Simple Nanochannel-Modified Electrode for Sensitive Detection of Alkaline Phosphatase Through Electrochemiluminescence Signal Quenching by Enzymatic Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Measurements and Instrumentation
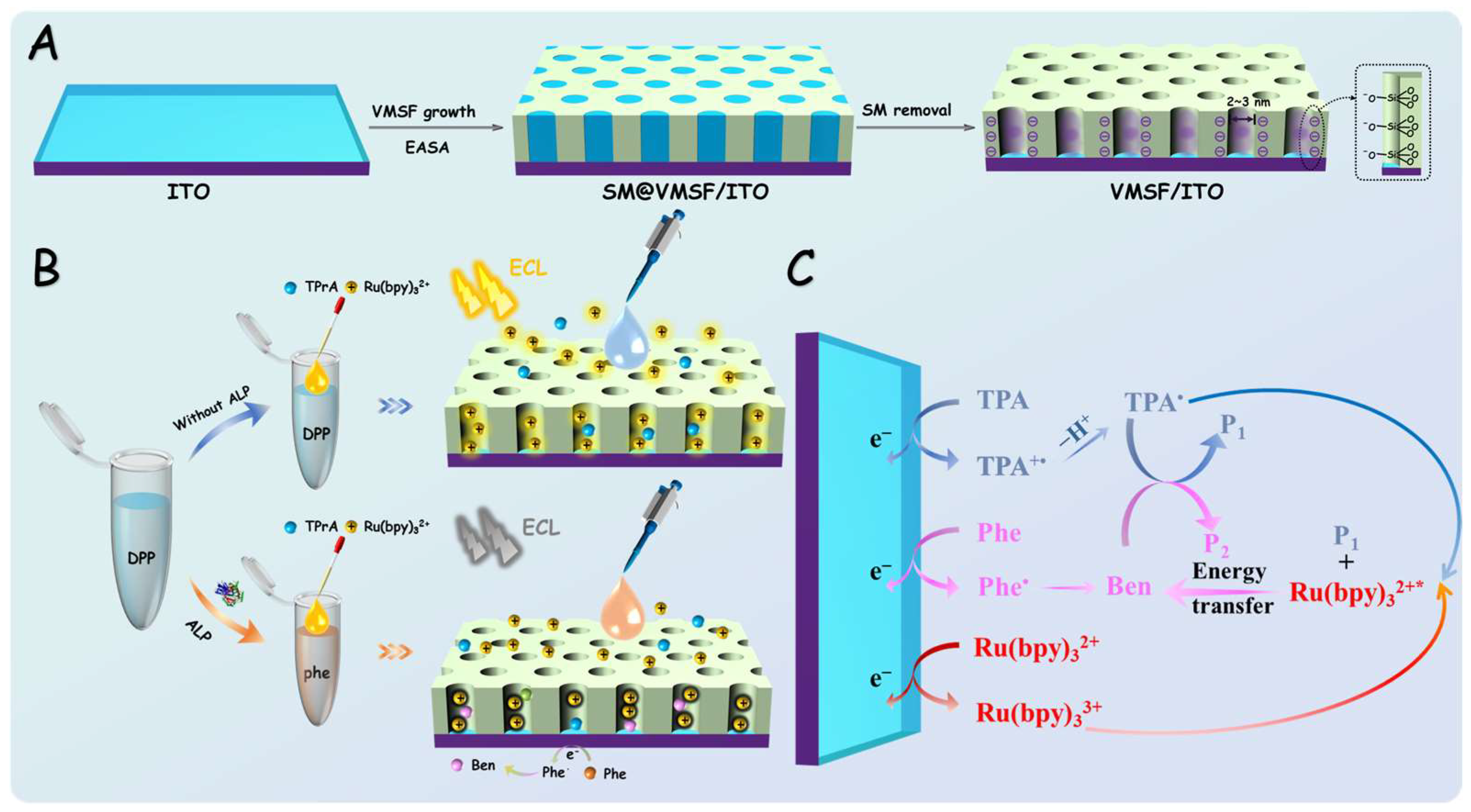
2.3. Preparation of VMSF/ITO Electrode
2.4. Detection of ALP
3. Results and Discussion
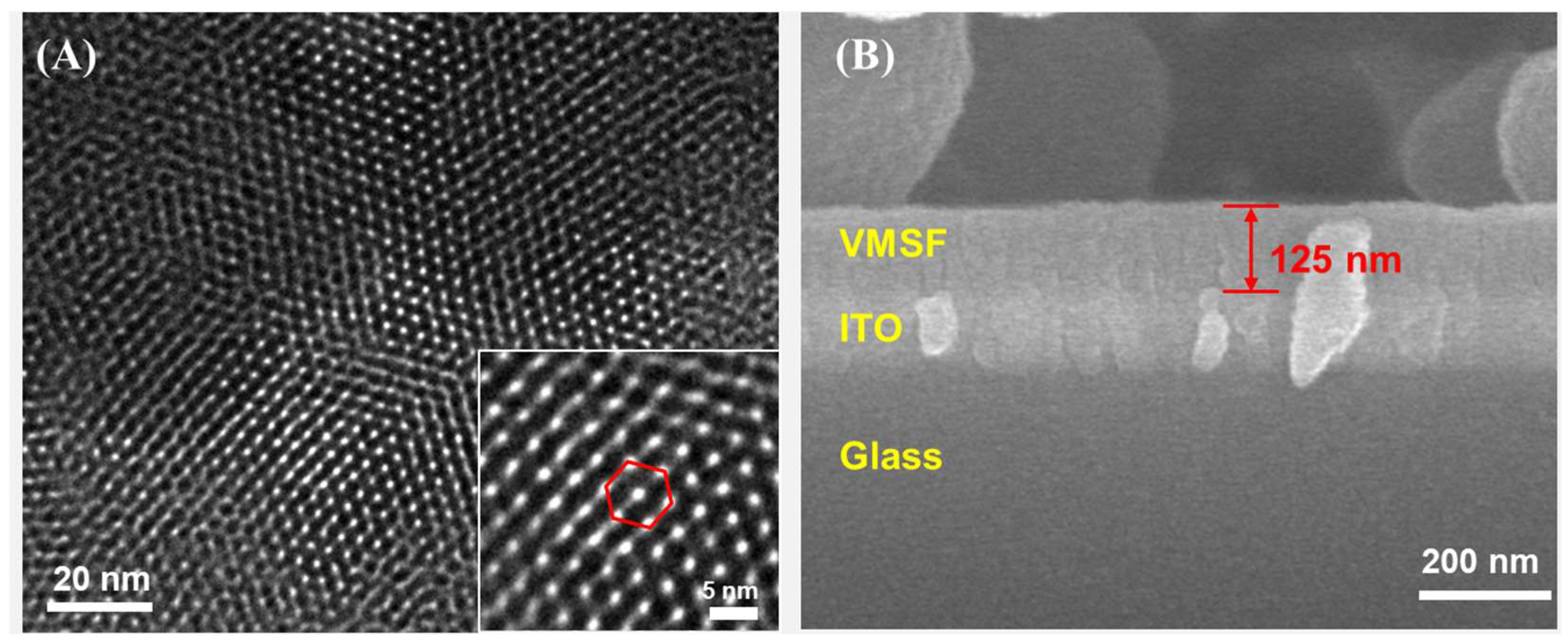
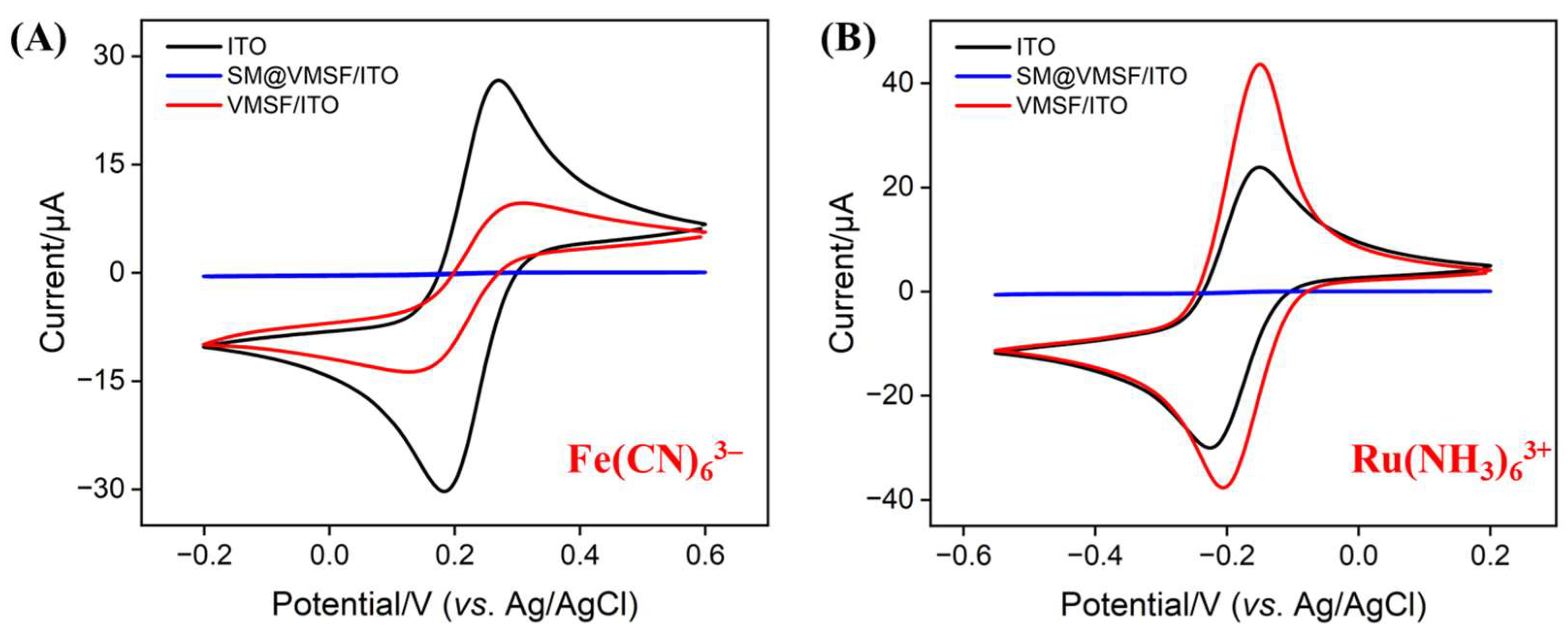
3.1. Characterization of VMSF-Modified Electrode
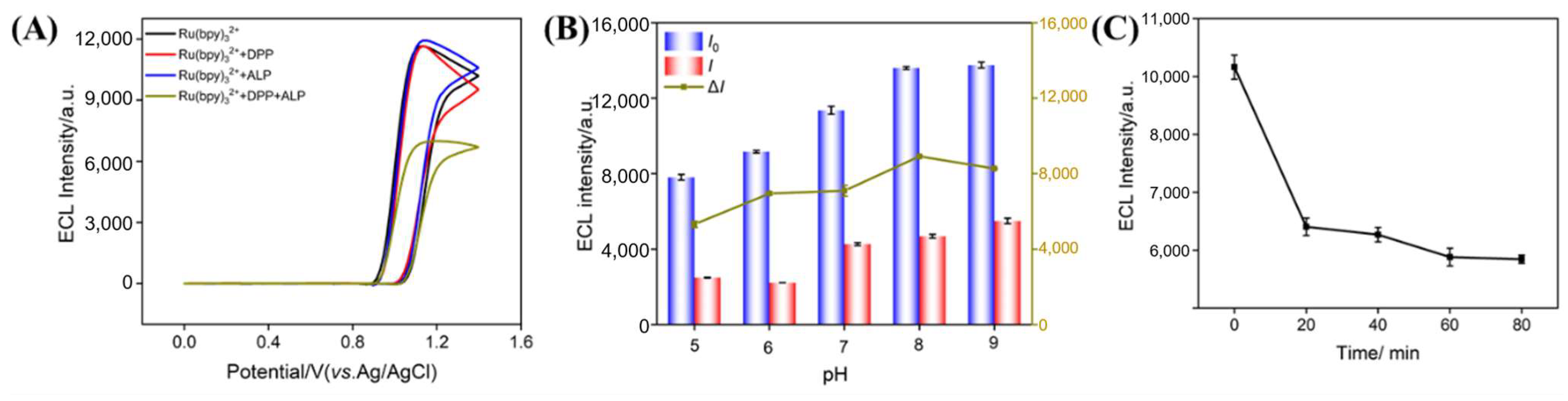
3.2. Feasibility Study and Condition Optimization for ALP Detection
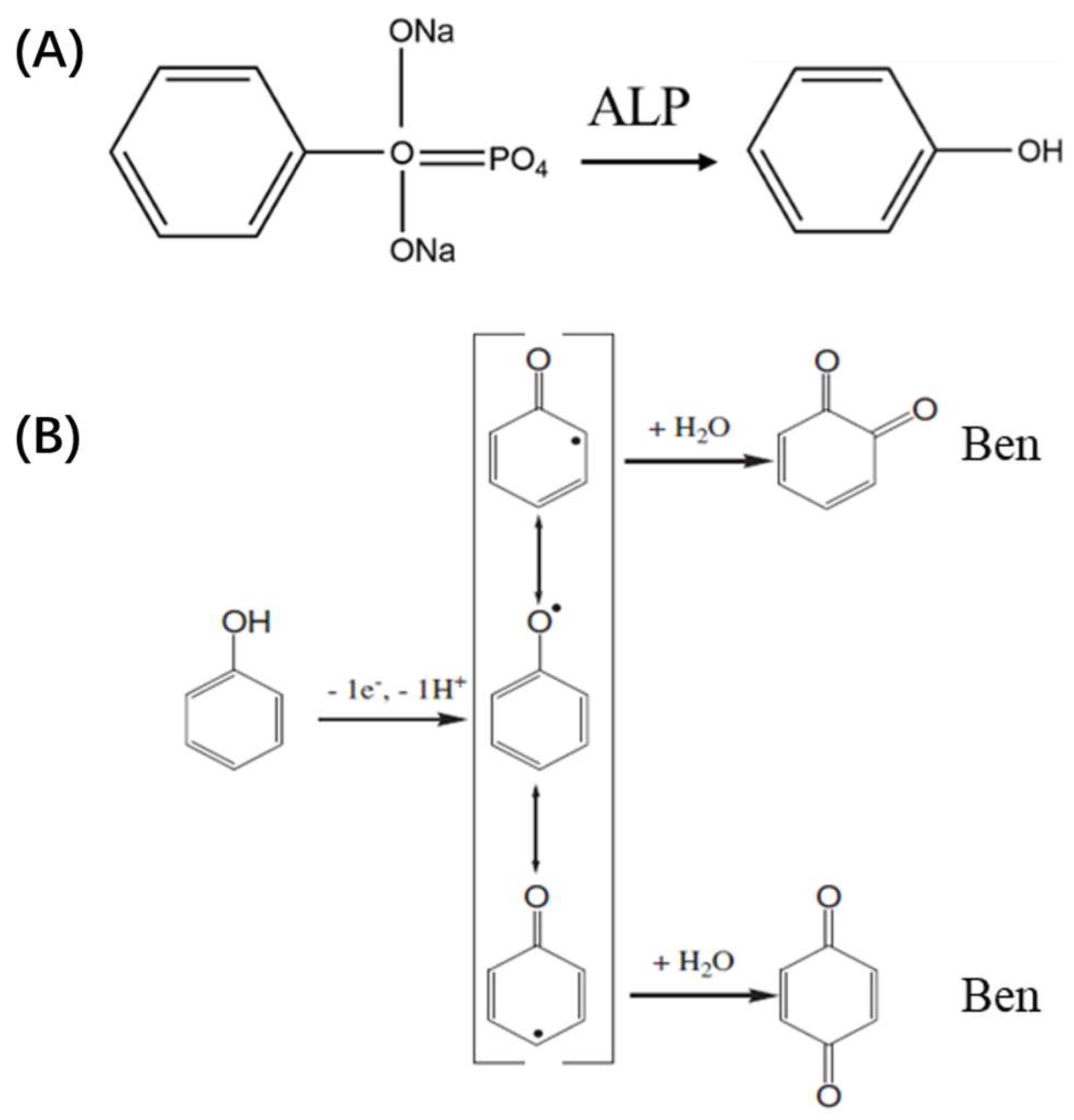
3.3. Mechanism of ECL Sensor for ALP Detection
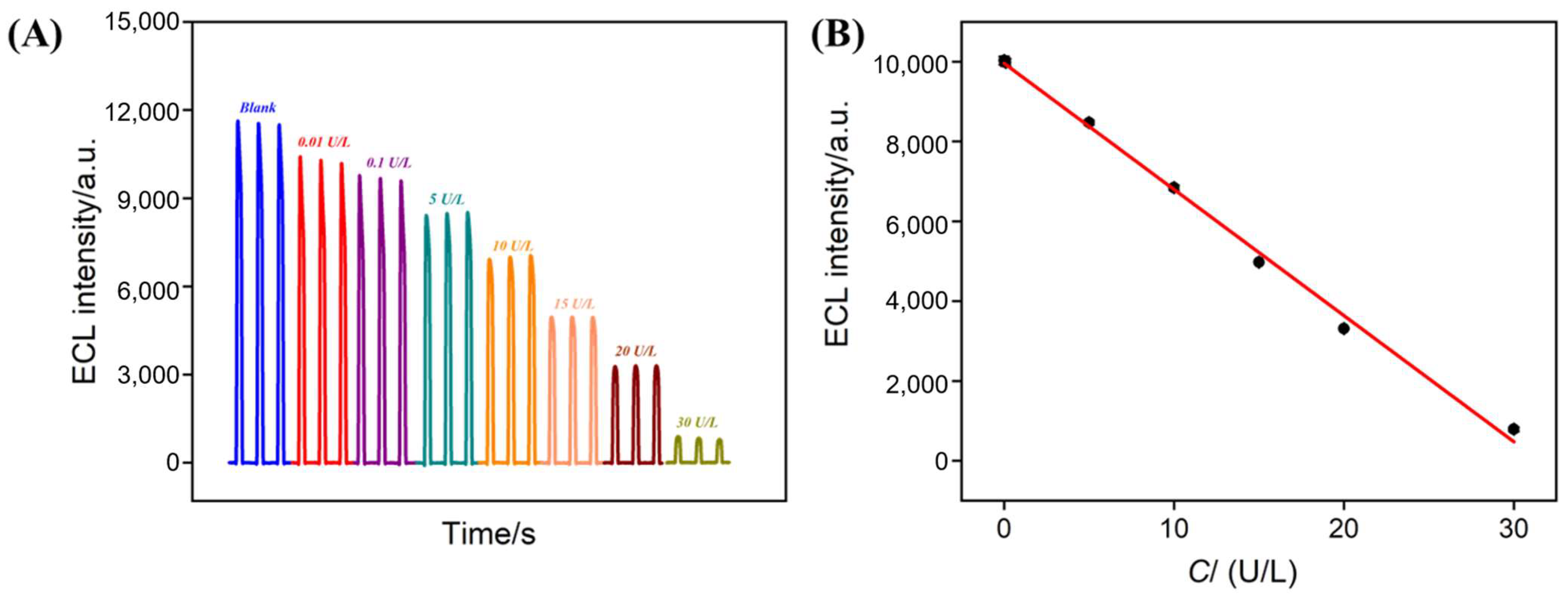
3.4. ECL Detection of ALP
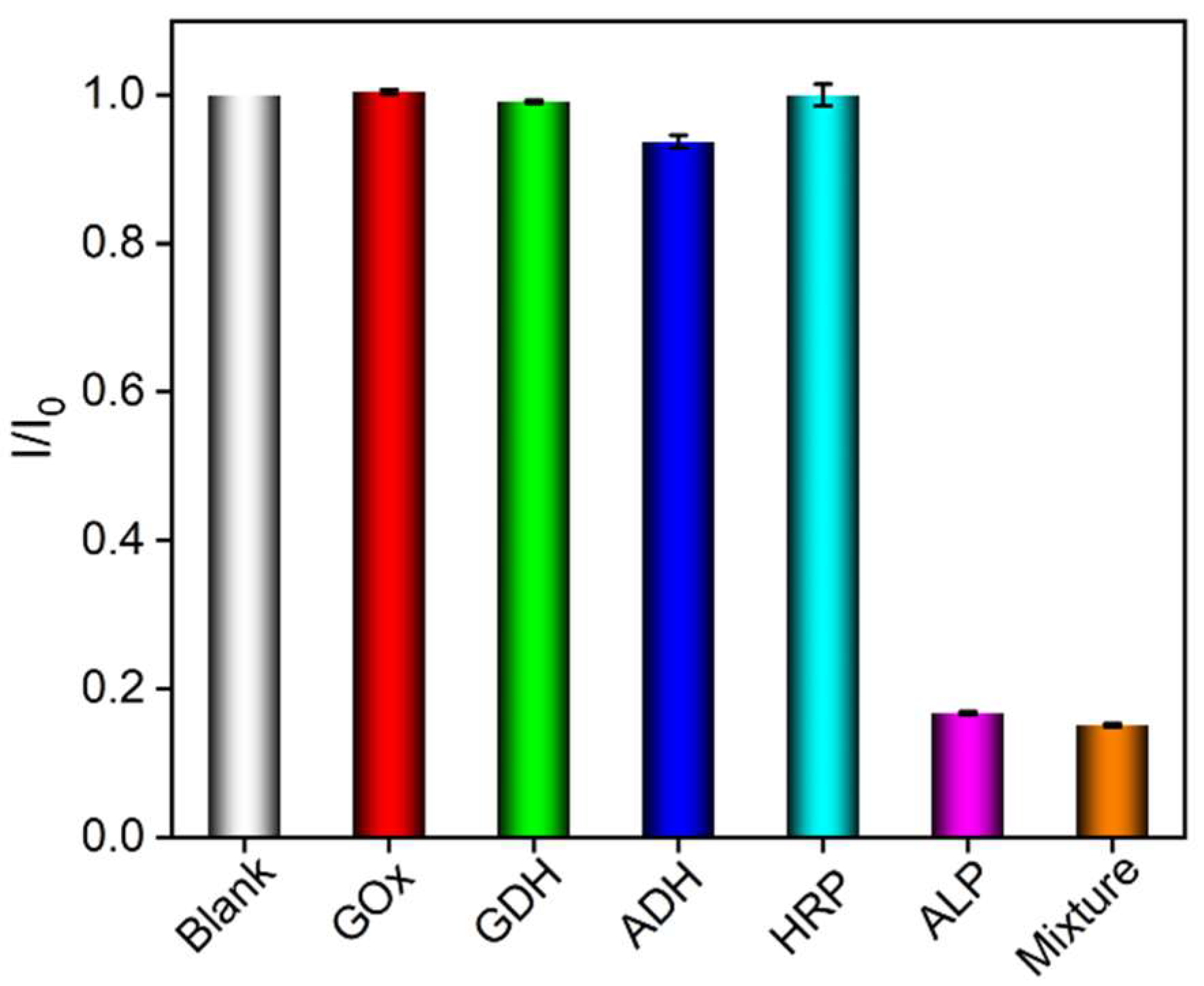
3.5. Selectivity of the Constructed ECL Sensors
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q.; Wang, X.; Peng, H.; Arabi, M.; Li, J.; Xiong, H.; Choo, J.; Chen, L. Ratiometric fluorescence and colorimetry dual-mode assay based on manganese dioxide nanosheets for visual detection of alkaline phosphatase activity. Sens. Actuators B Chem. 2020, 302, 127176. [Google Scholar] [CrossRef]
- Cheng, X.; Chai, Y.; Xu, J.; Wang, L.; Wei, F.; Xu, G.; Sun, Y.; Hu, Q.; Cen, Y. Enzyme cascade reaction-based ratiometric fluorescence probe for visual monitoring the activity of alkaline phosphatase. Sens. Actuators B Chem. 2020, 309, 127765. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Li, Z.; Chen, H.; Qiu, H. Recent progress and prospects of alkaline phosphatase biosensor based on fluorescence strategy. Biosens. Bioelectron. 2020, 148, 111811. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, R.W.; Liu, L.X.; Kot, A.; Liu, X.P.; Xiao, W.W.; Jia, J.J.; Li, Y.P.; Lam, K.S.; Yao, W. Identification of osteogenic progenitor cell-targeted peptides that augment bone formation. Nat. Commun. 2020, 11, 4278. [Google Scholar] [CrossRef]
- van Loo, B.; Bayer, C.D.; Fischer, G.; Jonas, S.; Valkov, E.; Mohamed, M.F.; Vorobieva, A.; Dutruel, C.; Hyvönen, M.; Hollfelder, F. Balancing specificity and promiscuity in enzyme evolution: Multidimensional activity transitions in the alkaline phosphatase superfamily. J. Am. Chem. Soc. 2019, 141, 370–387. [Google Scholar] [CrossRef]
