Fluorescent–Electrochemical–Colorimetric Triple-Model Immunoassays with Multifunctional Metal–Organic Frameworks for Signal Amplification
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Apparatus
2.3. Preparation and Modification of Cu-BDC MOFs
2.4. Modification of MB with Anti-AFP
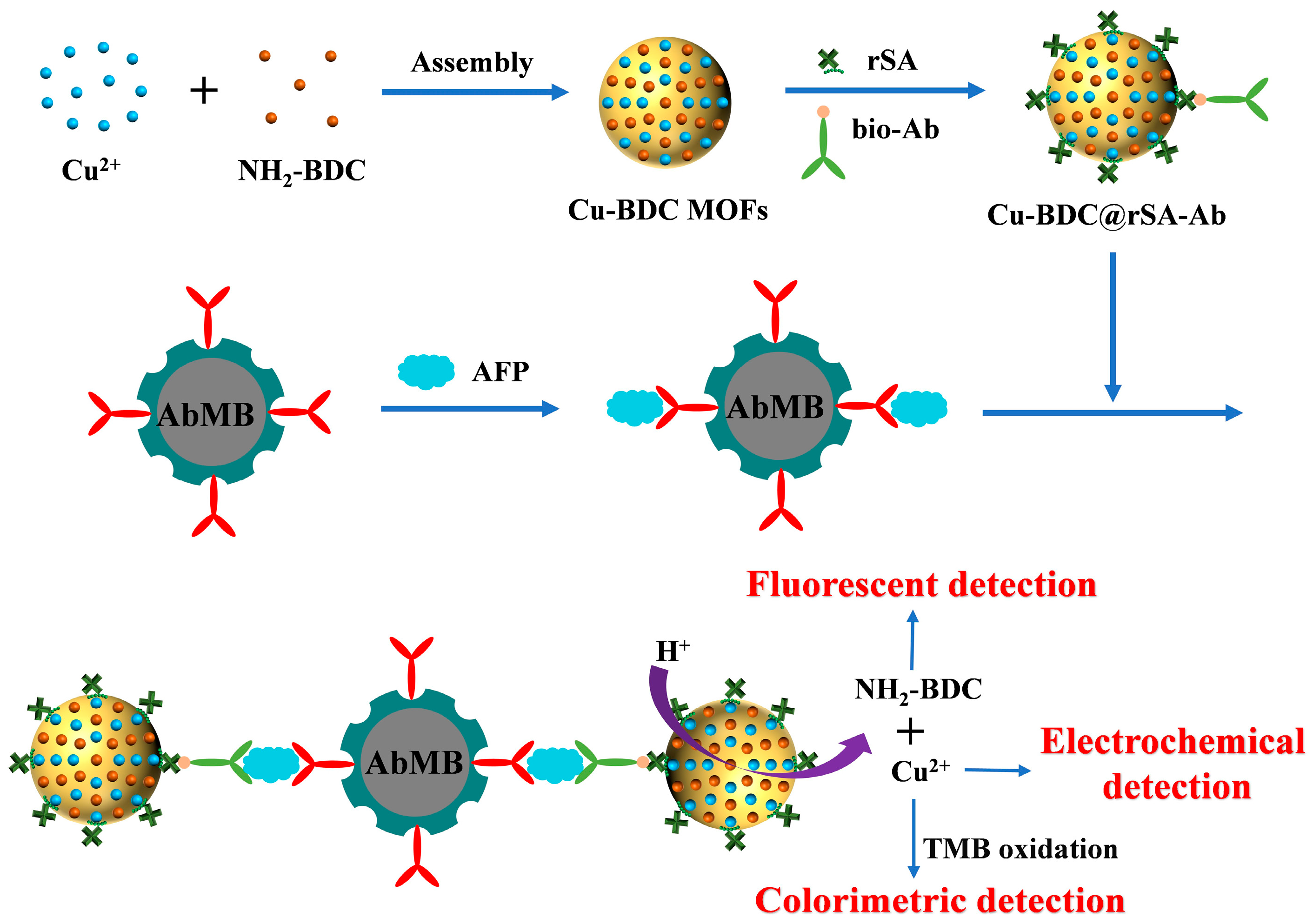
2.5. Procedure for Triple-Mode Immunoassays
- (i)
- The capture of AFP: A total of 20 μL of the prepared AbMB was mixed with a given concentration of AFP in 500 μL phosphate buffer (50 mM, pH 7.4) for 30 min.
