A Metal–Organic Hybrid Composed of Dual Quenching Cofactors as a Nanoquencher for the Fluorescent Determination of Protease Caspase-3
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Fluorescence Titration Experiments
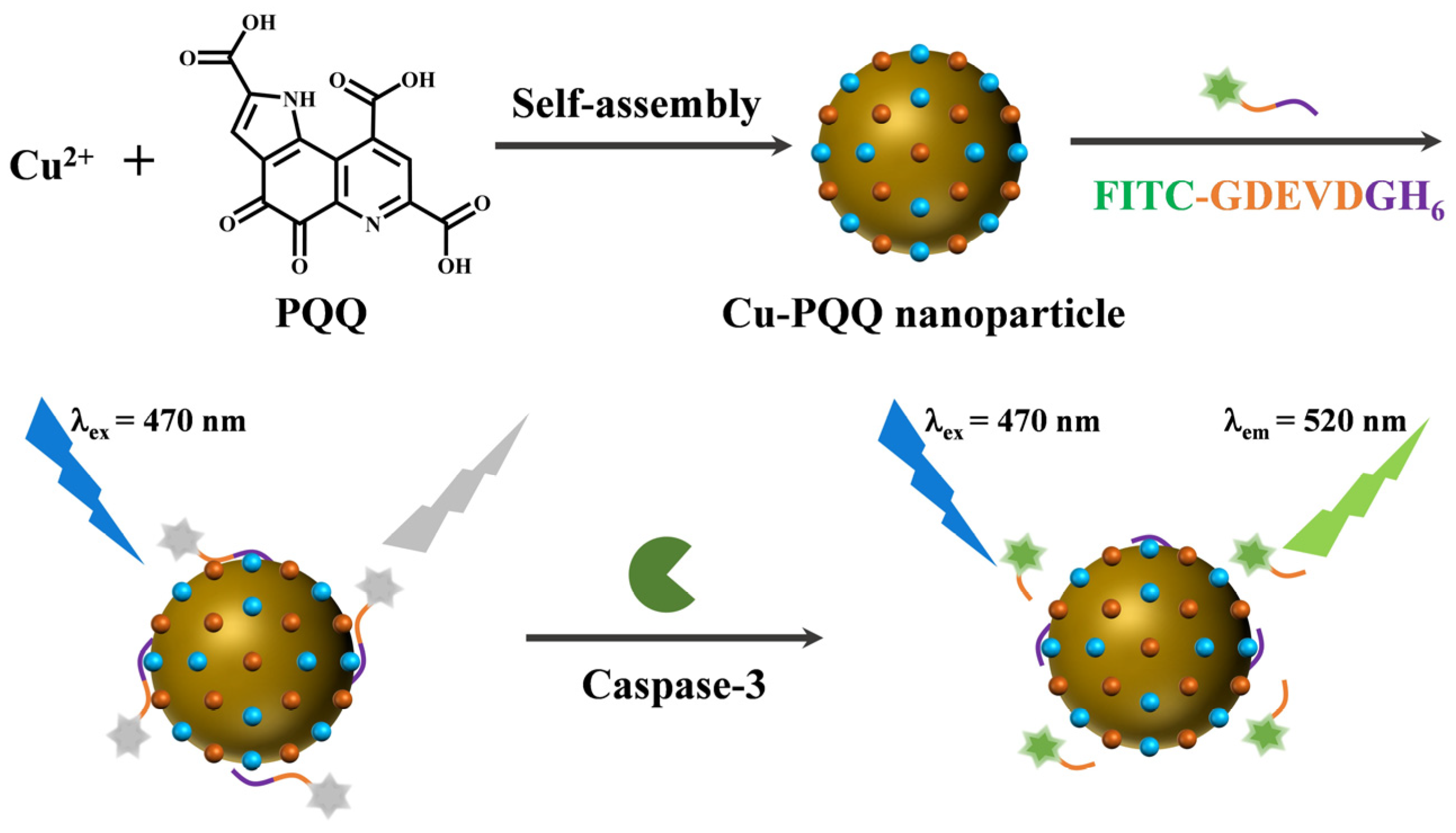
2.4. Synthesis of Cu-PQQ Nanoparticles and Cu-BTC MOFs
2.5. Detection of Caspase-3
2.6. Assays of Caspase-3 in Lysates
3. Results and Discussion
3.1. Characterization of Cu-PQQ Nanoparticles
3.2. Stability of Cu-PQQ Nanoparticles
3.3. Quenching Efficiency
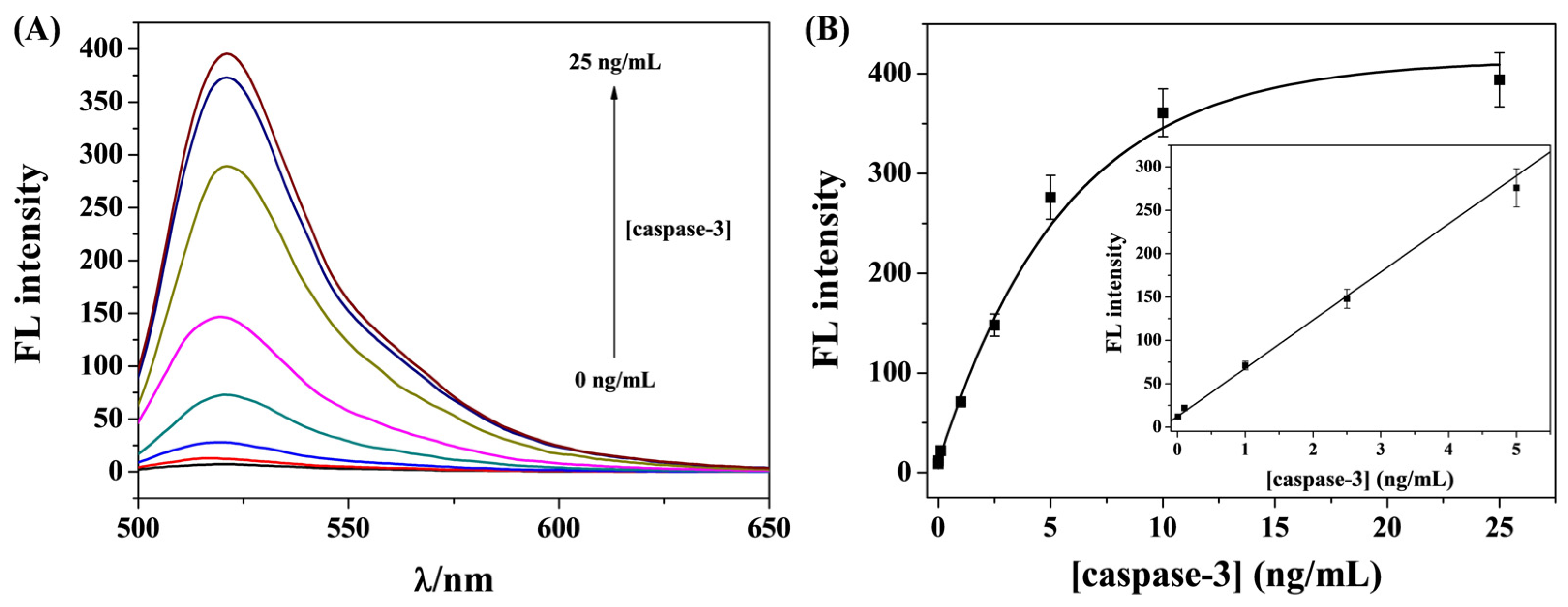
3.4. Sensitivity for Caspase-3 Detection
3.5. Selectivity
3.6. Inhibition Efficiency
3.7. Assays of Caspase-3 Activity in Cell Lysates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, I.L.H.; Yang, K.-L. Recent developments in protease activity assays and sensors. Analyst 2017, 142, 1867–1881. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ba, R.; Wang, W.; Zhang, Y.; Bao, B.; Chen, P.; Yao, W.; Zhu, J.-J.; Zhang, L.; Cheng, F.-F. Roles of nanomaterials in thrombin detection. TrAC-Trend. Anal. Chem. 2024, 175, 117734. [Google Scholar] [CrossRef]
- Suresh, V.; Sheik, D.A.; Detomasi, T.C.; Rajesh, U.C.; Zhao, T.; Zepeda, T.; Saladi, S.; Byers, K.; Craik, C.S.; Davisson, V.J. A prototype assay multiplexing SARS-CoV-2 3CL-protease and angiotensin-converting enzyme 2 for saliva-based diagnostics in COVID-19. Biosensors 2023, 13, 682. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, Y.; Wang, A.; Zhang, Y.; Cui, C.; Ye, S.; Mao, Q.; Feng, Y.; Li, J.; Xu, C.; et al. In vivo quantitative assessment of a radiation dose based on ratiometric photoacoustic imaging of tumor apoptosis. Anal. Chem. 2022, 94, 5149–5158. [Google Scholar] [CrossRef]
- Lei, Q.; Huang, X.; Zeng, W. Biosensors for Caspase-3: From chemical methodologies to biomedical applications. Talanta 2022, 240, 123198. [Google Scholar] [CrossRef]
- Liu, Q.T.; Wang, J.F.; Boyd, B.J. Peptide-based biosensors. Talanta 2015, 136, 114–127. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Zhang, C.-y. Label-free and homogenous detection of caspase-3-like proteases by disrupting homodimerization-directed bipartite tetracysteine display. Anal. Chem. 2017, 89, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Peptide based biosensors. TrAC-Trend. Anal. Chem. 2018, 107, 1–20. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, Z.; Liu, L.; Zeng, Y.; Ma, L.; Nie, Z.; Tian, Z.; Kai, D.; Zhang, F.; Liu, G.; et al. Intensity interrogation-based high-sensitivity surface plasmon resonance imaging biosensor for apoptosis detection in cancer. Biosensors 2023, 13, 946. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Saadatidizaji, Z.; Maleki, A.; de la Guardia, M.; Mahdavi, M.; Barzegar, S.; Ahadian, S. Recent progresses in development of biosensors for thrombin detection. Biosensors 2022, 12, 767. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, J.H.; Chung, S.J. Homogeneous detection of caspase-3 using intrinsic fluorescence resonance energy transfer (iFRET). Biosens. Bioelectron. 2015, 67, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Lee, E.J.; Hah, S.S. TAMRA- and Cy5-labeled probe for efficient kinetic characterization of caspase-3. Anal. Biochem. 2014, 446, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Liu, L.-H.; Cheng, H.; Li, B.; Qiu, W.-X.; Zhang, X.-Z. A dual-FRET-based fluorescence probe for the sequential detection of MMP-2 and caspase-3. Chem. Commun. 2015, 51, 14520. [Google Scholar] [CrossRef]
- den Hamer, A.; Dierickx, P.; Arts, R.; de Vries, J.S.P.M.; Brunsveld, L.; Merkx, M. Bright bioluminescent BRET sensor proteins for measuring intracellular caspase activity. ACS Sens. 2017, 2, 729–734. [Google Scholar] [CrossRef]
- Vuojola, J.; Syrjanpaa, M.; Lamminmaki, U.; Soukka, T. Genetically encoded protease substrate based on lanthanide binding peptide for time-gated fluorescence detection. Anal. Chem. 2013, 85, 1367–1373. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.; Min, D.-H. Graphene oxide for fluorescence-mediated enzymatic activity assays. J. Mater. Chem. B 2014, 2, 2452–2460. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Zhang, L.; Zhao, J.; Jiang, Z.; Wang, W. Peptide-derived biosensors and their applications in tumor immunology-related detection. Anal. Chem. 2022, 94, 431–441. [Google Scholar] [CrossRef]
- Chen, J.; Meng, H.; Tian, Y.; Yang, R.; Du, D.; Li, Z.; Qu, L.; Lin, Y. Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications. Nanoscale Horiz. 2019, 4, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Hussain, E.; Huang, X.; Zhang, Y.; Niu, N.; Shahzad, S.A.; Yu, C. Black phosphorus nanosheets based sensitive protease detection and inhibitor screening. Talanta 2019, 197, 270–276. [Google Scholar] [CrossRef]
