Laser-Induced Solid-Phase UV Fluorescence Spectroscopy for Rapid Detection of Polycyclic Aromatic Hydrocarbons in the Land Snail Bioindicator, Cantareus aspersus
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material (Snails)
2.2. Chemicals
2.3. Exposure Procedure
2.4. Spectrofluorescence Measurements
2.5. Statistical Analysis
3. Results
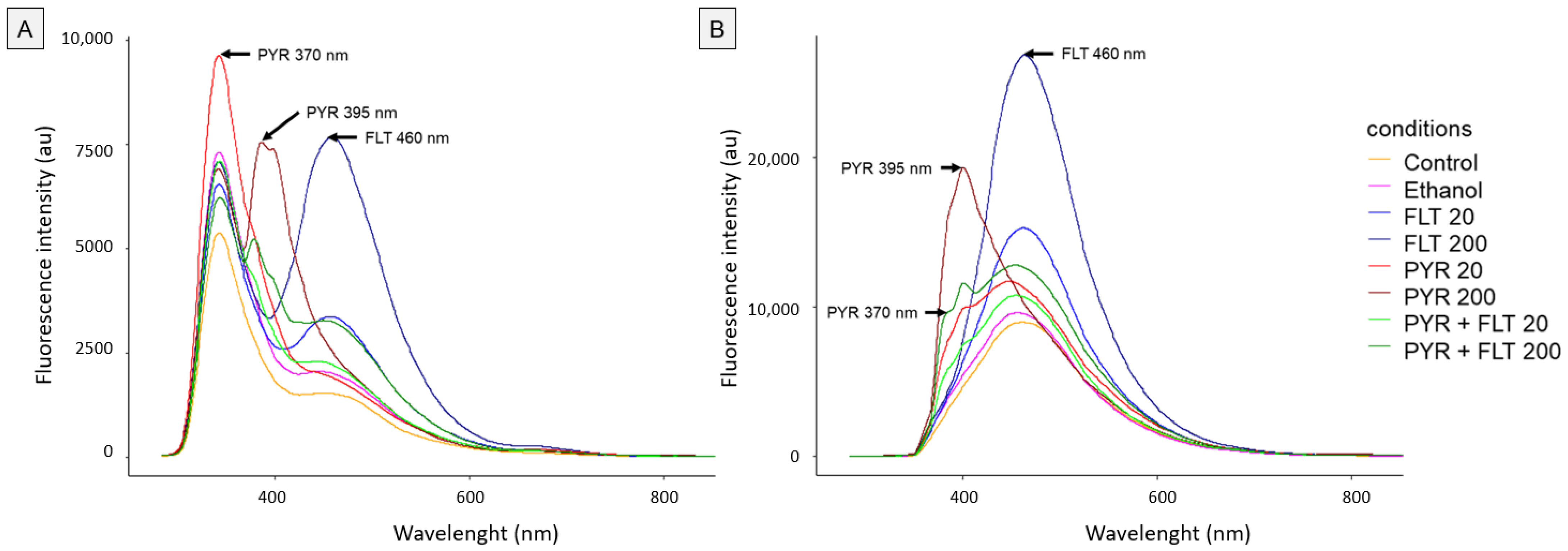
3.1. Fluorescence Spectrum Analysis
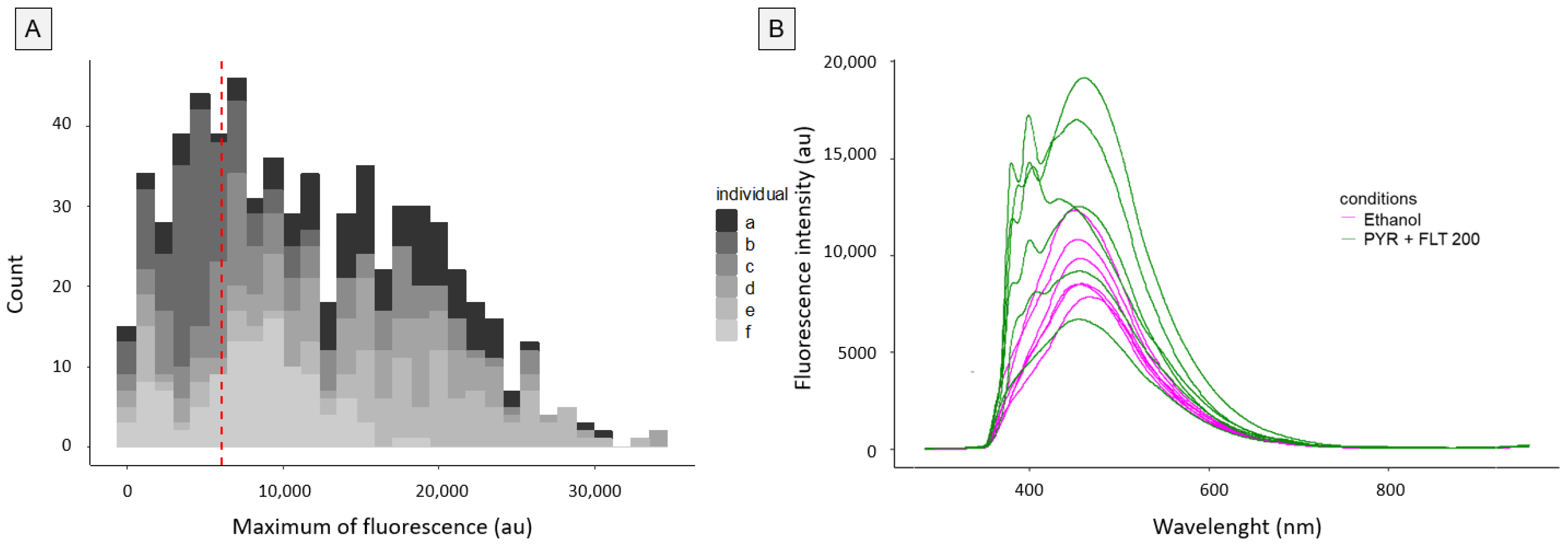
3.2. Inter-Individual and Intra-Individual Variability
3.3. Relationship Between Exposure Concentrations and Fluorescence Intensity
4. Discussion
4.1. Inter-Individual Variability of PAH Bioaccumulation
4.2. Application in Environmental Risk Assessment
4.3. Limitations of the Study
4.4. Future Milestones and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suter, G.; Cormier, S.; Barron, M. A weight of evidence framework for environmental assessments: Inferring quantities. Integr. Environ. Assess. Manag. 2017, 13, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Louzon, M.; Pauget, B.; Gimbert, F.; Morin-Crini, N.; Wong, J.W.Y.; Zaldibar, B.; Natal-da-Luz, T.; Neuwirthova, N.; Thiemann, C.; Sarrazin, B.; et al. In situ and ex situ bioassays with Cantareus aspersus for environmental risk assessment of metal(loid) and PAH-contaminated soils. Integr. Environ. Assess. Manag. 2021, 18, 539–554. [Google Scholar] [CrossRef] [PubMed]
- ISO 17402:2008; Soil Quality—Requirements and Guidance for the Selection and Application of Methods for the Assessment of Bioavailability of Contaminants in Soil and Soil Materials. ISO Standards: Geneva, Switzerland, 2008.
- ISO 19204:2017; Soil Quality—Procedure for Site-Specific Ecological Risk Assessment of Soil Contamination (Soil Quality TRIAD Approach). ISO Standards: Geneva, Switzerland, 2017.
- Louzon, M.; Pauget, B.; Gimbert, F.; Morin-Crini, N.; de Vaufleury, A. Ex situ environmental risk assessment of polluted soils using threshold guide values for the land snail Cantareus aspersus. Sci. Total Environ. 2020, 721, 137789. [Google Scholar] [CrossRef]
- Cachada, A.; Pereira, R.; Da Silva, E.F.; Duarte, A.C. The prediction of PAHs bioavailability in soils using chemical methods: State of the art and future challenges. Sci. Total Environ. 2014, 472, 463–480. [Google Scholar] [CrossRef]
- ISO 24032:2021; Soil Quality—In Situ Caging of Snails to Assess Bioaccumulation of Contaminants. ISO Standards: Geneva, Switzerland, 2021.
