CRISPR/Cas12a-Chemiluminescence Cascaded Bioassay for Amplification-Free and Sensitive Detection of Nucleic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
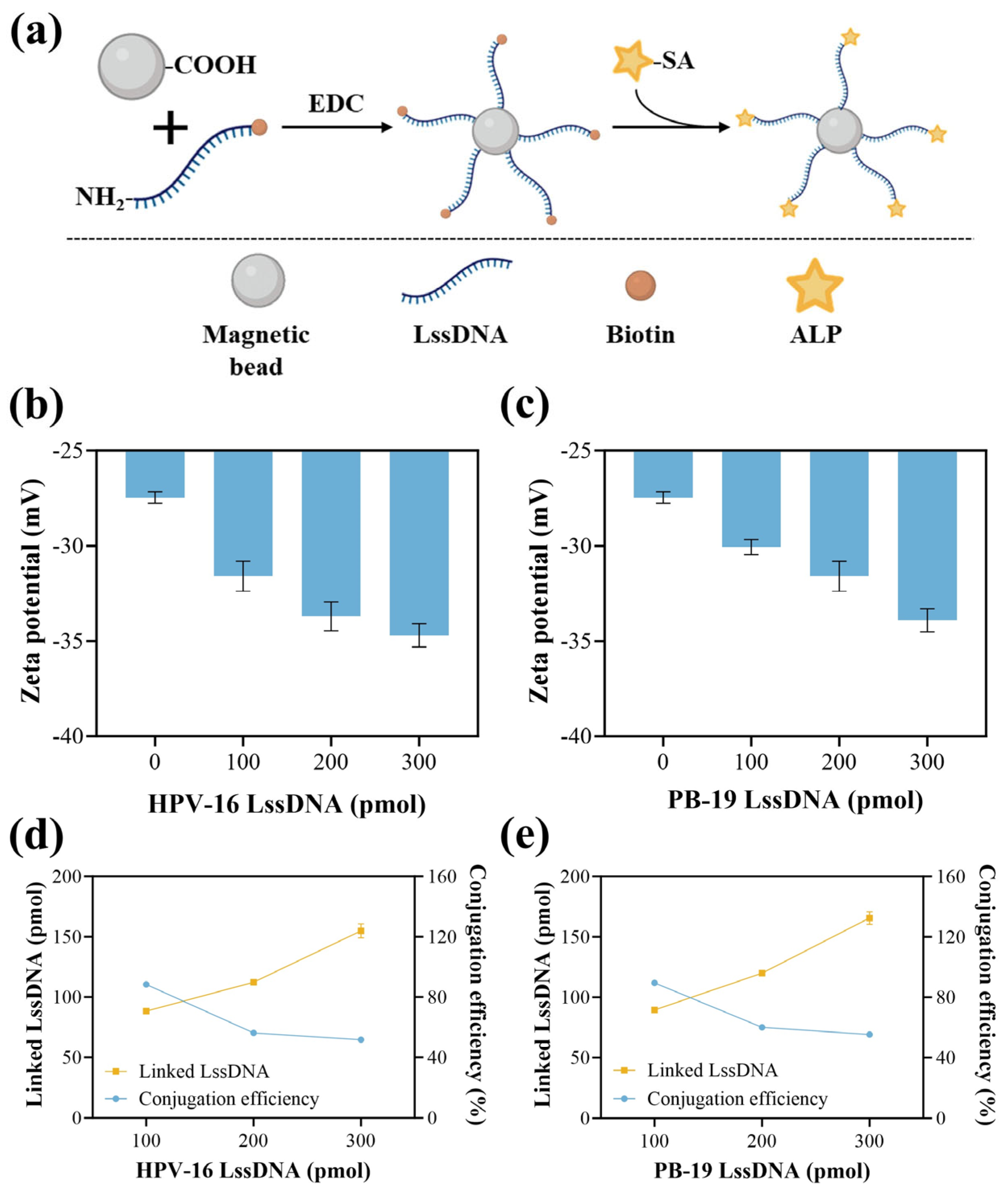
2.2. Preparation and Characterization of MB-Linking ssDNA-ALP
2.3. Procedure of Nucleic Acid Detection
2.4. Clinical Sample Collection and Processing
2.5. Data Analysis and Statistical Testing
3. Results and Discussion
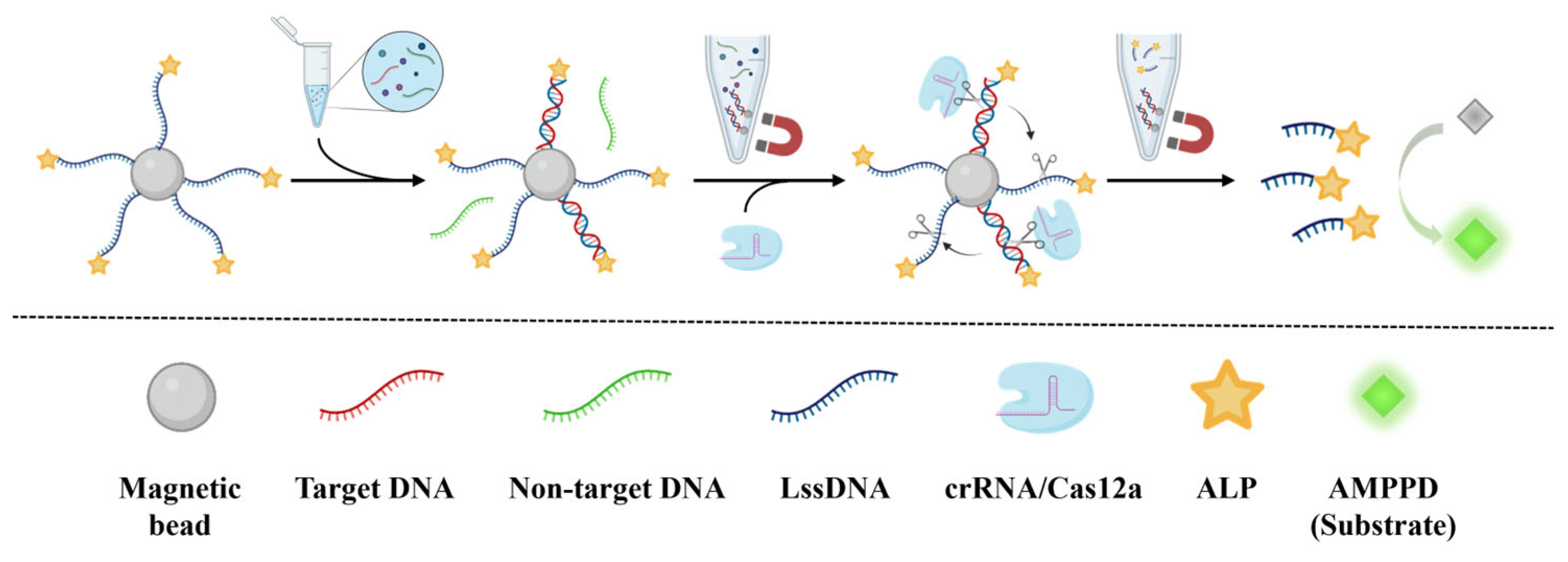
3.1. Principle of CCCB for Nucleic Acid Detection
3.2. Characterization of MB-LssDNA
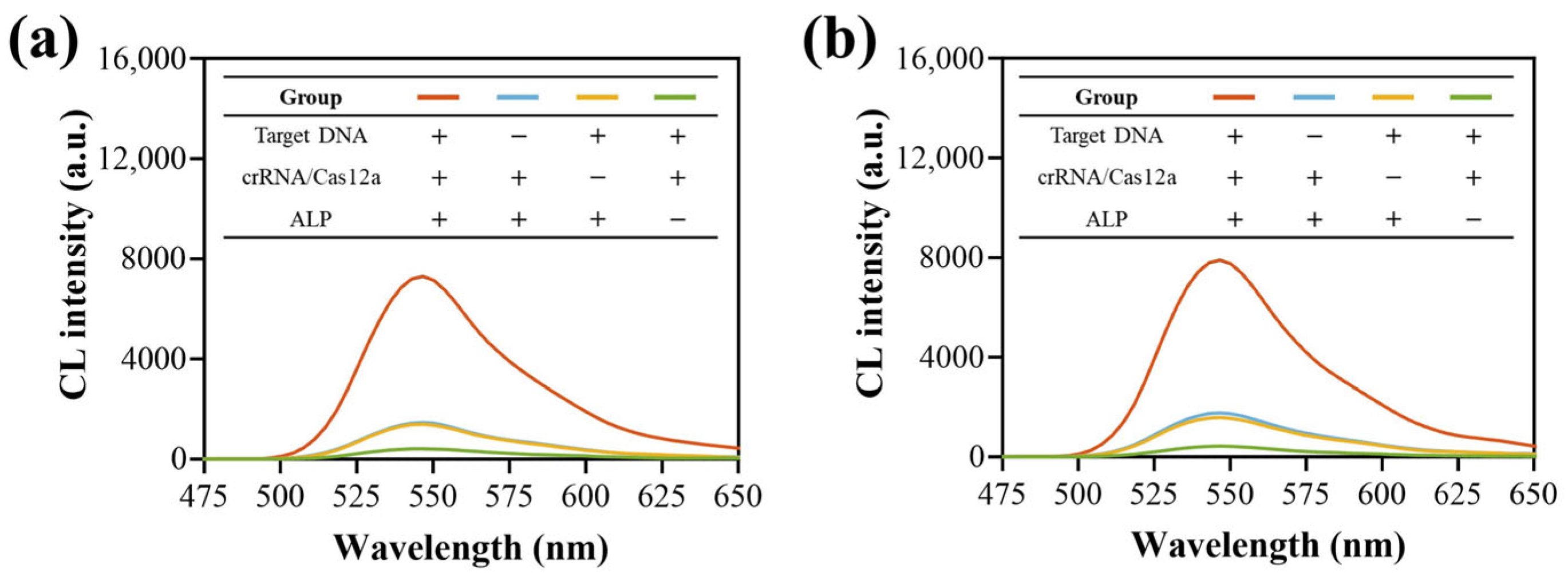
3.3. Feasibility Verification
3.4. Optimization of Experimental Conditions
3.5. Key Performance Verification
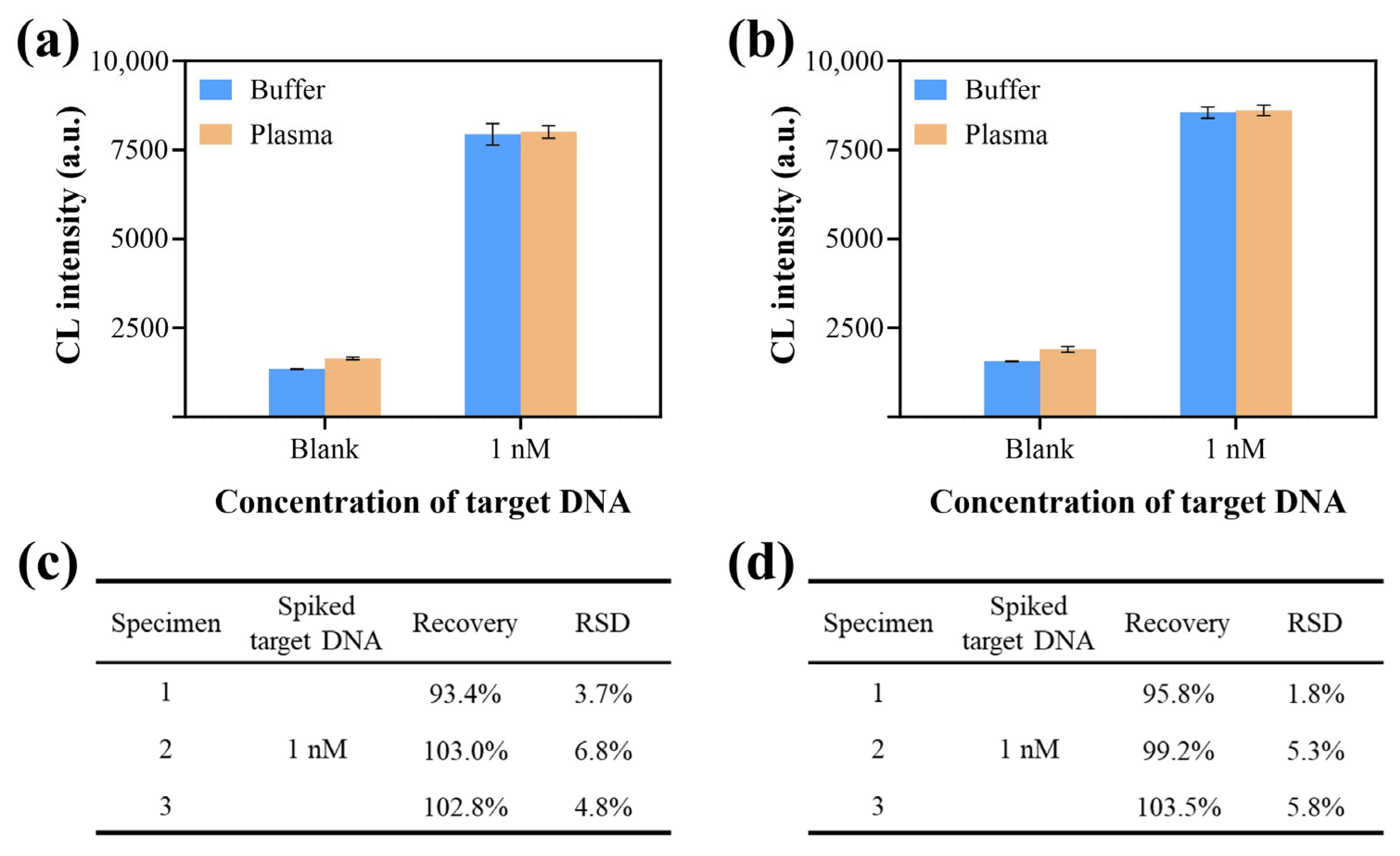
3.6. Application of CCCB in Clinical Specimens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hans, R.; Marwaha, N. Nucleic acid testing-benefits and constraints. Asian J. Transfus. Sci. 2014, 8, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Rowley, J.; Alemany, L.; Arbyn, M.; Giuliano, A.R.; Markowitz, L.E.; Broutet, N.; Taylor, M. Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, E1345–E1362. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses 2023, 15, 1440. [Google Scholar] [CrossRef] [PubMed]
- Nordholm, A.C.; Malled, F.T.; Ravn, S.F.; Sliirensen, L.F.; Moltke-Prehn, A.; Mollerup, J.E.; Funk, T.; Sperling, L.; Jeyaratnam, U.; Franck, K.T.; et al. Epidemic of parvovirus B19 and disease severity in pregnant people, Denmark, January to March 2024. Eurosurveillance 2024, 29, 2400299. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, F.P.; Guimaraes, C.D.; Peixoto, A.B.; Pontes, K.F.; Bonasoni, M.P.; Tonni, G.; Araujo, E., Jr. Parvovirus B19 Infection and Pregnancy: Review of the Current Knowledge. J. Pers. Med. 2024, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Kostiuk, M.; Biron, V.L. Molecular Detection Methods in HPV-Related Cancers. Front. Oncol. 2022, 12, 864820. [Google Scholar] [CrossRef] [PubMed]
