Advances of Nanozyme-Driven Multimodal Sensing Strategies in Point-of-Care Testing
Abstract
1. Introduction
2. Classification and Activity Regulation of Nanozymes for POCT
2.1. Classification of Nanozymes
| Classification Based on Core Structure | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Metal-based Nanozymes | Metal nanoparticles (e.g., gold, silver, copper) [40] | Excellent catalytic activity, good stability, and multifunctionality (such as conductivity and magnetism). | High metal costs, potential biological toxicity, and complex catalytic mechanisms. |
| Metal Oxide-based Nanozymes | TiO2, ZnO, CeO2 [43,44], Fe3O4, Fe2O3 [48] | Similar to metal nanozymes, but with higher biocompatibility. | Stability needs to be enhanced (such as aggregation, precipitation, or degradation). |
| Single-atom Nanozymes | Pt-N-C, Zn-N-C, Cu-N-C, Co-N-C, Fe-N-C [55], Se-N-C [56] | Low toxicity, high catalytic activity, distinct structure. | Hard to prepare and narrow substrate specificity. |
| Metal-Organic Framework-based Nanozymes | MOFs (e.g., ZIF-8 [51], UiO-66 [51] | Good biocompatibility, tunable properties, easy to functionalize, and multi-nanozyme mimicry. | Complex preparation, limited substrate specificity, and poor structural stability. |
| Carbon-based Nanozymes | Fullerene [60], Carbon Nanotubes (CNTs) [61], Graphene [62], Carbon Quantum Dots (CQDs) [64] | Good biocompatibility and degradability, abundant raw material sources, low cost, tunable structure, and surface chemical properties. | The catalytic activity still lags behind that of natural enzymes. |
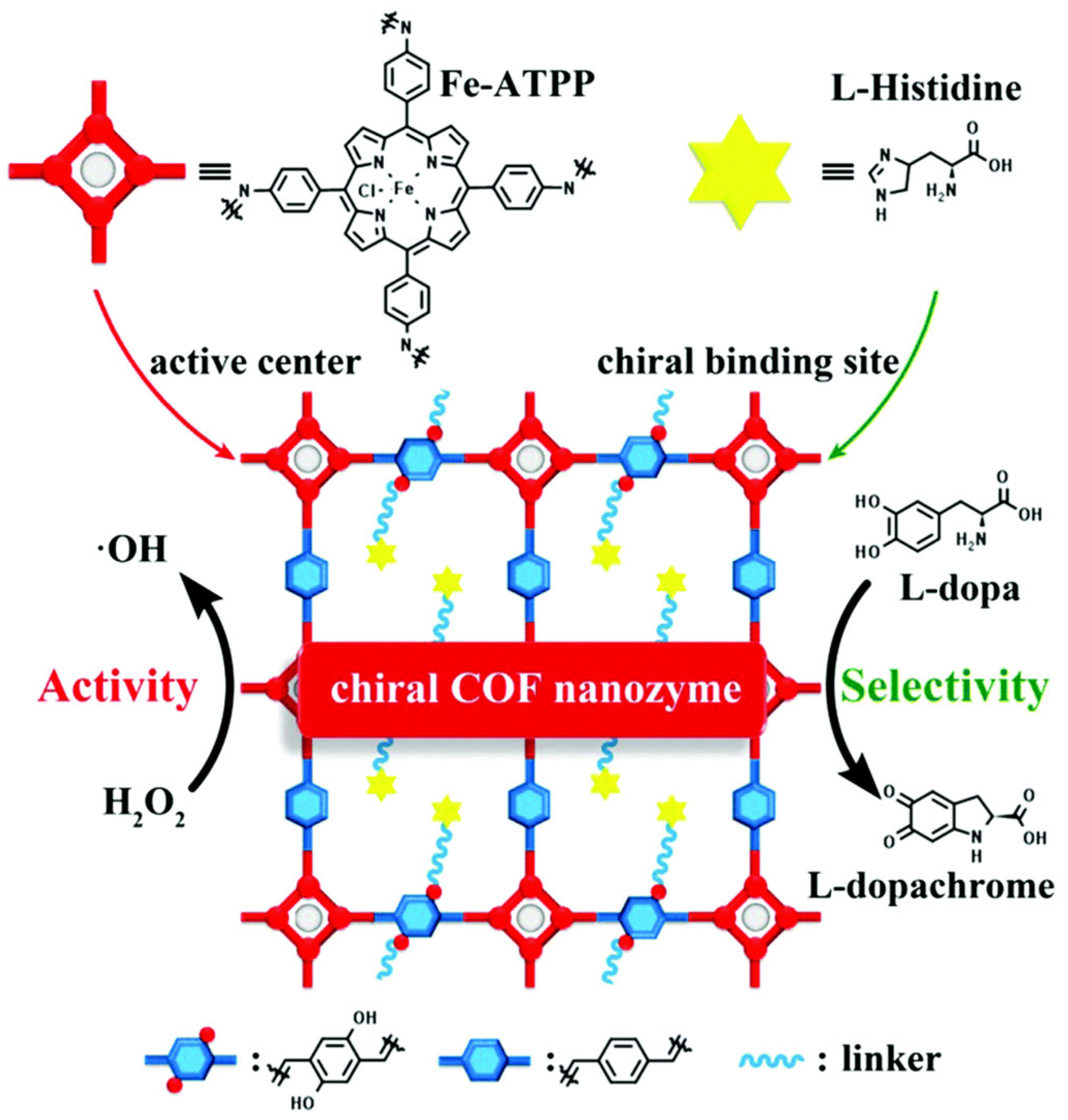
| Covalent organic framework, COF | l-His100@Fe-COF [63], CoP-TPE-COF, COF@Co3O4 | High specific surface area and porosity, easy to functionalize, structural designability, biocompatibility, and degradability. | Complex preparation process, cost and scalability issues, and stability problems. |
2.2. The Preparation of Nanozymes and Associated Challenges
2.3. Regulation of Nanozymes Activity
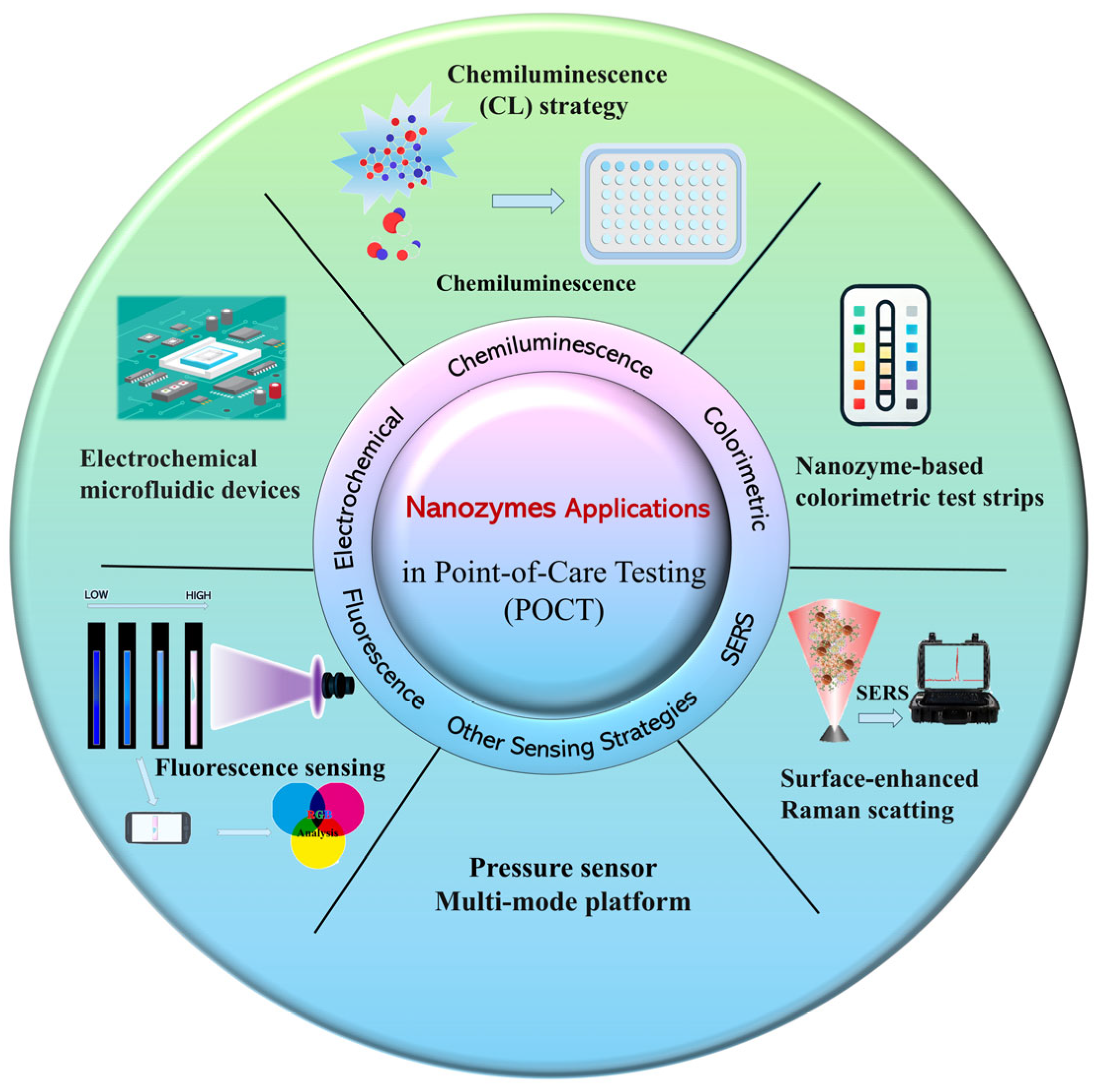
3. Application of Nanozymes in POCT
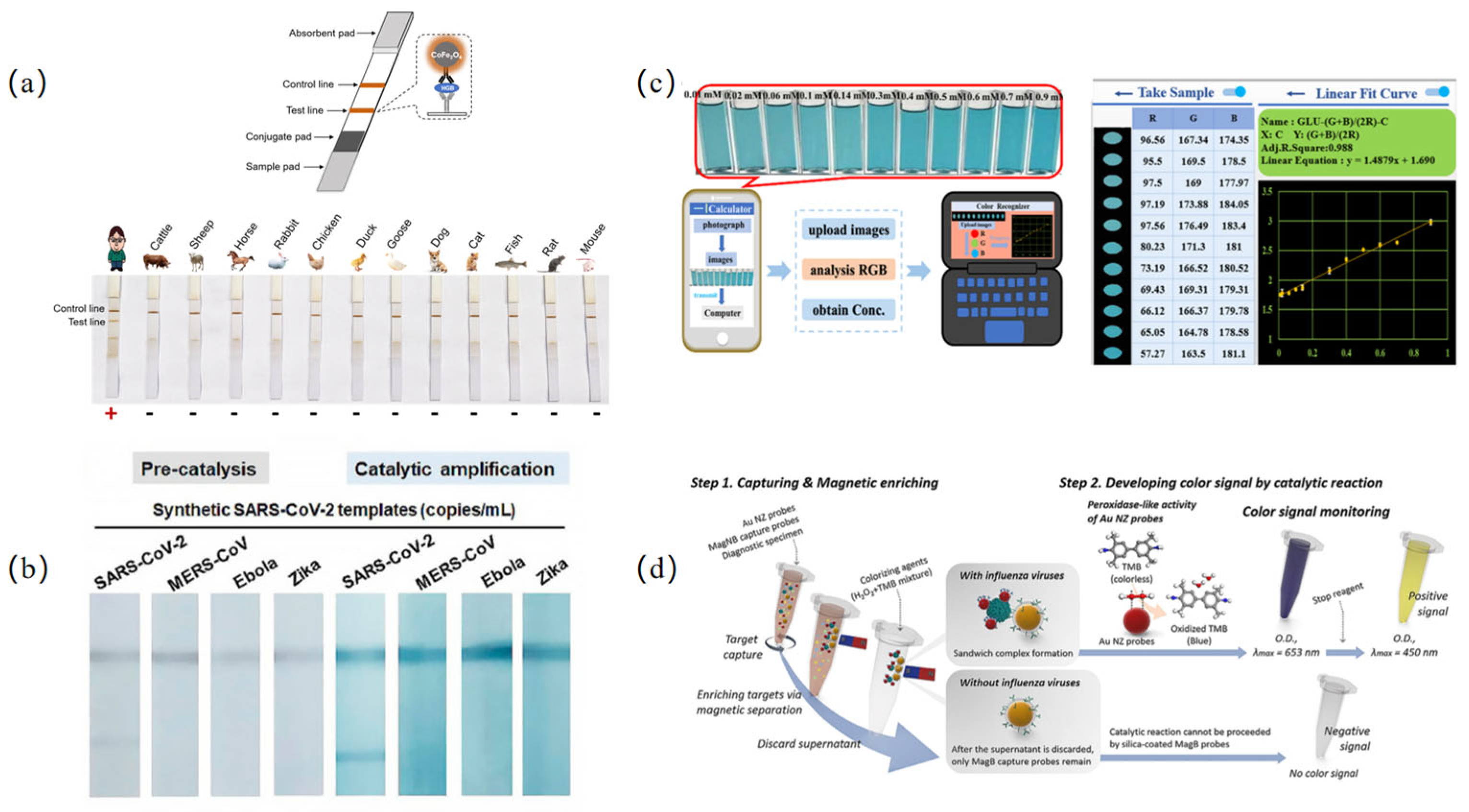
3.1. Colorimetric Sensing Based on Nanozymes
3.2. Electrochemical Sensing Based on Nanozymes
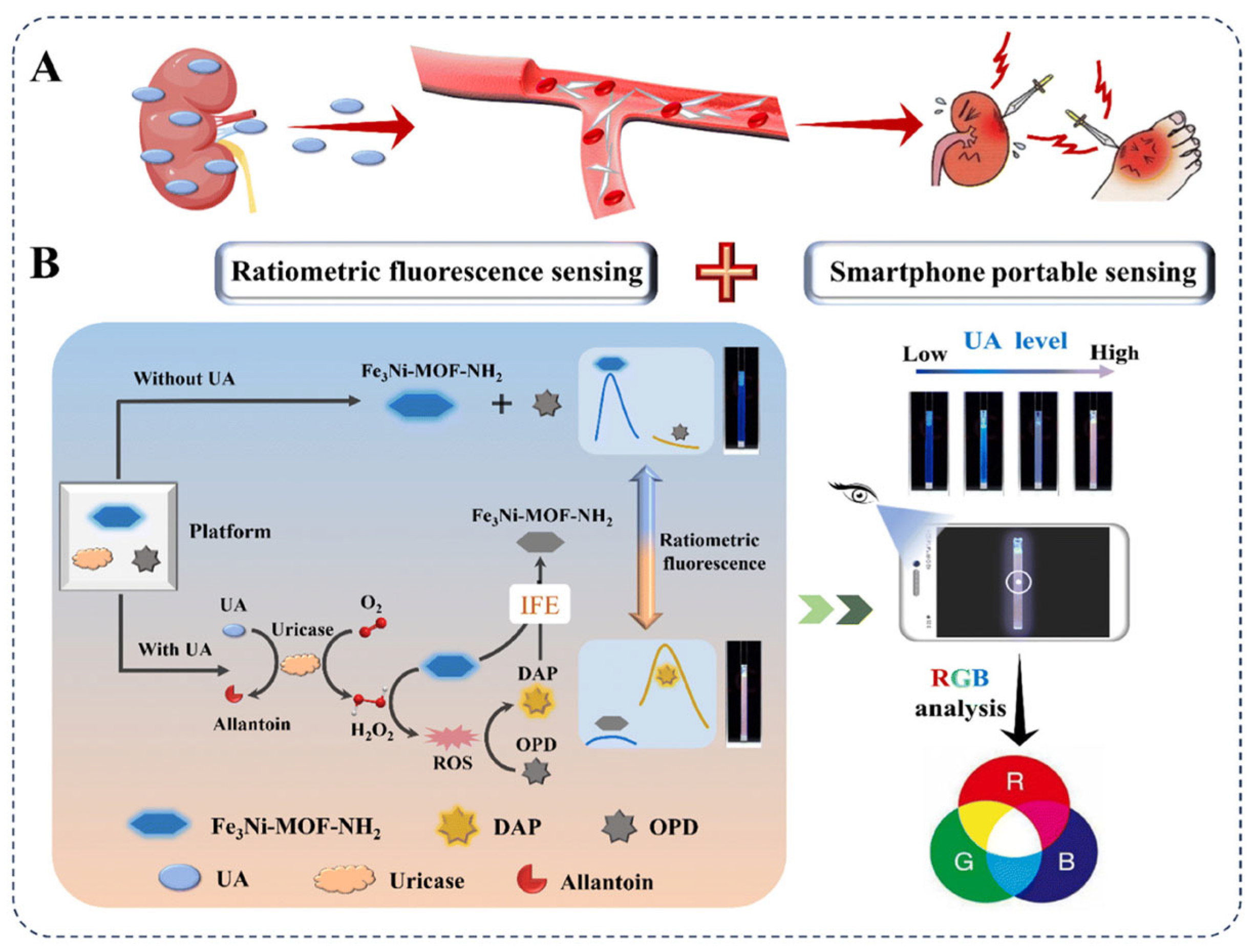
3.3. Fluorescent Analysis and Detection Based on Nanozymes
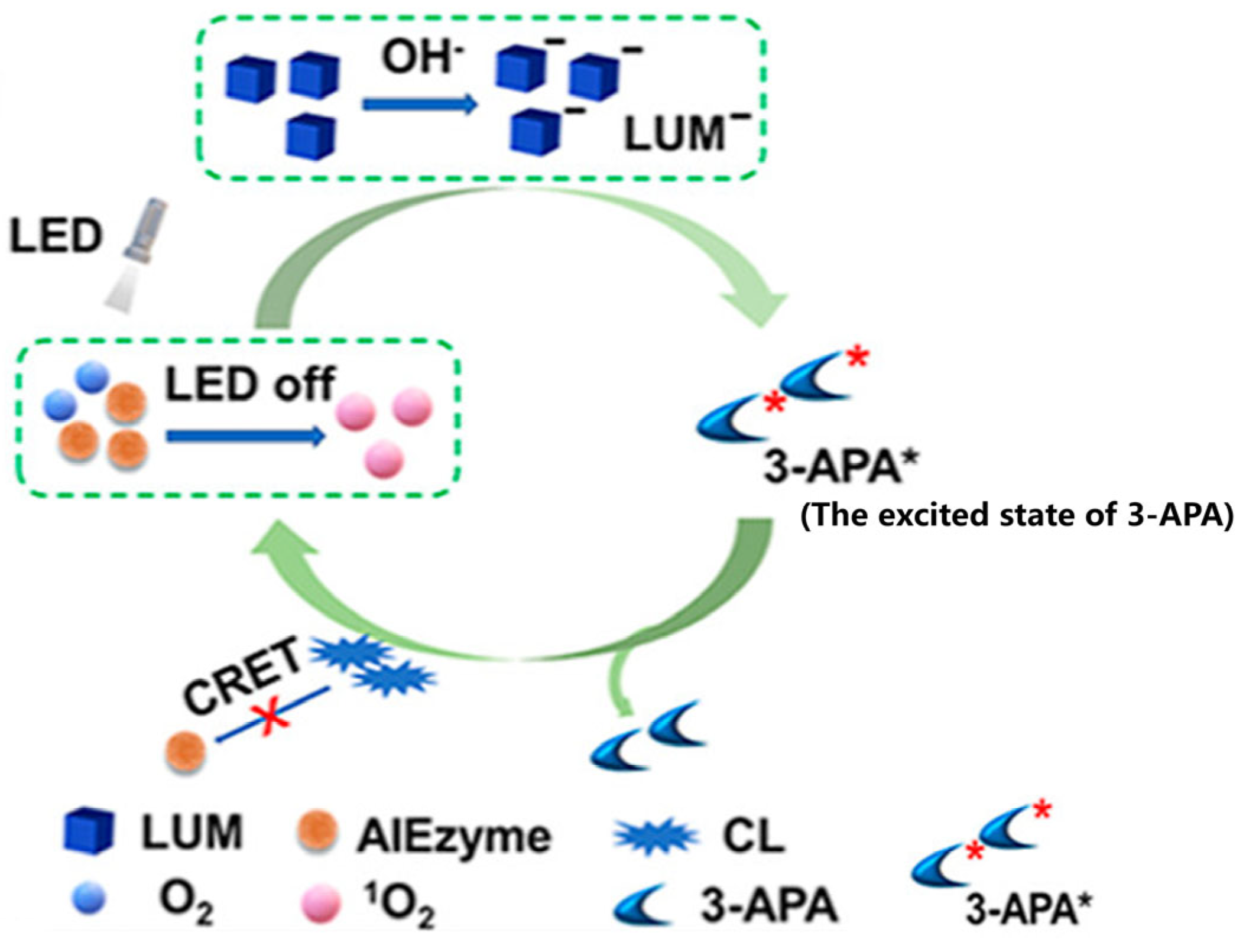
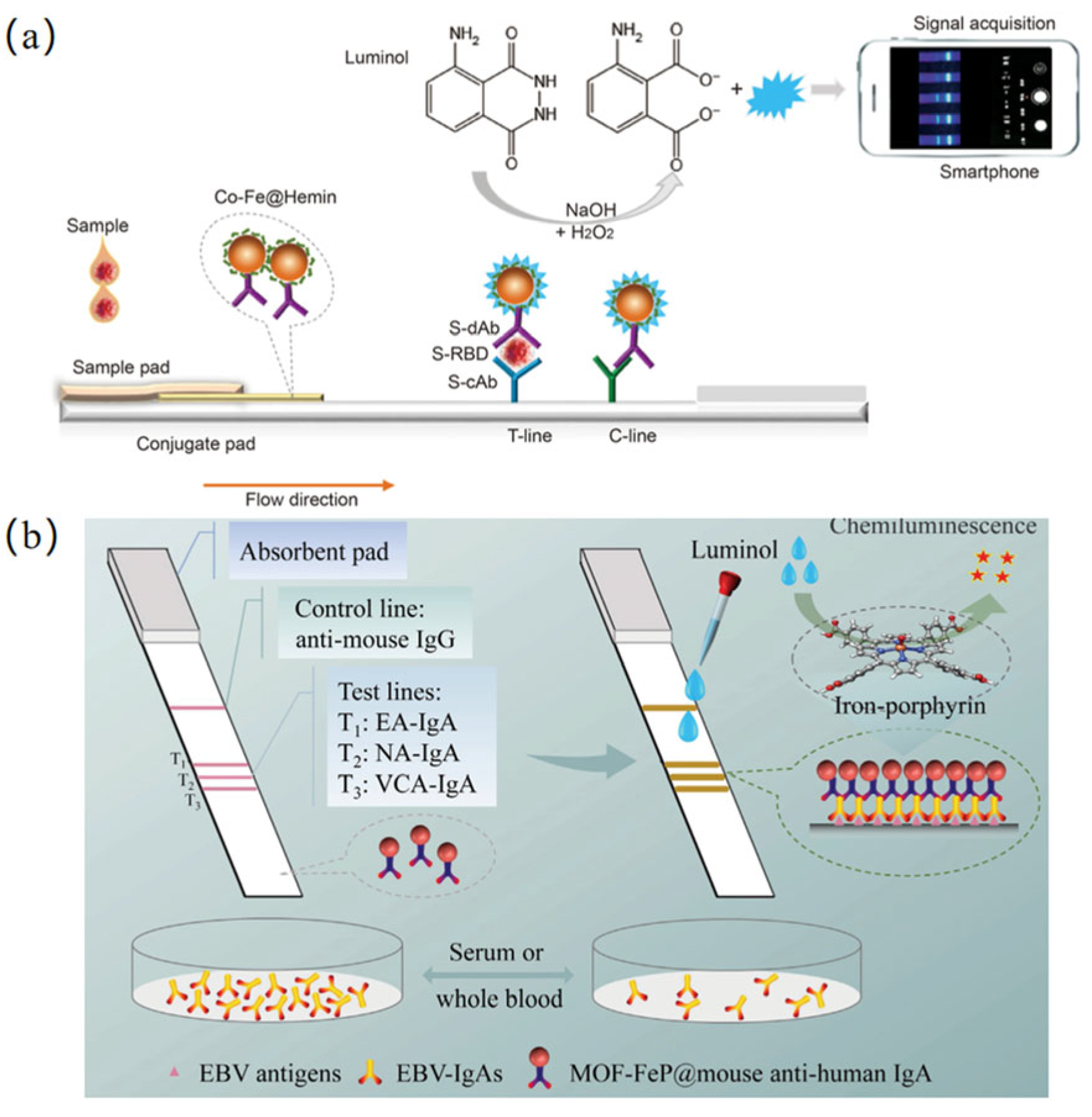
3.4. Chemiluminescence Detection Based on Nanozymes
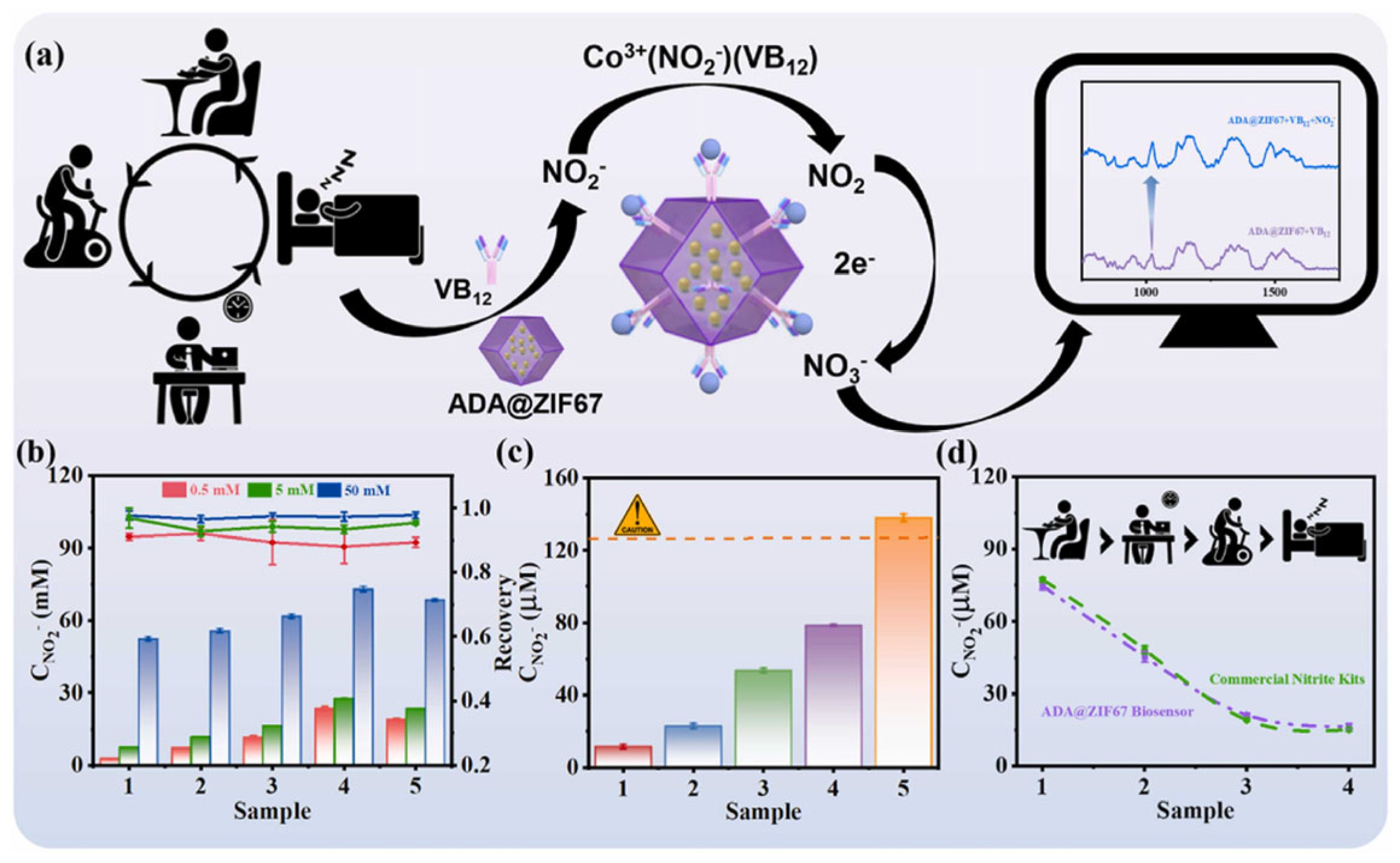
3.5. SERS Sensor Based on Nanozymes
3.6. Other Detection Methods and Sensing Strategies Based on Nanozymes
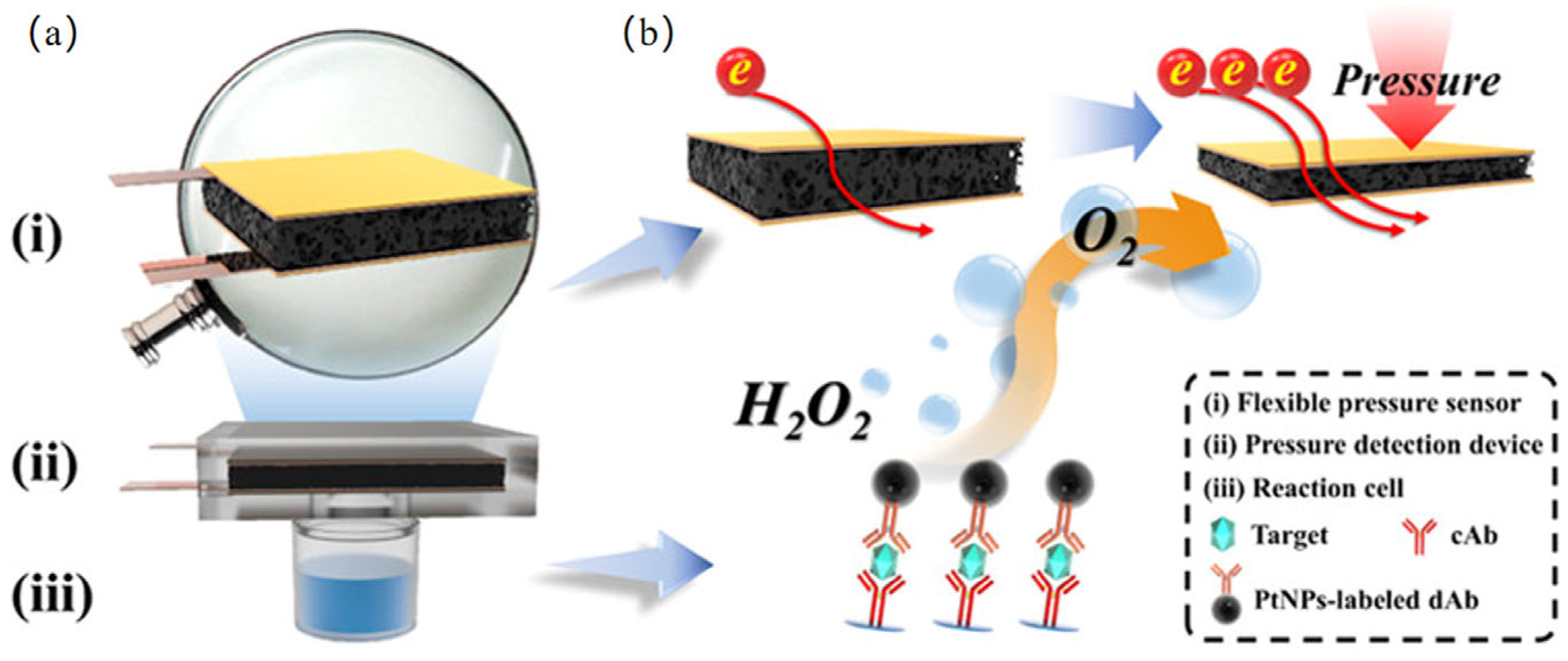
3.6.1. Pressure Sensor
3.6.2. Multi-Mode Platform
| Sensing Methods | Detected Substances | Detection Limits | Linear Range | References |
|---|---|---|---|---|
| Colorimetric | Human Hemoglobin | 1 ng·mL−1 | – | [87] |
| SARS-CoV-2 | 200 copies·mL−1 | – | [88] | |
| Glucose | 3.125 μM | 10–900 μM | [89] | |
| Influenza Viruses | 5.0 × 10−12 g·mL−1 | 5.0 × 10−15–5.0 × 10−6 g·mL−1 | [91] | |
| Electrochemical | H2O2 | 1.62 μM | 1 μM–3 mM | [96] |
| Fluorescent | Uric Acid | 24 nM | – | [101] |
| Captopril | 0.45 μM | 0.5–30 μM | [102] | |
| Escherichia coli | 1.74 cfu·mL−1 | 10–107 cfu·mL−1 | [103] | |
| Chemiluminescence | SARS-CoV-2 | 0.1 ng·mL−1 | 0.2–100 ng·mL−1 | [105] |
| Epstein–Barr virus | – | – | [106] | |
| SERS | Nitrite | 1.67 nM | 1–100 nM | [114] |
| Pressure | Carcinoembryonic Antigen | 0.13 ng·mL−1 | 0.2–60 ng/mL | [118] |
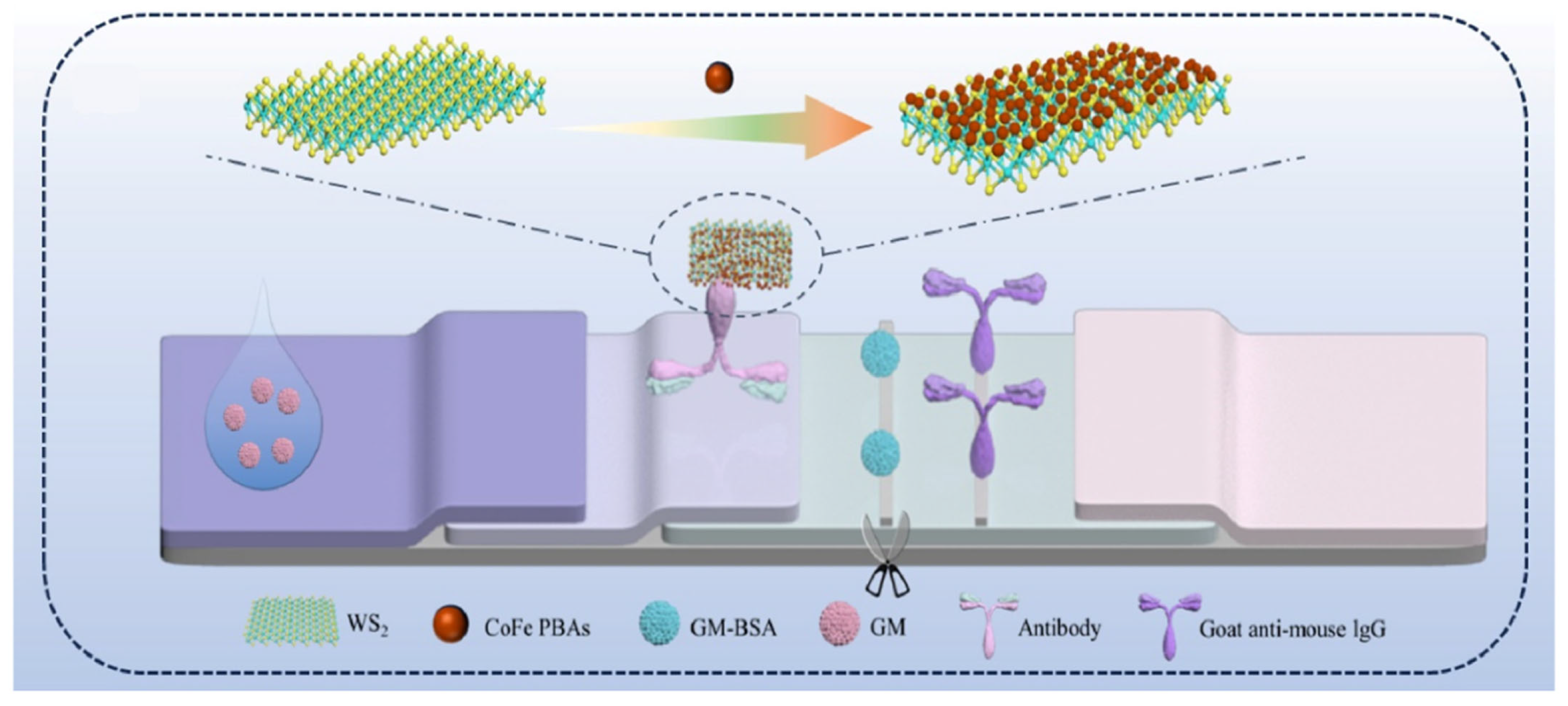
| Chemiluminescence | Gentamicin | 0.33 pg·mL−1 | 0.001–100 ng mL−1 | [120] |
| photothermal | Gentamicin | 16.67 pg/mL−1 | 0.05–100 ng mL−1 | [120] |
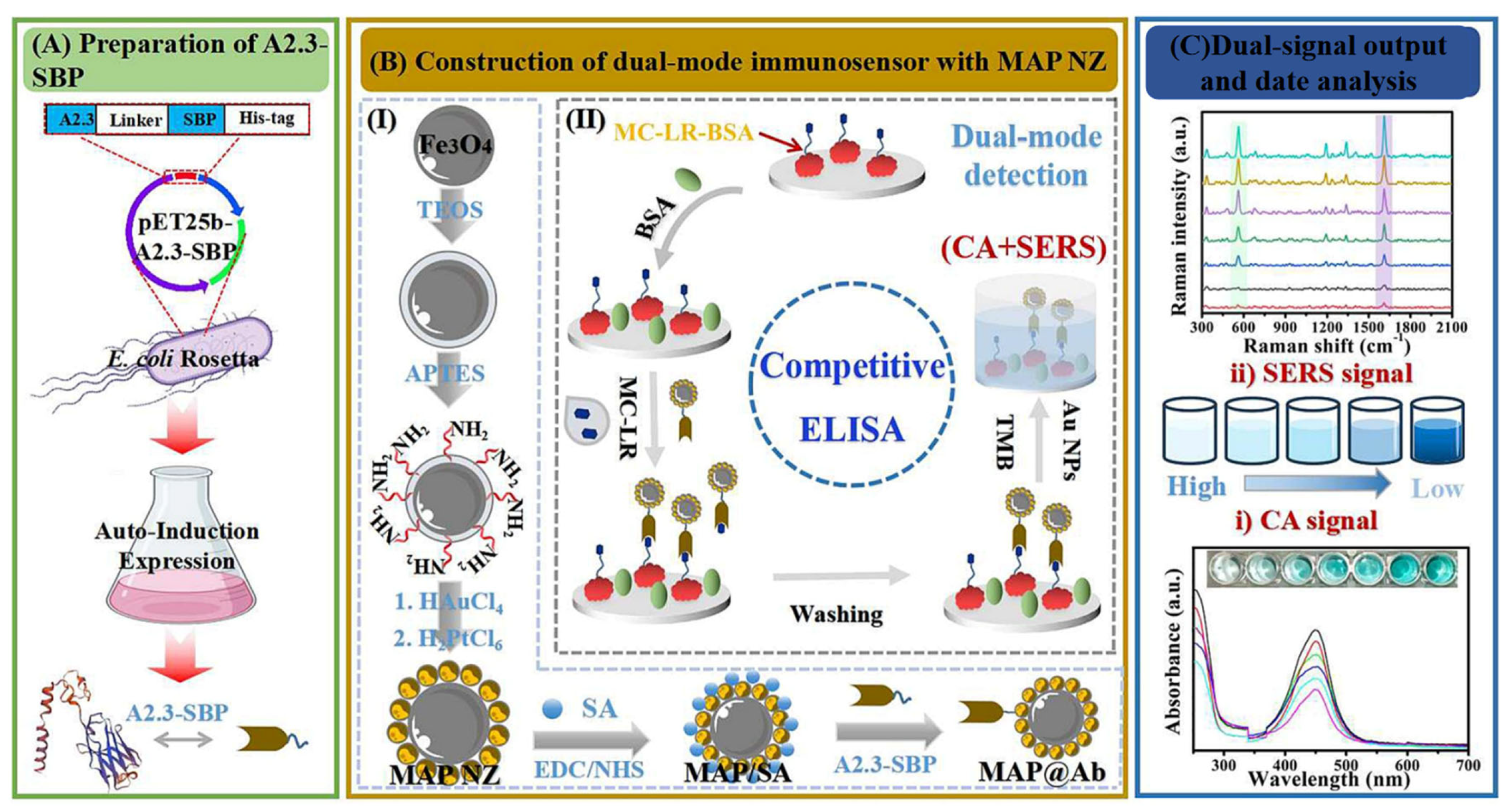
| Colorimetric | Microcystin-LR | 0.26 ng·mL−1 | – | [121] |
| SERS | Microcystin-LR | 0.032 ng·mL−1 | – | [121] |
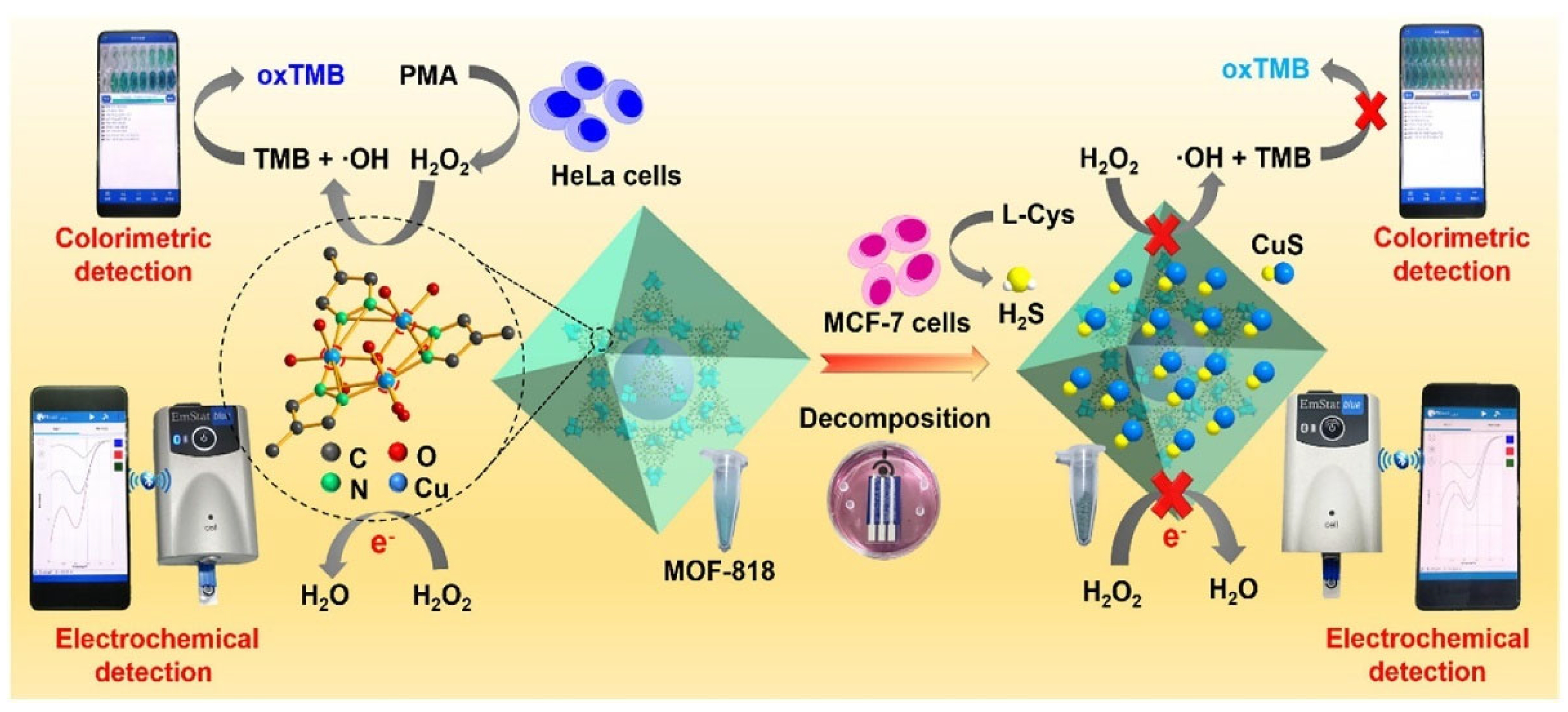
