Convenient Biochemical Testing Technologies for Oral Disease Risk Warning: Opportunities and Challenges
Abstract
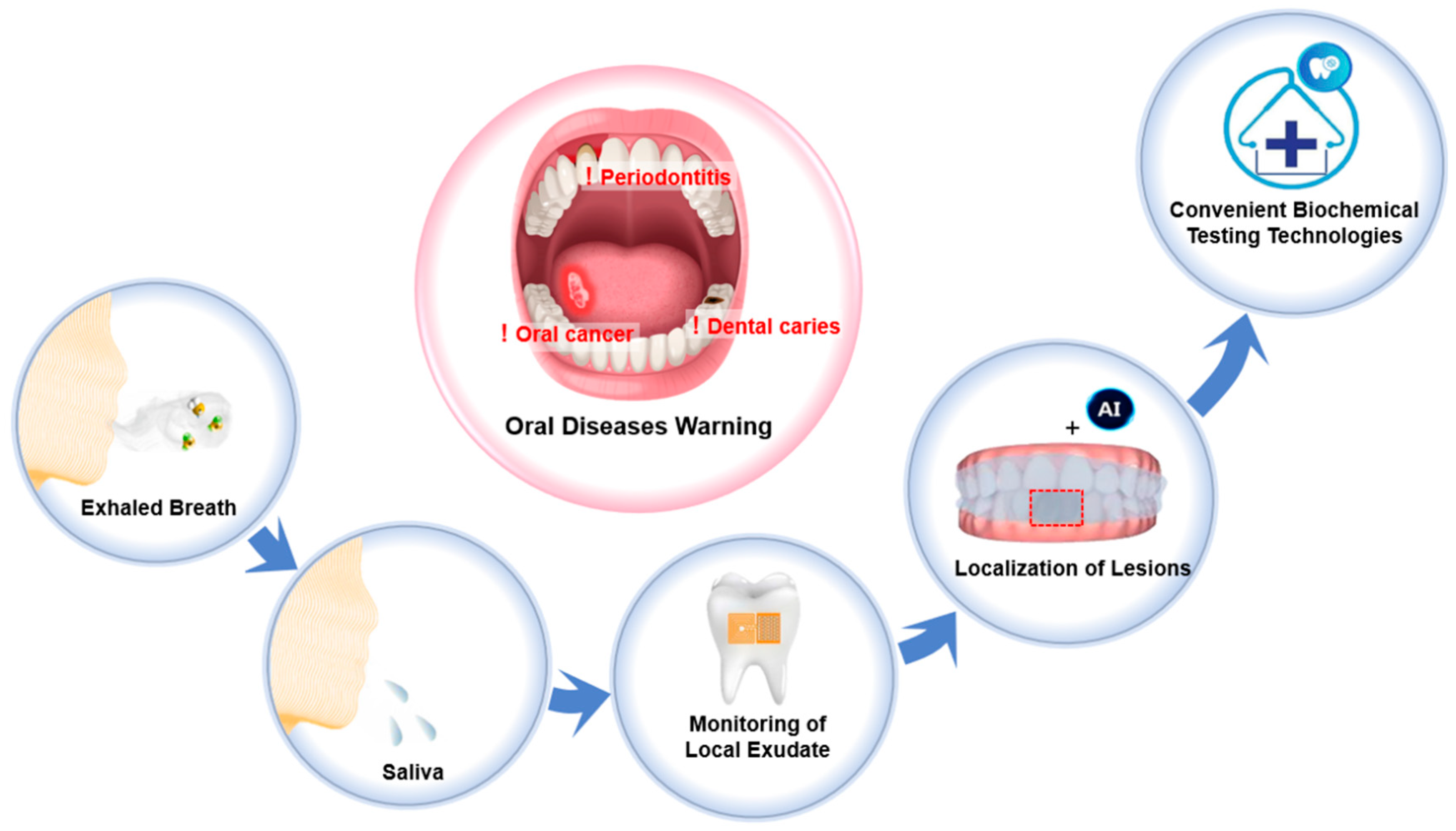
1. Introduction
2. Biochemical Testing Technologies for Oral Diseases Warning
2.1. Measuring Biomarkers in Exhaled Breath
2.2. Detecting Biomarkers in Whole Saliva
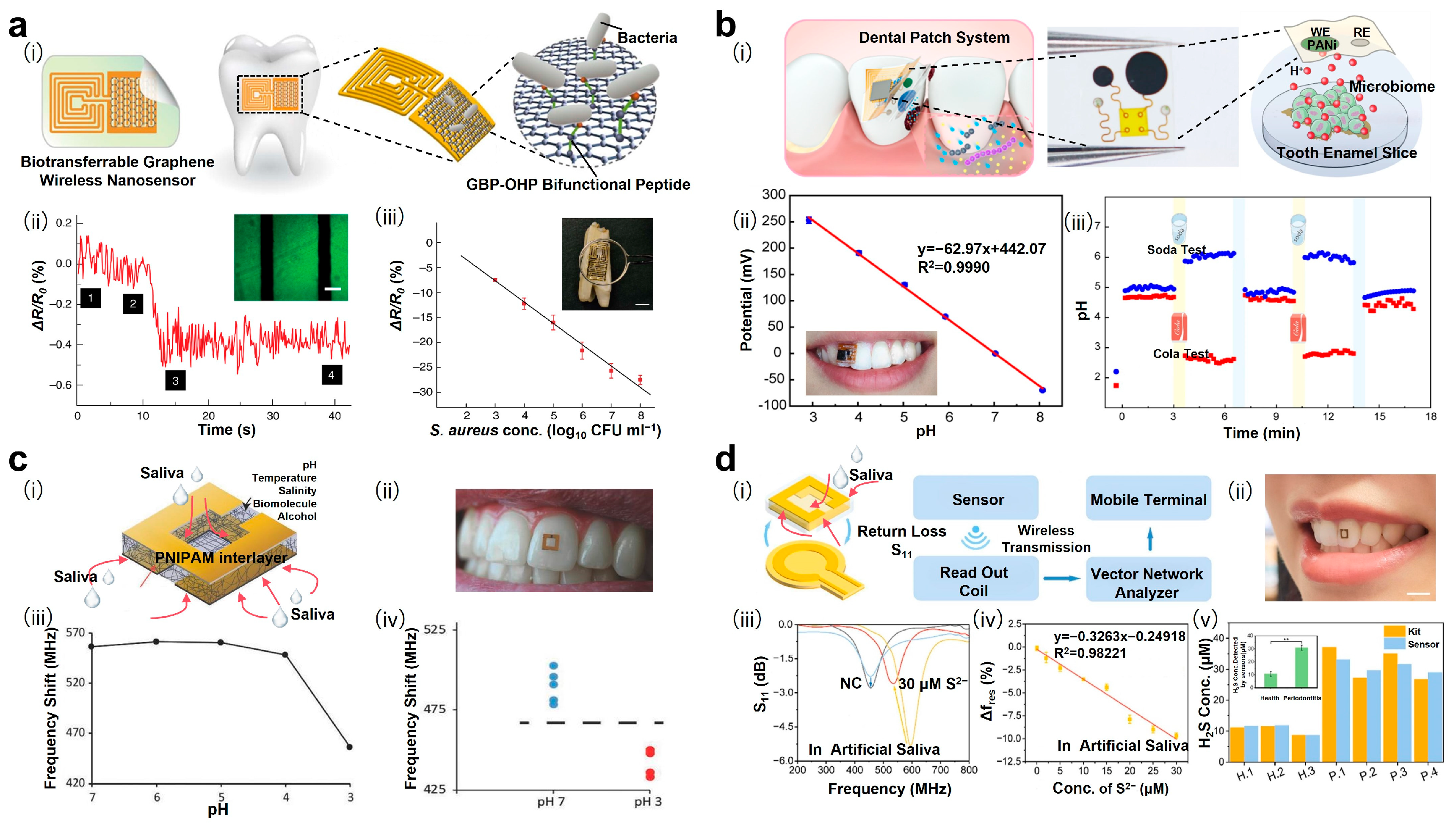
2.3. Monitoring Biomarkers in Local Exudate
2.4. Localizing Lesions Through Imaging Distribution of Biomarkers
3. Discussion
4. Opportunities and Perspectives
Funding
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C. Oral diseases: A global public health challenge. Lancet 2019, 394, 249. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the global burden of disease study 2019. J. Clin. Periodontol. 2021, 48, 1165. [Google Scholar] [CrossRef] [PubMed]
- Benzian, H.; Makino, Y.; Stauf, N.; Varenne, B.; Watt, R. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426. [Google Scholar] [CrossRef]
- Farrugia, C.; Stafford, G.P.; Potempa, J.; Wilkinson, R.N.; Chen, Y.; Murdoch, C.; Widziolek, M. Mechanisms of vascular damage by systemic dissemination of the oral pathogen Porphyromonas gingivalis. FEBS J. 2021, 288, 1479. [Google Scholar] [CrossRef]
- Tuganbaev, T.; Yoshida, K.; Honda, K. The effects of oral microbiota on health. Science 2022, 376, 934. [Google Scholar] [CrossRef]
- Brandini, D.A.; Takamiya, A.S.; Thakkar, P.; Schaller, S.; Rahat, R.; Naqvi, A.R. Covid-19 and oral diseases: Crosstalk, synergy or association? Rev. Med. Virol. 2021, 31, e2226. [Google Scholar] [CrossRef] [PubMed]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontology 2000 2020, 83, 90. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Tonmukayakul, U.; Le, L.K.D.; Calache, H.; Mihalopoulos, C. Economic evaluations of preventive interventions for dental caries and periodontitis: A systematic review. Appl. Health Econ. Health Policy 2023, 21, 53. [Google Scholar] [CrossRef]
- Amarasena, N.; Luzzi, L.; Brennan, D. Effect of different frequencies of dental visits on dental caries and periodontal disease: A scoping review. Int. J. Environ. Res. Public Health 2023, 20, 6858. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Yue, L.; Ling, J.; Fan, M.; Yang, D.; Huang, Z.; Niu, Y.; Liu, J.; Zhao, J. Expert consensus on dental caries management. Int. J. Oral Sci. 2022, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Hand, A.R.; Frank, M.E. Fundamentals of Oral Histology and Physiology; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Regezi, J.A.; Sciubba, J.; Jordan, R.C. Oral Pathology: Clinical Pathologic Correlations; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Warnakulasuriya, S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020, 102, 104550. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Roccuzzo, A.; Imber, J.C.; Stähli, A.; Klinge, B.; Lang, N.P. Clinical periodontal diagnosis. Periodontology 2000 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Casati, S.; Goldoni, R.; Thomaz, D.V.; Kehr, N.S.; Galimberti, D.; Del Fabbro, M.; Tartaglia, G.M. Salivary biomarkers: Novel noninvasive tools to diagnose chronic inflammation. Int. J. Oral Sci. 2023, 15, 27. [Google Scholar] [CrossRef]
- Ghaffari, R.; Rogers, J.A.; Ray, T.R. Recent progress, challenges, and opportunities for wearable biochemical sensors for sweat analysis. Sens. Actuators B Chem. 2021, 332, 129447. [Google Scholar] [CrossRef]
- Lamont, R.J.; Hajishengallis, G.N.; Koo, H.M.; Jenkinson, H.F. Oral Microbiology and Immunology; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Li, Y.; Tang, H.; Liu, Y.; Qiao, Y.; Xia, H.; Zhou, J. Oral wearable sensors: Health management based on the oral cavity. Biosens. Bioelectron. X 2022, 10, 100135. [Google Scholar] [CrossRef]
