Sensitivity and Cross-Reactivity Analysis of Serotype-Specific Anti-NS1 Serological Assays for Dengue Virus Using Optical Modulation Biosensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sample Collection
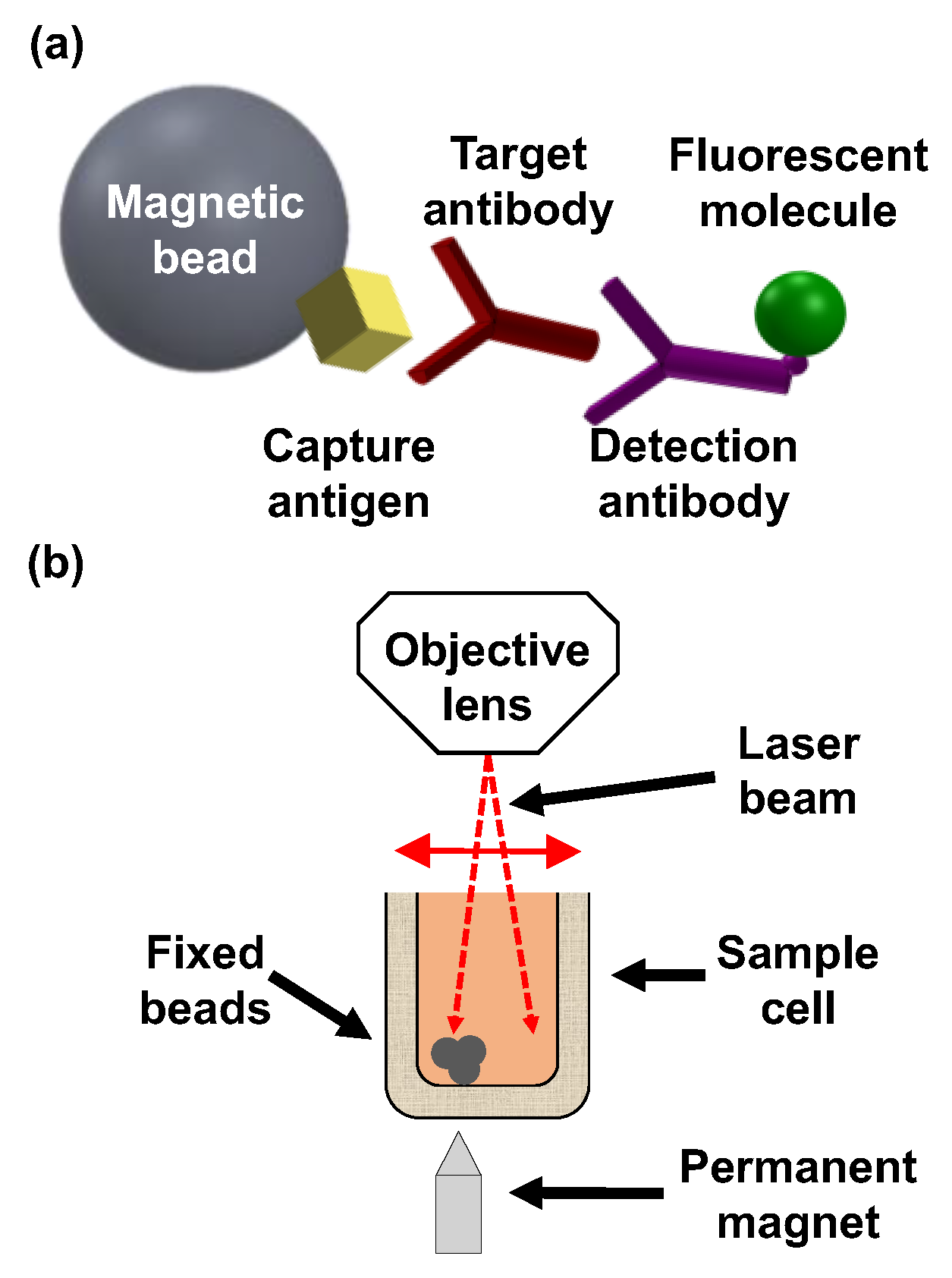
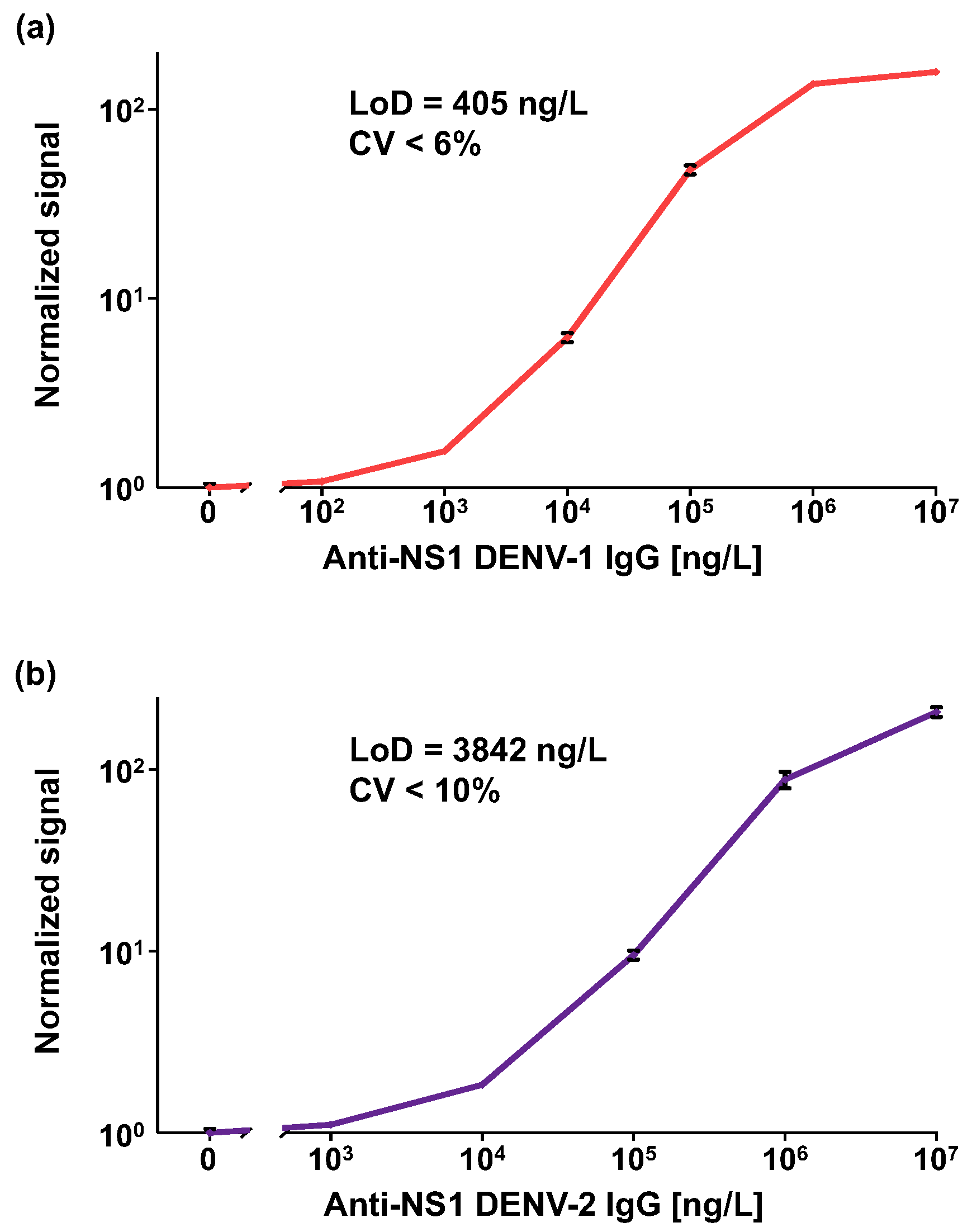
2.3. Analytical Performance of the OMB-Based Anti-DENV-1–2 NS1 IgG Assays
2.4. Clinical Sensitivity and Specificity of the OMB-Based Anti-DENV-1–3 NS1 IgG Serological Assays
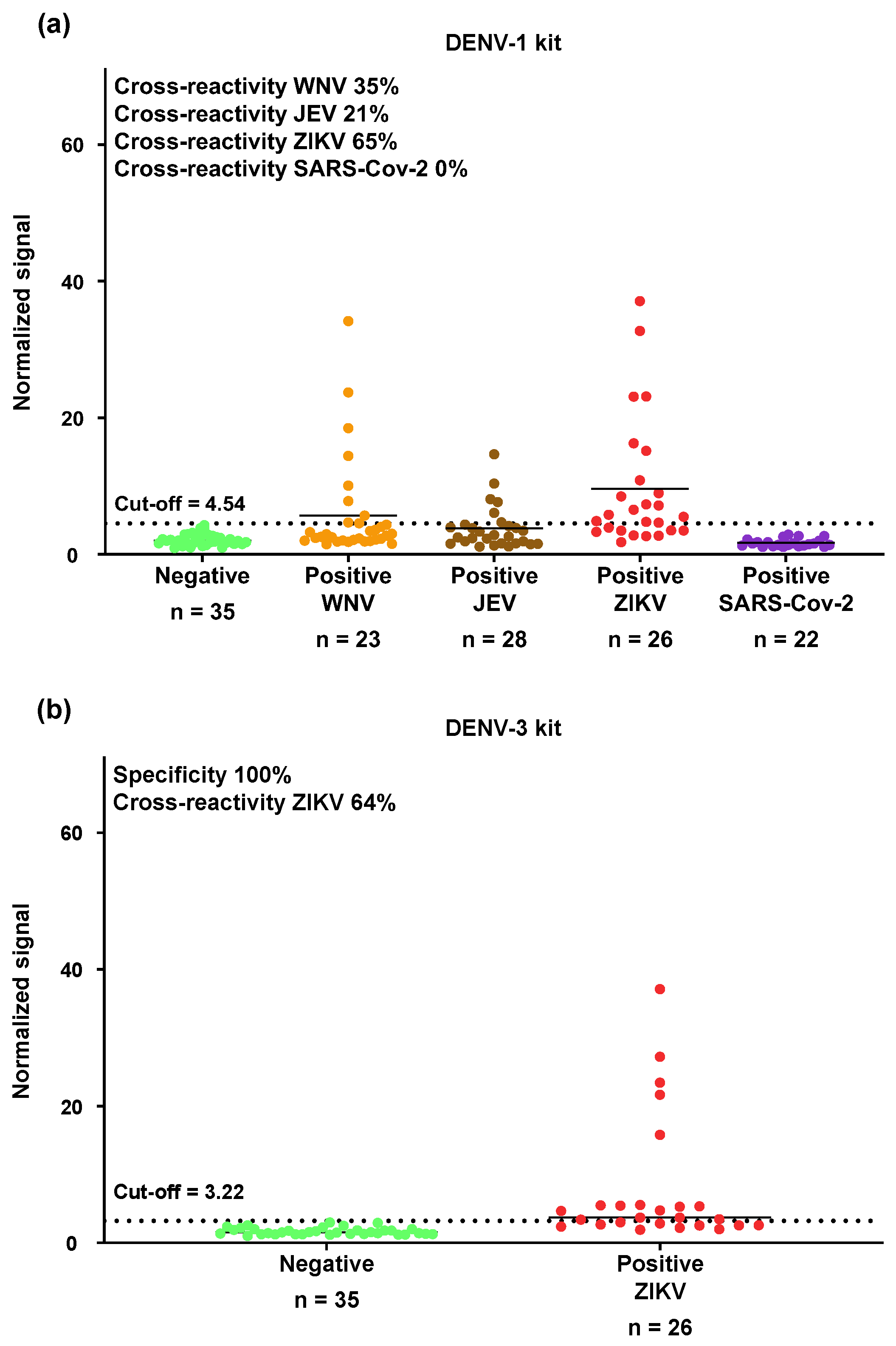
2.5. Cross-Reactivity of the OMB-Based Anti-DENV-1 and Anti-DENV-3 NS1 IgG Serological Assays with WNV, JEV, ZIKV, and SARS-CoV-2
2.6. Data Analysis
3. Results
3.1. Analytical Performance of the OMB-Based Anti-DENV-1–2 NS1 IgG Serological Assays
3.2. Evaluation of Clinical Sensitivity and Specificity of the OMB-Based Anti-DENV-1–3 NS1 IgG Serological Assays
3.3. Cross-Reactivity of the OMB-Based Anti-DENV-1 NS1 IgG Serological Assay with WNV, JEV, ZIKV, and SARS-CoV-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OMB | Optical modulation biosensing |
| LoD | Limit of detection |
| LoB | Limit of blank |
| CV | Coefficient of variance |
| DENV | Dengue virus |
| WNV | West Nile virus |
| ZIKV | Zika virus |
| JEV | Japanese encephalitis virus |
| ELISA | Enzyme-linked immunosorbent assays |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| RBD | Receptor-binding domain |
References
- WHO. Dengue and Severe Dengue. World Helath Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 9 July 2025).
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Jentes, E.S.; Lash, R.R.; Johansson, M.A.; Sharp, T.M.; Henry, R.; Brady, O.J.; Sotir, M.J.; Hay, S.I.; Margolis, H.S.; Brunette, G.W. Evidence-based risk assessment and communication: A new global dengue-risk map for travellers and clinicians. J. Travel. Med. 2016, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef]
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef]