- Al-Alam, J.; Baroudi, F.; Chbani, A.; Fajloun, Z.; Millet, M. A multiresidue method for the analysis of pesticides, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls in snails used as environmental biomonitors. J. Chromatogr. A 2020, 1621, 461006. [Google Scholar] [CrossRef]
- Kim, L.; Lee, D.; Cho, H.-K.; Choi, S.-D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, e00063. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Brunt, M.W.; Kreiberg, H.; von Keyserlingk, M.A.G. Invertebrate research without ethical or regulatory oversight reduces public confidence and trust. Humanit. Soc. Sci. Commun. 2022, 9, 250. [Google Scholar] [CrossRef]
- Milori, D.M.B.P.; Galeti, H.V.A.; Martin-Neto, L.; Dieckow, J.; González-Pérez, M.; Bayer, C.; Salton, J. Organic Matter Study of Whole Soil Samples Using Laser-Induced Fluorescence Spectroscopy. Soil Sci. Soc. Am. J. 2006, 70, 57–63. [Google Scholar] [CrossRef]
- Taylor, A.T.; Lai, E.P.C. Current State of Laser-Induced Fluorescence Spectroscopy for Designing Biochemical Sensors. Chemosensors 2021, 9, 275. [Google Scholar] [CrossRef]
- Longchamps, L.; Mandal, D.; Khosla, R. Assessment of Soil Fertility Using Induced Fluorescence and Machine Learning. Sensors 2022, 22, 4644. [Google Scholar] [CrossRef] [PubMed]
- Zacharioudaki, D.-E.; Fitilis, I.; Kotti, M. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef] [PubMed]
- Schultze, R.H.; Lemke, M.; Löhmannsröben, H.-G. Laser-Induced Fluorescence (LIF) Spectroscopy for the In Situ Analysis of Petroleum Product-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Motelay-Massei, A.; Ollivon, D.; Garban, B.; Teil, M.J.; Blanchard, M.; Chevreuil, M. Distribution and spatial trends of PAHs and PCBs in soils in the Seine River basin, France. Chemosphere 2004, 55, 555–565. [Google Scholar] [CrossRef]
- Garagnon, J.; Perrette, Y.; Naffrechoux, E.; Pons-Branchu, E. Polycyclic aromatic hydrocarbon record in an urban secondary carbonate deposit over the last three centuries (Paris, France). Sci. Total Environ. 2023, 905, 167429. [Google Scholar] [CrossRef]
- Wallet, T. Complémentarité des Méthodes Spectroscopiques en Phase Solide et des Modèles Chimiométriques Pour la Quantification In Situ des HAPs Dans les Sols; Université Savoie Mont Blanc: Chambéry, France, 2024. [Google Scholar]
- Chen, S.; Inskeep, W.P.; Williams, S.A.; Callis, P.R. Fluorescence lifetime measurements of fluoranthene, 1-naphthol, and napropamide in the presence of dissolved humic acid. Environ. Sci. Technol. 1994, 28, 1582–1588. [Google Scholar] [CrossRef]