- (ii)
- The capture of Cu-BDC@rSA-Ab: After magnetic separation and washing three times with water, the AFP@AbMB was supplemented with 50 μL Cu-BDC@rSA-Ab conjugates to incubate for 30 min.
- (iii)
- The dissolution of Cu-MOFs: The resulting Cu-BDC@rSA-Ab@AFP@AbMB conjugates were separated by magnet and washed three times with water. This was followed by the addition of 10 mM hydrochloric acid solution containing 10% DMF (25 μL) to release NH2-BDC and Cu2+ from the conjugates.
- (iv)
- Fluorescence and electrochemical measurement: A total of 100 μL of 50 mM acetic acid buffer solution containing 0.1 NaCl was added to dilute the supernatant for the fluorescence assay and DPV measurement. The fluorescence spectra were collected with an excitation wavelength of 340 nm. The DPV responses were recorded from 0.25 to –0.05 V.
- (v)
- Colorimetric assay: A total of 10 μL of 2.5 mM TMB and 10 μL of 1 M H2O2 were successively added to 80 μL of the released supernatant. After reacting for 15 min, color change was monitored by the naked eye and the UV–vis absorption spectrum was determined using the spectrophotometer.
3. Results and Discussion
3.1. Principle of Triple-Mode Immunoassays
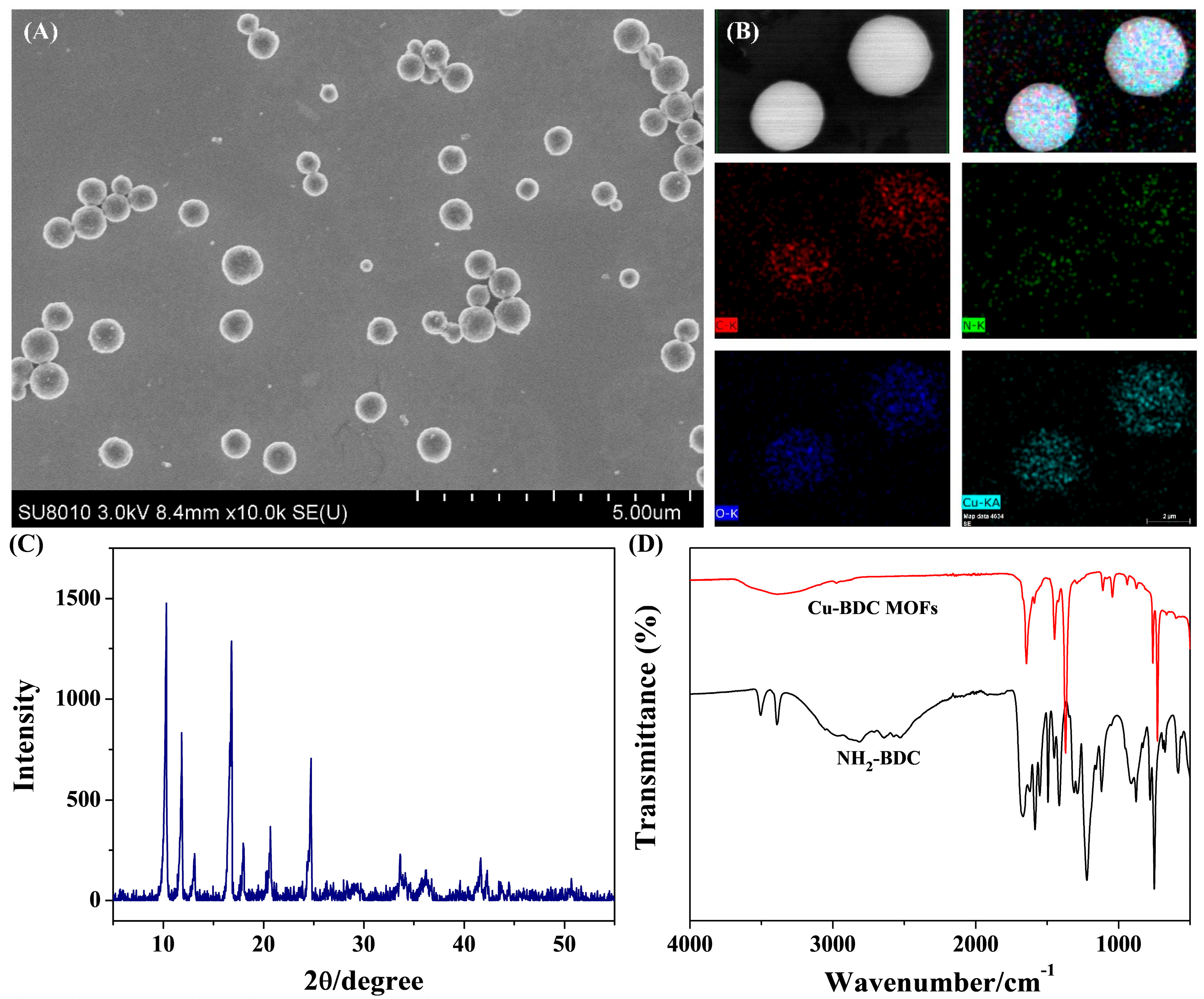
3.2. Characterization of Cu-BDC MOFs
3.3. Feasibility of Triple-Mode Immunoassays
3.4. Sensitivity of Triple-Mode Immunoassays
3.5. Selectivity and Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusa, V.; Millet, M.; Coscolla, C.; Roca, M. Analytical methods for human biomonitoring of pesticides. A review. Anal. Chim. Acta 2015, 891, 15–31. [Google Scholar] [CrossRef]
- Yeo, W.-H.; Kim, J.-H. Recent advances in portable biosensors for biomarker detection in body fluids. Biosensors 2020, 10, 127. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Zhang, Q.; Yang, L.; Zhou, M.; Song, D. Advances in carcinoembryonic antigen detection: A review of clinical applications and standardization. Anal. Bioanal. Chem. 2025, 417, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnkova, L.; Skládal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F. Optical immunoassays methods in protein analysis: An overview. Chemosensors 2022, 10, 326. [Google Scholar] [CrossRef]
- Chen, M.; Qileng, A.; Liang, H.; Lei, H.; Liu, W.; Liu, Y. Advances in immunoassay-based strategies for mycotoxin detection in food: From single-mode immunosensors to dual-mode immunosensors. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1285–1311. [Google Scholar] [CrossRef]
- Ma, X.; Ge, Y.; Xia, N. Overview of the design and application of dual-signal immunoassays. Molecules 2024, 29, 4551. [Google Scholar] [CrossRef]
- Saleh, R.O.; Almajidi, Y.Q.; Mansouri, S.; Hammoud, A.; Rodrigues, P.; Mezan, S.O.; Maabreh, H.G.; Deorari, M.; Shakir, M.N.; Alasheqi, M.Q. Dual-mode colorimetric and fluorescence biosensors for the detection of foodborne bacteria. Clin. Chim. Acta 2024, 553, 117741. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, H.; Wang, J. Multi-mode/signal biosensors: Electrochemical integrated sensing techniques. Adv. Funct. Mater. 2024, 34, 2403122. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, H.; Du, C.; Zhang, X.; Chen, J. Multifunctional ethyl violet@NH2-MIL-88B(Fe) hybrids: CRISPR Cas12a-assisted PEC-FL-CL triple-mode sensitive detection of HPV-16. Anal. Chem. 2024, 96, 15657–15664. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Y.; Wang, C.; Qiang, L.; Gao, J.; Wang, Y.; Liu, H.; Han, L.; Zhang, Y. Rapid and sensitive triple-mode detection of causative SARS-CoV-2 virus specific genes through interaction between genes and nanoparticles. Anal. Chim. Acta 2021, 1154, 338330. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.X.; Wang, J.; Diao, L.L.; Li, C.P. Construction of multi-mode photoelectrochemical immunoassays for accurate detection of cancer markers: Assisted with MOF-confined plasmonic nanozyme. Anal. Chem. 2024, 96, 1336–1344. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Yang, H.; Zhou, Y. Triple-readout immunoassay based on copper ion trigger for the detection of ochratoxin A. Anal. Chim. Acta 2025, 1345, 343750. [Google Scholar] [CrossRef]
- Ghosh, A.; Kallungal, S.F.T.; Ramaprabhu, S. 2D Metal-organic frameworks: Properties, synthesis, and applications in electrochemical and optical biosensors. Biosensors 2023, 13, 123. [Google Scholar] [CrossRef]
- Chang, J.; Hu, R.; Zhang, J.; Hou, T.; Li, F. Two-dimensional metal-organic framework nanozyme-mediated portable paper-based analytical device for dichlorophen assay. Biosens. Bioelectron. 2024, 255, 116271. [Google Scholar] [CrossRef] [PubMed]
- Leoi, M.W.N.; Zheng, X.T.; Yu, Y.; Gao, J.; Ong, D.H.S.; Koh, C.Z.H.; Chen, P.; Yang, L. Redefining metal organic frameworks in biosensors: Where are we now? ACS Appl. Mater. Interfaces 2025, 17, 13246–13278. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Y.; Chen, X. Recent advances in metal-organic frameworks for biomacromolecule sensing. Chemosensors 2022, 10, 412. [Google Scholar] [CrossRef]
- Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and application of electrochemical sensors with metal-organic frameworks as the electrode materials or signal tags. Nanomaterials 2022, 12, 3248. [Google Scholar] [CrossRef]
- Mohanty, B.; Kumari, S.; Yadav, P.; Kanoo, P.; Chakraborty, A. Metal-organic frameworks (MOFs) and MOF composites based biosensors. Coord. Chem. Rev. 2024, 519, 216102. [Google Scholar] [CrossRef]
- Dourandish, Z.; Tajik, S.; Beitollahi, H.; Jahani, P.M.; Nejad, F.G.; Sheikhshoaie, I.; Bartolomeo, A.D. A comprehensive review of metal–organic framework: Synthesis, characterization, and investigation of their application in electrochemical biosensors for biomedical analysis. Sensors 2022, 22, 2238. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Zhang, J.; Liu, J.; Wang, A.; Ding, L. Recent development of copper-based nanozymes for biomedical applications. Adv. Healthc. Mater. 2024, 13, 2302023. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Liu, G.; Zhang, S.; Shang, Z.; Yang, Y.; Li, Y.; Liu, L. Oxidase-mimicking peptide-copper complexes and their applications in sandwich affinity biosensors. Anal. Chim. Acta 2022, 1214, 339965. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, D.; Zhang, L.; Ouyang, H.; Fu, Z. Alkaline hydrolysis behavior of metal-organic frameworks NH2-MIL-53(Al) employed for sensitive immunoassay via releasing fluorescent molecules. ACS Appl. Mater. Interfaces 2019, 11, 35597–35603. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, Z.; Sun, C.; Long, Y.; Zheng, H. Bifunctional antibody and copper-based metal-organic framework nanocomposites for colorimetric α-fetoprotein sensing. Microchim. Acta 2020, 187, 465. [Google Scholar] [CrossRef]