- Kamath, V.; Olakkengil, N.D. Original Antigenic Sin in Dengue—Hoskins Effect. APIK J. Intern. Med. 2023, 11, 3. [Google Scholar] [CrossRef]
- Guzman, M.G.; Alvarez, M.; Halstead, S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013, 158, 1445–1459. [Google Scholar] [CrossRef]
- Back, A.T.; Lundkvist, A. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar]
- Fares, R.C.; Souza, K.P.; Anez, G.; Rios, M. Epidemiological Scenario of Dengue in Brazil. BioMed Res. Int. 2015, 2015, 321873. [Google Scholar] [CrossRef]
- Mardekian, S.K.; Roberts, A.L. Diagnostic Options and Challenges for Dengue and Chikungunya Viruses. BioMed Res. Int. 2015, 2015, 834371. [Google Scholar] [CrossRef]
- Chakravarti, A.; Matlani, M.; Jain, M. Immunodiagnosis of dengue virus infection using saliva. Curr. Microbiol. 2007, 55, 461–464. [Google Scholar] [CrossRef]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of diagnostic tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S38. [Google Scholar] [CrossRef] [PubMed]
- Moureau, G.; Temmam, S.; Gonzalez, J.P.; Charrel, R.N.; Grard, G.; de Lamballerie, X. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector Borne Zoonotic Dis. 2007, 7, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Calisher, C.H.; Gubler, D.J.; Chang, G.J.; Vorndam, A.V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 545–551. [Google Scholar] [CrossRef]
- Fisher, R.; Lustig, Y.; Sklan, E.H.; Schwartz, E. The Role of NS1 Protein in the Diagnosis of Flavivirus Infections. Viruses 2023, 15, 2. [Google Scholar] [CrossRef]
- Pal, S.; Dauner, A.L.; Mitra, I.; Forshey, B.M.; Garcia, P.; Morrison, A.C.; Halsey, E.S.; Kochel, T.J.; Wu, S.J.L. Evaluation of Dengue NS1 Antigen Rapid Tests and ELISA Kits Using Clinical Samples. PLoS ONE 2014, 9, 11. [Google Scholar] [CrossRef]
