Mn-Doped CeO2 Nanozyme-Integrated Mesoporous Interfaces for High-Sensitivity Antifouling Electrochemiluminescence Biosensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentations and Materials
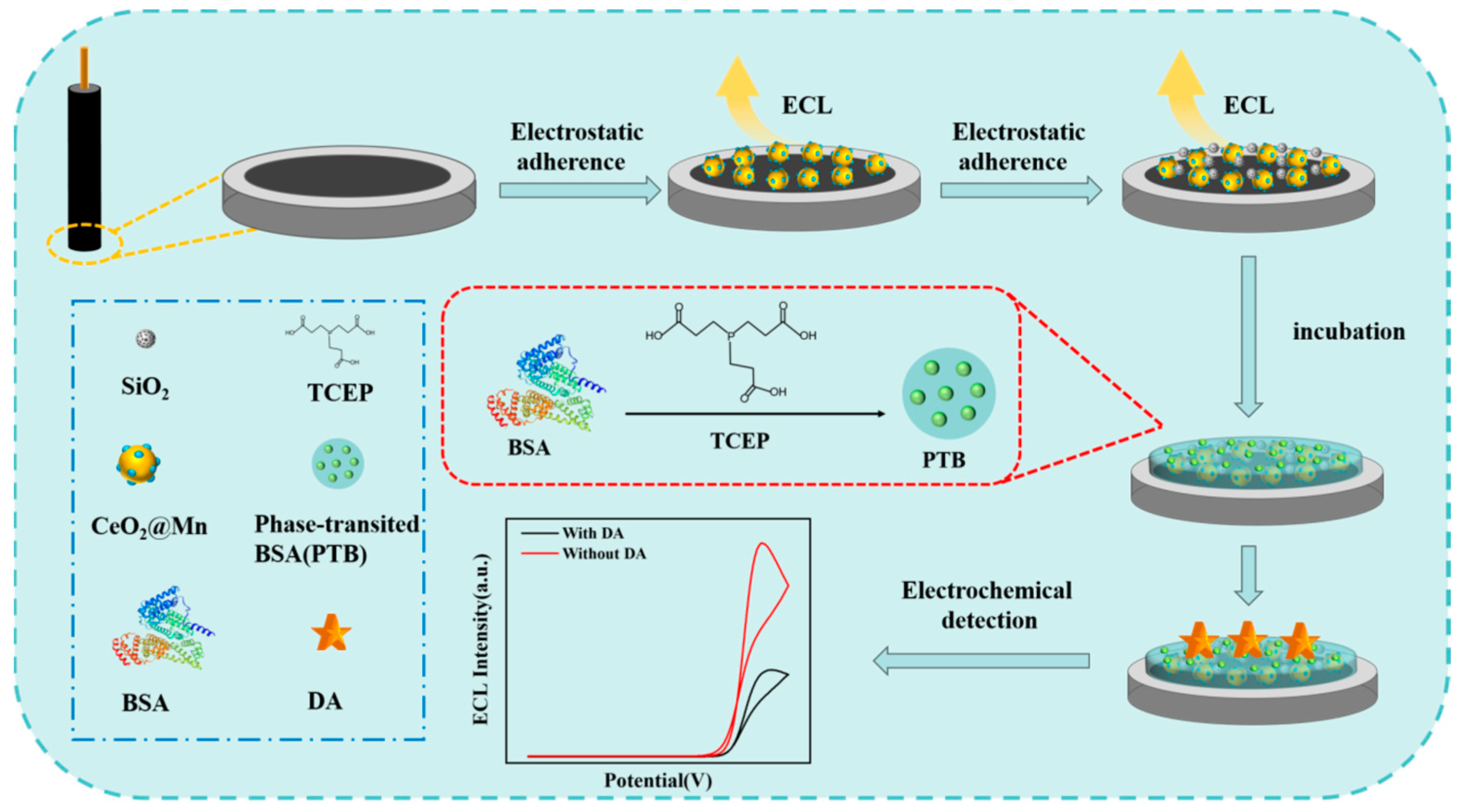
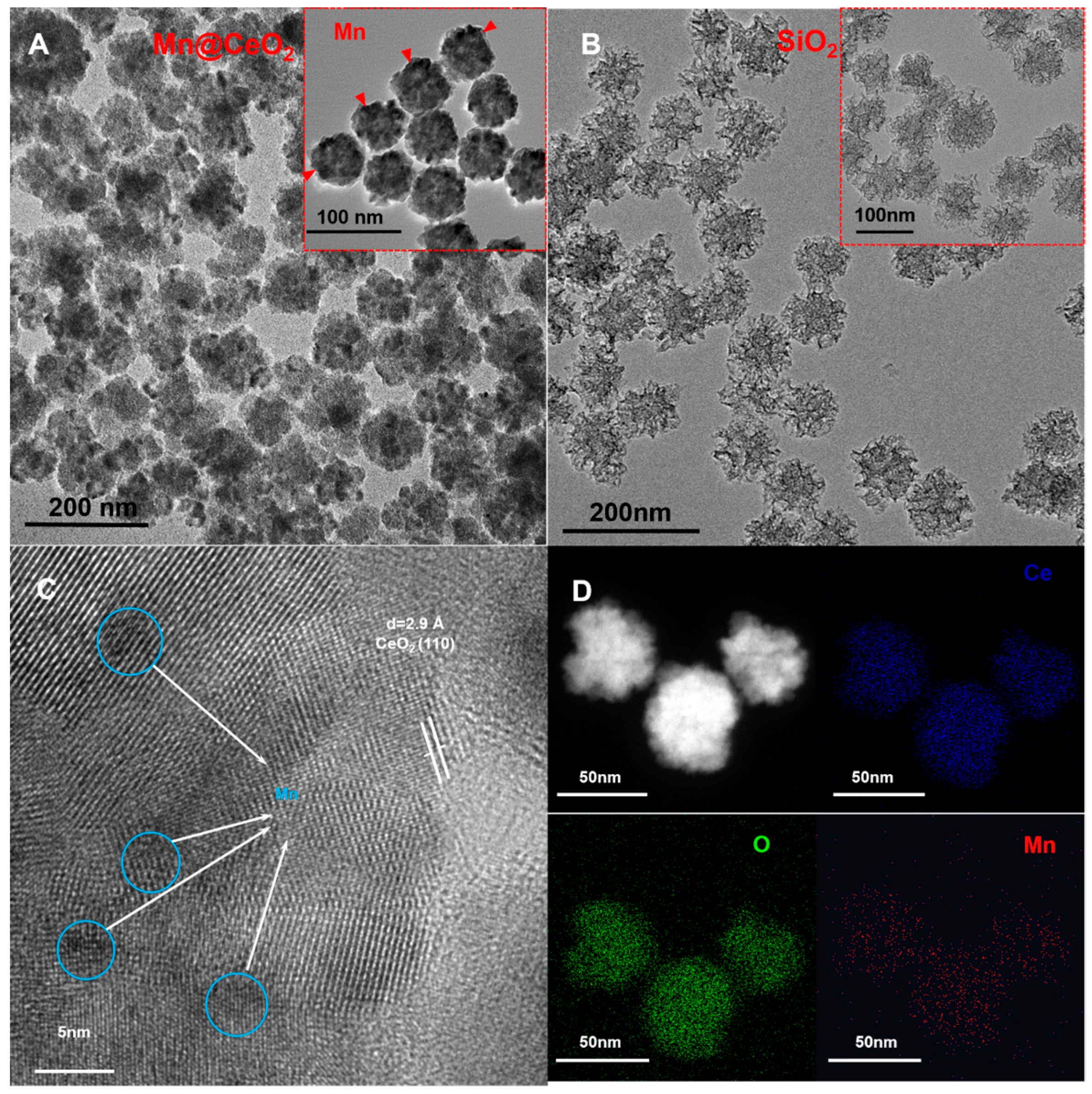
2.2. Preparation of CeO2@Mn
2.3. Preparation of the SiO2
2.4. Preparation of the CTS
2.5. Preparation of the PTB Coated Electrode
2.6. Establishment of the ECL Sensors
2.7. Electrochemical Measurements
3. Results and Discussions
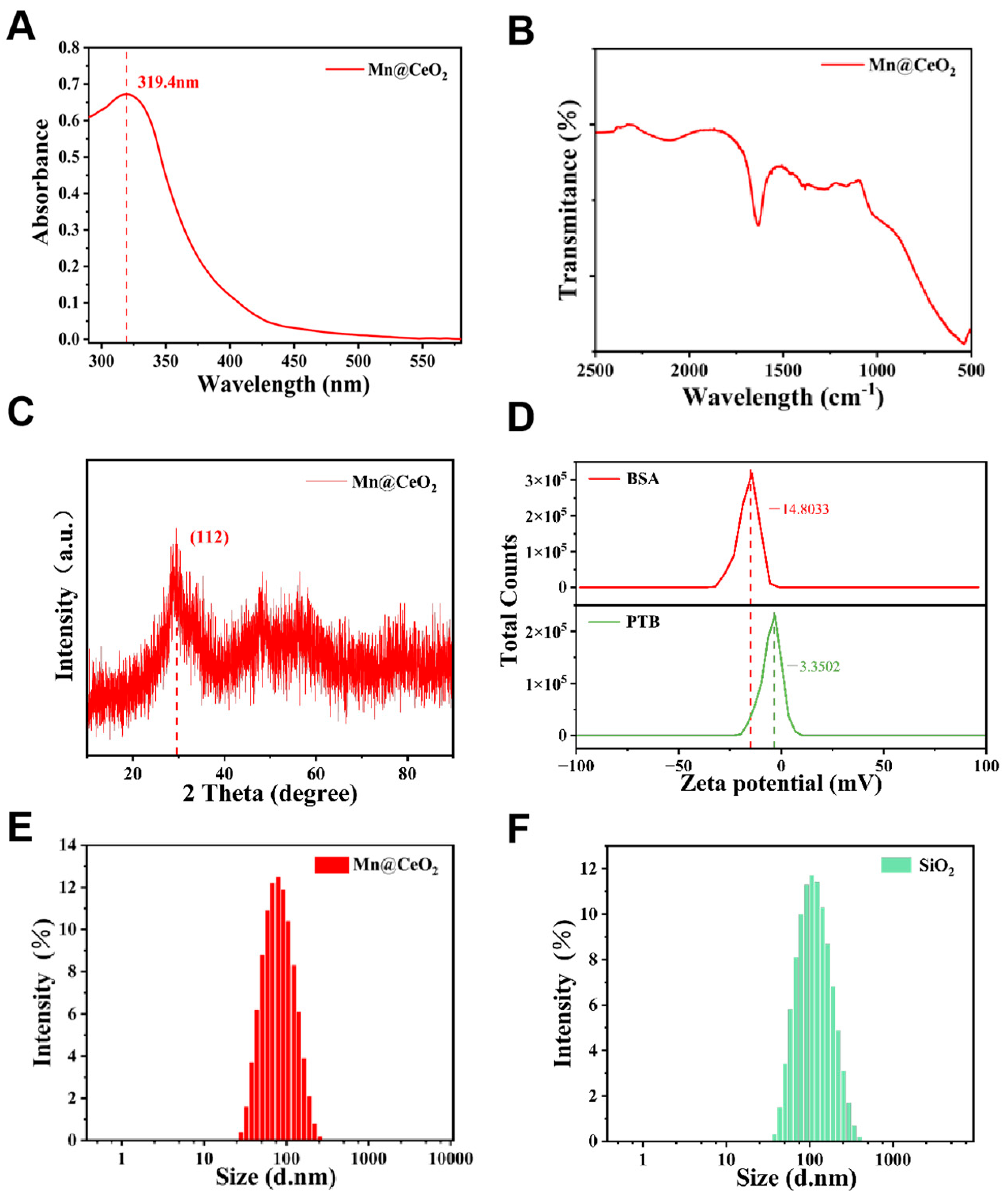
3.1. Characterization of Mn@CeO2 and SiO2
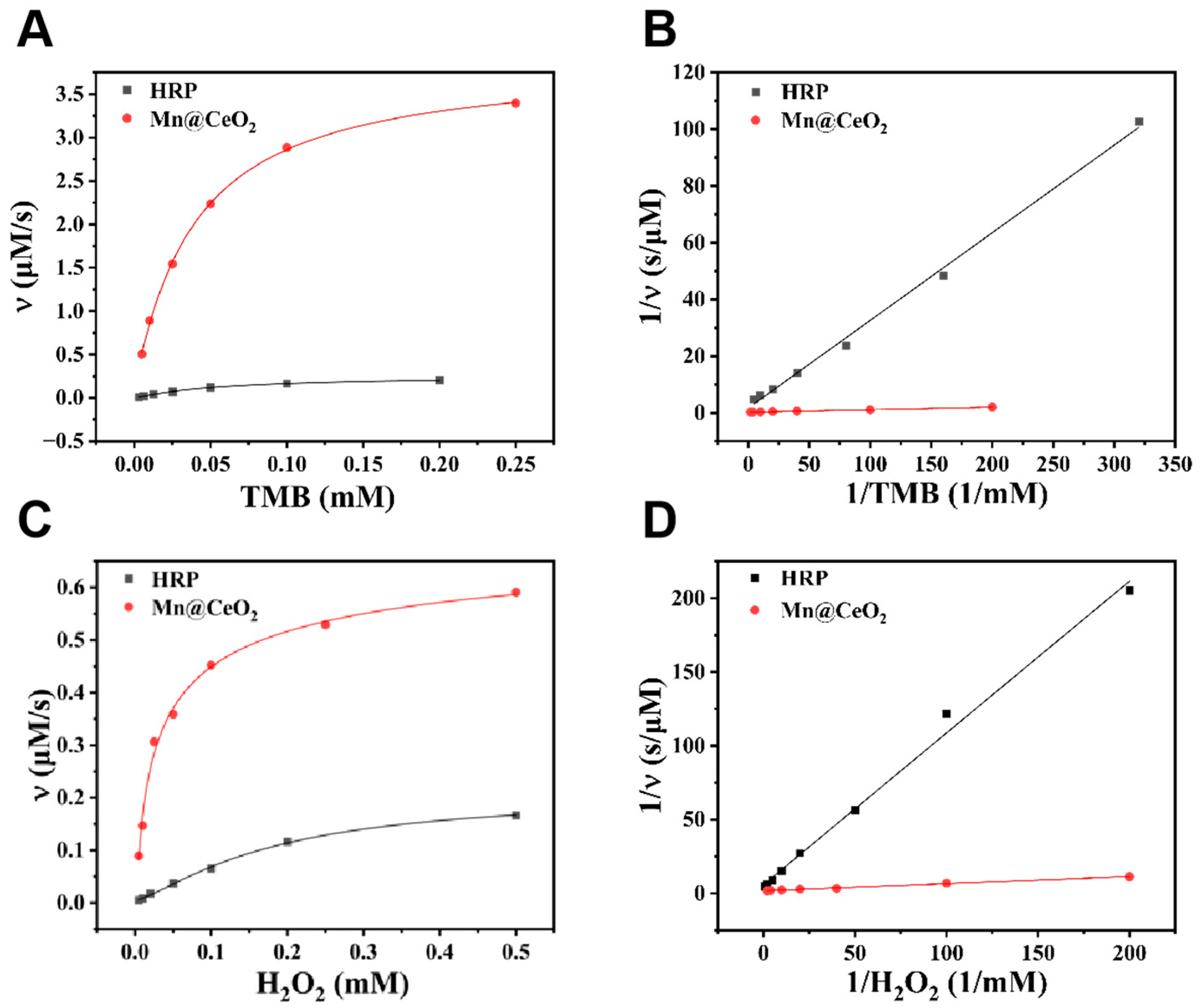
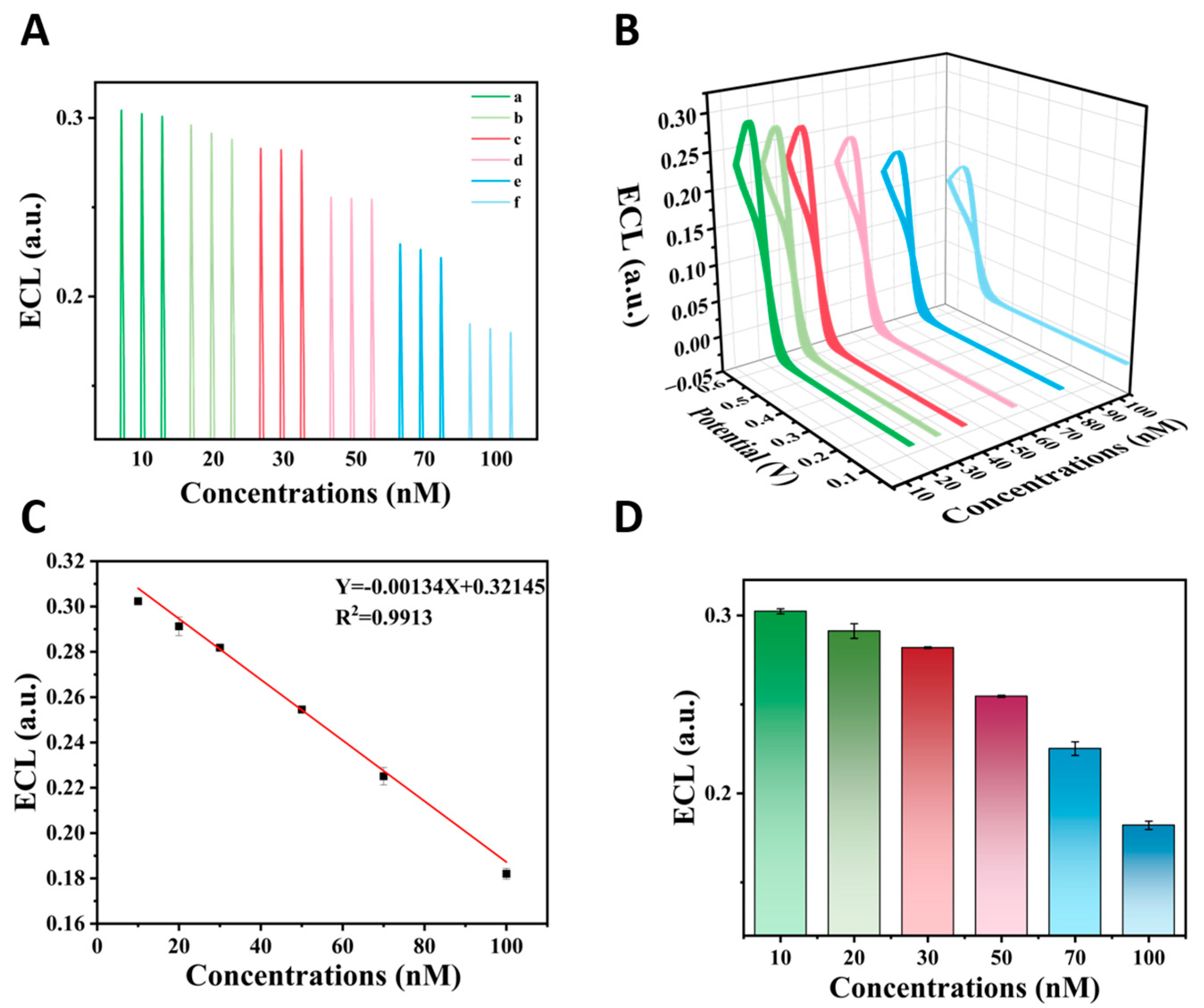
3.2. Electrochemical Sensing Interface Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vacher, M.; Galván, I.; Ding, B.; Schramm, S.; Berraud-Pache, R.; Naumov, P.; Ferré, N.; Liu, Y.; Navizet, I.; Roca-Sanjuán, D.; et al. Chemi- and Bioluminescence of Cyclic Peroxides. Chem. Rev. 2018, 118, 6927–6974. [Google Scholar] [CrossRef] [PubMed]
- Babarniri, B.; Bahari, D.; Salimi, A. Highly sensitive bioaffinity electrochemiluminescence sensors: Recent advances and future directions. Biosens. Bioelectron. 2019, 142, 111530. [Google Scholar]
- Jia, Z.; Zhang, H.; Chen, Y.; Fang, Y.; Zhang, J.; Hu, S. Perovskite-based electrochemiluminescence analysis of H2O2. RSC Adv. 2024, 14, 19744–19751. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, R.; Gao, Y.; Zhou, Y.; Zhu, J. Advances of Electrochemical and Electrochemiluminescent Sensors Based on Covalent Organic Frameworks. Nanomicro Lett. 2024, 16, 37. [Google Scholar] [CrossRef]
