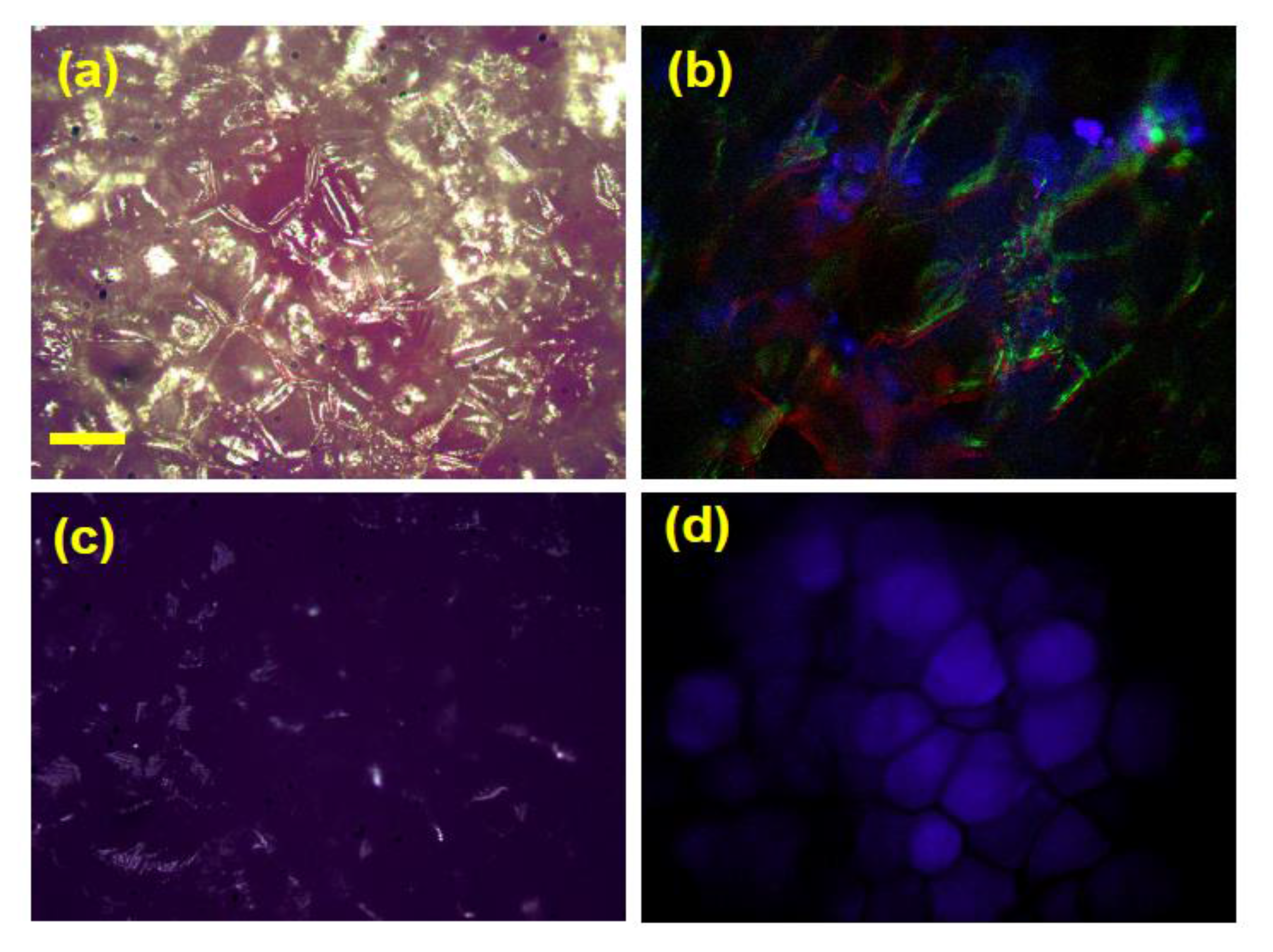
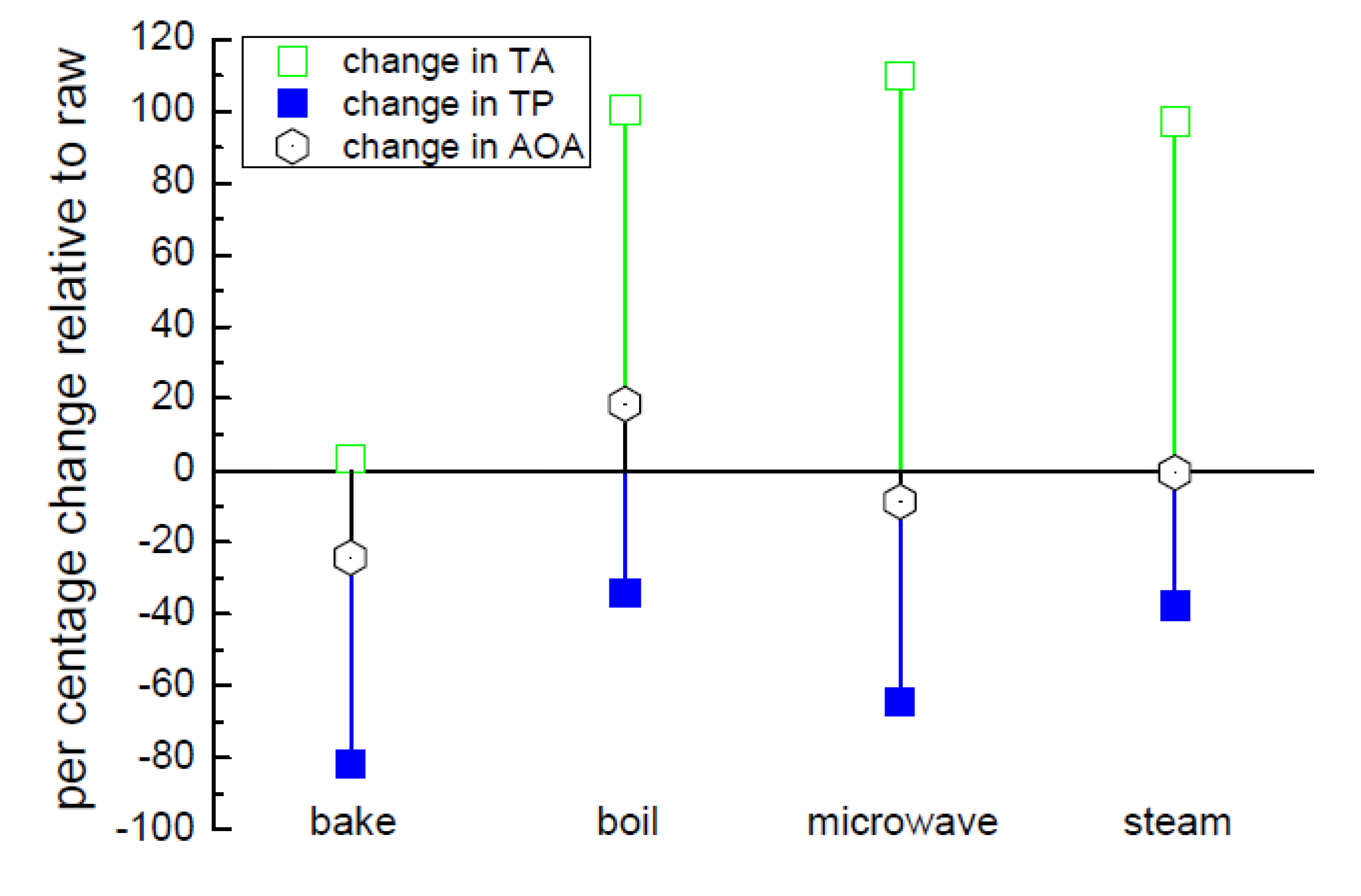
3.5. Interaction of Curcuminoids with Serum Albumin (Kinetic TCSPC)

4. Summary
Conflicts of Interest
References
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Rush, D.K.; Holm, D.G.; Stushnoff, C. Functional food characteristics of potato cultivars (Solanum tuberosum L.). Phytochemical composition and inhibition of 1-methyl-1-nitrosourea induced breast cancer in rats. J. Food Comp. Anal. 2009, 22, 571–576. [Google Scholar] [CrossRef]
- Dziri, S.; Hassen, I.; Fatnassi, S.; Mrabet, Y.; Casabianca, H.; Hanchi, B.; Hosni, K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J. Funct. Foods 2012, 4, 423–432. [Google Scholar] [CrossRef]
- Sancho, R.A.S.; Pastore, G.M. Evaluation of the effects of anthocyanins in type 2 diabetes. Rev. Food Res. Int. 2012, 46, 378–386. [Google Scholar] [CrossRef]
- Negi, P.S.; Namitha, K.K. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phyotochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Porrini, M.; Riso, P.; Brusamolini, A.; Berti, C.; Guarnieri, S.; Visioli, F. Daily intake of a formulated tomato drink affects carotenoid plasma and lymphocyte concentration and improves cellular antioxidant protection. Brit. J. Nutr. 2005, 93, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Goyal, C.K. Dietary lycopene: Its properties and anticancerogenic effects. Comp. Rev. Food Sci. Food Saf. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin-A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (solanum lycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims-a comprehensive review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Mordente, A.; Guantario, B.; Meucci, E.; Silvestrini, A.; Lombardi, E.; Martorana, G.E.; Giardina, B.; Böhm, V. Lycopene and cardiovascular diseases: An update. Curr. Med. Chem. 2011, 18, 1146–1163. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Tomatoes, tomato based products, lycopene, and cancer: Review of the epidemiologic literature. J. Nat. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: New York, NY, USA, 2002. [Google Scholar]
- O’Connor, D.V.; Phillips, D. Time-Correlated Single-Photon Counting; Academic Press: London, UK, 1984. [Google Scholar]
- Birch, D.J.S.; Imhof, R.E. Time-domain fluorescence spectroscopy using time-correlated single photon counting. In Topics in Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Plenum Press: New York, NY, USA, 1991; Volume 1, pp. 1–95. [Google Scholar]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suling, K. Molecular rotor measures viscosity of live cells via fluorescence lifetime imaging. J. Am. Chem. Soc. 2008, 130, 6672–6673. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, G.; Allison, A.; McLoskey, D.; Kuimova, M.K.; Yahioglu, G.; Suhling, K. Monitoring sol to gel transitions via fluorescence lifetime determination using viscosity sensitive fluorescent probes. J. Phys. Chem. B 2009, 113, 12067–12074. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.L.; Páez- Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajsekharan, K.N.; et al. Biological activities of curcumin and its analogues (congeners) made by man and mother nature. Biochem. Pharm. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Prather, K.L.J.; Klusskens, L.D.; Rodrigues, L.R. Heterogeneous production of curcuminoids. Microbiol. Mol. Biol. Rev. 2015, 79, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Han, K.-Y.; Sekikawa, M.; Shimada, K.-I.; Hashimoto, M.; Hashimoto, N.; Noda, T.; Tanaka, H.; Fukushima, M. Anthocyanin-rich purple potato flake extract has antioxidant capacity and improves antioxidant potential in rats. Br. J. Nutr. 2006, 96, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Oufir, M.; Guignard, C.; Hoffman, L.; Hausman, J.-F.; Evers, D.; Lardondelle, Y. Antioxidant profiling of native Andean potato tubers (solanum tuberosum L.) reveals cultivars with high levels of β-tocopherol, chlorogenic acid and petanin. J. Argic. Food Chem. 2007, 55, 10839–10849. [Google Scholar] [CrossRef] [PubMed]
- Shipp, J.; Abdel-Aal, E.-S.M. Food applications and physiological effects of anthocyanins as functional food ingredients. Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Stushnoff, C.; Holm, D.; Thompson, M.D.; Jiang, W.; Thompson, H.J.; Joyce, N.I.; Wilson, P. Antioxidant properties of cultivars and selections from the Colorado potato breeding program. Am. J. Pot. Res. 2008, 85, 267–276. [Google Scholar] [CrossRef]
- Fossen, T.; Andersen, O.M. Anthocyanins from tubers and shoots of the purple potato, solanum tubersom. J. Hort. Sci. Biotechnol. 2000, 75, 360–363. [Google Scholar]
- Pavaković, D.; Krsnik-Rasol, M. Complex biochemistry and biotechnological production of betalains. Food Technol. Biotechnol. 2011, 49, 145–155. [Google Scholar]
- Rabasović, M.S.; Šević, D.; Terzić, M.; Marinković, B.P. Comparison of beetroot extracts originating from several sites using time-resolved laser-induced fluorescence spectroscopy. Phys. Scr. 2012, T149. [Google Scholar] [CrossRef]
- Sandquist, C.; McHale, J.L. Improved efficiency of betanin-based dye-sensitized solar cells. J. Photochem. Photobiol. A 2011, 221, 90–97. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Mutka, A.M.; Bart, R.S. Image-based phenotyping of plant disease symptoms. Front. Plant Sci. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tan, J. Recent advances in the application of chlorophyll a fluorescence from photosystem II. Photochem. Photobiol. 2015, 91, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Holt, N.E.; Kennis, J.T.M.; Dall’Osto, L.; Bassi, R.; Fleming, G.R. Carotenoid to chlorophyll energy transfer in light harvesting complex II from arabidopsis thaliana probed by femtosecond upconversion. Chem. Phys. Lett. 2003, 379, 305–313. [Google Scholar] [CrossRef]