- Wang, M.; Lei, C.; Nie, Z.; Guo, M.; Huang, Y.; Yao, S. Label-free fluorescent detection of thrombin activity based on a recombinant enhanced green fluorescence protein and nickel ions immobilized nitrilotriacetic acid-coated magnetic nanoparticles. Talanta 2013, 116, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Chen, Z.; Ge, C.; Hu, W.; Wang, N.; Yang, L.; Luan, M.; Xu, J. Simultaneous visualization of MiRNA-221 and caspase-3 in cancer cells for investigating the feasibility of miRNA-targeted therapy with a dual-color fluorescent nanosensor. Biosensors 2022, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H. Proteolytic biosensors with functional nanomaterials: Current approaches and future challenges. Biosensors 2023, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh-Panahi, P.; Belali, S.; Sohrabi, H.; Oroojalian, F.; Hashemzaei, M.; Mokhtarzadeh, A.; de la Guardia, M. Metal-organic frameworks conjugated with biomolecules as efficient platforms for development of biosensors. TrAC-Trend. Anal. Chem. 2021, 141, 116285. [Google Scholar] [CrossRef]
- Qin, L.; Lin, L.X.; Fang, Z.P.; Yang, S.P.; Qiu, G.H.; Chen, J.X.; Chen, W.H. A water-stable metal-organic framework of a zwitterionic carboxylate with dysprosium: A sensing platform for Ebolavirus RNA sequences. Chem. Commun. 2016, 52, 132–135. [Google Scholar] [CrossRef]
- Chen, G.; Bai, W.; Jin, Y.; Zheng, J. Fluorescence and electrochemical assay for bimodal detection of lead ions based on Metal-Organic framework nanosheets. Talanta 2021, 232, 122405–122414. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, H.; Wei, X.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G. Metal-organic framework (MOF): A novel sensing platform for biomolecules. Chem. Commun. 2013, 49, 1276–1278. [Google Scholar] [CrossRef]
- Yang, S.-P.; Chen, S.-R.; Liu, S.-W.; Tang, X.-Y.; Qin, L.; Qiu, G.-H.; Chen, J.-X.; Chen, W.-H. Platforms formed from a three-dimensional Cu-based Zwitterionic metal−organic framework and probe ss-DNA: Selective fluorescent biosensors for human immunodeficiency virus 1 ds-DNA and sudan virus RNA sequences. Anal. Chem. 2015, 87, 12206–12214. [Google Scholar] [CrossRef]
- Qiu, G.-H.; Weng, Z.-H.; Hu, P.-P.; Duan, W.-J.; Xie, B.-P.; Sun, B.; Tang, X.-Y.; Chen, J.-X. Synchronous detection of ebolavirus conserved RNA sequences and ebolavirus-encoded miRNA-like fragment based on a zwitterionic copper (II) metal–organic framework. Talanta 2018, 180, 396–402. [Google Scholar] [CrossRef]
- Qiu, Q.; Chen, H.; Ying, S.; Sharif, S.; You, Z.; Wang, Y.; Ying, Y. Simultaneous fluorometric determination of the DNAs of Salmonella enterica, Listeria monocytogenes and Vibrio parahemolyticus by using an ultrathin metal-organic framework (type Cu-TCPP). Microchim. Acta 2019, 186, 93. [Google Scholar] [CrossRef]