- Bunzen, H. Chemical stability of metal-organic frameworks for applications in drug delivery. ChemNanoMat 2021, 7, 998. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, B.; Xiao, K.; Duan, H.; Zhao, H. The chemical stability of metal-organic frameworks in water treatments: Fundamentals, effect of water matrix and judging methods. Chem. Eng. J. 2022, 450, 138215. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Jin, X.; Liu, H.; Sun, K.; Wang, X.; Li, M.; Wang, P.; Chang, Y.; Wang, T.; et al. An encounter between metal ions and natural products: Natural products-coordinated metal ions for the diagnosis and treatment of tumors. J. Nanobiotech. 2024, 22, 726. [Google Scholar] [CrossRef]
- Röder, R.; Preiß, T.; Hirschle, P.; Steinborn, B.; Zimpel, A.; Höhn, M.; Rädler, J.O.; Bein, T.; Wagner, E.; Wuttke, S.; et al. Multifunctional nanoparticles by coordinative self-assembly of His tagged units with metal−organic frameworks. J. Am. Chem. Soc. 2017, 139, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, L.; Rub, F.A.; Hajja, A.; Alodhaibi, I.; Arai, M.; Alfuwais, M.; Makhzoum, T.; Yaqinuddin, A.; Al-Kattan, K.; Broering, D.C.; et al. Biosensing of alpha-fetoprotein: A key direction toward the early detection and management of hepatocellular carcinoma. Biosensors 2024, 14, 235. [Google Scholar] [CrossRef]
- Shan, C.-W.; Chen, Z.; Han, G.-C.; Feng, X.-Z.; Kraatz, H.-B. Electrochemical immuno-biosensors for the detection of the tumor marker alpha-fetoprotein: A review. Talanta 2024, 271, 125638. [Google Scholar] [CrossRef]
- Zhu, L.; Chang, Y.; Li, Y.; Qiao, M.; Liu, L. Biosensors based on the binding events of nitrilotriacetic acid–metal complexes. Biosensors 2023, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liu, G.; Li, S.; Liu, L.; Song, Q. Biorecognition element-free electrochemical detection of recombinant glycoproteins using metal-organic frameworks as signal tags. Anal. Chim. Acta 2023, 1273, 341540. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Yang, L.; Tan, Y.; Xie, Q.; Yao, S. Copper-based metal–organic framework nanoparticles with peroxidase-like activity for sensitive colorimetric detection of staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 24440–24445. [Google Scholar] [CrossRef]
- Geißler, D.; Wegner, K.D.; Fischer, C.; Resch-Genger, U. Exploring simple particle-based signal amplification strategies in a heterogeneous sandwich immunoassay with optical detection. Anal. Chem. 2024, 96, 5078–5085. [Google Scholar] [CrossRef]
- Shao, F.; Zhang, L.; Jiao, L.; Wang, X.; Miao, L.; Li, H.; Zhou, F. Enzyme-free immunosorbent assay of prostate specific antigen amplified by releasing pH indicator molecules entrapped in mesoporous silica nanoparticles. Anal. Chem. 2018, 90, 8673–8679. [Google Scholar] [CrossRef]