| Colorimetric | H2S | 0.8 μM | – | [122] |
| Electrochemical | H2S | 0.76 nM | – | [122] |
| Colorimetric | Escherichia coli | – | 102–106 CFU mL−1 | [123] |
| fluorescence | Escherichia coli | – | 102–106 CFU mL−1 | [123] |
| Colorimetric | Acetylcholinesterase | 0.003 U L−1 | – | [124] |
| Photothermal | Acetylcholinesterase | 0.003 U L−1 | – | [124] |
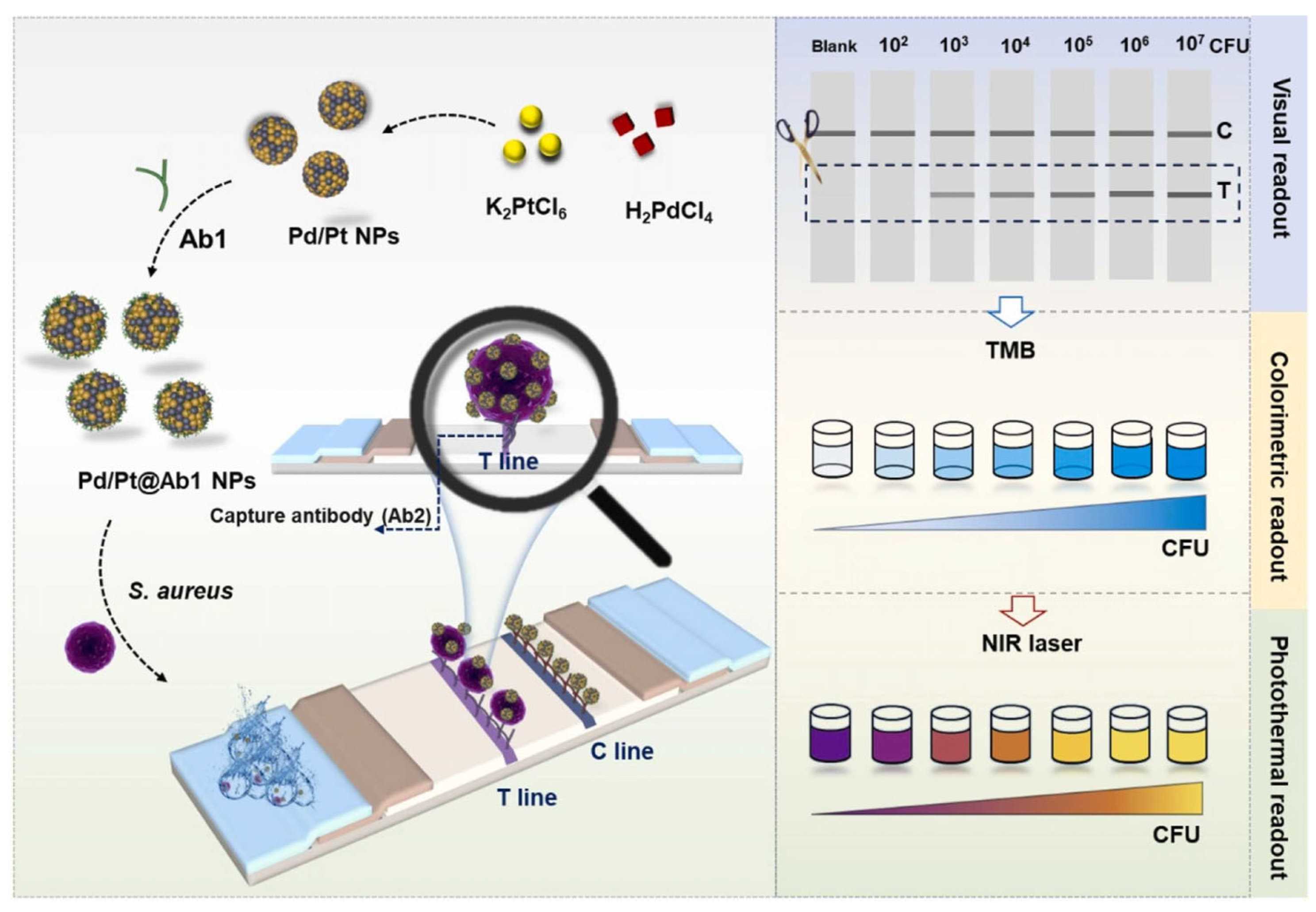
| Visual–colorimetric–photothermal LFIA | Staphylococcus aureus | 4 CFU mL−1 | 102–107 CFU mL−1 | [125] |
4. Nanozymes for POCT: Challenges and Perspectives
4.1. Challenges Associated with Insufficient Catalytic Activity in Nanozymes
4.2. Challenges in the Uniformity and Dispersibility of Nanozymes
4.3. Challenges in the Mass Production of Nanozymes
4.4. Prospects for Nanozyme-Based POCT Technologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Feng, Y.; Li, D.; Li, Y.; Zhao, A.; Zhang, B.; Cheng, J.; Cui, J. Technologies Research Status and Clinical Applications of Point of Care Testing. Hans J. Biomed. 2021, 11, 23. [Google Scholar] [CrossRef]
- Yang, S.-M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic point-of-care (POC) devices in early diagnosis: A review of opportunities and challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.H. Utilizing point-of-care testing to optimize patient care. Ejifcc 2021, 32, 140. [Google Scholar] [PubMed]
- Luppa, P.B.; Junker, R. Point-of-Care Testing: Principles and Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Poschenrieder, A.; Thaler, M.; Junker, R.; Luppa, P.B. Recent advances in immunodiagnostics based on biosensor technologies—From central laboratory to the point of care. Anal. Bioanal. Chem. 2019, 411, 7607–7621. [Google Scholar] [CrossRef]
- Bai, F.; Bu, T.; Wang, Z.; Shao, B. Integration of a new generation of immunochromatographic assays: Recent advances and future trends. Nano Today 2024, 57, 102403. [Google Scholar] [CrossRef]
- Yin, B.; Qian, C.; Wan, X.; Sohan, A.M.F.; Lin, X. Tape integrated self-designed microfluidic chip for point-of-care immunoassays simultaneous detection of disease biomarkers with tunable detection range. Biosens. Bioelectron. 2022, 212, 114429. [Google Scholar] [CrossRef]
- Wang, D.; Chan, H.N.; Liu, Z.; Micheal, S.; Li, L.; Baniani, D.B.; Tan, M.J.; Huang, L.; Wang, J.; Wu, H. Recent developments in microfluidic-based point-of-care testing (Poct) diagnoses. In Nanotechnology and Microfluidics; Wiley: Hoboken, NJ, USA, 2020; pp. 239–280. [Google Scholar]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Fan, K.; Gao, L.; Wei, H.; Jiang, B.; Wang, D.; Zhang, R.; He, J.; Meng, X.; Wang, Z.; Fan, H.; et al. Nanozyme. Prog. Chem. 2023, 35, 1–87. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Fan, K.; Hou, Y.; Zhang, R.; Yan, X. Nanozymes: A new choice for disease treatment. Sci. Sin. Vitae 2020, 50, 311–328. [Google Scholar] [CrossRef]
- Du, P.; Gao, L.; Jiao, J.; Fan, K.; Yan, X. Nanozyme: Combining power of natural enzymes and artificial catalysis. Bulletin of Chin. Acad. Sci. (Chin. Version) 2024, 39, 809–820. [Google Scholar]
- Wei, H.; Gao, L.; Fan, K.; Liu, J.; He, J.; Qu, X.; Dong, S.; Wang, E.; Yan, X. Nanozymes: A clear definition with fuzzy edges. Nano Today 2021, 40, 101269. [Google Scholar] [CrossRef]
- Fu, J.; Shen, T.; Wu, J.; Wang, C. Nanozyme: A new strategy combating bacterial. J. Inorg. Mater. 2021, 36, 257–268. [Google Scholar] [CrossRef]
- Ren, Y.; Bi, X.; He, Y.; Zhang, L.; Luo, L.; Li, L.; You, T. Research progress and applications of iron-based nanozymes in colorimetric sensing of agricultural pollutants. Biosens. Bioelectron. 2025, 271, 116999. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X. Reserach Advances on Nanozyme-Guided Therapy of Inflammatory Bowel Diseases. Acta Chim. Sin. 2023, 81, 1043. [Google Scholar] [CrossRef]