- Hou, Y.; Lv, C.; Liu, W.; Guo, Y.; Jin, Y.; Li, B.; Zhang, Y.; Liu, Y. In situ synthesis of copper metal-organic framework on paper-based device for dual-mode detection of volatile sulfur compounds in exhaled breath. Sens. Actuators B Chem. 2022, 352, 131008. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Z.; Zou, Y.; Zhang, J.; Xia, W.; Zhang, R.; He, Z.; Cai, X.; Lin, Y.; Duan, S.-Z. Engineering DNA–nanozyme interfaces for rapid detection of dental bacteria. ACS Appl. Mater. Interfaces 2019, 11, 30640. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, B.; Li, Y.; Pang, X.; Fu, Q.; Xiao, Z.; Shi, Z.; Li, X.; Luo, C.; Zhou, Z.K.; et al. Au@ Ag nanorods-PDMS wearable mouthguard as a visualized detection platform for screening dental caries and periodontal diseases. Adv. Healthc. Mater. 2022, 11, e2102682. [Google Scholar] [CrossRef]
- World Health Organization. Biomarkers in Risk Assessment: Validity and Validation; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Marsh, P.D. Dental plaque as a biofilm: The significance of pH in health and caries. Compend. Contin. Dducation Dent. 2009, 30, 76. [Google Scholar]
- Ferrer, M.D.; Pérez, S.; Lopez, A.L.; Sanz, J.L.; Melo, M.; Llena, C.; Mira, A. Evaluation of clinical, biochemical and microbiological markers related to dental caries. Int. J. Environ. Res. Public Health 2021, 18, 6049. [Google Scholar] [CrossRef]
- Sivapathasundharam, B.; Raghu, A. Shafer’s Textbook of Oral Pathology, 9th ed.; [An Adaptation of a Textbook of Oral Pathology, 1983, 4e, Elsevier Inc.]; Elsevier RELX India Pvt Ltd.: New Delhi, India, 2020; Volume 369. [Google Scholar]
- Alqahtani, A.A.; Alhalabi, F.; Alam, M.K. Salivary elemental signature of dental caries: A systematic review and meta-analysis of ionomics studies. Odontology 2024, 112, 27. [Google Scholar] [CrossRef]
- Welch, J.L.M.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791. [Google Scholar]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, S.S.; Gupta, M.; Snehi, S.K. Dental caries and lactobacillus: Role and ecology in the oral cavity. Int. J. Pharm. Sci. Res. 2019, 11, 4818. [Google Scholar]
- Lee, Y.H.; Shin, S.I.; Hong, J.Y. Investigation of volatile sulfur compound level and halitosis in patients with gingivitis and periodontitis. Sci. Rep. 2023, 13, 13175. [Google Scholar] [CrossRef]
- Passoja, A.; Puijola, I.; Knuuttila, M.; Niemelä, O.; Karttunen, R.; Raunio, T.; Tervonen, T. Serum levels of interleukin-10 and tumour necrosis factor-α in chronic periodontitis. J. Clin. Periodontol. 2010, 37, 881. [Google Scholar] [CrossRef]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1β is a potential therapeutic target for periodontitis: A narrative review. Int. J. Oral Sci. 2020, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Kiili, M.; Cox, S.; Chen, H.; Wahlgren, J.; Maisi, P.; Eley, B.; Salo, T.; Sorsa, T. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: Molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J. Clin. Periodontol. 2002, 29, 224. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, A.; Oyaizu, K.; Van Dyke, T.E. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: Findings from localized aggressive periodontitis. J. Periodontol. 2003, 74, 66. [Google Scholar] [CrossRef]
- Nauseef, W.M. Proteases, neutrophils, and periodontitis: The NET effect. J. Clin. Investig. 2014, 124, 4237. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T. The role of gingipains in the pathogenesis of periodontal disease. J. Periodontol. 2003, 74, 111. [Google Scholar] [CrossRef]
