Electrochemical Sensor Platform for Rapid Detection of Foodborne Toxins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. ATR-IR Spectroscopy Setup
2.3. Modification of Electrode Sensing Platform
2.4. Statistical Analysis
3. Results and Discussion
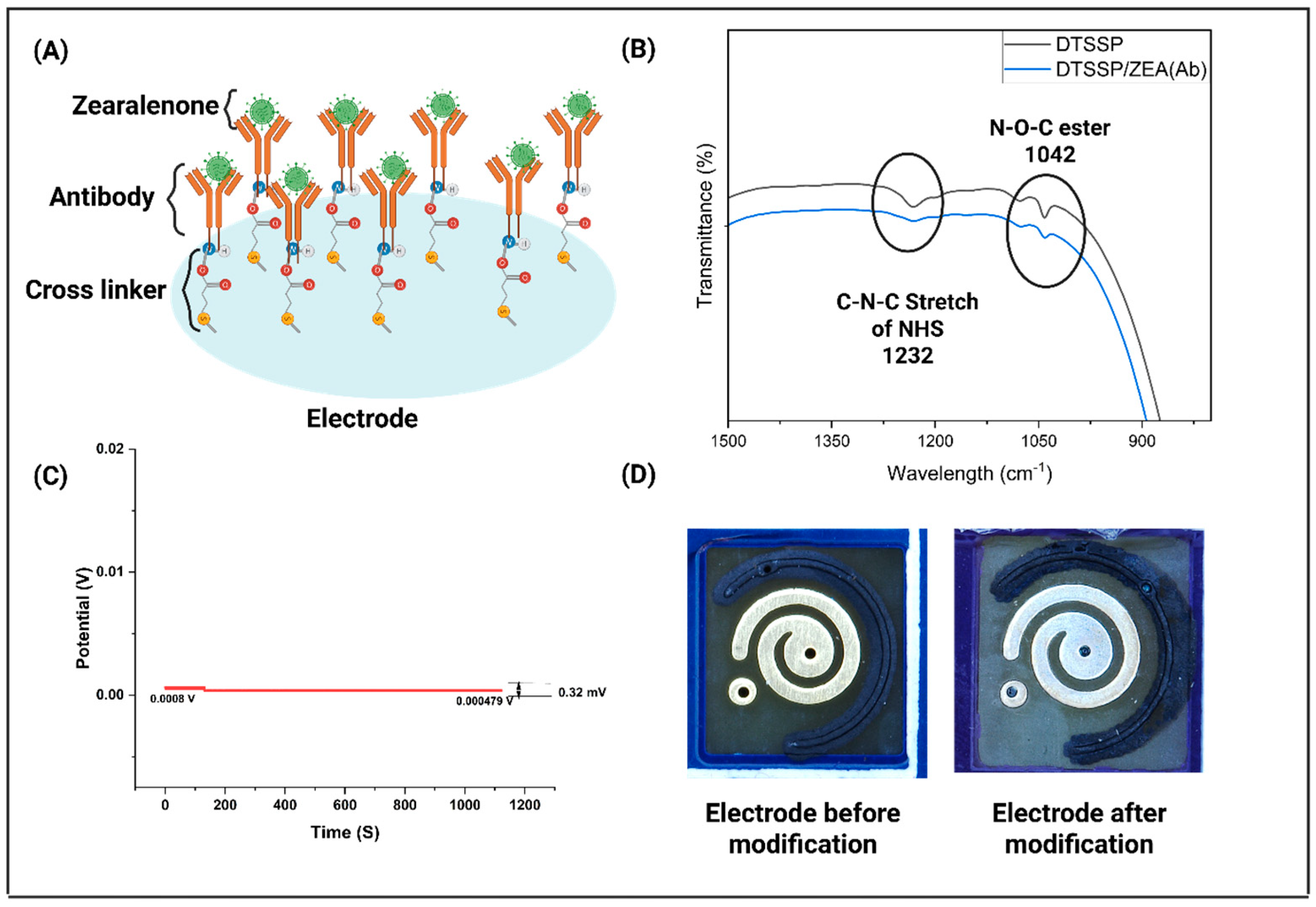
3.1. Characterization of the Sensor Platform
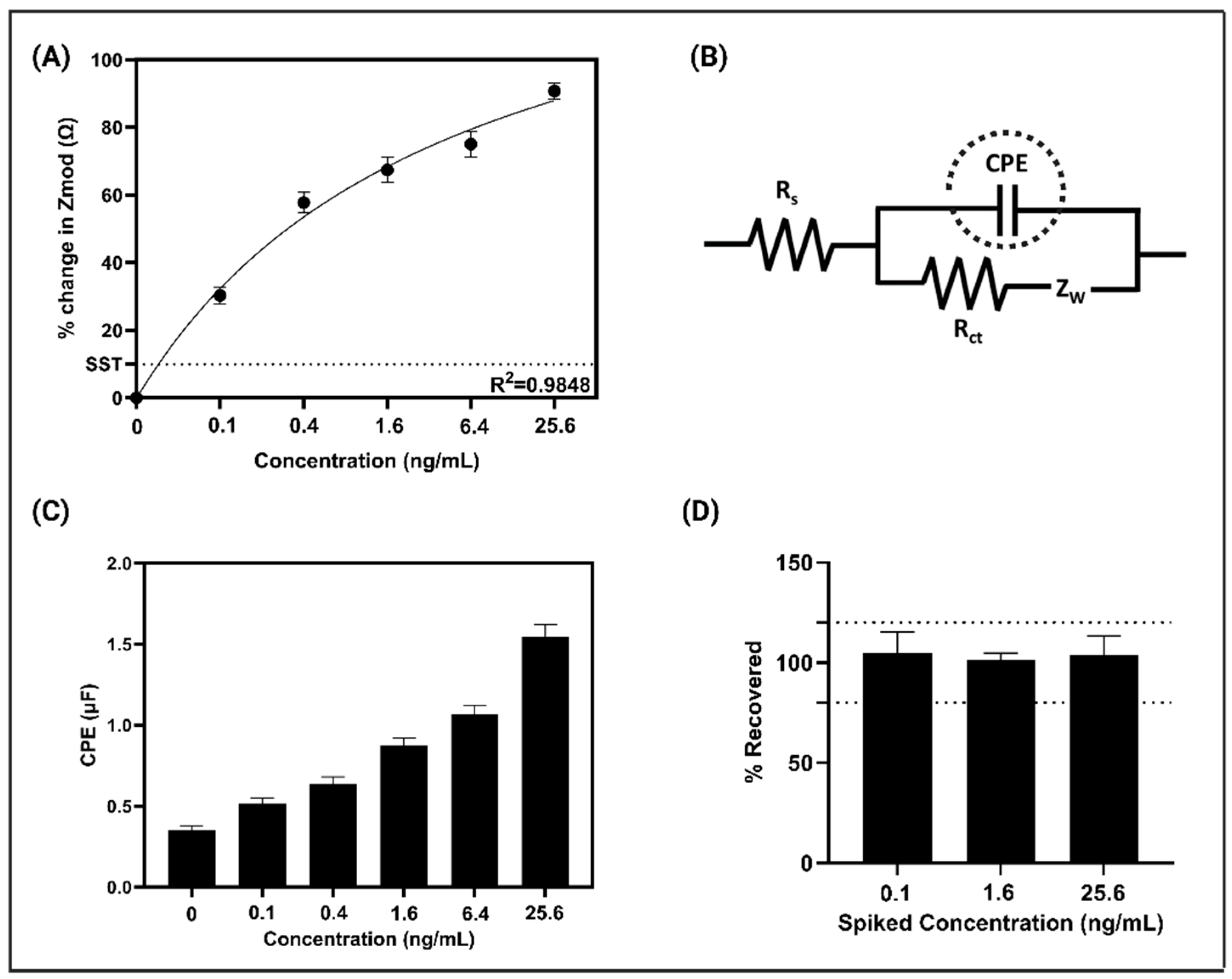
3.2. Electrochemical Signal Response on the Modified Sensor Platform and Spike and Recovery Study
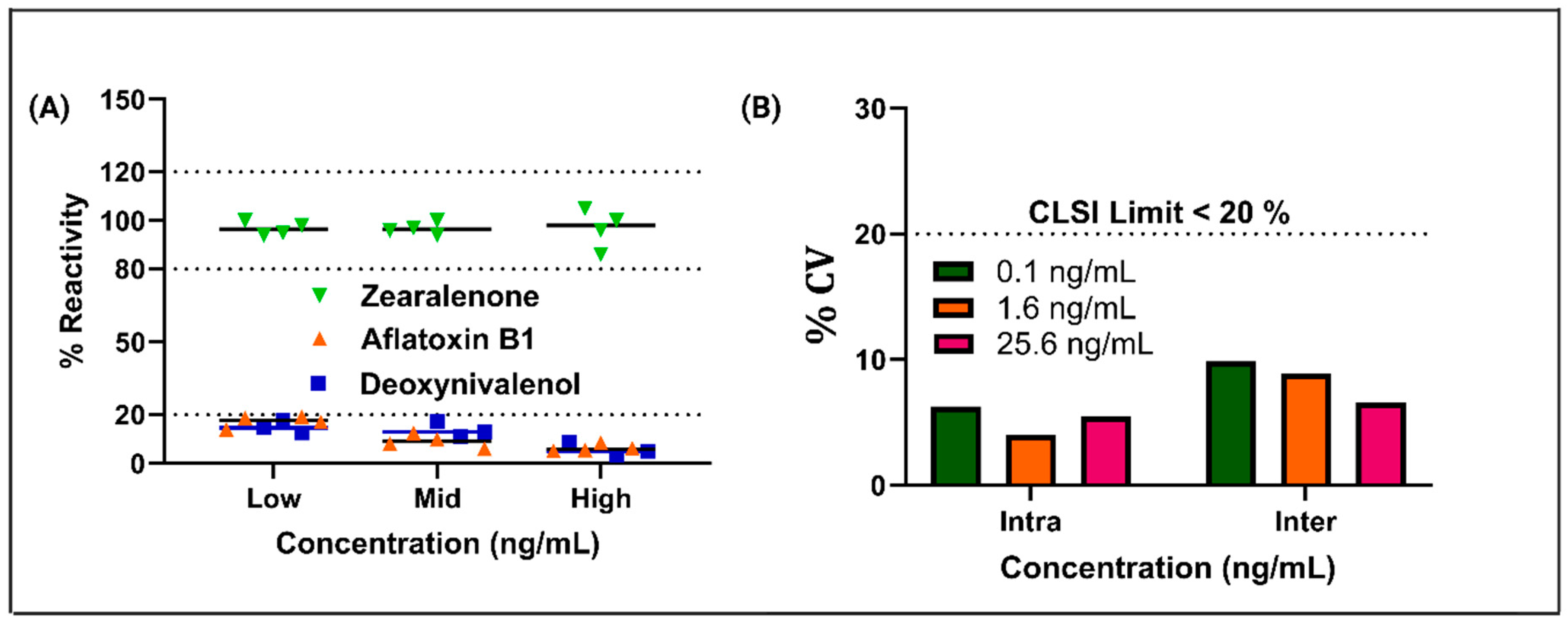
3.3. Cross-Reactivity, Reproducibility, and Repeatability
3.4. Correlation Study Between the Benchtop and Portable Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, V.; Basu, M.S.; Rajendran, T.P. Mycotoxin Research and Mycoflora in Some Commercially Important Agricultural Commodities. Crop Prot. 2008, 27, 891–905. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Shier, W.T. An Overview of Mycotoxin Contamination in Foods and Its Implications for Human Health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Richard, J.L. Some Major Mycotoxins and Their Mycotoxicoses—An Overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Şahin, S.; Üstündağ, Z. Detection Strategies of Zearalenone for Food Safety: A Review. Crit. Rev. Anal. Chem. 2022, 52, 294–313. [Google Scholar] [CrossRef]
- Dohlman, E. Mycotoxin Hazards and Regulations; U.S. Department of Agriculture: Washington, DC, USA, 2003. [Google Scholar]
- EFSA; ECDC. European Food Safety Authority and European Centre for Disease Prevention and Control. EFSA J. 2015, 13, 4329. [Google Scholar]