- Ritz, T.; Damjanović, A.; Schulten, K.; Zhang, J.-P.; Koyama, Y. Efficient light arvesting through carotenoids. Photosynth. Res. 2000, 66, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.-W.; Khoo, H.-E.; Prasad, K.N.; Ismail, A.; Tan, C.-P.; Rajab, N.F. Revealing the power of the natural red pigment lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Castón, M.J.P.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knutsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Speranza, L.; Pesce, M.; Cusenza, S.; D’Orazio, N.; Glade, M.J. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition 2012, 28, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; de Vos, R.C.H.; Hall, R.D. The effect of industrial food processing on potentially health-beneficial tomato antioxidants. Crit. Rev. Food Sci. Nutr. 2010, 50, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W., Jr.; Ford, N.A.; Lindshield, B.L. Are the health attributes of lycopene related to its antioxidant function? Arch. Biochem. Biophys. 2009, 483, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Bian, W.; Barsan, C.; Jauneau, A.; Pech, J.-C.; Latché, A.; Li, Z.; Chervin, C. Chloroplast to chromoplast transition in tomato fruit: Spectral confocal microscopy analyses of carotenoids and chlorophylls in isolated plastids and time-lapse recording on intact live tissue. Ann. Bot. 2011, 108, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.A.; Hungerford, G. The binding of curcuma longa extract with bovine serum albumin monitored via time-resolved fluorescence. Photochem. Photobiol. 2013, 89, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Horiba Scientific Time-Resolved Fluorescence Application Note TRFA-13, Monitoring Whole Leaf Fluorescence Using Time-Resolved Techniques. Available online: http://www.horiba.com/uk/scientific/products/fluorescence-spectroscopy/application-notes/ (accessed on 20 June 2015).
- Lemos, M.A.; Aliyu, M.M.; Hungerford, G. Influence of cooking on the levels of bioactive compounds in purple majesty potato observed via chemical and spectroscopic means. Food Chem. 2015, 173, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.A.; Aliyu, M.M.; Hungerford, G. Observation of the location and form of anthocyanin in purple potato using time-resolved fluorescence. Innov. Food Sci. Emerg. Technol. 2012, 16, 61–68. [Google Scholar] [CrossRef]
- Bot, F.; Anese, M.; Lemos, M.A.; Hungerford, G. Use of time-resolved spectroscopy as a method to monitor carotenoids present in tomato extract obtained using ultrasound treatment. 2015; in press. [Google Scholar]
- Wang, W.-D.; Xu, S.-Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Usha, K.; Singh, B. Potential applications of remote sensing in horticulture—A review. Sci. Hort. 2013, 153, 71–83. [Google Scholar] [CrossRef]