- Krsek, A.; Baticic, L.; Braut, T.; Sotosek, V. The Next Chapter in Cancer Diagnostics: Advances in HPV-Positive Head and Neck Cancer. Biomolecules 2024, 14, 925. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Guan, X.T.; Sun, S.Q. Microfluidic Biosensors: Enabling Advanced Disease Detection. Sensors 2025, 25, 1936. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Li, Y.C.; Liu, R.R.; Wei, W.P.; Ding, C.P. Development of a sensitive and specific nanoparticle-assisted PCR assay for detecting HPV-16 and HPV-18 DNA. J. Med. Virol. 2020, 92, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Pauschinger, M.; Noutsias, M.; Seeberg, B.; Bock, T.; Lassner, D.; Poller, W.; Kandolf, R.; Schultheiss, H.P. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, R.; Farshadpour, F.; Norozi, M. Molecular Evaluation of Human Parvovirus B19 Infection and Associated Risk Factors among Pregnant Women in Bushehr Province, Southern Iran. Am. J. Trop. Med. Hyg. 2022, 106, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.H.; Zhou, J.; Jiang, Y.J.; An, P.; Luo, B.; Lan, F.; Ying, B.W.; Wu, Y. DNA tetrahedron-based CRISPR bioassay for treble-self-amplified and multiplex HPV-DNA detection with elemental tagging. Biosens. Bioelectron. 2023, 229, 115229. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.P.; Peng, L.J.; Wu, J.; Khan, A.; Sun, Y.F.; Shen, H.; Li, Z.Y. Clustered Regularly Interspaced Short Palindromic Repeats-Associated Proteins13a combined with magnetic beads, chemiluminescence and reverse transcription-recombinase aided amplification for detection of avian influenza a (H7N9) virus. Front. Bioeng. Biotechnol. 