- Zhao, J.; Han, F.; Cheng, C.; Wang, H.; Zhao, G.; Jia, P.; Zhang, N.; Wang, Y.; Zhang, J.; Wei, Q. Recent progress in noble metal-based single-atom nanozymes for biomedical applications. Microchem. J. 2024, 207, 111731. [Google Scholar] [CrossRef]
- Ma, L.; Fan, K. The finding and application of the novel properties of nanozyme and ferritin. Chin. J. Nat. 2020, 42, 1–11. [Google Scholar]
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in point-of-care diagnosis: An emerging futuristic approach for biosensing. Nano-Micro Lett. 2021, 13, 193. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Z.-M.; Du, Y. Recent Progress of Nanozyme-Based Sensors in Point-of-Care Testing. Chin. J. Anal. Chem. 2023, 51, 800–810. [Google Scholar]
- Wang, K.; Meng, X.; Yan, X.; Fan, K. Nanozyme-based point-of-care testing: Revolutionizing environmental pollutant detection with high efficiency and low cost. Nano Today 2024, 54, 102145. [Google Scholar] [CrossRef]
- Xia, J.; Li, Z.; Ding, Y.; Shah, L.A.; Zhao, H.; Ye, D.; Zhang, J. Construction and Application of Nanozyme Sensor Arrays. Anal. Chem. 2024, 96, 8221–8233. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Li, Z.; Feng, K.; Zhang, W.; Ma, M.; Gu, N.; Zhang, Y. Catalytic mechanism and application of nanozymes. Chin. Sci. Bull. 2018, 63, 2128–2139. [Google Scholar] [CrossRef]
- Strzepa, A.; Pritchard, K.A.; Dittel, B.N. Myeloperoxidase: A new player in autoimmunity. Cell. Immunol. 2017, 317, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, W.; Dong, B.; Liu, D.; Xin, T. Application status of nanozymes in reactive oxygen species-related brain diseases. J. Clin. Neurosurg. 2024, 21, 341. [Google Scholar]
- Li, J.L.; Xu, F.; Liao, X. Advances in the application of antioxidant nanomaterials in the treatment of acute kidney injury. Emerg. Med. 2022, 42, 72–75. [Google Scholar]
- Xu, D.; Wu, L.; Yao, H.; Zhao, L. Catalase-like nanozymes: Classification, catalytic mechanisms, and their applications. Small 2022, 18, 2203400. [Google Scholar] [CrossRef]
- Yang, L.; Xu, X.; Song, Y.; Huang, J.; Xu, H. Research progress of nanozymes in colorimetric biosensing: Classification, activity and application. Chem. Eng. J. 2024, 487, 150612. [Google Scholar] [CrossRef]
- Niu, J.; Sun, S.; Liu, P.; Zhang, X.; Mu, X. Copper-based Nanozymes: Properties and Applications in Biomedicine. J. Inorg. Mater. 2023, 38, 489–502. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, W.; Tan, X.; Wu, J.; Yang, Q.; Hou, X. Aptamer sensor based on AuPtRh nanozyme for rapid detection of tetrodotoxin. J. Food Saf. Qual. 2022, 13, 7183. [Google Scholar]
- Mu, X.; Wang, J.; Li, Y.; Xu, F.; Long, W.; Ouyang, L.; Liu, H.; Jing, Y.; Wang, J.; Dai, H.; et al. Redox trimetallic nanozyme with neutral environment preference for brain injury. ACS Nano 2019, 13, 1870–1884. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, Y.; Shi, Y.; Liu, Y.; Liu, T.; Zhou, H.; Niu, W.; Zhang, L.; Zhang, J.; Xu, G. Triple-enzyme-mimicking AuPt3Cu hetero-structural alloy nanozymes towards cascade reactions in chemodynamic therapy. Chem. Eng. J. 2023, 463, 142494. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, H.; Zhao, J.; Wang, Y.; Zhao, G.; Zhang, Y.; Liu, X.; Wang, Y. Advances in the application of metal oxide nanozymes in tumor detection and treatment. Colloids Surf. B Biointerfaces 2024, 235, 113767. [Google Scholar] [CrossRef]
- Fu, Z.; Zeng, W.; Cai, S.; Li, H.; Ding, J.; Wang, C.; Chen, Y.; Han, N.; Yang, R. Porous Au@ Pt nanoparticles with superior peroxidase-like activity for colorimetric detection of spike protein of SARS-CoV-2. J. Colloid Interface Sci. 2021, 604, 113–121. [Google Scholar] [CrossRef]
- Wen, H. Basic Research on the Application of Carbonic Anhydrase Hybrid Nanoflowers and Their PVA/CS Gelase Membranes in CO2 Transformation. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2020. [Google Scholar]