- Xie, B.-P.; Qiu, G.-H.; Hu, P.-P.; Liang, Z.; Liang, Y.-M.; Sun, B.; Bai, L.-P.; Jiang, Z.-H.; Chen, J.-X. Simultaneous detection of Dengue and Zika virus RNA sequences with a three-dimensional Cu-based zwitterionic metal–organic framework, comparison of single and synchronous fluorescence analysis. Sens. Actuators B Chem. 2018, 254, 1133–1140. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, S.; Peng, Y.; Wang, M.; Jalalah, M.; Al-Assiri, M.S.; Harraz, F.A.; Yang, J.; Li, G. Sensor array for rapid pathogens identification fabricated with peptide conjugated 2D metal-organic framework nanosheets. Chem. Eng. J. 2021, 405, 126707. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, G.; Weng, W.; Qiu, B.; Guo, L.; Lin, Z.; Chen, G. Signal on fluorescence biosensor for MMP-2 based on FRET between semiconducting polymer dots and a metal organic framework. RSC Adv. 2014, 4, 58852–58857. [Google Scholar] [CrossRef]
- Teng, Q.; Zhou, K.; Zhu, C.; Yu, K.; Wang, Z.; Zhang, X.; Dai, Z. Principal component analysis-assisted zirconium-based metal-organic frameworks/DNA biosensor for the analysis of various phosphates. Talanta 2024, 271, 12573. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, L.; Wu, Y.-X.; Zhang, K.; Cao, Y.; Zhou, Y.; Wu, D.; Hu, F.; Gan, N. A two dimensional metaleorganic framework nanosheets-based fluorescence resonance energy transfer aptasensor with circular strand-replacement DNA polymerization target-triggered amplification strategy for homogenous detection of antibiotics. Anal. Chim. Acta 2018, 1020, 1–8. [Google Scholar] [CrossRef]
- Liang, L.; Chen, M.; Tong, Y.; Tan, W.; Chen, Z. Detection of Mycobacterium Tuberculosis IS6110 gene fragment by fluorescent biosensor based on FRET between two-dimensional metal-organic framework and quantum dots-labeled DNA probe. Anal. Chim. Acta 2021, 1186, 339090. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-based metal–organic frameworks for biomedical applications. Angew. Chem. Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Liu, B.; Meng, Q.; Yuan, M.; Ma, X.; Wang, J.; Wang, M.; Li, K.; Ma, P.A.; et al. Facile synthesis of Fe-based metal-quinone networks for mutually enhanced mild photothermal therapy and ferroptosis. Angew. Chem. Int. Ed. 2024, 64, e202414879. [Google Scholar] [CrossRef]
- Li, Y.; Miao, Y.; Yang, L.; Zhao, Y.; Wu, K.; Lu, Z.; Hu, Z.; Guo, J. Recent advances in the development and antimicrobial applications of metal–phenolic networks. Adv. Sci. 2022, 9, 2202684. [Google Scholar] [CrossRef]