- Yin, F.; Sun, Q.; Huang, X.; Wu, G.; Zhang, Y.; Shen, Y. Recent progress in signal enhancement of nanomaterials-based electrochemiluminescence systems. TrAC-Trends Anal. Chem. 2023, 169, 117376. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Wang, B.; Liu, X.; Lu, C. Understanding role of microstructures of nanomaterials in electrochemiluminescence properties and their applications. TrAC-Trends Anal. Chem. 2023, 162, 117030. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, X.; Yang, Q.; Chen, H.; Hao, N.; Hu, S. A perovskite-based electrochemiluminescence aptasensor for tetracycline screening. Luminescence 2024, 39, e4717. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J. Recent progress on nanozymes in electrochemical sensing. J. Electroanal. Chem. 2023, 936, 117391. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Wang, H.; Gu, X.; Zhou, Y.; Xi, F. Enhanced electrochemiluminescence of luminol at neutral medium using nanochannel-confined Co3O4 nanozyme for highly sensitive detection of tumor biomarker. Microchem. J. 2025, 209, 112903. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, Z.; Zhai, W.; Qu, Y. Insights on catalytic mechanism of CeO2 as multiple nanozymes. Nano Res. 2022, 15, 10328–10342. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, X.; Wang, Y.; Shen, B.; Yang, Y.; Huang, H. Heteroatom-doped nanozyme progress and perspectives: From synthesis strategies to biomedical applications. Chem. Eng. J. 2023, 468, 143703. [Google Scholar] [CrossRef]
- Guan, H.; Zhao, Y.; Cheng, J.; Zhang, Y.; Yang, Q.; Zhang, B. Fabrication of Pt/CeO2/NCNFs with embedded structure as high-efficiency nanozyme for electrochemical sensing of hydrogen peroxide. Synth. Met. 2020, 270, 116604. [Google Scholar] [CrossRef]