- Chen, W.; Li, S.; Wang, J.; Sun, K.; Si, Y. Metal and metal-oxide nanozymes: Bioenzymatic characteristics, catalytic mechanism, and eco-environmental applications. Nanoscale 2019, 11, 15783–15793. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A review on metal-and metal oxide-based nanozymes: Properties, mechanisms, and applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Q.; Zhu, J.; Luo, L.; Pu, S.; Zhang, W.; Zhu, W.; Sun, J.; Wang, J. Portable colorimetric detection of mercury (II) based on a non-noble metal nanozyme with tunable activity. Inorg. Chem. 2019, 58, 1638–1646. [Google Scholar] [CrossRef]
- Singh, N. Antioxidant metal oxide nanozymes: Role in cellular redox homeostasis and therapeutics. Pure Appl. Chem. 2021, 93, 187–205. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Liu, R.; Ding, G.; Huang, H. Cerium Oxide Nanozymes Improve Skeletal Muscle Function in Gestational Diabetic Offspring by Attenuating Mitochondrial Oxidative Stress. ACS Omega 2024, 9, 21851–21863. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Sun, S.; Wang, J.; Li, Q.; Yan, R.; Gao, Y.; Liu, H.; Liu, S.; Hao, W.; et al. Catalytic patch with redox Cr/CeO2 nanozyme of noninvasive intervention for brain trauma. Theranostics 2021, 11, 2806. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, S.; Gu, D.; Zhu, B.; Liu, H.; Wu, W.; Wu, J.; Wei, H.; Miao, L. Cerium oxide nanozyme attenuates periodontal bone destruction by inhibiting the ROS–NFκB pathway. Nanoscale 2022, 14, 2628–2637. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, M.; Geng, H.; Zhang, P.; Zheng, Z.; Zhou, Y.; He, W. NIR enhanced peroxidase-like activity of Au@ CeO2 hybrid nanozyme by plasmon-induced hot electrons and photothermal effect for bacteria killing. Appl. Catal. B Environ. 2021, 295, 120317. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Sensors and biosensors based on metal oxide nanomaterials. TrAC Trends Anal. Chem. 2019, 121, 115690. [Google Scholar] [CrossRef]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef]
- Sarangi, M.K.; Patel, L.; Rath, G.; Nanda, S.S.; Yi, D.K. Metal organic framework modulated nanozymes tailored with their biomedical approaches. Chin. Chem. Lett. 2024, 35, 109381. [Google Scholar] [CrossRef]
- Lian, Z.; Lu, C.; Zhu, J.; Zhang, X.; Wu, T.; Xiong, Y.; Sun, Z.; Yang, R. Mo@ ZIF-8 nanozyme preparation and its antibacterial property evaluation. Front. Chem. 2022, 10, 1093073. [Google Scholar] [CrossRef] [PubMed]
- Loosen, A.; Simms, C.; Smolders, S.; De Vos, D.E.; Parac-Vogt, T.N. Bimetallic Ce/Zr UiO-66 metal–organic framework nanostructures as peptidase and oxidase nanozymes. ACS Appl. Nano Mater. 2021, 4, 5748–5757. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–organic framework nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Wang, X.; Niu, R.; Chen, X.; Zhu, Y.; Sun, Z.; Yang, J.; Liu, G.; Luo, Y. Intelligent dual-lock deoxyribonucleic acid automatons boosting precise tumor imaging. ACS Appl. Mater. Interfaces 2023, 15, 3826–3838. [Google Scholar] [CrossRef]
- Peng, C.; Pang, R.; Li, J.; Wang, E. Current Advances on the Single-Atom Nanozyme and Its Bioapplications. Adv. Mater. 2024, 36, 2211724. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Jiang, B.; Hao, H.; Chen, Y.; Dong, J.; Mao, Y.; Zhang, Z.; Gao, R.; Chen, W.; Zhang, R.; et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat. Catal. 2021, 4, 407–417. [Google Scholar] [CrossRef]
- Cheng, J.; Li, L.; Jin, D.; Zhang, Y.; Yu, W.; Yu, J.; Zou, J.; Dai, Y.; Zhu, Y.; Liu, M.; et al. A non-metal single atom nanozyme for cutting off the energy and reducing power of tumors. Angew. Chem. 2024, 136, e202319982. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, Z.N.; Liang, X.; Sun, H.b.; Xin, H.; Liang, Q. Recent advances in nanozymes: From matters to bioapplications. Adv. Funct. Mater. 2022, 32, 2110432. [Google Scholar] [CrossRef]
- Jozdani, S.M.B.; Hashemian, Z.; Damavandi, S.E.; Elyasigorji, Z.; Vosough, M. Emerging Trends in the Biomedical Application of Carbon-based Nanomaterials. Nano Biomed. Eng. 2024, 16, 357–369. [Google Scholar] [CrossRef]