- Tan, M.; Cao, G.; Wang, R.; Cheng, L.; Huang, W.; Yin, Y.; Ma, H.; Ho, S.-H.; Wang, Z.; Zhu, M.; et al. Metal-ion-chelating phenylalanine nanostructures reverse immune dysfunction and sensitize breast tumour to immune checkpoint blockade. Nat. Nanotechnol. 2024, 19, 1903–1913. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Y.; Ma, J.; Li, Y.; Fan, L.; Li, X. Visual sensor array for multiple aromatic amines via specific ascorbic acid oxidase mimic triggered schiff-base chemistry. Anal. Chem. 2024, 96, 13131–13139. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gao, F.; Yi, X.; La, M. Optical bioassays based on the signal amplification of redox cycling. Biosensors 2024, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, M.; Ye, D.; Zhang, N.; Lim, E.; Lu, J.; Jiang, C. Tumor cell membrane-targeting pH-dependent electron donor acceptor fluorescence systems with low background signals. Biomaterials 2014, 35, 2952–2960. [Google Scholar] [CrossRef]
- Satheeshkumar, K.; Kumar, P.S.; Nandhini, C.; Shanmugapriya, R.; Vennila, K.N.; Elango, K.P. A simple metal ion displacement-type turn-on fluorescent probe for the detection of halide ions in 100% water—Spectroscopic and TD-DFT investigations. Inorg. Chem. Commun. 2022, 139, 109299. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Zhang, J.; Liu, J.; Wang, A.; Ding, L. Recent development of copper-based nanozymes for biomedical applications. Adv. Healthc. Mater. 2024, 13, 2302023. [Google Scholar] [CrossRef]
- Röder, R.; Preiß, T.; Hirschle, P.; Steinborn, B.; Zimpel, A.; Höhn, M.; Rädler, J.O.; Bein, T.; Wagner, E.; Wuttke, S.; et al. Multifunctional nanoparticles by coordinative self-assembly of His tagged units with metal−organic frameworks. J. Am. Chem. Soc. 2017, 139, 2359–2368. [Google Scholar] [CrossRef]
- Brunner, J.; Kraemer, R. Copper(II)-quenched oligonucleotide probes for fluorescent DNA sensing. J. Am. Chem. Soc. 2004, 126, 13626–13627. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kwok, R.T.; Liu, J.; Xing, B.; Tang, B.Z.; Liu, B. Real-time monitoring of cell apoptosis and drug screening using fluorescent light-up probe with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 134, 17972–17981. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.; Liu, C.; Qiu, M.; Ma, X.; Mao, Z.; Sun, Y.; Liu, Z. Recent advances in the development of activatable multifunctional probes for in vivo imaging of caspase-3. Chin. Chem. Lett. 2021, 32, 168–178. [Google Scholar] [CrossRef]