- Ruan, H.; Zhang, S.; Wang, H.; Pei, J.; Zhao, R.; Mu, X.; Wang, H.; Zhang, X. Single-Atom Pd/CeO2 Nanostructures for Mimicking Multienzyme Activities. ACS Appl. Nano Mater. 2022, 5, 6564–6574. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Lin, X.; Gao, X.; Wang, Q.; Huang, R.; Ruan, Y.; Xu, H.; Tian, L.; Ling, C.; et al. Mn-Ce Symbiosis: Nanozymes with Multiple Active Sites Facilitate Scavenging of Reactive Oxygen Species (ROS) Based on Electron Transfer and Confinement Anchoring. Angew. Chem. Int. Ed. 2025, 64, e202416686. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Zhu, X.; Liu, M.; Cheng, M.; Fan, K.; Zhao, Z.; Huang, Q.; Zhang, X.; Han, Z. Molecularly imprinted polymer-based electrochemical sensor for rapid detection of masked deoxynivalenol with Mn-doped CeO2 nanozyme as signal amplifier. J. Hazard. Mater. 2024, 477, 135366. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhong, S.; Hu, X.; Koh, K.; Chen, H. Perspectives and trends in advanced optical and electrochemical biosensors based on engineered peptides. Mikrochim. Acta 2023, 190, 327. [Google Scholar] [CrossRef]
- Jiang, X.; Yao, T.; Wang, S.; Han, H.; Ma, Z.; Yang, H. Novel 2D CuFe-MOF-based immunoprobe: Addressing antifouling electrochemical immunosensing inadequate sensitivity challenge. Sens. Actuators B Chem. 2024, 406, 135410. [Google Scholar] [CrossRef]
- Pu, X.; Niu, M.; Fan, X.; Sun, L.; Gu, Y.; Wang, S. A zwitterionic phosphorylcholine-based antifouling electrochemical aptasensor for aflatoxin B1 detection in food. J. Agric. Food Chem. 2025, 139, 107178. [Google Scholar]