- European Union. Official Journal of the European Union; European Union: Brussels, Belgium, 2005. [Google Scholar]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an Endocrine Disruptor in Humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef]
- Luo, S.; Liu, Y.; Guo, Q.; Wang, X.; Tian, Y.; Yang, W.; Li, J.; Chen, Y. Determination of Zearalenone and Its Derivatives in Feed by Gas Chromatography–Mass Spectrometry with Immunoaffinity Column Cleanup and Isotope Dilution. Toxins 2022, 14, 764. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and Its Metabolites: Effect on Human Health, Metabolism and Neutralisation Methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for Detection of Aflatoxins in Agricultural Food Crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- Do, J.H.; Choi, D.-K. Aflatoxins: Detection, Toxicity, and Biosynthesis. Biotechnol. Bioprocess Eng. 2007, 12, 585–593. [Google Scholar] [CrossRef]
- Shan, S.; Liu, D.; Guo, Q.; Wu, S.; Chen, R.; Luo, K.; Hu, L.; Xiong, Y.; Lai, W. Sensitive Detection of Escherichia Coli O157: H7 Based on Cascade Signal Amplification in ELISA. J. Dairy Sci. 2016, 99, 7025–7032. [Google Scholar] [CrossRef]
- Abdulmawjood, A.; Bülte, M.; Cook, N.; Roth, S.; Schönenbrücher, H.; Hoorfar, J. Toward an International Standard for PCR-Based Detection of Escherichia Coli O157: Part 1. Assay Development and Multi-Center Validation. J. Microbiol. Methods 2003, 55, 775–786. [Google Scholar] [CrossRef]