- Bosch, I.; Reddy, A.; de Puig, H.; Ludert, J.E.; Perdomo-Celis, F.; Narváez, C.F.; Versiani, A.; Fandos, D.; Nogueira, M.L.; Singla, M.; et al. Serotype-specific detection of dengue viruses in a nonstructural protein 1-based enzyme-linked immunosorbent assay validated with a multi-national cohort. PLoS Negl. Trop. Dis. 2020, 14, e0008203. [Google Scholar] [CrossRef]
- Poltep, K.; Nakayama, E.E.; Sasaki, T.; Kurosu, T.; Takashima, Y.; Phadungsombat, J.; Kosoltanapiwat, N.; Hanboonkunupakarn, B.; Suwanpakdee, S.; Imad, H.A.; et al. Development of a Dengue Virus Serotype-Specific Non-Structural Protein 1 Capture Immunochromatography Method. Sensors 2021, 21, 7809. [Google Scholar] [CrossRef]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- Calidonio, J.M.; Hamad-Schifferli, K. Biophysical and biochemical insights in the design of immunoassays. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2023, 1867, 130266. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.B.Y.; How, C.H.; Ng, C.W. Definitive tests for dengue fever: When and which should I use? Singap. Med. J. 2017, 58, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; Diamond, M.S. Defining the levels of secreted non-structural protein NS1 after west nile virus infection in cell culture and mice. J. Med. Virol. 2008, 80, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Bonaparte, M.; Zheng, L.; Garg, S.; Guy, B.; Lustig, Y.; Schwartz, E.; DiazGranados, C.A.; Savarino, S.; Ataman-Önal, Y. Evaluation of rapid diagnostic tests and conventional enzyme-linked immunosorbent assays to determine prior dengue infection. J. Travel Med. 2019, 26, taz078. [Google Scholar] [CrossRef]
- World Health, O. Dengue vaccine: WHO position paper, July 2016—Recommendations. Vaccine 2017, 35, 1200–1201. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Smith, P.G.; Luo, R.; Kelly-Cirino, C.; Curry, D.; Larson, H.; Durbin, A.; Chu, M.; Tharmaphornpilas, P.; Ng, L.C.; et al. Pre-vaccination screening strategies for the use of the CYD-TDV dengue vaccine: A meeting report. Vaccine 2019, 37, 5137–5146. [Google Scholar] [CrossRef]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; WHO: Geneva, Switzerland, 2009; pp. 1–147. [Google Scholar]