- Schwarz, F.P.; Wasik, S.P. Fluorescence measurements of benzene, naphthalene, anthracene, pyrene, fluoranthene, and benzo[e]pyrene in water. Anal. Chem. 1976, 48, 524–528. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Louzon, M.; Amiot, C.; de Vaufleury, A. QuEChERS applicability to measure land snail polycyclic aromatic hydrocarbons for risk assessment. Toxicol. Environ. Chem. 2020, 102, 209–223. [Google Scholar] [CrossRef]
- Beach, D.G.; Hellou, J. Bioaccumulation and biotransformation of 1-hydroxypyrene by the marine whelk Neptunea lyrata. Int. J. Environ. Anal. Chem. 2011, 91, 1227–1243. [Google Scholar] [CrossRef]
- Beach, D.G.; Quilliam, M.A.; Rouleau, C.; Croll, R.P.; Hellou, J. Bioaccumulation and biotransformation of pyrene and 1-hydroxypyrene by the marine whelk Buccinum undatum. Environ. Toxicol. Chem. 2009, 29, 779–788. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L. Sorption of Polycyclic Aromatic Hydrocarbons to Carbohydrates and Lipids of Ryegrass Root and Implications for a Sorption Prediction Model. Environ. Sci. Technol. 2009, 43, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, P.; Chandler, G. A laboratory and field comparison of sediment polycyclic aromatic hydrocarbon bioaccumulation by the cosmopolitan estuarine polychaete Streblospio benedicti (Webster). Mar. Environ. Res. 1998, 45, 387–401. [Google Scholar] [CrossRef]
- Beninger, P.G. Seasonal variations of the major lipid classes in relation to the reproductive activity of two species of clams raised in a common habitat: Tapes decussatus L. (Jeffreys, 1863) and T. philippinarum (Adams & Reeve, 1850). J. Exp. Mar. Biol. Ecol. 1984, 79, 79–90. [Google Scholar] [CrossRef]
- Meador, J.P.; Stein, J.E.; Reichert, W.L.; Varanasi, U. Bioaccumulation of Polycyclic Aromatic Hydrocarbons by Marine Organisms; Springer: New York, NY, USA, 1995. [Google Scholar]
- Narváez, M.; Freites, L.; Guevara, M.; Mendoza, J.; Guderley, H.; Lodeiros, C.J.; Salazar, G. Food availability and reproduction affects lipid and fatty acid composition of the brown mussel, Perna perna, raised in suspension culture. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2008, 149, 293–302. [Google Scholar] [CrossRef]
- Ismert, M.; Oster, T.; Bagrel, D. Effects of atmospheric exposure to naphthalene on xenobiotic-metabolising enzymes in the snail Helix aspersa. Chemosphere 2002, 46, 273–280. [Google Scholar] [CrossRef]