- Kolber, Z.; Klimov, D.; Ananyev, G.; Rascher, U.; Berry, J.; Osmond, B. Measuring photosynthetic parameters at a distance: Laser induced fluorescence transient (LIFT) method for remote measurements of photosynthesis in terrestrial vegetation. Photosynth. Res. 2005, 84, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sayed, O.H. Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 2003, 41, 321–330. [Google Scholar] [CrossRef]
- Harbinson, J.; Prinzenburg, A.E.; Kruijer, W.; Aarts, M.G.M. High throughput screening with chlorophyll fluorescence imaging and its use in crop improvement. Curr. Opin. Biotechnol. 2012, 23, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Seifert, B.; Pflanz, M.; Zude, M. Spectral shift as advanced index for fruit chlorophyll breakdown. Food Bioprocess Technol. 2014, 7, 2050–2059. [Google Scholar] [CrossRef]
- Buschmann, C.; Grumbach, K.H. Herbicides which inhibit electron transport or produce chlorosis and their effect on chloroplast development in raddish seedlings II. Pigment excitation, chlorophyll fluorescence and pigment-protein complexes. Z. Naturforsch. 1982, 37c, 632–641. [Google Scholar]
- Hunsche, M.; Bürling, K.; Noga, G. Spectral and time-resolved fluorescence signature of four weed species as affected by selected herbicides. Pesticide Biochem. Physiol. 2011, 101, 39–47. [Google Scholar] [CrossRef]
- Cho, B.-K.; Kim, M.S.; Baek, I.-S.; Kim, D.-Y.; Lee, W.-H.; Kim, J.; Bae, H.; Kim, Y.-S. Detection of cuticle defects on cherry tomatoes using hyperspectral fluorescence imagery. Postharvest Biol. Technol. 2013, 76, 40–49. [Google Scholar] [CrossRef]
- Guzmán, E.; Baeten, V.; Pierna, J.A.F.; García-Mesa, J.A. Evaluation of the overall quality of olive using fluorescence spectroscopy. Food Chem. 2015, 173, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Khmelinskii, I.V.; Sikorski, M.; Caponio, F.; Bilancia, M.T.; Pasqualone, A.; Gomes, T. Fluorescence spectroscopy in monitoring of extra virgin olive oil during storage. Int. J. Food Sci. Technol. 2008, 43, 52–61. [Google Scholar] [CrossRef]
- Franck, F.; Juneau, P.; Popovic, R. Resolution of the photosystem I and photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature. Biochim. Biophys. Acta 2002, 1556, 239–246. [Google Scholar] [CrossRef]
- Keuper, H.J.K.; Sauer, K. Effect of photosystem II reaction center closure on nanosecond fluorescence relaxation kinetics. Photosynth. Res. 1989, 20, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Mazzinghi, P.; Fusi, F.; Ambrosini, I. The F685/F730 chlorophyll fluorescence ratio as a tool in plant physiology: Response to physiological and environmental factors. J. Plant Physiol. 1995, 145, 228–238. [Google Scholar] [CrossRef]