- Lietzan, A.D.; Simpson, J.B.; Walton, W.G.; Jariwala, P.B.; Xu, Y.; Boynton, M.H.; Liu, J.; Redinbo, M.R. Microbial b-glucuronidases drive human periodontal disease etiology. Sci. Adv. 2023, 9, eadg3390. [Google Scholar] [CrossRef]
- Usui, M.; Iwasaki, M.; Ariyoshi, W.; Kobayashi, K.; Kasai, S.; Yamanaka, R.; Nakashima, K.; Nishihara, T. The ability of a novel trypsin-like peptidase activity assay kit to detect red-complex species. Diagnostics 2022, 12, 2172. [Google Scholar] [CrossRef]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768. [Google Scholar] [CrossRef]
- Reyes, L. Porphyromonas gingivalis. Trends Microbiol. 2021, 29, 376. [Google Scholar] [CrossRef]
- He, W.; You, M.; Wan, W.; Xu, F.; Li, F.; Li, A. Point-of-care periodontitis testing: Biomarkers, current technologies, and perspectives. Trends Biotechnol. 2018, 36, 1127. [Google Scholar] [CrossRef]
- AlAli, A.; Walsh, T.; Maranzano, M. CYFRA 21-1 and MMP-9 as salivary biomarkers for the detection of oral squamous cell carcinoma: A systematic review of diagnostic test accuracy. Int. J. Oral Maxillofac. Surg. 2020, 49, 973. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef]
- Silva, L.M.; Doyle, A.D.; Greenwell-Wild, T.; Dutzan, N.; Tran, C.L.; Abusleme, L.; Juang, L.J.; Leung, J.; Chun, E.M.; Lum, A.G. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science 2021, 374, eabl5450. [Google Scholar] [CrossRef] [PubMed]
- Omori, H.; Nishio, M.; Masuda, M.; Miyachi, Y.; Ueda, F.; Nakano, T.; Sato, K.; Mimori, K.; Taguchi, K.; Hikasa, H. YAP1 is a potent driver of the onset and progression of oral squamous cell carcinoma. Sci. Adv. 2020, 6, eaay3324. [Google Scholar] [CrossRef]
- Poljak, M.; Cuschieri, K.; Alemany, L.; Vorsters, A. Testing for human papillomaviruses in urine, blood, and oral specimens: An update for the laboratory. J. Clin. Microbiol. 2023, 61, e01403. [Google Scholar] [CrossRef]
- Cheng, C.S.; Ou, B.R.; Lung, F.D. Developing a biosensor-based immunoassay to detect HPV E6 oncoprotein in the saliva rinse fluid of oral cancer patients. J. Pers. Med. 2022, 12, 594. [Google Scholar] [CrossRef]
- Vasilescu, A.; Hrinczenko, B.; Swain, G.M.; Peteu, S.F. Exhaled breath biomarker sensing. Biosens. Bioelectron. 2021, 182, 113193. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Deng, Y.; Yang, H.; Liao, Y.; Cheng, X.; Zou, Y.; Wu, L.; Deng, Y. Functionalization of mesoporous semiconductor metal oxides for gas sensing: Recent advances and emerging challenges. Adv. Sci. 2023, 10, 2204810. [Google Scholar] [CrossRef]
- Voss, A.; Schroeder, R.; Schulz, S.; Haueisen, J.; Vogler, S.; Horn, P.; Stallmach, A.; Reuken, P. Detection of liver dysfunction using a wearable electronic nose system based on semiconductor metal oxide sensors. Biosensors 2022, 12, 70. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206. [Google Scholar] [CrossRef]
- Das, S.; Mojumder, S.; Saha, D.; Pal, M. Influence of major parameters on the sensing mechanism of semiconductor metal oxide based chemiresistive gas sensors: A review focused on personalized healthcare. Sens. Actuators B Chem. 2022, 352, 131066. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Shi, F.; Han, Q.; Shi, Y.; Yang, L.; Wang, K.; Dong, B.; Wang, L.; Xu, L. Nanometric surface-selective regulation of Au/In2O3 nanofibers as an exhaled H2S chemiresistor for periodontitis diagnosis. ACS Sens. 2022, 7, 3530. [Google Scholar] [CrossRef]