- Song, Z.; Li, Y.; Li, R.; Fan, G.C.; Luo, X. Robust Electrochemical Biosensors Based on Antifouling Peptide Nanoparticles for Protein Quantification in Complex Biofluids. ACS Sens. 2024, 9, 1525–1532. [Google Scholar] [CrossRef]
- Yang, H.; Wang, P.; Geng, F.; Wu, Q.; Song, F.; Ding, C. Copolymerization of zwitterionic sulfobetaine and hydrophobic acrylamide based antifouling electrochemical biosensors for detection of CA125 in clinical serum samples. Sens. Actuators B Chem. 2023, 387, 133820. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Huang, Y.; Nahar, S.; Alam, M.M.; Hu, S.; McVicar, D.W.; Yang, D. Reactive Oxygen Species-Sensitive Biodegradable Mesoporous Silica Nanoparticles Harboring TheraVac Elicit Tumor-Specific Immunity for Colon Tumor Treatment. ACS Nano 2023, 17, 19740–19752. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, R.; Yang, X.; Ambrosi, A.; Luo, X. Ultralow fouling electrochemical detection of uric acid directly in serum based on phase-transited bovine serum albumin and conducting polymer. Chin. Chem. Lett. 2023, 34, 108314. [Google Scholar] [CrossRef]
- Ansari, M.; Gouadria, S.; Khosa, R.; Manzoor, S.; Abdullah, M.; Abid, A.; Ahmad, N.; Aman, S.; Farid, H. Nanospheres type Morphology for regulating the electrochemical property of CeO2 nanostructures for energy storage system. J. Sol-Gel Sci. Technol. 2023, 106, 121–130. [Google Scholar] [CrossRef]
- Rajesh, K.; Sakthivel, P.; Santhanam, A.; Venugobal, J. Structural, morphological and optical behavior of Zn2+:CeO2 nanoparticles annealed under Ar atmosphere. Rom. Rep. Phys. 2021, 73, 504. [Google Scholar]