- Pedrós, R.; Moya, I.; Goulas, Y.; Jacquemoud, S. Chlorophyll fluorescence emission spectrum inside a leaf. Photochem. Photobiol. Sci. 2008, 7, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Belgio, E.; Johnson, M.P.; Jurić, S.; Ruban, A.V. Higher plant photosystem II light-harvesting antenna, not the reaction center, determines the excited-state lifetime—Both the maximum and the nonphotochemically quenched. Biophys. J. 2012, 102, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (cucurbita spp.): A review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [PubMed]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrasonics Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Mirolo, G.; Beraldo, P.; Lippe, G. Effect of ultrasound treatments of tomato pulp on microstructure and lycopene in vitro bioaccessibility. Food Chem. 2013, 136, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Bot, F.; Panozzo, A.; Mirolo, G.; Lippe, G. Effect of ultrasound treatment, oil addition and storage time on lycopene stability and in vitro bioaccessibility of tomato pulp. Food Chem. 2015, 172, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.W.; Anderson, D.G. Biosynthesis of carotenes. Annu. Rev. Plant Physiol. 1967, 18, 197–228. [Google Scholar] [CrossRef]
- Fraser, P.D.; Truesdale, M.R.; Bird, C.R.; Schuch, W.; Bramley, P.M. Carotenoid biosynthesis during tomato fruit development. Evidence for tissue-specific gene expression. Plant Physiol. 1994, 105, 405–413. [Google Scholar] [PubMed]
- Bramley, P.M. Regulation of carotenoid formation during tomato fruit ripening and development. J. Expt. Biol. 2002, 53, 2107–2113. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. Lycopene; chemistry, biosysnthesis, metabolism and degradation under various abiotic parameters. J. Food Sci. Technol. 2015, 52, 41–53. [Google Scholar] [CrossRef]
- Gillbro, T.; Andersson, P.O.; Liu, R.S.H.; Asato, A.E.; Takaishi, S.; Cogdell, R.J. Location of the carotenoid 2Ag—state and its role in photosynthesis. Photochem. Photobiol. 1993, 57, 44–48. [Google Scholar] [CrossRef]
- Billsten, H.H.; Herek, J.L.; Garcia-Asua, G.; Hashøj, L.; Polívka, T.; Hunter, C.N.; Sundström, V. Dynamics of energy transfer from lycopene to bacteriochlorophyll in genetically-modified LH2 complexes of rhodobacter sphaeroides. Biochemistry 2002, 41, 4127–4136. [Google Scholar] [CrossRef]
- Schouten, R.E.; Farneti, B.; Tijskens, L.M.M.; Alarcón, A.A.; Woltering, E.J. Quantifying lycopene synthesis and chlorophyll breakdown in tomato fruit using remittance VIS spectroscopy. Postharvest Biol. Technol. 2014, 96, 53–63. [Google Scholar] [CrossRef]
- Davis, J.A.; Cannon, E.; van Dao, L.; Hannaford, P.; Quiney, H.M.; Nugent, K.A. Long-lived coherence in carotenoids. New J. Phys. 2010, 12. [Google Scholar] [CrossRef]
- Fujii, R.; Onaka, K.; Nagae, H.; Koyama, Y.; Watanabe, Y. Fluorescence spectroscopy of all-trans-lycopene: Comparison of the energy and the potential displacements of its 2Ag- state with those of neurosporene and spheroidene. J. Lumin. 2001, 92, 213–222. [Google Scholar] [CrossRef]
- Andersson, P.O.; Takaichi, S.; Cogdell, R.J.; Gillbro, T. Photochem. Photobiol. 2001, 74, 549–557. [CrossRef]