- Zhao, J.; He, H.; Guo, J.; He, Z.; Zhao, C.; Wang, H.; Gao, Z.; Song, Y. Target-driven Z-scheme heterojunction formation for ppb H2S detection from exhaled breath at room temperature. ACS Sens. 2023, 8, 2824. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhou, J.; Zhang, J.; Zhao, Y.; Xie, C.; Yin, W.; Xie, J.; Li, H.; Xu, X.; Zhao, L. A structural color hydrogel for diagnosis of halitosis and screening of periodontitis. Mater. Horiz. 2024, 11, 519. [Google Scholar] [CrossRef]
- Krespi, Y.P.; Shrime, M.G.; Kacker, A. The relationship between oral malodor and volatile sulfur compound–producing bacteria. Otolaryngol.—Head Neck Surg. 2006, 135, 671. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Edlund, M.B.; Claesson, R.; Carlsson, J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 1990, 5, 195. [Google Scholar] [CrossRef]
- Abarca-Vargas, R.; Malacara, C.F.P.; Petricevich, V.L. Characterization of chemical compounds with antioxidant and cytotoxic activities in bougainvillea x buttiana holttum and standl, (var. rose) extracts. Antioxidants 2016, 5, 45. [Google Scholar] [CrossRef]
- Yoshida, A.; Yoshimura, M.; Ohara, N.; Yoshimura, S.; Nagashima, S.; Takehara, T.; Nakayama, K. Hydrogen sulfide production from cysteine and homocysteine by periodontal and oral bacteria. J. Periodontol. 2009, 80, 1845. [Google Scholar] [CrossRef]
- Chen, H.; Lv, L.; Xue, K.; Zhang, P.; Du, L.; Cui, G. Oral exhalation H2S sensor based on Cu2O/ZnO heterostructures. ACS Sens. 2025, 10, 2579. [Google Scholar] [CrossRef]
- Tereshkov, M.; Dontsova, T.; Saruhan, B.; Krüger, S. Metal oxide-based sensors for ecological monitoring: Progress and perspectives. Chemosensors 2024, 12, 42. [Google Scholar] [CrossRef]
- Huang, Y.; Yen, T.; Shi, M.; Hung, Y.; Chen, W.; Wu, C.; Hung, K.; Lo, K. Competition between oxygen and water molecules on SiO2/P-doped Si surface: The electrical dipole evolution on water/oxygen-adsorbed oxide surface. Sens. Actuators B Chem. 2023, 376, 133011. [Google Scholar] [CrossRef]
- Tokura, Y.; Nakada, G.; Moriyama, Y.; Oaki, Y.; Imai, H.; Shiratori, S. Ultrasensitive detection of methylmercaptan gas using layered manganese oxide nanosheets with a quartz crystal microbalance sensor. Anal. Chem. 2017, 89, 12123. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, H.; Zheng, J.; Li, Q.; Xu, R.; Xu, J.; Song, Y.; Song, P.; Gao, Z.; Zhao, C. Integrating vacancies and defect levels in heterojunctions to synergistically enhance the performance of H2S chemiresistors for periodontitis diagnosis. ACS Sens. 2025, 10, 3072. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Saito, J.; Munakata, M.; Shibata, Y. Hydrogen sulfide as a novel biomarker of asthma and chronic obstructive pulmonary disease. Allergol. Int. 2021, 70, 181. [Google Scholar] [CrossRef]
- Van den Velde, S.; van Steenberghe, D.; Van Hee, P.; Quirynen, M. Detection of odorous compounds in breath. J. Dent. Res. 2009, 88, 285. [Google Scholar] [CrossRef]
- Du, P.; Tseng, Y.; Liu, P.; Zhang, H.; Huang, G.; Hu, C.E.; Chen, J. Role of exhaled hydrogen sulfide in the diagnosis of colorectal cancer. BMJ Open Gastroenterol. 2024, 11, e001229. [Google Scholar] [CrossRef]
- Kassinos, S.C.; Sznitman, J. Multiscale modeling of respiratory transport phenomena and intersubject variability. Annu. Rev. Fluid Mech. 2025, 57, 141. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Géli, V.; Nabet, N. Saliva, a molecular reflection of the human body? implications for diagnosis and treatment. Cell Stress 2024, 8, 59. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chung, J.W.; Kim, Y.K.; Chung, S.C.; Kho, H.S. Comparison of the composition of oral mucosal residual saliva with whole saliva. Oral Dis. 2007, 13, 550. [Google Scholar] [CrossRef]