- Marchand, S.; Merchiers, M.; Messens, W.; Coudijzer, K.; De Block, J. Thermal inactivation kinetics of alkaline phosphatase in equine milk. Int. Dairy J. 2009, 19, 763–767. [Google Scholar] [CrossRef]
- Dumitrascu, L.; Stanciuc, N.; Aprodu, I.; Ciuciu, A.M.; Alexe, P.; Bahrim, G.E. Monitoring the heat-induced structural changes of alkaline phosphatase by molecular modeling, fluorescence spectroscopy and inactivation kinetics investigations. J. Food Sci. Tech. Mys. 2015, 52, 6290–6300. [Google Scholar] [CrossRef][Green Version]
- Yu, L.; Shi, Z.; Fang, C.; Zhang, Y.; Liu, Y.; Li, C. Disposable lateral flow-through strip for smartphone-camera to quantitatively detect alkaline phosphatase activity in milk. Biosens. Bioelectron. 2015, 69, 307–315. [Google Scholar] [CrossRef]
- Park, C.S.; Ha, T.H.; Kim, M.; Raja, N.; Yun, H.S.; Sung, M.J.; Kwon, O.S.; Yoon, H.; Lee, C.S. Fast and sensitive near-infrared fluorescent probes for ALP detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosens. Bioelectron. 2018, 105, 151–158. [Google Scholar] [CrossRef]
- Mukaiyama, K.; Kamimura, M.; Uchiyama, S.; Ikegami, S.; Nakamura, Y.; Kato, H. Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin. Exp. Res. 2015, 27, 413–418. [Google Scholar] [CrossRef]
- Li, G.; Fu, H.; Chen, X.; Gong, P.; Chen, G.; Xia, L.; Wang, H.; You, J.; Wu, Y. Facile and sensitive fluorescence sensing of alkaline phosphatase activity with photoluminescent carbon dots based on inner filter effect. Anal. Chem. 2016, 88, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Chai, L.; Tang, C.; Huang, Y.; Chen, J.; Feng, H. Carbon quantum dots-based recyclable real-time fluorescence assay for alkaline phosphatase with adenosine triphosphate as substrate. Anal. Chem. 2015, 87, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Lee, C.Y.; Park, H.G. A sensitive dual colorimetric and fluorescence system for assaying the activity of alkaline phosphatase that relies on pyrophosphate inhibition of the peroxidase activity of copper ions. Analyst 2014, 139, 4691–4695. [Google Scholar] [CrossRef]