- Yu, Z.-J.; Yang, T.-T.; Liu, G.; Deng, D.-H.; Liu, L. Gold nanoparticles-based colorimetric immunoassay of carcinoembryonic antigen with metal–organic framework to load quinones for catalytic oxidation of cysteine. Sensors 2024, 24, 6701. [Google Scholar] [CrossRef]
- Xia, N.; Sun, Z.; Ding, F.; Wang, Y.; Sun, W.; Liu, L. Protease biosensor by conversion of a homogeneous assay into a surface-tethered electrochemical analysis based on streptavidin-biotin interactions. ACS Sens. 2021, 6, 1166–1173. [Google Scholar] [CrossRef]
- Suzuki, S.; Sakurai, T.; Itoh, S.; Ohshiro, Y. Preparation and characterization of ternary copper(II) complexes containing coenzyme PQQ and bipyridine or terpyridine. Inorg. Chem. 1988, 27, 591–592. [Google Scholar] [CrossRef]
- Wang, H.-S.; Liu, H.-L.; Wang, K.; Ding, Y.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Insight into the unique fluorescence quenching property of metal organic frameworks upon DNA binding. Anal. Chem. 2017, 89, 11366–11371. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, L.; Luo, F.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G. Fluorescence biosensor for the H5N1 antibody based on a metal–organic framework platform. J. Mater. Chem. B 2013, 1, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, Q.; Gao, X.; Xu, K.; Tang, B. Monitoring the activation of caspases-1/3/4 for describing the pyroptosis pathways of cancer cells. Anal. Chem. 2021, 93, 12022–12031. [Google Scholar] [CrossRef]
- Liu, X.; Song, X.; Luan, D.; Hu, B.; Xu, K.; Tang, B. Real-time in situ cisualizing of the sequential activation of caspase cascade using a multicolor gold-selenium bonding fluorescent nanoprobe. Anal. Chem. 2019, 91, 5994–6002. [Google Scholar] [CrossRef]
- Yang, Y.; He, Y.; Deng, Z.; Li, J.; Huang, J.; Zhong, S. Intelligent nanoprobe: Acid-responsive drug release and in situ evaluation of its own therapeutic effect. Anal. Chem. 2020, 92, 12371–12378. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Shi, X.; He, X.; Wei, W.; Ma, N.; Chen, H. Highly sensitive detection of caspase-3 activities via a nonconjugated gold nanoparticle-quantum dot pair mediated by an inner-filter effect. ACS Appl. Mater. Interfaces 2013, 5, 9798–9802. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yi, C.; Zhang, Z.; Zhang, H.; Li, M.; Yang, M.; Jiang, Q. Peptide-bridged assembly of hybrid nanomaterial and its application for caspase-3 detection. ACS Appl. Mater. Interfaces 2013, 5, 6494–6501. [Google Scholar] [CrossRef]