- Allwinn, R.; Doerr, H.W.; Emmerich, P.; Schmitz, H.; Preiser, W. Cross-reactivity in flavivirus serology: New implications of an old finding? Med. Microbiol. Immunol. 2002, 190, 199–202. [Google Scholar] [CrossRef]
- Endale, A.; Medhin, G.; Darfiro, K.; Kebede, N.; Legesse, M. Magnitude of Antibody Cross-Reactivity in Medically Important Mosquito-Borne Flaviviruses: A Systematic Review. Infect. Drug Resist. 2021, 14, 4291–4299. [Google Scholar] [CrossRef]
- L’Huillier, A.G.; Hamid-Allie, A.; Kristjanson, E.; Papageorgiou, L.; Hung, S.; Wong, C.F.; Stein, D.R.; Olsha, R.; Goneau, L.W.; Dimitrova, K.; et al. Evaluation of Euroimmun Anti-Zika Virus IgM and IgG Enzyme-Linked Immunosorbent Assays for Zika Virus Serologic Testing. J. Clin. Microbiol. 2017, 55, 2462–2471. [Google Scholar] [CrossRef]
- Steinhagen, K.; Probst, C.; Radzimski, C.; Schmidt-Chanasit, J.; Emmerich, P.; van Esbroeck, M.; Schinkel, J.; Grobusch, M.P.; Goorhuis, A.; Warnecke, J.M.; et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: A multicohort study of assay performance, 2015 to 2016. Eurosurveillance 2016, 21, 30426. [Google Scholar] [CrossRef] [PubMed]
- Michelson, Y.; Lustig, Y.; Avivi, S.; Schwartz, E.; Danielli, A. Highly Sensitive and Specific Zika Virus Serological Assays Using a Magnetic Modulation Biosensing System. J. Infect. Dis. 2019, 219, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.J.M.; George, J.K.; Velasco, M.; Bonaparte, M.I.; Zheng, L.; DiazGranados, C.A.; Marques, E.T.A.; Huleatt, J.W. Development of an anti-dengue NS1 IgG ELISA to evaluate exposure to dengue virus. J. Virol. Methods 2018, 257, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, D.; Gomes, L.; Jeewandara, C.; Jayarathna, G.S.B.; Herath, D.; Perera, P.A.; Fernando, S.; Wijewickrama, A.; Hardman, C.S.; Ogg, G.S.; et al. Role of NS1 antibodies in the pathogenesis of acute secondary dengue infection. Nat. Commun. 2018, 9, 5242. [Google Scholar] [CrossRef]