- Xu, X.; Xu, X.; Wu, T.; Wang, J.; Wang, G. CeO2@Mn Nanozymes for Colorimetric and Fluorescence Detection of Iodide Ions in Urine and Blood. ACS Appl. Nano Mater. 2025, 8, 1956–1964. [Google Scholar]
- Chen, S.; Cao, Z.; Jiang, S. Ultra-low fouling peptide surfaces derived from natural amino acids. Biomaterials 2009, 30, 5892–5896. [Google Scholar] [CrossRef]
- Hu, X.; Tian, J.; Li, C.; Su, H.; Qin, R.; Wang, Y.; Cao, X.; Yang, P. Amyloid-Like Protein Aggregates: A New Class of Bioinspired Materials Merging an Interfacial Anchor with Antifouling. Adv. Mater. 2020, 32, e2000128. [Google Scholar] [CrossRef]
- Zhong, X.; Deng, Y.; Yang, Q.; Yi, S.; Qiu, H.; Chen, L.; Hu, S. An extracellular electron transfer enhanced electrochemiluminescence aptasensor for Escherichia coli analysis. Analyst 2023, 148, 4414–4420. [Google Scholar] [CrossRef]
- Padha, B.; Verma, S.; Mahajan, P.; Arya, S. Electrochemical Impedance Spectroscopy (EIS) Performance Analysis and Challenges in Fuel Cell Applications. J. Electrochem. Sci. Technol. 2022, 13, 167–176. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Siroma, Z.; Mineshige, A.; Takeno, M.; Fukutsuka, T.; Abe, T.; Uchida, S. Electrochemical Impedance Spectroscopy Part 1: Fundamentals. Electrochemistry 2022, 90, 102007. [Google Scholar] [CrossRef]
- Huang, X.; Xia, F.; Nan, Z. Fabrication of FeS2/SiO2 Double Mesoporous Hollow Spheres as an Artificial Peroxidase and Rapid Determination of H2O2 and Glutathione. ACS Appl. Mater. Interfaces 2020, 12, 46539–46548. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, S.; Liu, X.; Zhu, X.; Gao, Y.; Liu, Q. CeO2/Co3O4@N-doped hollow carbon microspheres with improved peroxidase-like activity for the determination of quercetin. Anal. Bioanal. Chem. 2022, 414, 4767–4775. [Google Scholar] [CrossRef]