- Fan, K.; Xi, J.; Fan, L.; Wang, P.; Zhu, C.; Tang, Y.; Xu, X.; Liang, M.; Jiang, B.; Yan, X.; et al. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun. 2018, 9, 1440. [Google Scholar] [CrossRef]
- Devi, N.; Kumar, R.; Chen, Y.-S.; Singh, R.K. Carbon-based nanomaterials: Carbon nanotube, fullerene, and carbon dots. In Nanomaterials: Advances and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 27–57. [Google Scholar]
- Yang, Y.; Li, T.; Qin, Y.; Zhang, L.; Chen, Y. Construct of carbon nanotube-supported Fe2O3 hybrid nanozyme by atomic layer deposition for highly efficient dopamine sensing. Front. Chem. 2020, 8, 564968. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Xu, D.; Zheng, X.; Huang, Q. A green and facile approach to a graphene-based peroxidase-like nanozyme and its application in sensitive colorimetric detection of l-cysteine. Anal. Bioanal. Chem. 2021, 413, 4013–4022. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Ren, J.; Qu, X. A chiral covalent organic framework (COF) nanozyme with ultrahigh enzymatic activity. Mater. Horiz. 2020, 7, 3291–3297. [Google Scholar] [CrossRef]
- Jin, J.; Li, L.; Zhang, L.; Luan, Z.; Xin, S.; Song, K. Progress in the application of carbon dots-based nanozymes. Front. Chem. 2021, 9, 748044. [Google Scholar] [CrossRef]
- Qin, L.; Hu, Y.; Wei, H. Nanozymes: Preparation and characterization. In Nanozymology: Connecting Biology and Nanotechnology; Springer: Singapore, 2020; pp. 79–101. [Google Scholar]
- Lin, Y.; Ren, J.; Qu, X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef]
- Li, X.; Lin, G.; Zhou, L.; Prosser, O.; Malakooti, M.H.; Zhang, M. Green synthesis of iron-doped graphene quantum dots: An efficient nanozyme for glucose sensing. Nanoscale Horiz. 2024, 9, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, R.; Fan, K.; Gao, L.; Yan, X. Exploring the specificity of nanozymes. ACS Nano 2024, 18, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wang, Z.; Wang, S.; Wang, G.; Dong, H.; He, H.; Wu, H.; Ma, M.; Gao, X.; Zhang, Y. Elucidating the catalytic mechanism of Prussian blue nanozymes with self-increasing catalytic activity. Nat. Commun. 2024, 15, 5908. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Tong, Y.; Zhang, M.; Wu, X.; Yue, L. Boron-doped and ketonic carbonyl group-enriched graphdiyne as a dual-site carbon nanozyme with enhanced peroxidase-like activity. Anal. Chem. 2022, 94, 17272–17278. [Google Scholar] [CrossRef]
- Tao, X.-S.; Liu, Y.; Gan, Y.; Li, Y.-T.; Sha, J.; Cao, A.-M. A template-free assembly of Cu, N-codoped hollow carbon nanospheres as low-cost and highly efficient peroxidase nanozymes. Analyst 2022, 147, 5419–5427. [Google Scholar] [CrossRef]
- Fan, K.; Wang, H.; Xi, J.; Liu, Q.; Meng, X.; Duan, D.; Gao, L.; Yan, X. Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem. Commun. 2017, 53, 424–427. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A single-atom nanozyme for wound disinfection applications. Angew. Chem. 2019, 131, 4965–4970. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Wang, Z.; Li, M.; Bu, H.; Zia, D.S.; Dai, P.; Liu, J. Nanomaterials for molecular recognition: Specific adsorption and regulation of nanozyme activities. Mater. Chem. Front. 2023, 7, 3625–3640. [Google Scholar] [CrossRef]
- Lu, J.-T.; Zou, J.-H.; Zhao, B.-L.; Zhang, Y.-W. Research Progress on the Application of Inorganic Nanoparticle Enzyme in the Field of Analytical Sensing. Chin. J. Appl. Chem. 2024, 41, 60–86. [Google Scholar]
- Yu, J.; Wang, D.; Tipparaju, V.V.; Tsow, F.; Xian, X. Mitigation of humidity interference in colorimetric sensing of gases. ACS Sens. 2020, 6, 303–320. [Google Scholar] [CrossRef]