- Galula, J.U.; Salem, G.M.; Destura, R.V.; Remenyi, R.; Chao, D.-Y. Comparable Accuracies of Nonstructural Protein 1- and Envelope Protein-Based Enzyme-Linked Immunosorbent Assays in Detecting Anti-Dengue Immunoglobulin G Antibodies. Diagnostics 2021, 11, 741. [Google Scholar] [CrossRef]
- Tyson, J.; Tsai, W.Y.; Tsai, J.J.; Brites, C.; Mässgård, L.; Ha Youn, H.; Pedroso, C.; Drexler, J.F.; Stramer, S.L.; Balmaseda, A.; et al. Combination of Nonstructural Protein 1-Based Enzyme-Linked Immunosorbent Assays Can Detect and Distinguish Various Dengue Virus and Zika Virus Infections. J. Clin. Microbiol. 2019, 57, e01464-18. [Google Scholar] [CrossRef]
- Tsai, W.-Y.; Youn, H.H.; Brites, C.; Tsai, J.-J.; Tyson, J.; Pedroso, C.; Drexler, J.F.; Stone, M.; Simmons, G.; Busch, M.P.; et al. Distinguishing Secondary Dengue Virus Infection from Zika Virus Infection with Previous Dengue by a Combination of 3 Simple Serological Tests. Clin. Infect. Dis. 2017, 65, 1829–1836. [Google Scholar] [CrossRef]
- Lustig, Y.; Keler, S.; Kolodny, R.; Ben-Tal, N.; Atias-Varon, D.; Shlush, E.; Gerlic, M.; Munitz, A.; Doolman, R.; Asraf, K.; et al. Potential Antigenic Cross-reactivity Between Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Dengue Viruses. Clin. Infect. Dis. 2020, 73, e2444–e2449. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chao, C.H.; Lai, Y.C.; Hsieh, K.H.; Wang, J.R.; Wan, S.W.; Huang, H.J.; Chuang, Y.C.; Chuang, W.J.; Yeh, T.M. Antibodies against the SARS-CoV-2 S1-RBD cross-react with dengue virus and hinder dengue pathogenesis. Front. Immunol. 2022, 13, 941923. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Recombinant Protein-Based Dengue Vaccines. Front. Immunol. 2018, 9, 1919. [Google Scholar] [CrossRef]