- Hartenbach, F.A.R.R.; Velasquez, É.; Nogueira, F.C.; Domont, G.B.; Ferreira, E.; Colombo, A.P.V. Proteomic analysis of whole saliva in chronic periodontitis. J. Proteom. 2020, 213, 103602. [Google Scholar] [CrossRef] [PubMed]
- Gug, I.T.; Tertis, M.; Hosu, O.; Cristea, C. Salivary biomarkers detection: Analytical and immunological methods overview. TrAC Trends Anal. Chem. 2019, 113, 301. [Google Scholar] [CrossRef]
- Matzeu, G.; Naveh, G.R.; Agarwal, S.; Roshko, J.A.; Ostrovsky-Snider, N.A.; Napier, B.S.; Omenetto, F.G. Functionalized mouth-conformable interfaces for pH evaluation of the oral cavity. Adv. Sci. 2021, 8, 2003416. [Google Scholar] [CrossRef] [PubMed]
- Wignarajah, S.; Suaifan, G.A.; Bizzarro, S.; Bikker, F.J.; Kaman, W.E.; Zourob, M. Colorimetric assay for the detection of typical biomarkers for periodontitis using a magnetic nanoparticle biosensor. Anal. Chem. 2015, 87, 12161. [Google Scholar] [CrossRef]
- Alhogail, S.; Suaifan, G.A.; Bizzarro, S.; Kaman, W.E.; Bikker, F.J.; Weber, K.; Cialla-May, D.; Popp, J.; Zourob, M. On site visual detection of Porphyromonas gingivalis related periodontitis by using a magnetic-nanobead based assay for gingipains protease biomarkers. Microchim. Acta 2018, 185, 1. [Google Scholar] [CrossRef]
- Şenel, S. An overview of physical, microbiological and immune barriers of oral mucosa. Int. J. Mol. Sci. 2021, 22, 7821. [Google Scholar] [CrossRef]
- Korgaonkar, J.; Tarman, A.Y.; Koydemir, H.C.; Chukkapalli, S.S. Periodontal disease and emerging point-of-care technologies for its diagnosis. Lab A Chip 2024, 24, 3326. [Google Scholar] [CrossRef]
- West, N.X.; Joiner, A. Enamel mineral loss. J. Dent. 2014, 42, S2. [Google Scholar] [CrossRef]
- Song, Y.; Wei, W.; Qu, X. Colorimetric biosensing using smart materials. Adv. Mater. 2011, 23, 4215. [Google Scholar] [CrossRef]
- Babu, V.; Hegde, K.S.; Bhat, S.; Sargod, S. Evaluation of efficacy of three different commercially available kit for chairside cariogenic bacteria test–caries risk test, saliva-check mutans and cariscreen. Cureus 2019, 11, e6504. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, Y.; Ren, X.; Zhang, R.; Cheng, R.; Hu, T. A method for Porphyromonas gingivalis based on recombinase polymerase amplification and lateral flow strip technology. Anal. Biochem. 2024, 687, 115425. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Kim, I. Recent developments in innovative magnetic nanoparticles-based immunoassays: From improvement of conventional immunoassays to diagnosis of COVID-19. BioChip J. 2022, 16, 351. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.Q.; Lee, E.M.; Dang, T.T.T.; Kim, E.R.; Ko, Y.; Gu, M.B. An IoT-based aptasensor biochip for the diagnosis of periodontal disease. Biosens. Bioelectron. 2024, 251, 116097. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.D.; Menezes, L.; Baeten, K.; Walsh, L.J.; Whitfield, B.C.; Batstone, M.D.; Kenny, L.; Frazer, I.H.; Scheper, G.C.; Punyadeera, C. Oral HPV16 prevalence in oral potentially malignant disorders and oral cavity cancers. Biomolecules 2020, 10, 223. [Google Scholar] [CrossRef]
- Giraldi, L.; Collatuzzo, G.; Hashim, D.; Franceschi, S.; Herrero, R.; Chen, C.; Schwartz, S.M.; Smith, E.; Kelsey, K.; McClean, M. Infection with Human Papilloma Virus (HPV) and risk of subsites within the oral cancer. Cancer Epidemiol. 2021, 75, 102020. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Day, A.T.; Alvarez, V.M.; Chirinos, S.R.; Santillana, G.; Guo, M.; Anderson, K.S.; Sturgis, E.M. Circulating tumor HPV DNA, antibodies to HPV16 early proteins, and oral HPV16 DNA as biomarkers for HPV-related oropharyngeal cancer screening. Cancer Biomark. 2025, 42, 18758592241313323. [Google Scholar] [CrossRef]