- Jampaiah, D.; Srinivasa, R.T.; Coyle, V.E.; Nafady, A.; Bhargava, S.K. Co3O4@CeO2 hybrid flower-like microspheres: A strong synergistic peroxidase-mimicking artificial enzyme with high sensitivity for glucose detection. J. Mater. Chem. B 2017, 5, 720–730. [Google Scholar] [CrossRef]
- Hao, J.; Feng, J.; Sun, S.; Cao, Z.; Xu, W.; Hu, L.; Yao, W.; Yan, Z. Reliable ratiometric colorimetric monitoring of dopamine in practice based on the catalytic signal amplification of nano CeO2/CuO modified carboxylated chitosan. Chem. Eng. Sci. 2024, 295, 120193. [Google Scholar] [CrossRef]
- Chen, F.; Ma, X.; Cao, X.; Dou, Y.; Guan, S.; Qiu, X.; Han, J. An effective antioxidant to mitigate reperfusion injury by tailoring CeO2 electronic structure on layered double hydroxide nanosheets. Chem. Eng. J. 2023, 475, 146190. [Google Scholar] [CrossRef]
- Dadigala, R.; Bandi, R.; Han, S.Y.; Kwon, G.J.; Lee, S.H. Rapid in-situ growth of enzyme-mimicking Pd nanoparticles on TEMPO-oxidized nanocellulose for the efficient detection of ascorbic acid. Int. J. Biol. Macromol. 2023, 234, 123657. [Google Scholar] [CrossRef]
- Das, D.P.; Boruah, P.K.; Sarmah, P.; Dutta, R.; Boukherroub, D.R.; Das, D.M.R. A Facile Preparation of Reduced Graphene Oxide Capped AuAg Bimetallic Nanoparticles: A Selective Nanozyme for Glutathione Detection. Chemistryselect 2022, 7, e202203415. [Google Scholar] [CrossRef]