- Qian, Z.S.; Chai, L.J.; Huang, Y.Y.; Tang, C.; Jia Shen, J.; Chen, J.R.; Feng, H. A real-time fluorescent assay for the detection of alkaline phosphatase activity based on carbon quantum dots. Biosens. Bioelectron. 2015, 68, 675–680. [Google Scholar] [CrossRef]
- Jin, L.; Dong, Y.; Wu, X.; Cao, G.; Wang, G. Versatile and amplified biosensing through enzymatic cascade reaction by coupling alkaline phosphatase in situ generation of photoresponsive nanozyme. Anal. Chem. 2015, 87, 10429–10436. [Google Scholar] [CrossRef]
- Magnusson, P.; Löfman, O.; Larsson, L. Determination of alkaline phosphatase isoenzymes in serum by high-performance liquid chromatography with post-column reaction detection. J. Chromatogr. B 1992, 576, 79–86. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, J.; Lu, Y.; Sun, J.; Yang, X. Fluorescence immunoassay based on the phosphate-triggered fluorescence turn-on detection of alkaline phosphatase. Anal. Chem. 2018, 90, 3505–3511. [Google Scholar] [CrossRef]
- Wang, J.R.; Xia, C.; Yang, L.; Li, Y.F.; Li, C.M.; Huang, C.Z. DNA Nanofirecrackers Assembled through Hybridization Chain Reaction for Ultrasensitive SERS Immunoassay of Prostate Specific Antigen. Anal. Chem. 2020, 92, 4046–4052. [Google Scholar] [CrossRef]
- Mahato, K.; Chandra, P. Paper-based miniaturized immunosensor for naked eye ALP detection based on digital image colorimetry integrated with smartphone. Biosens. Bioelectron. 2019, 128, 9–16. [Google Scholar] [CrossRef]
- Hu, L.; Xu, G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010, 39, 3275–3304. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Wang, H.; Gu, X.; Zhou, Y.; Xi, F. Enhanced electrochemiluminescence of luminol at neutral medium using nanochannel-confined Co3O4 nanozyme for highly sensitive detection of tumor biomarker. Microchem. J. 2025, 209, 112903. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, T.; Wang, S.; Jiang, Y.; Zhao, Y.; Yan, F.; Xi, F. A dual-functional antibiofouling and signal amplification sensing platform enabling accurate analysis in complicated biological samples. Sens. Actuators B Chem. 2025, 439, 137856. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Ru, H.; Yan, F.; Liu, J. Silica nanochannels as nanoreactors for the confined synthesis of Ag NPs to boost electrochemical stripping chemiluminescence of the luminol-O2 system for the sensitive aptasensor. Anal. Chem. 2024, 96, 10264–10273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, Y.; Liu, J. Sensitive detection of biomarker in gingival crevicular fluid based on enhanced electrochemiluminescence by nanochannel-confined Co3O4 nanocatalyst. Biosensors 2025, 15, 63. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, J.; Han, Q.; Hu, W.; Yan, F.; Liu, J. Nanogold amplified electrochemiluminescence/electrochemistry in bipolar silica nanochannel array for ultrasensitive detection of SARS-CoV-2 pseudoviruses. Talanta 2024, 277, 126319. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Liu, J.; Mou, Y. Nanochannel confined graphene quantum dots/platinum nanoparticles boosts electrochemiluminescence of luminal-O2 system for sensitive immunoassay. Talanta 2025, 285, 127223. [Google Scholar] [CrossRef]
- Nie, F.; Luo, K.; Zheng, X.; Zheng, J.; Song, Z. Novel preparation and electrochemiluminescence application of luminol functional-Au nanoclusters for ALP determination. Sens. Actuators B 2015, 218, 152–159. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Guo, X.; Kang, Q.; Shen, D.; Zou, G. Sensitive and selective determining ascorbic acid and activity of alkaline phosphatase based on electrochemiluminescence of dual-stabilizers-capped CdSe quantum dots in carbon nanotube-nafion composite. Talanta 2016, 154, 175–182. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Wu, T.; Liu, Y. Highly active electrochemiluminescence of ruthenium complex co-assembled chalcogenide nanoclusters and the application for label-free detection of alkaline phosphatase. Anal. Chem. 2021, 93, 15794–15801. [Google Scholar] [CrossRef]
- Zhou, X.; Gu, X.; Zhang, S.; Zou, Y.; Yan, F. Magnetic graphene oxide and vertically-ordered mesoporous silica film for universal and sensitive homogeneous electrochemiluminescence aptasensor platform. Microchem. J. 2024, 200, 110315. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Zheng, Y.; Liu, J. Solid-phase electrochemiluminescence enzyme electrodes based on nanocage arrays for highly sensitive detection of cholesterol. Biosensors 2024, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, T.; Chen, P.; Yan, F.; Liu, J. Bipolar silica nanochannel array for dual-mode electrochemiluminescence and electrochemical immunosensing platform. Sens. Actuators B Chem. 2022, 368, 132086. [Google Scholar] [CrossRef]
- Walcarius, A. Electroinduced surfactant self-assembly driven to vertical growth of oriented mesoporous films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yao, L.; Chen, K.; Su, B. Silica Nanochannel Membranes for Electrochemical Analysis and Molecular Sieving: A comprehensive review. Crit. Rev. Anal. Chem. 2019, 50, 424–444. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L.; Wang, H.; Huang, L.; Lin, J.; Gao, X.; Xi, F. Nanochannel-confined Ni(OH)2-CeO2 composite nanozyme boosts electrochemiluminescence of luminol-dissolved oxygen for immunosensing. Biosens. Bioelectron. 2025, 280, 117451. [Google Scholar] [CrossRef]
- Fan, X.; Wu, J.; Zhang, T.; Liu, J. Electrochemical/electrochemiluminescence sensors based on vertically-ordered mesoporous silica films for biomedical analytical applications. ChemBioChem 2024, 25, e202400320. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical silica nanochannels supported by nanocarbon composite for simultaneous detection of serotonin and melatonin in biological fluids. Sens. Actuators B Chem. 2022, 353, 131101. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, T.; Wang, M.; Yan, F.; Liu, J. Disposable electrochemical sensors for highly sensitive detection of chlorpromazine in human whole blood based on the silica nanochannel array modified screen-printed carbon electrode. Molecules 2022, 27, 8200. [Google Scholar] [CrossRef]
- Wang, M.; Lin, J.; Gong, J.; Ma, M.; Tang, H.; Liu, J.; Yan, F. Rapid and sensitive determination of doxorubicin in human whole blood by vertically-ordered mesoporous silica film modified electrochemically pretreated glassy carbon electrodes. RSC Adv. 2021, 11, 9021–9028. [Google Scholar] [CrossRef]