- Hamzan, N.I.; Rahman, N.A.; Suraiya, S.; Mohamad, I.; Kalarakkal, T.G.; Mohamad, S. Real-time loop-mediated isothermal amplification assay for rapid detection of Human Papillomavirus 16 in oral squamous cell carcinoma. Arch. Oral Biol. 2021, 124, 105051. [Google Scholar] [CrossRef]
- Ghouneimy, A.; Ali, Z.; Aman, R.; Jiang, W.; Aouida, M.; Mahfouz, M. CRISPR-based multiplex detection of human papillomaviruses for one-pot point-of-care diagnostics. ACS Synth. Biol. 2024, 13, 837. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-McAuley, C.; Compton, R.G. Chemical analysis in saliva and the search for salivary biomarkers—A tutorial review. Analyst 2018, 143, 81. [Google Scholar] [CrossRef]
- Prodan, A.; Brand, H.S.; Ligtenberg, A.J.; Imangaliyev, S.; Tsivtsivadze, E.; van der Weijden, F.; Crielaard, W.; Keijser, B.J.; Veerman, E.C. Interindividual variation, correlations, and sex-related differences in the salivary biochemistry of young healthy adults. Eur. J. Oral Sci. 2015, 123, 149. [Google Scholar] [CrossRef]
- Quintana, M.; Palicki, O.; Lucchi, G.; Ducoroy, P.; Chambon, C.; Salles, C.; Morzel, M. Inter-individual variability of protein patterns in saliva of healthy adults. J. Proteom. 2009, 72, 822. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, F.; Wang, K.; Zhang, W.; Li, Y.; Sun, Y.; Sun, X.; Li, C.; Dong, B.; Wang, L. Smart biosensors and intelligent devices for salivary biomarker detection. TrAC Trends Anal. Chem. 2021, 140, 116281. [Google Scholar] [CrossRef]
- Shi, Z.; Lu, Y.; Shen, S.; Xu, Y.; Shu, C.; Wu, Y.; Lv, J.; Li, X.; Yan, Z.; An, Z. Wearable battery-free theranostic dental patch for wireless intraoral sensing and drug delivery. npj Flex. Electron. 2022, 6, 49. [Google Scholar] [CrossRef]
- Tseng, P.; Napier, B.; Garbarini, L.; Kaplan, D.L.; Omenetto, F.G. Functional, RF-trilayer sensors for tooth-mounted, wireless monitoring of the oral cavity and food consumption. Adv. Mater. 2018, 30, 1703257. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, X.; Sun, R.; Xu, Y.; Shi, Z.; Dai, C.; Wen, H.; Han, R.P.; Ye, Q.; Zhang, F. Hydrogel-based radio frequency H2S sensor for in situ periodontitis monitoring and antibacterial treatment. Biosens. Bioelectron. 2024, 259, 116404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, C.; Fu, Q.; Zhou, C.; Ruelas, M.; Wang, Y.; He, J.; Wang, Y.; Zhang, Y.S.; Zhou, J. A transparent, wearable fluorescent mouthguard for high-sensitive visualization and accurate localization of hidden dental lesion sites. Adv. Mater. 2020, 32, e2000060. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Qu, X.; Li, Y.; Zhou, C.; Xie, D.; Dong, J.; Ji, L.; Xu, J.; Zhou, J. Highly-sensitive ultra-thin dental patches assisted with artificial-intelligence recognition for mapping hidden periodontitis lesions. Sens. Actuators B Chem. 2025, 435, 137648. [Google Scholar] [CrossRef]
- Sujith, A.; Sajja, G.S.; Mahalakshmi, V.; Nuhmani, S.; Prasanalakshmi, B. Systematic review of smart health monitoring using deep learning and Artificial Intelligence. Neurosci. Inform. 2022, 2, 100028. [Google Scholar] [CrossRef]
- Pitchika, V.; Büttner, M.; Schwendicke, F. Artificial Intelligence and personalized diagnostics in periodontology: A narrative review. Periodontology 2000 2024, 95, 220. [Google Scholar] [CrossRef]
- Adra Corporation. (Ed: U.S. Food and Drug Administration). U.S. Food and Drug Administration, 10903 New Hampshire Avenue Silver Spring, MD 20993 2023. 510(k) Premarket Notification. K223296. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K223296 (accessed on 8 May 2025).