- Giessing, A.M.; Mayer, L.M.; Forbes, T.L. Synchronous fluorescence spectrometry of 1-hydroxypyrene: A rapid screening method for identification of PAH exposure in tissue from marine polychaetes. Mar. Environ. Res. 2003, 56, 599–615. [Google Scholar] [CrossRef]
- Madrid, F.; Florido, M.C.; Rubio-Bellido, M.; Villaverde, J.; Morillo, E. Dissipation of a mix of priority PAHs in soils by using availability enhancers. Effect of aging and pollutant interactions. Sci. Total Environ. 2022, 837, 155744. [Google Scholar] [CrossRef]
- Louzon, M.; Devalloir, Q.; Gimbert, F.; Pauget, B.; Rieffel, D.; de Vaufleury, A. From bioavailability to risk assessment of polluted soil using snails: Link between excess transfer and inhibition of sexual maturation. Environ. Sci. Pollut. Res. 2021, 28, 17343–17354. [Google Scholar] [CrossRef]
- Louzon, M.; Gimbert, F.; Belly, T.; Amiot, C.; Pauget, B.; de Vaufleury, A.; Capelli, N. From environmental bioavailability of metal(loid)s to their ecogenotoxicological effects in land snails. Environ. Sci. Pollut. Res. 2021, 28, 43629–43642. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green Analytical Chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F. Chemometric Methods for Spectroscopy-Based Pharmaceutical Analysis. Front. Chem. 2018, 6, 576. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Mamede, R.; Carreiras, J.; Duarte, I.A.; Caçador, I.; Reis-Santos, P.; Vasconcelos, R.P.; Gameiro, C.; Ré, P.; Tanner, S.E.; et al. Harnessing the Full Power of Chemometric-Based Analysis of Total Reflection X-ray Fluorescence Spectral Data to Boost the Identification of Seafood Provenance and Fishing Areas. Foods 2022, 11, 2699. [Google Scholar] [CrossRef]
- El Maouardi, M.; Mansouri, M.A.; De Braekeleer, K.; Bouklouze, A.; Heyden, Y.V. Evaluation of Multivariate Filters on Vibrational Spectroscopic Fingerprints for the PLS-DA and SIMCA Classification of Argan Oils from Four Moroccan Regions. Molecules 2023, 28, 5698. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, Y.; Choe, Y.; Kim, S.H.; Cho, J.; Kim, C.; Kim, K.-N. Exposure to polycyclic aromatic hydrocarbons, heavy metals, and per- and polyfluoroalkyl substances and their associations with serum lipid profiles in the general Korean adult population. Environ. Health 2025, 24, 30. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Keinänen, M.; Vuontisjärvi, H.; Baršienė, J.; Broeg, K.; Förlin, L.; Gercken, J.; Kopecka, J.; Köhler, A.; Parkkonen, J.; et al. Use of biliary PAH metabolites as a biomarker of pollution in fish from the Baltic Sea. Mar. Pollut. Bull. 2006, 53, 479–487. [Google Scholar] [CrossRef]