2023, 10, 1094028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Li, L.L.; Cao, X.L.; Li, P.; Du, J.; Zou, M.J.; Wang, L.L. Clinical application of amplification-based versus amplification-free metagenomic next-generation sequencing test in infectious diseases. Front. Cell. Infect. Microbiol. 2023, 13, 1138174. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Wang, S.Y.; Deng, X.B.; Zhang, C.H. Catalytic Hairpin Assembly-Based Self-Ratiometric Gel Electrophoresis Detection Platform for Reliable Nucleic Acid Analysis. Biosensors 2024, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Liu, Y.J.; Tang, X.Q.; Qiao, J.L.; Kou, J.; Man, S.L.; Zhu, L.; Ma, L. CRISPR/Cas-Powered Amplification-Free Detection of Nucleic Acids: Current State of the Art, Challenges, and Futuristic Perspectives. ACS Sens. 2023, 8, 4420–4441. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, J.; Xiao, B.; Chen, A.L. Microfluidic-assisted integrated nucleic acid test strips for POCT. Talanta 2024, 267, 125150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.Y.; Wang, J.; Liu, G. CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.T.; Zhao, J.R.; Sha, Z.; Liang, Y.J.; Huang, J.H.; Zhang, J.; Sun, S.Q. CRISPR/Cas12a and aptamer-chemiluminescence based analysis for the relative abundance determination of tumor-related protein positive exosomes for breast cancer diagnosis. Biosens. Bioelectron. 2024, 259, 116380. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Zhao, K.R.; Liu, Z.J.; Wang, L.; Ye, S.Y.; Liang, G.X. Cas12a-based electrochemiluminescence biosensor for target amplification-free DNA detection. Biosens. Bioelectron. 2021, 176, 112954. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.H.; Yang, T.T.; Zhang, D.C.; Xu, L.L.; Cheng, X.X.; Ding, S.J.; Cheng, W. An all-in-one telomerase assay based on CRISPR-Cas12a trans-cleavage while telomere synthesis. Anal. Chim. Acta 2021, 1159, 338404. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Wen, R.Q.; Ma, P.; Gu, K.; Li, C.; Zhou, C.Y.; Lei, C.W.; Tang, Y.Z.; Wang, H.N. Immunocapture Magnetic Beads Enhanced the LAMP-CRISPR/Cas12a Method for the Sensitive, Specific, and Visual Detection of Campylobacter jejuni. Biosensors 2022, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.H.; Yang, N.N.; Xiong, Y.F.; Shi, M.M.; Wang, L.; Nie, F.P.; Huo, D.Q.; Hou, C.J. Completely Free from PAM Limitations: Asymmetric RPA with CRISPR/Cas12a for Nucleic Acid Assays. ACS Sens. 2023, 8, 4655–4663. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Shin, M.; Yang, L.T.; Conley, B.; Yoon, J.; Lee, S.N.; Lee, K.B.; Choi, J.W. Clustered Regularly Interspaced Short Palindromic Repeats-Mediated Amplification-Free Detection of Viral DNAs Using Surface-Enhanced Raman Spectroscopy-Active Nanoarray. ACS Nano 2021, 15, 13475–13485. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Zhan, X.H.; Shui, S.X.; Liu, Y.C.; Li, Z.Y.; Peng, Y.; Ying, B.W.; Yi, Q.Y.; Lan, F.; Wu, Y. High-activity PtIr@Fe-MOF nanozyme with low-hindrance PEA-CRISPR strategy for ultrasensitive lateral flow assay of AMI diagnosis. Chem. Eng. J. 2025, 511, 162022. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.B.; Harrington, L.B.; Da Costa, M.; Tian, X.R.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Xu, J.; Xiong, L.; Wang, S.L.; Yu, C.Z.; Lv, J.T.; Lin, J.M. Recent development of chemiluminescence for bioanalysis. Trac-Trends Anal. Chem. 2023, 166, 117213. [Google Scholar] [CrossRef]