- Adam Foresman. (Ed: U.S. Food and Drug Administration). U.S. Food and Drug Administration, 10903 New Hampshire Avenue Silver Spring, MD 20993 2023. 510(k) Premarket Notification. K232440. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K232440 (accessed on 8 May 2025).
- Shorten, C.; Khoshgoftaar, T.M. A survey on image data augmentation for deep learning. J. Big Data 2019, 6, 1. [Google Scholar] [CrossRef]
- Wang, H.; Jin, Q.; Li, S.; Liu, S.; Wang, M.; Song, Z. A comprehensive survey on deep active learning in medical image analysis. Med. Image Anal. 2024, 95, 103201. [Google Scholar] [CrossRef]
- Krishnan, R.; Rajpurkar, P.; Topol, E.J. Self-supervised learning in medicine and healthcare. Nat. Biomed. Eng. 2022, 6, 1346. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, G.; Han, S.; Shi, L.; Xie, Y. Model compression and hardware acceleration for neural networks: A comprehensive survey. Proc. IEEE 2020, 108, 485. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Bai, H.; Yu, B.; Li, W.; Gao, Y. A survey on federated learning. Knowl.-Based Syst. 2021, 216, 106775. [Google Scholar] [CrossRef]
- de Almeida e Bueno, L.; Kwong, M.T.; Bergmann, J.H. Performance of oral cavity sensors: A systematic review. Sensors 2023, 23, 588. [Google Scholar] [CrossRef]
- Patel, J.; Wallace, J.; Doshi, M.; Gadanya, M.; Yahya, I.B.; Roseman, J.; Srisilapanan, P. Oral health for healthy ageing. Lancet Healthy Longev. 2021, 2, e521. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, F.; Wang, Z.; Meng, G.; Gu, Y.; Wu, H.; Liu, D.; Niu, K. Analysis of the microbial community diversity in various regions of the healthy oral cavity. BMC Oral Health 2024, 24, 978. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Zhu, Z.; Zhao, L.; Wang, H.; Song, C.; Chen, Y.; Zhao, Q.; Yang, J.; Pei, Y. A comprehensive review on synergy of multi-modal data and ai technologies in medical diagnosis. Bioengineering 2024, 11, 219. [Google Scholar] [CrossRef]
- Murala, D.K.; Panda, S.K.; Dash, S.P. MedMetaverse: Medical care of chronic disease patients and managing data using artificial intelligence, blockchain, and wearable devices state-of-the-art methodology. IEEE Access 2023, 11, 138954. [Google Scholar] [CrossRef]
- Williamson, S.; Prybutok, V. Balancing privacy and progress: A review of privacy challenges, systemic oversight, and patient perceptions in AI-driven healthcare. Appl. Sci. 2024, 14, 675. [Google Scholar] [CrossRef]



| Disease | Lesion Location | Pathological Process | Biomarkers |
|---|---|---|---|
| Dental caries | Teeth (enamel, dentin, pulp, etc.) | Microorganisms adhering to the tooth surface metabolize sugars from food, producing organic acids. These acids lead to the dissolution of minerals, such as calcium and phosphorus, from the enamel, resulting in its demineralization. | pH [28], lactate [29], acetate [30], Ca2+ [31], H2O2 [32], Streptococcus mutans (S. mutans) [33], and Lactobacillus [34] |
| Periodontitis | Periodontal supporting tissues (gingiva, periodontal ligament, alveolar bone, cementum, etc.) | Microorganisms in dental plaque proliferate, producing toxins and enzymes that trigger inflammatory responses in gingival tissue. Then, the inflammatory mediators cause both vasodilation and increased vascular permeability. Immune cells, including neutrophils and lymphocytes, migrate to the lesion site, releasing pro-inflammatory factors and enzymes. | H2S [35], TNF-α [36], IL-1β [37], MMP-8 [38], human neutrophil hydrolase (HNE) [39], cathepsin-G [40], gingipain [41], β-glucuronidase [42], typsin-like enzyme [43], PI3K [44], Porphyromonas gingivalis (P. gingivalis) [45], and Actinobacillus actinomycetemcomitans [46] |