- Burg, S.; Roth, S.; Cohen, M.; Avivi-Mintz, S.; Margulis, M.; Rohana, H.; Peretz, A.; Danielli, A. High throughput optical modulation biosensing for highly sensitive and rapid detection of biomarkers. Talanta 2022, 248, 123624. [Google Scholar] [CrossRef] [PubMed]
- Margulis, M.; Rohana, H.; Erster, O.; Mandelboim, M.; Biber, A.; Schwartz, E.; Peretz, A.; Danielli, A. Highly sensitive extraction-free saliva-based molecular assay for rapid diagnosis of SARS-CoV-2. J. Clin. Microbiol. 2024, 62, e0060024. [Google Scholar] [CrossRef] [PubMed]
- Avivi-Mintz, S.; Lustig, Y.; Indenbaum, V.; Schwartz, E.; Danielli, A. Highly Sensitive and Specific SARS-CoV-2 Serological Assay Using a Magnetic Modulation Biosensing System. Biosensors 2022, 12, 7. [Google Scholar]
- Margulis, M.; Cohen, M.; Burg, S.; Avivi-Mintz, S.; Danielli, A. Optical modulation biosensing system for rapid detection of biological targets at low concentrations. Biomed. Opt. Express 2021, 12, 5338–5350. [Google Scholar] [CrossRef]
- Hamilton, R.G. Assay Performance Parameters. In Encyclopedia of Medical Immunology: Allergic Diseases; Mackay, I.R., Rose, N.R., Ledford, D.K., Lockey, R.F., Eds.; Springer: New York, NY, USA, 2014; pp. 80–84. [Google Scholar]
- Musso, D.; Desprès, P. Serological Diagnosis of Flavivirus-Associated Human Infections. Diagnostics 2020, 10, 302. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Diagnostic tests. 1: Sensitivity and specificity. BMJ 1994, 308, 1552. [Google Scholar] [CrossRef]
- Borysiak, M.D.; Thompson, M.J.; Posner, J.D. Translating diagnostic assays from the laboratory to the clinic: Analytical and clinical metrics for device development and evaluation. Lab Chip 2016, 16, 1293–1313. [Google Scholar] [CrossRef]
- Matsunaga, K.I.; Kimoto, M.; Lim, V.W.; Thein, T.L.; Vasoo, S.; Leo, Y.S.; Sun, W.; Hirao, I. Competitive ELISA for a serologic test to detect dengue serotype-specific anti-NS1 IgGs using high-affinity UB-DNA aptamers. Sci. Rep. 2021, 11, 18000. [Google Scholar] [CrossRef]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef]