- Gilgen, M.; Hübner, P.; Höfelein, C.; Lüthy, J.; Candrian, U. PCR-Based Detection of Verotoxin-Producing Escherichia Coli (VTEC) in Ground Beef. Res. Microbiol. 1998, 149, 145–154. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Mukhopadhyay, U.K. Novel Multiplex PCR Approaches for the Simultaneous Detection of Human Pathogens: Escherichia Coli 0157: H7 and Listeria Monocytogenes. J. Microbiol. Methods 2007, 68, 193–200. [Google Scholar] [CrossRef]
- Wang, H.; Gill, C.O.; Yang, X. Development of a Real-Time PCR Procedure for Quantification of Viable Escherichia Coli in Populations of E. Coli Exposed to Lactic Acid, and the Acid Tolerance of Verotoxigenic E. coli (VTEC) from Cattle Hides. Food Control 2014, 43, 104–109. [Google Scholar] [CrossRef]
- Jacangelo, J.G.; Askenaizer, D.J.; Schwab, K. Research Needs in Drinking Water: A Basis in Regulations in the United States. J. Water Health 2006, 4, 1–9. [Google Scholar] [CrossRef]
- Anand, R.; Mishra, K.K.; Bharadvaja, N. Introduction to Bio-Nanotechnology. In Biogenic Nanomaterials for Environmental Sustainability: Principles, Practices, and Opportunities; Shah, M.P., Bharadvaja, N., Kumar, L., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–11. ISBN 978-3-031-45956-6. [Google Scholar]
- Poudyal, D.C.; Dhamu, V.N.; Paul, A.; Samson, M.; Muthukumar, S.; Prasad, S. A Novel Single Step Method to Rapidly Screen for Metal Contaminants in Beverages, a Case Study with Aluminum. Environ. Technol. Innov. 2022, 28, 102691. [Google Scholar] [CrossRef]