- Ko, A.; Liao, C. based colorimetric sensors for point-of-care testing. Anal. Methods 2023, 15, 4377–4404. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, G.; Chai, H.; Qu, L.; Zhang, X. Flexible biosensors based on colorimetry, fluorescence, and electrochemistry for point-of-care testing. Front. Bioeng. Biotechnol. 2021, 9, 753692. [Google Scholar] [CrossRef]
- VS, A.P.; Joseph, P.; SCG, K.D.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Zhu, W.; Li, L.; Zhou, Z.; Yang, X.; Hao, N.; Guo, Y.; Wang, K. A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, S.; Guan, Y.; Wu, C.; Qian, Y.; Zhou, H.; Qian, Y.; Yue, Y.; Yue, W. Ultrasmall CuMn-His Nanozymes with Multienzyme Activity at Neutral pH: Construction of a Colorimetric Sensing Array for Biothiol Detection and Disease Identification. ACS Appl. Mater. Interfaces 2024, 16, 34538–34548. [Google Scholar] [CrossRef]
- Jin, R.; Xing, Z.; Kong, D.; Yan, X.; Liu, F.; Gao, Y.; Sun, P.; Liang, X.; Lu, G. Sensitive colorimetric sensor for point-of-care detection of acetylcholinesterase using cobalt oxyhydroxide nanoflakes. J. Mater. Chem. B 2019, 7, 1230–1237. [Google Scholar] [CrossRef]
- Xiong, Y.; Su, L.; Ye, F.; Zhao, S. Ultrasmall phosphatase-mimicking nanoceria with slight self-colour for nonredox nanozyme-based colorimetric sensing. Anal. Chim. Acta 2022, 1200, 339604. [Google Scholar] [CrossRef]
- Hong, J.; Guo, Z.; Duan, D.; Zhang, Y.; Chen, X.; Li, Y.; Tu, Z.; Feng, L.; Chen, L.; Yan, X.; et al. Highly sensitive nanozyme strip: An effective tool for forensic material evidence identification. Nano Res. 2024, 17, 1785–1791. [Google Scholar] [CrossRef]
- Meng, X.; Zou, S.; Li, D.; He, J.; Fang, L.; Wang, H.; Yan, X.; Duan, D.; Gao, L. Nanozyme-strip for rapid and ultrasensitive nucleic acid detection of SARS-CoV-2. Biosens. Bioelectron. 2022, 217, 114739. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Cai, T.; Wang, R.; Yang, G.; Wen, T.; Peng, H. Magnetic and N–doped nanofibrous carbon microsphere with hierarchical porosity as a nanozyme for on-site and visual detection of glucose. J. Taiwan Inst. Chem. Eng. 2023, 152, 105171. [Google Scholar] [CrossRef]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza virus and SARS-CoV-2: Pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.; Tran, V.T.; Lee, D.K.; Ahmed, S.R.; Hong, J.C.; Lee, J.; Park, E.Y.; Lee, J. Magnetic nanozyme-linked immunosorbent assay for ultrasensitive influenza A virus detection. ACS Appl. Mater. Interfaces 2018, 10, 12534–12543. [Google Scholar] [CrossRef]
- Mohan, J.M.; Amreen, K.; Javed, A.; Dubey, S.K.; Goel, S. Emerging trends in miniaturized and microfluidic electrochemical sensing platforms. Curr. Opin. Electrochem. 2022, 33, 100930. [Google Scholar] [CrossRef]
- Hai, X.; Li, Y.; Zhu, C.; Song, W.; Cao, J.; Bi, S. DNA-based label-free electrochemical biosensors: From principles to applications. TrAC Trends Anal. Chem. 2020, 133, 116098. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- He, L.; Huang, R.; Xiao, P.; Liu, Y.; Jin, L.; Liu, H.; Li, S.; Deng, Y.; Chen, Z.; Li, Z.; et al. Current signal amplification strategies in aptamer-based electrochemical biosensor: A review. Chin. Chem. Lett. 2021, 32, 1593–1602. [Google Scholar] [CrossRef]
- Ko, E.; Tran, V.-K.; Son, S.E.; Hur, W.; Choi, H.; Seong, G.H. Characterization of Au@ PtNP/GO nanozyme and its application to electrochemical microfluidic devices for quantification of hydrogen peroxide. Sens. Actuators B Chem. 2019, 294, 166–176. [Google Scholar] [CrossRef]
- Mu, Z.; Guo, J.; Li, M.; Wu, S.; Zhang, X.; Wang, Y. A sensitive fluorescence detection strategy for H2O2 and glucose by using aminated Fe–Ni bimetallic MOF as fluorescent nanozyme. Microchim. Acta 2023, 190, 81. [Google Scholar] [CrossRef]