- Rastogi, M.; Sharma, N.; Singh, S.K. Flavivirus NS1: A multifaceted enigmatic viral protein. Virol. J. 2016, 13, 131. [Google Scholar] [CrossRef]
- Thomas, S.J. Is new dengue vaccine efficacy data a relief or cause for concern? npj Vaccines 2023, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Tricou, V.; Sáez-Llorens, X.; Yu, D.; Rivera, L.; Jimeno, J.; Villarreal, A.C.; Dato, E.; Saldaña de Suman, O.; Montenegro, N.; DeAntonio, R.; et al. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2–17 years: A randomised, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1434–1443. [Google Scholar] [CrossRef]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Ismail, H.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Balmaseda, A.; Stettler, K.; Medialdea-Carrera, R.; Collado, D.; Jin, X.; Zambrana, J.V.; Jaconi, S.; Cameroni, E.; Saborio, S.; Rovida, F.; et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 8384–8389. [Google Scholar] [CrossRef] [PubMed]



| DENV Kit | No. of IgG+/Total Samples (%) in Different Serum Panels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Naive | Positive DENV-1 | Positive DENV-2 ISR | Positive DENV-2 VIE | Positive DENV-2 Combine | Positive DENV-1 +DENV-2 Combine | Positive WNV | Positive JEV | Positive ZIKV | Positive SARS-CoV-2 | |
| DENV-1 kit | 0/35 (0) | 14/14 (100) | 7/9 (78) | 41/51 (80) | 48/60 (80) | 62/74 (84) | 8/23 (35) | 6/28 (21) | 17/26 (65) | 0/22 (0) |
| DENV-2 kit | 0/35 (0) | 9/11 (82) | 9/9 (100) | 20/20 (100) | 29/29 (100) | 38/40 (95) | Not tested | Not tested | Not tested | Not tested |
| DENV-3 kit | 0/35 (0) | 7/11 (64) | 8/8 (100) | 18/21 (86) | 26/29 (90) | 33/40 (83) | Not tested | Not tested | 16/26 (62) | Not tested |
| Paper Author | Assay and Technology Type | Limit of Detection |
|---|---|---|
| Tsai et al. (2017) [39] | Anti-DENV-1 NS1 IgG ELISA | Not reported |
| Nascimento et al. (2018) [35] | Pan-DENV anti-NS1 IgG ELISA | 2.33 EU/mL |