| Oral cancer | Oral mucosa (tongue, buccal mucosa, gums, floor of mouth, hard palate, soft palate, etc.) | Abnormal changes, such as leukoplakia, erythema, and lichen planus, can present in the oral mucosa. In these regions, epithelial cells proliferate abnormally, leading to morphological changes, such as enlarged nuclei, prominent nucleoli, and disrupted cell polarity. | CYFRA-21-1 [47], IL-8 [48], fibrin [49], and YAP1 [50], HPV DNA [51], HPV E6 antibody [52] |
| Sample Resources | Sensor | Limit of Detection (LOD) | Sensitivity | Selectiveness | Cost | Ease of Use |
|---|---|---|---|---|---|---|
| Exhaled Breath | Au/In2O3-MPTES [58] | 10 ppb H2S | At 10 ppm H2S, Ra/Rg = 1505.3 | High | High | Low |
| Manganese Oxide-Nanosheets [68] | 20 ppb MM | 40 Hz/1000 ppb | Middle | High | Low | |
| Cu2O/ZnO heterojunctions [65] | 10 ppb H2S | At 0.01 ppm H2S, Ig/Ia = 62 | High | High | Middle | |
| Co-MOFs heterojunctions [59] | 1.3 ppb H2S | At 10 ppm H2S, Ra/Rg = 32 | Middle | High | Middle | |
| Fe-MoO3-x/TiO2 [69] | 0.34 ppb H2S | At 0.2 ppm H2S, Ra/Rg = 4.9 | Middle | High | Middle | |
| BISS-PAAm based structural color hydrogel [60] | 61 ppb VSCs | 177.87 nm/mM | Middle | Middle | High | |
| Cu-TATB@paper [23] | 8 nM VSCs | 148 a.u./μM | High | Middle | High | |
| Whole Saliva | Anthocyanins-based paper points [79] | / | −20 ± 0.5 | Middle | Low | High |
| MNPs [81] | 49 CFU/mL P. gingivalis | / | High | Middle | Middle | |
| MNPs [80] | 1 pg/mL HNE, 100 fg/mL Cathepsin-G | / | High | Middle | Middle | |
| RPA-LF strip [87] | 6.40 × 10−4 μg/mL P. gingivalis DNA | / | High | Middle | Middle | |
| Fe3O4 NPs-based DNA-Nanozyme Interfaces [24] | 12 CFU/mL S. mutans | / | High | Middle | Middle | |
| PMMA-based Aptasensor [89] | 0.011 nM ODAM | 3854.2/nM | High | High | Low | |
| Local Exudate from Si-ngle Site | Bifunctional graphene [25] | single bacterium | At 100 CFU/mL bacterium, ∆R/R0 = −0.05 | High | Middle | Middle |
| PANi-based dental patch [99] | / | −62.97 mV/pH | Middle | Middle | Middle | |
| Modified PNIPAM hydrogel RF-Trilayer Sensor [100] | / | 6 MHz/1000 (mg/dL) | Middle | Middle | Middle | |
| AG-AgNPs-CHL hydrogel RF sensor [101] | 1.2 μM H2S | 0.62/μM | High | Middle | Middle | |
| Local Exudate from the Whole Dentition | Nitrazine-Yellow based dental floss [79] | / | −33.8 ± 1.5 | Middle | Low | High |
| ZnO-PDMS Mouthguard [102] | / | / | High | Low | High | |
| Au@Ag Nanorods-PDMS Mouthguard [26] | 7.86 ppm H2S | 0.34/ppm | High | Low | High | |
| ZnO QDs-PDMS dental patches [103] | 20.3 μM H2S | −1255.4 a.u./μM | High | Low | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, J.; Wang, S.; Li, Y.; Ji, L.; Xie, D.; Zhou, J. Convenient Biochemical Testing Technologies for Oral Disease Risk Warning: Opportunities and Challenges. Biosensors 2025, 15, 327. https://doi.org/10.3390/bios15050327
Liu Y, Xu J, Wang S, Li Y, Ji L, Xie D, Zhou J. Convenient Biochemical Testing Technologies for Oral Disease Risk Warning: Opportunities and Challenges. Biosensors. 2025; 15(5):327. https://doi.org/10.3390/bios15050327
Chicago/Turabian StyleLiu, Ying, Jincheng Xu, Siyuan Wang, Yuanfang Li, Li Ji, Dong Xie, and Jianhua Zhou. 2025. "Convenient Biochemical Testing Technologies for Oral Disease Risk Warning: Opportunities and Challenges" Biosensors 15, no. 5: 327. https://doi.org/10.3390/bios15050327
APA StyleLiu, Y., Xu, J., Wang, S., Li, Y., Ji, L., Xie, D., & Zhou, J. (2025). Convenient Biochemical Testing Technologies for Oral Disease Risk Warning: Opportunities and Challenges. Biosensors, 15(5), 327. https://doi.org/10.3390/bios15050327