- Wan, W.; Hua, D.; Le, J.; He, T.; Yan, Z.; Zhou, C. Study of laser-induced chlorophyll fluorescence lifetime and its correction. Measurement 2015, 60, 64–70. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Fujii, R.; Qian, P.; Inaba, T.; Mizoguchi, T.; Koyama, Y.; Onaka, K.; Watanabe, Y. Mechanism of the carotenoid-to-bacteriochlorophyll energy transfer via the S1 state in the LH2 complexes from purple bacteria. J. Phys. Chem. B 2000, 104, 3683–3691. [Google Scholar] [CrossRef]
- Khorshidi, S.; Dvarynejad, G.; Tehranifar, A.; Fallahi, E. Effect of modified atmosphere packaging on chemical composition, antioxidant activity, anthocyanins and total phenolic content of cherry fruits. Hortic. Environ. Biotechnol. 2011, 53, 471–481. [Google Scholar] [CrossRef]
- Osorni, M.M.L.; Chaves, A.R. Quality changes in stored raw grated beetroots as affected by temperature and packaging film. J. Food Sci. 1998, 63, 327–330. [Google Scholar]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Mills, A. Oxygen indicators and intelligent inks for packaging food. Chem. Soc. Rev. 2005, 34, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Wendel, M.; Szot, D.; Starzak, K.; Tuwalska, D.; Prukala, D.; Pedzinski, T.; Sikorski, M.; Wybraniec, S.; Burdzinski, G. Photophysical properties of indicaxanthin in aqueous and alcoholic solutions. Dyes Pigments 2015, 113, 634–639. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. Fluorescence pigments: New perspectives in betalain research and applications. Food Res. Int. 2005, 38, 879–884. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Šulc, M.; Orsák, M.; Pivec, V.; Hejtmankova, A.; Čepl, J. Cultivar differences of total anthocyanins and anthocyanidins in red and purple fleshed potatoes and their relation to antioxidant activity. Food Chem. 2009, 114, 836–843. [Google Scholar] [CrossRef]
- Brown, C.R. Antioxidants in potato. Am. J. Pot. Res. 2005, 82, 163–175. [Google Scholar] [CrossRef]
- Burmeister, A.; Bondiek, S.; Apel, L.; Kuhne, C.; Hillebrand, S.; Fleischmann, P. Comparison of carotenoid and anthocyanin profiles of raw and boiled Solanum tuberosum and Solanum phureja tubers. J. Food Comp. Anal. 2011, 24, 865–872. [Google Scholar] [CrossRef]
- Mori, M.; Hayashi, K.; Takada, A.O.; Watanuki, H.; Katahira, R.; Ono, H.; Terahara, N. Anthocyanin from skins and fleshes of potato varieties. Food Sci. Technol. Res. 2010, 16, 115–122. [Google Scholar] [CrossRef]
- Harborne, J.B. Plant polyphenols 1. anthocyanin production in the cultivated potato. Biochem. J. 1960, 74, 262–269. [Google Scholar] [PubMed]
- Wigand, M.C.; Dangles, O.; Brouillard, R. Complexation of a fluorescent anthocyanin with purines and polyphenols. Phytochemistry 1992, 31, 4317–4324. [Google Scholar] [CrossRef]
- Rodrigues, R.F.; da Silva, P.F.; Shimizu, K.; Freitas, A.A.; Kovalenko, S.A.; Ernsting, N.P.; Quina, F.H.; Maçanita, A. Ultrafast internal conversion in a model anthocyanin-poyphenol complex: Implications for the biological role of anthocyanins in vegetative tissues of plants. Chem. Eur. J. 2009, 15, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Hejtmankova, K.; Pazderu, K.; Dvořak, P.; Čepl, J. Impact of selected factors—Cultivar, storage, cooking and baking on the content of anthocyanins in coloured-flesh potatoes. Food Chem. 2012, 133, 1107–1116. [Google Scholar] [CrossRef]