- Poudyal, D.C.; Dhamu, V.N.; Samson, M.; Muthukumar, S.; Prasad, S. Pesticide Analytical Screening System (PASS): A Novel Electrochemical System for Multiplex Screening of Glyphosate and Chlorpyrifos in High-Fat and Low-Fat Food Matrices. Food Chem. 2023, 400, 134075. [Google Scholar] [CrossRef]
- Poudyal, D.C.; Dhamu, V.N.; Samson, M.; Muthukumar, S.; Prasad, S. Portable Pesticide Electrochem-Sensor: A Label-Free Detection of Glyphosate in Human Urine. Langmuir 2022, 38, 1781–1790. [Google Scholar] [CrossRef]
- Mishra, K.K.; Dhamu, V.N.; Jophy, C.; Muthukumar, S.; Prasad, S. Electroanalytical Platform for Rapid E. coli O157:H7 Detection in Water Samples. Biosensors 2024, 14, 298. [Google Scholar] [CrossRef]
- Rawat, B.; Mishra, K.K.; Barman, U.; Arora, L.; Pal, D.; Paily, R.P. Two-Dimensional MoS2-Based Electrochemical Biosensor for Highly Selective Detection of Glutathione. IEEE Sens. J. 2020, 20, 6937–6944. [Google Scholar] [CrossRef]
- Mishra, K.K.; Dhamu, V.N.; Kokala, A.; Muthukumar, S.; Prasad, S. Advancing Food Safety: Two-Plex Electrochemical Biosensor for Mycotoxin Detection in Food Matrices. Biosens Bioelectron X 2025, 25, 100626. [Google Scholar] [CrossRef]
- Mishra, K.K.; Dhamu, V.N.; Poudyal, D.C.; Muthukumar, S.; Prasad, S. PathoSense: A Rapid Electroanalytical Device Platform for Screening Salmonella in Water Samples. Microchim. Acta 2024, 191, 146. [Google Scholar] [CrossRef]
- Wilkowska, A.; Biziuk, M. Determination of Pesticide Residues in Food Matrices Using the QuEChERS Methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Spiro, J.C.K.; Mishra, K.K.; Dhamu, V.N.; Bhatia, A.; Muthukumar, S.; Prasad, S. Development of a Portable Electrochemical Sensing Platform for Impedance Spectroscopy-Based Biosensing Using an ARM-Based Microcontroller. Sens. Diagn. 