- Huang, J.; Xu, S.; Yan, F.; Liu, J. Electrochemiluminescence enzyme biosensors for ultrasensitive determination of glucose using glucose dehydrogenase immobilized on vertical silica nanochannels. Sens. Actuators B Chem. 2024, 402, 135119. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, Y.; Liu, J. Solid electrochemiluminescence sensor by immobilization of emitter ruthenium(ii)tris(bipyridine) in bipolar silica nanochannel film for sensitive detection of oxalate in serum and urine. Nanomaterials 2024, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, J.; Zhang, R.; Yan, F. Dual-mode electrochemiluminescence and electrochemical sensor for alpha-fetoprotein detection in human serum based on vertically ordered mesoporous silica films. Front. Chem. 2022, 10, 1023998. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zheng, Y.; Yan, F.; Xu, L. Enzyme-based solid-phase electrochemiluminescence sensors with stable, anchored emitters for sensitive glucose detection. Biosensors 2025, 15, 332. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Huang, H.; Lv, N.; Liu, J.; Liu, Y. Nanochannel array on electrochemically polarized screen printed carbon electrode for rapid and sensitive electrochemical determination of clozapine in human whole blood. Molecules 2022, 27, 2739. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Liu, J.; Qin, D. Label-free homogeneous electrochemical aptasensor based on size exclusion/charge-selective permeability of nanochannel arrays and 2D nanorecognitive probe for sensitive detection of alpha-fetoprotein. Molecules 2023, 28, 6935. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, H.; Liu, J. Highly sensitive electrochemical immunosensor based on methylene blue-reduced graphene oxide nanocomposites as signal probes for IL-6 detection in gingival crevicular fluid samples. Front. Chem. 2025, 13, 1549927. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Yan, F. In situ growth of Au NPs on nitrogen-doped graphene quantum dots decorated graphene composites for the construction of an electrochemical immunosensor and its application in CEA detection. Molecules 2025, 30, 1347. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Yan, F.; Liu, J. Vertical silica nanochannels and o-phenanthroline chelator for the detection of trace Fe(II). ACS Appl. Nano Mater. 