- Ren, R.; Cai, G.; Yu, Z.; Zeng, Y.; Tang, D. Metal-polydopamine framework: An innovative signal-generation tag for colorimetric immunoassay. Anal. Chem. 2018, 90, 11099–11105. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Yan, H.; Xu, W.; Wu, Y.; Gu, W.; Li, H.; Du, D.; Lin, Y.; Zhu, C. Self-assembly of all-inclusive allochroic nanoparticles for the improved ELISA. Anal. Chem. 2019, 91, 8461–8465. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.E.; Wright, D.W. Sensitive method for biomolecule detection utilizing signal amplification with porphyrin nanoparticles. Anal. Chem. 2016, 88, 5928–5933. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Xiong, S.; Li, X.; Zhan, S.; Zeng, L.; Xiong, Y. Dual-mode fluorescent and colorimetric immunoassay for the ultrasensitive detection of alpha-fetoprotein in serum samples. Anal. Chim. Acta 2018, 1038, 112–119. [Google Scholar] [CrossRef]
- Xie, Z.; Hou, J. Industrialising metal−organic frameworks bridging laboratory innovation and future applications. Ind. Eng. Chem. Res. 2025, 64, 7941–7955. [Google Scholar] [CrossRef]
- Salehipour, M.; Rezaei, S.; Rezaei, M.; Yazdani, M.; Mogharabi-Manzari, M. Opportunities and challenges in biomedical applications of metal–organic frameworks. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4443–4462. [Google Scholar] [CrossRef]
- Sun, J.; Hu, T.; Chen, C.; Zhao, D.; Yang, F.; Yang, X. Fluorescence immunoassay system via enzyme-enabled in situ synthesis of fluorescent silicon nanoparticles. Anal. Chem. 2016, 88, 9789–9795. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, M.; Lv, S.; Zhang, K.; Lu, M.; Tang, D. In situ synthesis of fluorescent polydopamine nanoparticles coupled with enzyme-controlled dissolution of MnO2 nanoflakes for a sensitive immunoassay of cancer biomarkers. J. Mater. Chem. B 2017, 5, 8506–8513. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, J.; Lu, Y.; Sun, J.; Yang, X. Fluorescence immunoassay based on the phosphate-triggered fluorescence turn-on detection of alkaline phosphatase. Anal. Chem. 2018, 90, 3505–3511. [Google Scholar] [CrossRef]
- Sun, Z.H.; Zhang, X.X.; Xu, D.; Liu, J.; Yu, R.J.; Jing, C.; Han, H.X.; Ma, W. Silver-amplified fluorescence immunoassay via aggregation-induced emission for detection of disease biomarker. Talanta 2021, 225, 121963–121968. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Chen, L.; Liang, M.; Xu, H.; Tang, S.; Yang, H.-H.; Song, H. Sensitive fluorescence immunoassay of alpha-fetoprotein through copper ions modulated growth of quantum dots in-situ. Sens. Actuat. B Chem. 2017, 247, 408–413. [Google Scholar] [CrossRef]
- Yang, T.; Li, C.M.; He, J.H.; Chen, B.; Li, Y.F.; Huang, C.Z. Ratiometrically fluorescent electrospun nanofibrous film as a Cu2+-mediated solid-phase immunoassay platform for biomarkers. Anal. Chem. 2018, 90, 9966–9974. [Google Scholar] [CrossRef]
- Gao, F.; Chang, Y.; Xia, N.; Li, Y.; Liu, L. Multifunctional self-assembled nanoparticles serving as the signal labels of fluorescent immunoassays. Microchem. J. 2024, 207, 111921. [Google Scholar] [CrossRef]
- Yu, L.; Cui, X.; Li, H.; Lu, J.; Kang, Q.; Shen, D. A ratiometric electrochemical sensor for multiplex detection of cancer biomarkers using bismuth as an internal reference and metal sulfide nanoparticles as signal tags. Analyst 2019, 144, 4073–4080. [Google Scholar] [CrossRef]
- Meng, W.; Li, M.; Zhang, Y. Adriamycin coated silica microspheres as labels for cancer biomarker alpha-fetoprotein detection. Anal. Methods 2021, 13, 2665–2670. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, R.; Yu, H.; An, S.; Han, J.; Xie, G.; Chen, S. Thi-Au-Fe3O4 confined in ZIF-8 nanoreactor as signal-amplifying tag for constructing high-efficiency electrochemical platform. Sensor. Actuator. B Chem. 2020, 305, 127496. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Ma, C.; Song, Y.; Qiao, X.; Hong, C. A sandwich-type electrochemical immunosensor for ultrasensitive detection of multiple tumor markers using an electrical signal difference strategy. Talanta 2020, 219, 121322. [Google Scholar] [CrossRef]