- Wu, M.-T.; Simpson, C.D.; Christiani, D.C.; Hecht, S.S. Relationship of Exposure to Coke-Oven Emissions and Urinary Metabolites of Benzo(a)pyrene and Pyrene in Coke-Oven Workers. Cancer Epidemiol. Biomark. Prev. 2002, 11, 311–314. [Google Scholar]
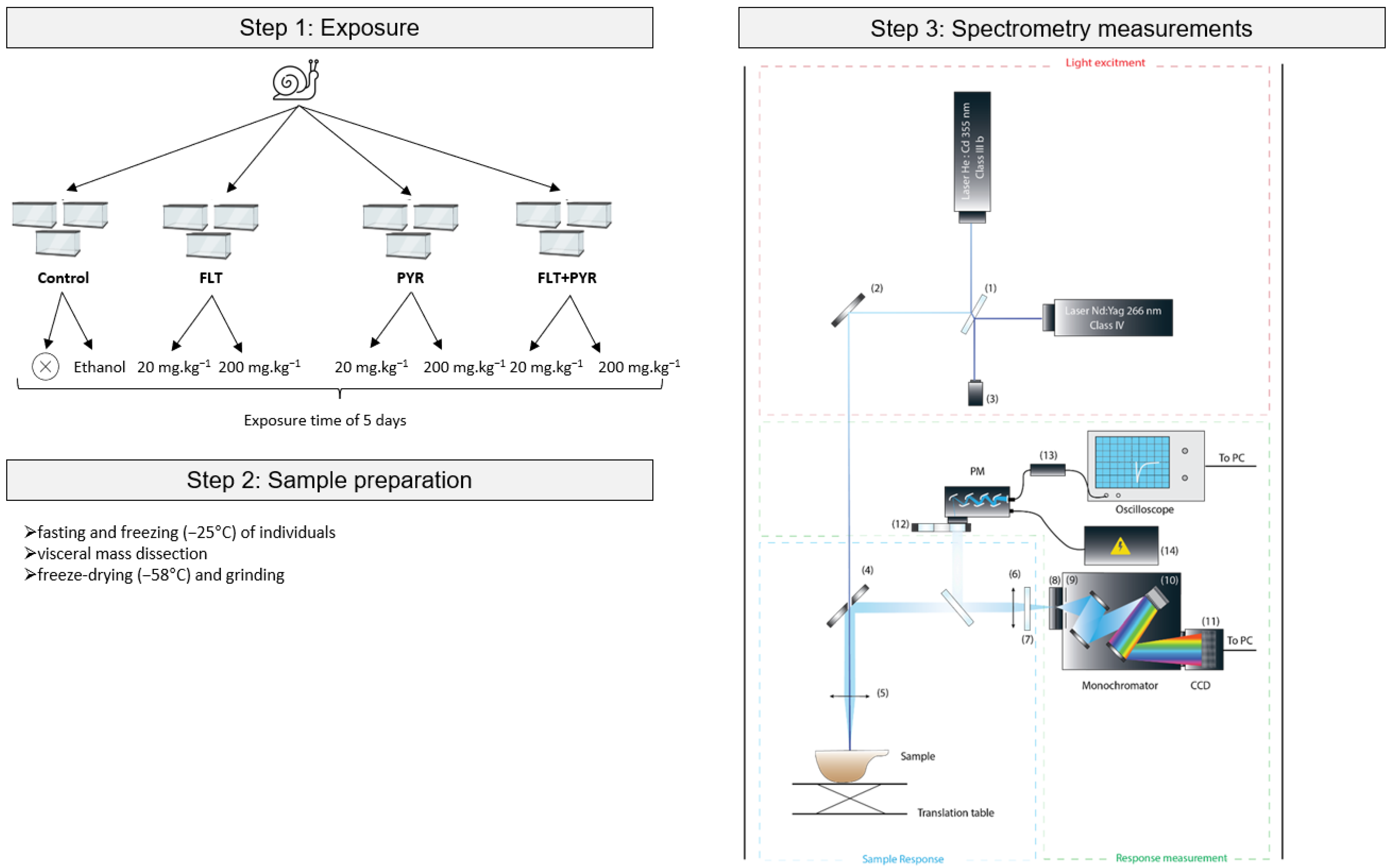


| Condition Names | Spiked Concentrations by the Dosed Addition Method (mg·kg−1 Dry Weight) | PAH Spiked | Ethanol Used for Spiking | Number of Replicates (Faunarium) | Number of Snails per Replicate (Faunarium) |
|---|---|---|---|---|---|
| Control | 0 | No | No | 3 | 6 |
| Ethanol | 0 | No | Yes | 3 | 6 |
| PYR20 | 20 | PYR | Yes | 3 | 6 |
| PYR200 | 200 | PYR | Yes | 3 | 6 |
| FLT20 | 20 | FLT | Yes | 3 | 6 |
| FLT200 | 200 | FLT | Yes | 3 | 6 |
| PYR+FLT20 | 20 | PYR+FLT | Yes | 3 | 6 |
| PYR+FLT200 | 200 | PYR+FLT | Yes | 3 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louzon, M.; Bertoncini, T.; Casañas, N.; Perrette, Y.; Plassart, G.; Quiers, M.; Wallet, T.; Kamel, M.; Aleya, L. Laser-Induced Solid-Phase UV Fluorescence Spectroscopy for Rapid Detection of Polycyclic Aromatic Hydrocarbons in the Land Snail Bioindicator, Cantareus aspersus. Biosensors 2025, 15, 450. https://doi.org/10.3390/bios15070450
Louzon M, Bertoncini T, Casañas N, Perrette Y, Plassart G, Quiers M, Wallet T, Kamel M, Aleya L. Laser-Induced Solid-Phase UV Fluorescence Spectroscopy for Rapid Detection of Polycyclic Aromatic Hydrocarbons in the Land Snail Bioindicator, Cantareus aspersus. Biosensors. 2025; 15(7):450. https://doi.org/10.3390/bios15070450
Chicago/Turabian StyleLouzon, Maxime, Thomas Bertoncini, Noah Casañas, Yves Perrette, Gaël Plassart, Marine Quiers, Tanguy Wallet, Mohamed Kamel, and Lotfi Aleya. 2025. "Laser-Induced Solid-Phase UV Fluorescence Spectroscopy for Rapid Detection of Polycyclic Aromatic Hydrocarbons in the Land Snail Bioindicator, Cantareus aspersus" Biosensors 15, no. 7: 450. https://doi.org/10.3390/bios15070450
APA StyleLouzon, M., Bertoncini, T., Casañas, N., Perrette, Y., Plassart, G., Quiers, M., Wallet, T., Kamel, M., & Aleya, L. (2025). Laser-Induced Solid-Phase UV Fluorescence Spectroscopy for Rapid Detection of Polycyclic Aromatic Hydrocarbons in the Land Snail Bioindicator, Cantareus aspersus. Biosensors, 15(7), 450. https://doi.org/10.3390/bios15070450