- Wang, N.; Shi, J.; Liu, Y.; Sun, W.; Su, X. Constructing bifunctional metal–organic framework based nanozymes with fluorescence and oxidase activity for the dual-channel detection of butyrylcholinesterase. Anal. Chim. Acta 2022, 1205, 339717. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Mu, Z.; Wu, S.; Wang, J.; Yang, Y.; Zhao, M.; Wang, Y. Label-free fluorescence detection of hydrogen peroxide and glucose based on the Ni-MOF nanozyme–induced self-ligand emission. Microchim. Acta 2022, 189, 219. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Bu, X.; Fu, Y.; Wang, Z.; Liu, Q. Recent advances in dual-emission ratiometric fluorescence probes for chemo/biosensing and bioimaging of biomarkers. Coord. Chem. Rev. 2019, 383, 82–103. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Y.; Lv, X.; Fan, D.; Dong, S. A facile, low-cost bimetallic iron–nickel MOF nanozyme-propelled ratiometric fluorescent sensor for highly sensitive and selective uric acid detection and its smartphone application. Nanoscale 2024, 16, 1394–1405. [Google Scholar] [CrossRef]
- Wang, N.; Li, Z.; Zhao, Y.; Wu, X.; Zhou, C.; Su, X. A novel robust hydrogel-assisted paper-based sensor based on fluorescence UiO-66-NH2@ ZIF-8 for the dual-channel detection of captopril. Talanta 2024, 277, 126400. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fang, Y.; Tian, L.; Liu, X.; Zhou, X.; Chen, X.; Gao, H.; Qin, H.; Liu, Y. AIE nanozyme-based long persistent chemiluminescence and fluorescence for POCT of pathogenic bacteria. ACS Sens. 2023, 8, 3205–3214. [Google Scholar] [CrossRef]
- Chang, J.; Yu, L.; Hou, T.; Hu, R.; Li, F. Direct and specific detection of glyphosate using a phosphatase-like nanozyme-mediated chemiluminescence strategy. Anal. Chem. 2023, 95, 4479–4485. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ju, C.; Han, C.; Shi, R.; Chen, X.; Duan, D.; Yan, J.; Yan, X. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 173, 112817. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Liu, D.; He, J.; Wang, M.; Huang, H.; Nie, G.; Ding, H.; Yan, X. Rapid and sensitive detection of Epstein-Barr virus antibodies in nasopharyngeal carcinoma by chemiluminescence strips based on iron-porphyrin single atom nanozyme. Nano Res. 2024, 17, 1827–1836. [Google Scholar] [CrossRef]
- Tripathy, S.; Chavva, S.; Coté, G.L.; Mabbott, S. Modular and handheld Raman systems for SERS-based point-of-care diagnostics. Curr. Opin. Biomed. Eng. 2023, 28, 100488. [Google Scholar] [CrossRef]
- Puravankara, V.; Manjeri, A.; Kim, Y.H.; Kitahama, Y.; Goda, K.; Dwivedi, P.K.; George, S.D. Surface-Enhanced Raman spectroscopy for Point-of-Care Bioanalysis: From lab to field. Chem. Eng. J. 2024, 498, 155163. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Wan, F.; Wang, J.; Hu, J. A review of cavity-enhanced Raman spectroscopy as a gas sensing method. Appl. Spectrosc. Rev. 2020, 55, 393–417. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, N.; Yao, Y.; Zhang, X.; Peng, X.; Zhao, L.; Wang, J.; Peng, L.; Wang, Z.; Mochizuki, K.; et al. Breaking the nanoparticle’s dispersible limit via rotatable surface ligands. Nat. Commun. 2022, 13, 3581. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, T.G.; Lee, S.H.; Kim, W.; Bang, A.; Moon, S.W.; Song, J.; Shin, J.-H.; Yu, J.S.; Choi, S. Label-free surface-enhanced Raman spectroscopy biosensor for on-site breast cancer detection using human tears. ACS Appl. Mater. Interfaces 2020, 12, 7897–7904. [Google Scholar] [CrossRef]
- Qu, L.; Han, J.; Huang, Y.; Yang, G.; Liu, W.; Long, Z.; Gu, Y.; Zhang, Q.; Gao, M.; Dong, X. Peroxidase-like nanozymes for Point-of-Care SERS sensing and wound healing. ACS Appl. Bio Mater. 2023, 6, 1272–1282. [Google Scholar] [CrossRef]