- Esmaeili, A.; Dehghan, G.; Dadakhani, S.; Fathinia, M.; Haghighat, H.; Khataee, A. Development of ATP-modified CoFe2O4@ZIF-8@ZIF-67 core-shell nanozyme for sensitive colorimetric detection of dopamine. Microchem. J. 2024, 204, 111027. [Google Scholar] [CrossRef]
- Alula, M.T.; Hendricks-Leukes, N.R. Silver nanoparticles loaded carbon-magnetic nanocomposites: A nanozyme for colorimetric detection of dopamine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 322, 124830. [Google Scholar]
- Zhu, Z.; Gong, L.; Miao, X.; Chen, C.; Su, S. Prussian Blue Nanoparticle Supported MoS2 Nanocomposites as a Peroxidase-Like Nanozyme for Colorimetric Sensing of Dopamine. Biosensors 2022, 12, 260. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, K.; Gao, M.; Zhang, T.; Wang, L.; Cui, Y.; Ji, X.; Ma, G.; Hu, J. Vancomycin-Stabilized Platinum Nanoparticles with Oxidase-like Activity for Sensitive Dopamine Detection. Biomolecules 2023, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Du, X.; Xu, G.; Song, P. Nanozyme catalyzed-SERRS sensor for the recognition of dopamine based on AgNPs@PVP with oxidase-like activity. Spectrochim. Acta A 2024, 307, 123606. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Yan, X.; Gu, Y.; Zhang, T.; Liu, Y.; Song, Y.; Xu, Z.; Xing, Y.; Li, X.; Zhang, Z.; et al. Cobalt-decorated 3D hybrid nanozyme: A catalytic amplification platform with intrinsic oxidase-like activity. Electrochim. Acta 2021, 395, 139197. [Google Scholar] [CrossRef]
- Singh, G.; Kushwaha, A.; Sharma, M. Electrochemistry of rGO-Cu3H2Mo2O10 cuboidal nanostructures: An effective detection of neurotransmitter dopamine in blood serum sample. J. Electroanal. Chem. 2021, 880, 114889. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Cai, G.; Li, M.; Tang, D. In situ formation of (001)TiO2/Ti3C2 heterojunctions for enhanced photoelectrochemical detection of dopamine. Electrochem. Commun. 2021, 125, 106987. [Google Scholar] [CrossRef]