- Li, L.; Wei, Y.; Zhang, S.; Chen, X.; Shao, T.; Feng, D. Electrochemical immunosensor based on metal ions functionalized CNSs@Au NPs nanocomposites as signal amplifier for simultaneous detection of triple tumor markers. J. Electroanal. Chem. 2021, 880, 114882. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, K.; Zhu, L.; Tang, D.; Niessner, R.; Knopp, D. H2-Based Electrochemical Biosensor with Pd Nanowires@ZIF-67 Molecular Sieve Bilayered Sensing Interface for Immunoassay. Anal. Chem. 2019, 91, 12055–12062. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Zhang, C.; Chen, Y.; Wang, Y.; Xie, M. Manganese dioxide nanoparticle-based colorimetric immunoassay for the detection of alpha-fetoprotein. Microchim. Acta 2017, 184, 2767–2774. [Google Scholar] [CrossRef]
- Deng, X.; Fang, Y.; Lin, S.; Cheng, Q.; Liu, Q.; Zhang, X. Porphyrin-based porous organic frameworks as a biomimetic catalyst for highly efficient colorimetric immunoassay. ACS Appl. Mater. Interfaces 2017, 9, 3514–3523. [Google Scholar] [CrossRef]
- Peng, M.-P.; Ma, W.; Long, Y.-T. Alcohol dehydrogenase-catalyzed gold nanoparticle seed-mediated growth allows reliable detection of disease biomarkers with the naked eye. Anal. Chem. 2015, 87, 5891–5896. [Google Scholar] [CrossRef]
- Su, B.; Xu, H.; Xie, G.; Chen, Q.; Sun, Z.; Cao, H.; Liu, X. Generation of ananobody-alkaline phosphatase fusion and its application in an enzyme cascade-amplified immunoassay for colorimetric detection of alpha fetoprotein in human serum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120088. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X.; Tang, M.; Liu, L.; Shi, Y.; Gao, C.; Liao, B.; Zheng, H. New optical method for the determination of β-galactosidase and α-fetoprotein based on oxidase-like activity of fluorescein. Talanta 2019, 194, 164–170. [Google Scholar] [CrossRef]
- Kim, J.U.; Kim, J.M.; Thamilselvan, A.; Nam, K.H.; Kim, M.I. Colorimetric and electrochemical dual-mode detection of thioredoxin 1 based on the efficient peroxidase-mimicking and electrocatalytic property of Prussian Blue nanoparticles. Biosensors 2024, 14, 185. [Google Scholar] [CrossRef]
- Seddaoui, N.; Attaallah, R.; Amine, A. Development of an optical immunoassay based on peroxidase-mimicking Prussian blue nanoparticles and a label-free electrochemical immunosensor for accurate and sensitive quantification of milk species adulteration. Microchim. Acta 2022, 189, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Al-Ogaidi, I.; Aguilar, Z.P.; Suri, S.; Gou, H.; Wu, N. Dual detection of cancer biomarker CA125 using absorbance and electrochemical methods. Analyst 2013, 138, 5647–5653. [Google Scholar] [CrossRef]
- Hong, W.; Lee, S.; Cho, Y. Dual-responsive immunosensor that combines colorimetric recognition and electrochemical response for ultrasensitive detection of cancer biomarkers. Biosens. Bioelectron. 2016, 86, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Son, S.E.; Hur, W.; Tran, V.K.; Lee, H.B.; Park, Y.; Han, D.K.; Seong, G.H. Electrochemical immunoassay for determination of glycated albumin using nanozymes. Sci. Rep. 2020, 10, 9513–9524. [Google Scholar] [CrossRef]