- Yi, Z.H.; Xiao, S.; Kang, X.Z.; Long, F.; Zhu, A.N. Bifunctional MOF-Encapsulated Cobalt-Doped Carbon Dots Nanozyme-Powered Chemiluminescence/Fluorescence Dual-Mode Detection of Aflatoxin B1. ACS Appl. Mater. Interfaces 2024, 16, 16494–16504. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.C.; Ye, R.F. One-Pot Synthesis of Enzyme and Antibody/CaHPO4 Nanoflowers for Magnetic Chemiluminescence Immunoassay of Salmonella enteritidis. Sensors 2023, 23, 2779. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Li, F.Z.; Liu, W.J.; Hu, J.; Liu, M.; Zhang, C.Y. An electrostatic force-independent dephosphorylation-driven chemiluminescent biosensor for sensitive and rapid detection of poly (ADP-ribose) polymerase-1 in human breast tissues. Chem. Eng. J. 2023, 460, 141776. [Google Scholar] [CrossRef]
- Chen, L.G.; Li, J.J.; Sun, L.; Wang, H.B. Ratiometric fluorometric assay triggered by alkaline phosphatase: Proof-of-concept toward a split-type biosensing strategy for DNA detection. Talanta 2024, 271, 125703. [Google Scholar] [CrossRef] [PubMed]
- Neumair, J.; Kröger, M.; Stütz, E.; Jerin, C.; Chaker, A.M.; Schmidt-Weber, C.B.; Seidel, M. Flow-Based CL-SMIA for the Quantification of Protein Biomarkers from Nasal Secretions in Comparison with Sandwich ELISA. Biosensors 2023, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.F.; Somoza, R.A.; Wang, L.; Welter, J.F.; Li, Y.; Caplan, A.I.; Liu, C.C. Exploring the Trans-Cleavage Activity of CRISPR-Cas12a (cpf1) for the Development of a Universal Electrochemical Biosensor. Angew. Chem. Int. Ed. 2019, 58, 17399–17405. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Ke, Y.Q.; Maboyi, N.; Zhi, X.; Yan, S.J.; Li, F.W.; Zhao, B.; Jia, X.L.; Song, S.P.; Ding, X.T. CRISPR/Cas12a Powered DNA Framework-Supported Electrochemical Biosensing Platform for Ultrasensitive Nucleic Acid Analysis. Small Methods 2021, 5, 2100935. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.X.; Wu, Y.L.; Zeng, R.J.; Zeng, Y.Y.; Liu, X.L.; Tang, D.A.P. CRISPR/Cas12a-mediated liposome-amplified strategy for the photoelectrochemical detection of nucleic acid. Chem. Commun. 