2024, 3, 1835–1842. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Karnam, J.B.; Muthukumar, S.; Prasad, S. COVID Severity Test (CoST Sensor)—An Electrochemical Immunosensing Approach to Stratify Disease Severity. Bioeng. Transl. Med. 2023, 8, e10566. [Google Scholar] [CrossRef]
- Kumar Mishra, K.; Narayanan Dhamu, V.; Muthukumar, S.; Prasad, S. Quick and Sensitive Two-Plex Electrochemical Platform for Pathogen Detection in Water. Nano Select 2025, 2025, e70017. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, K.K.; Thakkar, K.M.; Dhamu, V.N.; Muthukumar, S.; Prasad, S. Electrochemical Sensor Platform for Rapid Detection of Foodborne Toxins. Biosensors 2025, 15, 361. https://doi.org/10.3390/bios15060361
Mishra KK, Thakkar KM, Dhamu VN, Muthukumar S, Prasad S. Electrochemical Sensor Platform for Rapid Detection of Foodborne Toxins. Biosensors. 2025; 15(6):361. https://doi.org/10.3390/bios15060361
Chicago/Turabian StyleMishra, Kundan Kumar, Krupa M. Thakkar, Vikram Narayanan Dhamu, Sriram Muthukumar, and Shalini Prasad. 2025. "Electrochemical Sensor Platform for Rapid Detection of Foodborne Toxins" Biosensors 15, no. 6: 361. https://doi.org/10.3390/bios15060361
APA StyleMishra, K. K., Thakkar, K. M., Dhamu, V. N., Muthukumar, S., & Prasad, S. (2025). Electrochemical Sensor Platform for Rapid Detection of Foodborne Toxins. Biosensors, 15(6), 361. https://doi.org/10.3390/bios15060361