2024, 7, 7743–7752. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, S.; Zhang, S.; Wang, G.; Yi, H.; Xin, G.-Z.; Yang, X. Mesoporous silica-mediated controllable electrochemiluminescence quenching for immunosensor with simplicity, sensitivity and tunable detection range. Talanta 2021, 231, 122399. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Jin, H.; Wang, J.; Yan, F. Copper nanoparticles confined in a silica nanochannel film for the electrochemical detection of nitrate ions in water samples. Molecules 2023, 28, 7515. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, T.; Zhou, H.; Yan, F.; Liu, Y. Silica nanochannels boosting Ru(bpy)32+-mediated electrochemical sensor for the detection of guanine in beer and pharmaceutical samples. Front. Nutr. 2022, 9, 987442. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, S.; Liu, J.; Xing, J. Homogeneous electrochemical aptamer sensor based on two-dimensional nanocomposite probe and nanochannel modified electrode for sensitive detection of carcinoembryonic antigen. Molecules 2023, 28, 5186. [Google Scholar] [CrossRef]
- Zheng, H.; Zu, Y. Highly efficient quenching of coreactant electrogenerated chemiluminescence by phenolic compounds. J. Phys. Chem. B 2005, 109, 16047–16051. [Google Scholar] [CrossRef]
- Cho, K.G.; Lee, J.I.; Lee, S.J.; Hong, K.Y.; Kang, M.S.; Lee, K.H. Light-emitting devices based on electrochemiluminescence gels. Adv. Funct. Mater. 2020, 30, 1907936. [Google Scholar] [CrossRef]
- Qi, W.; Fu, Y.; Zhao, M.; He, H.; Tian, X.; Hu, L.; Zhang, Y. Electrochemiluminescence resonance energy transfer immunoassay for alkaline phosphatase using p-nitrophenyl phosphate as substrate. Anal. Chim. Acta 2020, 1097, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, X. Alkaline phosphatase-responsive anodic electrochemiluminescence of CdSe nanoparticles. Anal. Chem. 2012, 84, 6986–6993. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Wang, Y.; Pei, K.; Wu, D.; Qi, W. An electrochemiluminescence dual “turn-on” strategy for alkaline phosphatase detection using a dual quenching Ru(bpy)32+ encapsulated zeolite imidazole metal organic framework. Chem. Commun. 