- Aliyu, M.M. Effect of Different Cooking Techniques and Storage Methods on the Stability of Anthocyanin in Purple Majesty Potatoes. Master Thesis, University of Abertay Dundee, Dundee, UK, 2011. [Google Scholar]
- Brown, C.R.; Durst, R.W.; Wrolstad, R.; de Jong, W. Variability of phytonutrient content of potato in relation to growing location and cooking method. Pot. Res. 2008, 51, 259–270. [Google Scholar] [CrossRef]
- Navarre, D.A.; Shakya, R.; Holden, J.; Kumar, S. The effect of different cooking methods on phenolics and vitamin C in developmentally young potato tubers. Am. J. Pot. Res. 2010, 87, 350–359. [Google Scholar] [CrossRef]
- Moncada, M.C.; Pina, F.; Roque, A.; Parola, A.J.; Maestri, M.; Balzani, V. Tuning the photochromic properties of a flavylium compound by pH. Eur. J. Org. Chem. 2004, 2004, 302–312. [Google Scholar] [CrossRef]
- Quina, F.H.; Moreira, P.F., Jr.; Vaultier-Giongo, C.; Rettori, D.; Rodrigues, R.F.; Freitas, A.A.; Silva, P.F.; Maçanita, A.L. Photochemistry of anthocyanins and their biological role in plant tissues. Pure Appl. Chem. 2009, 81, 1687–1694. [Google Scholar] [CrossRef]
- Petrov, V.; Gomes, R.; Parola, A.J.; Pina, F. Flash photolysis and stopped flow studies of the 2'-methoxyflavylium network in aq. acidic and alkaline solution. Dyes Pigments 2009, 80, 149–155. [Google Scholar] [CrossRef]
- Fossen, T.; Cabrita, L.; Andersen, Ø.M. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chem. 1998, 63, 435–440. [Google Scholar] [CrossRef]
- Achir, N.; Vitrac, O.; Trystram, G. Direct observation of the surface structure of French fries by uv-vis confocal laser scanning microscopy. Food Res. Int. 2010, 43, 307–314. [Google Scholar] [CrossRef]
- Han, X.-Z.; Hamaker, B.R. Local of starch granule-associated proteins revealed by confocal laser scanning microscopy. J. Cereal. Sci. 2002, 35, 109–116. [Google Scholar] [CrossRef]
- Moreira, P.F., Jr.; Giestas, L.; Yihwa, C.; Vautier-Giongo, C.; Quina, F.H.; Maçanita, A.L.; Lima, J.C. The dynamics of ultrafast excited state proton transfer in anionic micelles. J. Phys. Chem. A 2003, 107, 4203–4210. [Google Scholar]
- Freitas, A.A.; Quina, F.H.; Fernandes, A.C.; Maçanita, A.A.L. Picosecond dynamics of the prototropic reactions of 7-hydroxyflavylium photoacids anchored at an anionic micellar surface. J. Phys. Chem. A 2010, 114, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chemico Biol. Int. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Nardo, L.; Paderno, R.; Andreoni, A.; Márson, M.; Haukvik, T.; Tønnesen, H.H. Role of H-bond formation in the photoreactivity of curcumin. Spectroscopy 2008, 22, 187–198. [Google Scholar] [CrossRef]