2021, 57, 8977–8980. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.X.; Ou, Y.J.; Lin, Y.; Hu, T. Enhanced chemiluminescence imaging sensor for ultrasensitive detection of nucleic acids based on HCR-CRISPR/Cas12a. Biosens. Bioelectron. 2022, 212, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Yan, Y.R.; Que, H.Y.; Yang, T.T.; Cheng, X.X.; Ding, S.J.; Zhang, X.M.; Cheng, W. CRISPR/Cas12a-Mediated Interfacial Cleaving of Hairpin DNA Reporter for Electrochemical Nucleic Acid Sensing. ACS Sens. 2020, 5, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.G.; Tian, Y.F.; Zhang, Y.J.; Lu, X.Y.; Xiao, D.; Zhou, C.S. One-step fast and label-free imaging array for multiplexed detection of trace avian influenza viruses. Anal. Chim. Acta 2021, 1171, 7. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Wang, P.; Wang, Y.; Sun, S. CRISPR/Cas12a-Chemiluminescence Cascaded Bioassay for Amplification-Free and Sensitive Detection of Nucleic Acids. Biosensors 2025, 15, 479. https://doi.org/10.3390/bios15080479
Guan X, Wang P, Wang Y, Sun S. CRISPR/Cas12a-Chemiluminescence Cascaded Bioassay for Amplification-Free and Sensitive Detection of Nucleic Acids. Biosensors. 2025; 15(8):479. https://doi.org/10.3390/bios15080479
Chicago/Turabian StyleGuan, Xiaotian, Peizheng Wang, Yi Wang, and Shuqing Sun. 2025. "CRISPR/Cas12a-Chemiluminescence Cascaded Bioassay for Amplification-Free and Sensitive Detection of Nucleic Acids" Biosensors 15, no. 8: 479. https://doi.org/10.3390/bios15080479
APA StyleGuan, X., Wang, P., Wang, Y., & Sun, S. (2025). CRISPR/Cas12a-Chemiluminescence Cascaded Bioassay for Amplification-Free and Sensitive Detection of Nucleic Acids. Biosensors, 15(8), 479. https://doi.org/10.3390/bios15080479