2022, 58, 12114–12117. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bian, X.; Chen, L.; Guo, L.; Qiu, B.; Lin, Z. Highly sensitive homogeneous electrochemiluminescence biosensor for alkaline phosphatase detection based on click chemistry-triggered branched hybridization chain reaction. Anal. Chem. 2021, 93, 10351–10357. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wu, M.-X.; Ding, S.-N. Anodic electrochemiluminescence from CsPbBr3 perovskite quantum dots for an alkaline phosphatase assay. Chem. Commun. 2020, 56, 8099–8102. [Google Scholar] [CrossRef]
- Bushira, F.A.; Kitte, S.A.; Wang, Y.; Li, H.; Wang, P.; Jin, Y. Plasmon-boosted Cu-doped TiO2 oxygen vacancy-rich luminol electrochemiluminescence for highly sensitive detection of alkaline phosphatase. Anal. Chem. 2021, 93, 15183–15191. [Google Scholar] [CrossRef]
| Sample | Added (U/L) | Detected (U/L) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| Pasteurized milk a | 0.100 | 0.107 | 107 | 4.7 |
| 1.00 | 0.962 | 96.2 | 2.9 | |
| 5.00 | 4.92 | 98.4 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Luo, X.; Xi, F.; Yang, N. Simple Nanochannel-Modified Electrode for Sensitive Detection of Alkaline Phosphatase Through Electrochemiluminescence Signal Quenching by Enzymatic Reaction. Biosensors 2025, 15, 377. https://doi.org/10.3390/bios15060377
Ma T, Luo X, Xi F, Yang N. Simple Nanochannel-Modified Electrode for Sensitive Detection of Alkaline Phosphatase Through Electrochemiluminescence Signal Quenching by Enzymatic Reaction. Biosensors. 2025; 15(6):377. https://doi.org/10.3390/bios15060377
Chicago/Turabian StyleMa, Tianjun, Xuan Luo, Fengna Xi, and Nuo Yang. 2025. "Simple Nanochannel-Modified Electrode for Sensitive Detection of Alkaline Phosphatase Through Electrochemiluminescence Signal Quenching by Enzymatic Reaction" Biosensors 15, no. 6: 377. https://doi.org/10.3390/bios15060377
APA StyleMa, T., Luo, X., Xi, F., & Yang, N. (2025). Simple Nanochannel-Modified Electrode for Sensitive Detection of Alkaline Phosphatase Through Electrochemiluminescence Signal Quenching by Enzymatic Reaction. Biosensors, 15(6), 377. https://doi.org/10.3390/bios15060377