- Adhikary, R.; Mukherjee, P.; Kee, T.W.; Petrich, J.W. Excited-state intramolecular hydrogen atom transfer and salvation dynamics of the medicinal pigment curcumin. J. Phys. Chem. B 2009, 113, 5255–5261. [Google Scholar] [CrossRef] [PubMed]
- Yeung, I.; Jin, S.M.; Ramanathan, V.; Kim, H.M.; Suh, Y.D.; Kim, S.K. Excited sate dynamics of curcumin and solvent hydrogen bonding. Bull. Korean Chem. Soc. 2011, 32, 3090–3093. [Google Scholar] [CrossRef]
- Khopde, S.M.; Priyadarsini, K.I.; Palit, D.K.; Mukherjee, T. Effect of solvent on the excited-state photophysical properties of curcumin. Photochem. Photobiol. 2000, 72, 625–631. [Google Scholar] [CrossRef]
- Barik, A.; Goel, N.K.; Priyadarsini, K.I.; Mohan, H. Effect of deuterated solvents on the excited state photophysical properties of curcumin. J. Photosci. 2004, 11, 95–99. [Google Scholar]
- Ghosh, R.; Mondal, J.A.; Palit, D.K. Ultrafast dynamics of the excited states of curcumin in solution. J. Phys. Chem. B 2010, 114, 12129–12143. [Google Scholar] [CrossRef] [PubMed]
- Kee, T.W.; Adhikary, R.; Carlson, P.J.; Mukherjee, P.; Petrich, J.W. Femtosecond fluorescence upconversion investigations on the excited-state photophysics of curcumin. Aust. J. Chem. 2011, 64, 23–30. [Google Scholar] [CrossRef]
- Mitra, S.P. Binding and stability of curcumin in presence of bovine serum albumin. J. Surf. Sci. Technol. 2007, 23, 91–110. [Google Scholar]
- Bourassa, P.; Kanakis, C.D.; Tarantillis, P.; Pollissiou, M.G.; Tajmir-Riahi, H.A. Resveratrol, genistein and curcumin bind bovine serum albumin. J. Phys. Chem. B 2010, 114, 3348–3354. [Google Scholar] [CrossRef] [PubMed]
- Barakat, C.; Patra, D. Combining time-resolved fluorescence with synchronous fluorescence spectroscopy to study bovine serum albumin-curcumin complex during unfolding and refolding processes. Luminescence 2013, 28, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Priyadarsini, K.I.; Mohan, H. Photophysical studies on binding of curcumin to bovine serum albumin. Photochem. Photobiol. 2003, 77, 597–603. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemos, M.A.; Sárniková, K.; Bot, F.; Anese, M.; Hungerford, G. Use of Time-Resolved Fluorescence to Monitor Bioactive Compounds in Plant Based Foodstuffs. Biosensors 2015, 5, 367-397. https://doi.org/10.3390/bios5030367
Lemos MA, Sárniková K, Bot F, Anese M, Hungerford G. Use of Time-Resolved Fluorescence to Monitor Bioactive Compounds in Plant Based Foodstuffs. Biosensors. 2015; 5(3):367-397. https://doi.org/10.3390/bios5030367
Chicago/Turabian StyleLemos, M. Adília, Katarína Sárniková, Francesca Bot, Monica Anese, and Graham Hungerford. 2015. "Use of Time-Resolved Fluorescence to Monitor Bioactive Compounds in Plant Based Foodstuffs" Biosensors 5, no. 3: 367-397. https://doi.org/10.3390/bios5030367
APA StyleLemos, M. A., Sárniková, K., Bot, F., Anese, M., & Hungerford, G. (2015). Use of Time-Resolved Fluorescence to Monitor Bioactive Compounds in Plant Based Foodstuffs. Biosensors, 5(3), 367-397. https://doi.org/10.3390/bios5030367