| Jayathilaka et al. (2018) [36] | Anti-DENV-1 and anti-DENV-2 NS1 IgG ELISA | Not reported |
| Tyson et al. (2019) [38] | Anti-DENV-2–4 NS1 IgG ELISA and Pan-DENV anti-NS1 IgG ELISA | Not reported |
| Galula et al. (2021) [37] | Pan-DENV anti-NS1 IgG ELISA | Indirect ELISA: 1030–1260 ng/L GAC-ELISA: 1380–1870 ng/L |
| Matsunaga et al. (2021) [51] | Anti-DENV-1–4 NS1 IgG ELISA and Pan-DENV anti-NS1 IgG ELISA Competitive ELISA | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terenteva, S.; Golani-Zaidie, L.; Avivi, S.; Lustig, Y.; Indenbaum, V.; Koren, R.; Hoa, T.M.; Tuyen, T.T.K.; Huyen, M.T.; Hoan, N.M.; et al. Sensitivity and Cross-Reactivity Analysis of Serotype-Specific Anti-NS1 Serological Assays for Dengue Virus Using Optical Modulation Biosensing. Biosensors 2025, 15, 453. https://doi.org/10.3390/bios15070453
Terenteva S, Golani-Zaidie L, Avivi S, Lustig Y, Indenbaum V, Koren R, Hoa TM, Tuyen TTK, Huyen MT, Hoan NM, et al. Sensitivity and Cross-Reactivity Analysis of Serotype-Specific Anti-NS1 Serological Assays for Dengue Virus Using Optical Modulation Biosensing. Biosensors. 2025; 15(7):453. https://doi.org/10.3390/bios15070453
Chicago/Turabian StyleTerenteva, Sophie, Linoy Golani-Zaidie, Shira Avivi, Yaniv Lustig, Victoria Indenbaum, Ravit Koren, Tran Mai Hoa, Tong Thi Kim Tuyen, Ma Thi Huyen, Nguyen Minh Hoan, and et al. 2025. "Sensitivity and Cross-Reactivity Analysis of Serotype-Specific Anti-NS1 Serological Assays for Dengue Virus Using Optical Modulation Biosensing" Biosensors 15, no. 7: 453. https://doi.org/10.3390/bios15070453
APA StyleTerenteva, S., Golani-Zaidie, L., Avivi, S., Lustig, Y., Indenbaum, V., Koren, R., Hoa, T. M., Tuyen, T. T. K., Huyen, M. T., Hoan, N. M., Hoi, L. T., Trung, N. V., Schwartz, E., & Danielli, A. (2025). Sensitivity and Cross-Reactivity Analysis of Serotype-Specific Anti-NS1 Serological Assays for Dengue Virus Using Optical Modulation Biosensing. Biosensors, 15(7), 453. https://doi.org/10.3390/bios15070453