- Xu, X.H.; Xia, J.; Song, S.; Liu, Y.; Chen, J.; Zhong, S.; Chen, H.; Zhang, Z.H. Dual-signal immuno-competitive determination of brain natriuretic peptide based on magnetic nanozyme. Electroanalysis 2023, 35, e202200500–e202200508. [Google Scholar] [CrossRef]
- Xie, W.; Tian, M.; Luo, X.; Jiang, Y.; He, N.; Liao, X.; Liu, Y. A dual-mode fluorescent and colorimetric immunoassay based on in situ ascorbic acid-induced signal generation from metal-organic frameworks. Sens. Actuat. B Chem. 2020, 302, 127180–127186. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Hu, H.; Feng, Y.; Zhu, S.; Li, C.; Feng, N. A dual-readout sandwich immunoassay based on biocatalytic perovskite nanocrystals for detection of prostate specific antigen. Biosens. Bioelectron. 2022, 203, 113979. [Google Scholar] [CrossRef]
- Zhuge, W.; Tan, X.; Zhang, R.; Li, H.; Zheng, G. Fluorescent and colorimetric immunoassay of nuclear matrix protein 22 enhanced by porous Pd nanoparticles. Chin. Chem. Lett. 2019, 30, 1307–1309. [Google Scholar] [CrossRef]
- Miao, L.; Jiao, L.; Tang, Q.; Li, H.; Zhang, L.; Wei, Q. A nanozyme-linked immunosorbent assay for dual-modal colorimetric and ratiometric fluorescent detection of cardiac troponin I. Sens. Actuat. B Chem. 2019, 288, 60–64. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Lu, S.; Bao, X.; Sun, J.; Yang, X. An enzyme cascade-triggered fluorogenic and chromogenic reaction applied in enzyme activity assay and immunoassay. Anal. Chem. 2018, 90, 7754–7760. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, D.; Wang, B.; Ni, P.; Jiang, Y.; Zhang, C.; Yang, F.; Lu, Y.; Sun, J. Alkaline phosphatase-triggered in situ formation of silicon-containing nanoparticles for a fluorometric and colorimetric dual-channel immunoassay. Anal. Chem. 2020, 92, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Rawat, K.; Bohidar, H.B.; Rajamani, P. Dual-probe (colorimetric and fluorometric) detection of ferritin using antibody-modified gold@carbon dot nanoconjugates. Microchim. Acta 2019, 186, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, Y.; Huang, P.; Wu, F.-Y. Carbon dot-encapsulated plasmonic core-satellite nanoprobes for sensitive detection of cancer biomarkers via dual-mode colorimetric and fluorometric immunoassay. ACS Appl. Nano Mater. 2022, 5, 11539–11548. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, Y.; Zhang, X.J.; Yang, X.T.; Tang, Y.Y. Colorimetric and fluorometric dual-channel detection of α-fetoprotein based on the use of ZnS-CdTe hierarchical porous nanospheres. Microchim. Acta 2019, 186, 124–132. [Google Scholar] [CrossRef]




| Method | Signal Label | Signal Output | Linear Range (ng/mL) | Detection Limit (pg/mL) | Ref. |
|---|---|---|---|---|---|
| FL | ALP/Si NPs | Enzyme catalysis | 1~60 | 1000 | [42] |
| FL | AOX–AuNPs/Mn2+ | Enzyme catalysis | 0.05~20 | 17.3 | [43] |
| FL | ALP/calcein-Ce3+ | Enzyme catalysis | 1~40 | 41 | [44] |
| FL | AgNPs/TPE-4TA | Signal probe | 0.01~5000 | 42 | [45] |
| FL | CuO NPs/CdS QDs | Signal probe | 1~80 | 450 | [46] |
| FL | CuO NPs/CdS QDs/ENFFs | Signal probe | 0.01~200 | 8.3 | [47] |