- Zeng, M.-H.; Zhang, C.; Yao, Q.-H.; Jin, J.-W.; Ye, T.-X.; Chen, X.-M.; Guo, Z.-Y.; Chen, X. Multifunction nanoenzyme-assisted ion-selective and oxidation catalysis SERS biosensors for point-of-care nitrite testing. Sens. Actuators B Chem. 2024, 405, 135352. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, X.; Chen, S.C.; Zhao, N. Emerging technologies of flexible pressure sensors: Materials, modeling, devices, and manufacturing. Adv. Funct. Mater. 2019, 29, 1808509. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Ma, Y.; Cheng, L.; Zhang, L.; Liu, Q.; Chen, J.; Zhao, Y.; Tu, K.; Zhang, M.; et al. Integrating Pt nanoparticles with carbon nanodots to achieve robust cascade superoxide dismutase-catalase nanozyme for antioxidant therapy. Nano Today 2023, 49, 101768. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, J.; Su, W.; Guo, J.; Deng, J.; Tang, L.; Li, G.; Liang, Y.; Zheng, L.; He, M.; et al. Modulating Pt nanozyme by using isolated cobalt atoms to enhance catalytic activity for alleviating osteoarthritis. Nano Today 2023, 49, 101809. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, G.; Liu, X.; Tang, D. Platinum nanozyme-triggered pressure-based immunoassay using a three-dimensional polypyrrole foam-based flexible pressure sensor. ACS Appl. Mater. Interfaces 2020, 12, 40133–40140. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yang, C.; Jin, Y. Advances in Gas Pressure-Based Portable Biosensing. TrAC Trends Anal. Chem. 2024, 180, 117897. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, Z.; Xue, J.; Zheng, L.; Liu, H.; Ouyang, H.; Fu, Z.; He, Y. Chemiluminescent/photothermal dual-mode lateral flow immunoassay based on CoFe PBAs/WS2 nanozyme for rapid and highly sensitive point-of-care testing of gentamicin. Biosens. Bioelectron. 2024, 265, 116711. [Google Scholar] [CrossRef]
- Wu, L.; Jiao, L.; Xue, D.; Li, Y.; Han, Y.; Ouyang, W.; Chen, Q. Nanozyme and bifunctional nanobody-based colorimetric-SERS dual-mode Immunosensor for microcystin-LR detection. Food Chem. 2024, 464, 141574. [Google Scholar] [CrossRef]
- Yu, K.; Li, M.; Chai, H.; Liu, Q.; Hai, X.; Tian, M.; Qu, L.; Xu, T.; Zhang, G.; Zhang, X. MOF-818 nanozyme-based colorimetric and electrochemical dual-mode smartphone sensing platform for in situ detection of H2O2 and H2S released from living cells. Chem. Eng. J. 2023, 451, 138321. [Google Scholar] [CrossRef]
- Yin, B.; Zhu, H.; Zeng, S.; Sohan, A.M.F.; Wan, X.; Liu, J.; Zhang, P.; Lin, X. Chip-based automated equipment for dual-mode point-of-care testing foodborne pathogens. Biosens. Bioelectron. 2024, 257, 116338. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, H.; Tao, H.; Liu, J.; Li, Y.; Li, Q.-X.; Yang, T.; Meng, S.; Yang, Y.; Hu, R. Dual-mode sensing platform based on an iodide ion synergistic covalent triazine frameworks (CTFs) for point-of-care testing (POCT) of acetylcholinesterase. Anal. Chim. Acta 2025, 1350, 343836. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Li, C.; Liu, S.; Lang, Y.; Zhang, X.; Zhang, B.; Liu, C. A “three-in-one” multifunctional palladium/platinum nanoparticles-driven multimodal lateral flow immunoassays for point-of-care testing of Staphylococcus aureus. Sens. Actuators B Chem. 2024, 413, 135877. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent advances in nanozyme research. Adv. Mater. 2019, 31, 1805368. [Google Scholar] [CrossRef]
- Somerville, S.V.; Li, Q.; Wordsworth, J.; Jamali, S.; Eskandarian, M.R.; Tilley, R.D.; Gooding, J.J. Approaches to improving the selectivity of nanozymes. Adv. Mater. 2024, 36, 2211288. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Z.; Fu, Q.; Wang, M.; Duan, D. Advances of Nanozyme-Driven Multimodal Sensing Strategies in Point-of-Care Testing. Biosensors 2025, 15, 375. https://doi.org/10.3390/bios15060375
Chang Z, Fu Q, Wang M, Duan D. Advances of Nanozyme-Driven Multimodal Sensing Strategies in Point-of-Care Testing. Biosensors. 2025; 15(6):375. https://doi.org/10.3390/bios15060375
Chicago/Turabian StyleChang, Ziyi, Qingjie Fu, Mengke Wang, and Demin Duan. 2025. "Advances of Nanozyme-Driven Multimodal Sensing Strategies in Point-of-Care Testing" Biosensors 15, no. 6: 375. https://doi.org/10.3390/bios15060375
APA StyleChang, Z., Fu, Q., Wang, M., & Duan, D. (2025). Advances of Nanozyme-Driven Multimodal Sensing Strategies in Point-of-Care Testing. Biosensors, 15(6), 375. https://doi.org/10.3390/bios15060375