- Ding, Y.X.; Zhao, X.L.; Wu, P.; Wang, R.R.; Xie, L.L.; Li, Z.H.; Zhu, Z.G.; Zhao, H.L.; Lan, M.B. ZIF-67 MOF derived Co-Based CeO2 electrochemical sensor for dopamine. Electrochim. Acta 2023, 463, 142802. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Qiu, H.; Chen, H.; Li, W.; Zhang, Y.; Huang, M.; Zhang, T.; Xu, X.; Hu, S. Mn-Doped CeO2 Nanozyme-Integrated Mesoporous Interfaces for High-Sensitivity Antifouling Electrochemiluminescence Biosensing. Biosensors 2025, 15, 411. https://doi.org/10.3390/bios15070411
Huang G, Qiu H, Chen H, Li W, Zhang Y, Huang M, Zhang T, Xu X, Hu S. Mn-Doped CeO2 Nanozyme-Integrated Mesoporous Interfaces for High-Sensitivity Antifouling Electrochemiluminescence Biosensing. Biosensors. 2025; 15(7):411. https://doi.org/10.3390/bios15070411
Chicago/Turabian StyleHuang, Guanze, Haiyan Qiu, Huiping Chen, Wanxuan Li, Yufei Zhang, Minfang Huang, Tingting Zhang, Xiaoxin Xu, and Shanwen Hu. 2025. "Mn-Doped CeO2 Nanozyme-Integrated Mesoporous Interfaces for High-Sensitivity Antifouling Electrochemiluminescence Biosensing" Biosensors 15, no. 7: 411. https://doi.org/10.3390/bios15070411
APA StyleHuang, G., Qiu, H., Chen, H., Li, W., Zhang, Y., Huang, M., Zhang, T., Xu, X., & Hu, S. (2025). Mn-Doped CeO2 Nanozyme-Integrated Mesoporous Interfaces for High-Sensitivity Antifouling Electrochemiluminescence Biosensing. Biosensors, 15(7), 411. https://doi.org/10.3390/bios15070411