| FL | bio-PyFNPs | Signal probe | 0.001~2.5 | 0.5 | [48] |
| EC | CdS QDs | Electrooxidation | 0.001~1 | 0.11 | [49] |
| EC | ADM@AuNPs@SiO2 | Electrooxidation | 0.0005~75 | 0.17 | [50] |
| EC | Thi-Au-Fe3O4@ZIF-8 | Electroreduction | Not reported | 0.003 | [51] |
| EC | MnO2 nanosheets | Electrocatalysis | 0.005~100 | 1 | [52] |
| EC | CNSs@AuNPs@Zn2+ | Electrocatalysis | 0.010~80 | 2.6 | [53] |
| EC | DPNs | Electrocatalysis | 0.1~50 | 40 | [54] |
| Color | MnO2 NPs | Nanocatalysis | 6.25~400 | 22 | [55] |
| Color | FePor-TFPA-COP | Nanocatalysis | 0.005~100 | 1 | [56] |
| Color | ADH | Enzyme catalysis | Not reported | 1 | [57] |
| Color | ALP | Enzyme catalysis | 1250~10,000 | 148 | [58] |
| Color | β-gal | Enzyme catalysis | 0.1~100 | 80 | [59] |
| Color | MPDA@TP | Color indicator | 0.01~1 | 2.3 | [36] |
| Method | Signal Label | Target | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Colorimetric–Electrochemical | PBNPs | TRX1 | 10~50 ng/mL | 9 and 6.5 ng/mL | [60] |
| PBNPs | IgG | 0.5~10 µg/mL | 34 ng/mL | [61] | |
| ALP | CA125 | 5~1000 and 50~1000 U/mL | 1.3 and 40 U/mL | [62] | |
| HRP-Ppy NPs | PSA | 0.001~40 ng/mL | 0.8 and 0.7 pg/mL | [63] | |
| uPtNZs | GA | 0.01~5 and 0.005~10 mg/mL | 9.2 and 3.8 µg/mL | [64] | |
| H-AuNPs | BNP | 0.001~0.2 and 5~25 ng/mL | 0.03 and 80.3 pg/mL | [65] | |
| Colorimetric–Fluorescent | Fe-MOFs | PSA | 1~20 and 0~15 ng/mL | 180 pg/mL | [66] |
| CsPbBr3 NCs | PSA | 0.1~15 and 0.01~80 ng/mL | 290 and 81 pg/mL | [67] | |
| porous Pd NPs | NMP22 | 0.001~0.5 ng/mL | 0.35 and 0.31 pg/mL | [68] | |
| nanoceria | cTnI | 0.001~10 ng/mL | 227 and 413 fg/mL | [69] | |
| ALP | cTnI | 0.2~80 and 0.05~4 ng/mL | 60 and 15 ng/mL | [70] | |
| ALP | PSA | 0.02~28 and 0.02~20 ng/mL | 4.1 and 9.6 pg/mL | [71] | |
| Au@CD | ferritin | 1~160 and 1~120 ng/mL | 20 and 64 ng/mL | [72] | |
| GOx | PSA | 0.005~20 ng/mL | 2.3 and 0.84 ng/mL | [73] | |
| QDs/ZnS NSs | AFP | 0.05~12 ng/mL | 7 and 10 pg/mL | [74] | |
| AuNF@Fluorescein | AFP | 5~5000 and 0.01~10 pg/mL | 17.7 and 0.029 fg/mL | [39] |
| Added (pg/mL) | Found by Fluorescence (pg/mL) | Found by DPV (pg/mL) | Found by UV-vis (pg/mL) |
|---|---|---|---|
| 25.0 | 25.6 ± 2.5 | 24.5 ± 2.6 | 26.8 ± 2.8 |
| 50.0 | 48.8 ± 4.6 | 47.2 ± 4.8 | 50.2 ± 5.2 |
| 100.0 | 103.5 ± 9.8 | 101.5 ± 10.2 | 104.5 ± 10.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, N.; Zheng, C.; Liu, G. Fluorescent–Electrochemical–Colorimetric Triple-Model Immunoassays with Multifunctional Metal–Organic Frameworks for Signal Amplification. Biosensors 2025, 15, 376. https://doi.org/10.3390/bios15060376
Xia N, Zheng C, Liu G. Fluorescent–Electrochemical–Colorimetric Triple-Model Immunoassays with Multifunctional Metal–Organic Frameworks for Signal Amplification. Biosensors. 2025; 15(6):376. https://doi.org/10.3390/bios15060376
Chicago/Turabian StyleXia, Ning, Chuye Zheng, and Gang Liu. 2025. "Fluorescent–Electrochemical–Colorimetric Triple-Model Immunoassays with Multifunctional Metal–Organic Frameworks for Signal Amplification" Biosensors 15, no. 6: 376. https://doi.org/10.3390/bios15060376
APA StyleXia, N., Zheng, C., & Liu, G. (2025). Fluorescent–Electrochemical–Colorimetric Triple-Model Immunoassays with Multifunctional Metal–Organic Frameworks for Signal Amplification. Biosensors, 15(6), 376. https://doi.org/10.3390/bios15060376