- Yang, J.; Ma, M.; Wang, N.; Liu, L.; Zhao, C.; Li, J.; Chen, Y.; Ma, P.; Song, D. Spindle monitor: A tool for real-time assessment and concurrent treatment of postoperative tumor prognosis. Anal. Chem. 2023, 95, 17654–17661. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Huang, X.; Zhu, Z.; Zhu, H.; Gao, Y.; Zhu, Z.; Chen, H. Cell membrane-coated gold nanoparticles for apoptosis imaging in living cells based on fluorescent determination. Microchim. Acta 2020, 187, 175. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Chu, X.; Chen, T.; Ge, J.; Yu, R. Graphene oxide–peptide conjugate as an intracellular protease sensor for caspase-3 activation imaging in live cells. Angew. Chem. Int. Ed. 2011, 50, 7065–7069. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Luo, Y.; Huang, L.; Feng, Y.; Ju, H.; Yu, B.-Y. Pegylated folate and peptide-decorated graphene oxide nanovehicle for in vivo targeted delivery of anticancer drugs and therapeutic self-monitoring. Biosens. Bioelectron. 2016, 80, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, H.; Zhang, W.; Zhao, C.; Tao, X.; Tong, C.; Liu, B. Visual monitoring of cisplatin-regulated caspase-3 activity in living cells based on a reduced graphene oxide-loaded fluorescent probe. Analyst 2024, 149, 5073–5080. [Google Scholar] [CrossRef]
- Shen, Y.; Xin, Z.; Zhu, Y.; Wang, J. Mesoporous carbon nanospheres featured multifunctional fluorescent nanoprobe: Simultaneous activation and tracing of caspase-3 involved cell apoptosis. Sens. Actuat. B Chem. 2022, 358, 131485. [Google Scholar] [CrossRef]
- Yin, X.; Yang, B.; Chen, B.; He, M.; Hu, B. Multifunctional gold nanocluster decorated metal−organic framework for real-time monitoring of targeted drug delivery and quantitative evaluation of cellular therapeutic response. Anal. Chem. 2019, 91, 10596–10603. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Qiu, Q.; Wen, Q.; Zhang, Q.; Yang, W.; Yuwen, L.; Weng, L.; Wang, L. Efficient biofunctionalization of MoS2 nanosheets with peptides as intracellular fluorescent biosensor for sensitive detection of caspase-3 activity. J. Colloid Interface Sci. 2019, 543, 96–105. [Google Scholar] [CrossRef]
- Dong, X.; Ong, S.Y.; Zhang, C.; Chen, W.; Du, S.; Xiao, Q.; Gao, L.; Yao, S.Q. Broad-spectrum polymeric nanoquencher as an efficient fluorescence sensing platform for biomolecular detection. ACS Sens. 2021, 6, 3102–3111. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Song, D.; Wang, Z. An upconversion nanoparticle-based fluorescence resonance energy transfer system for effectively sensing caspase-3 activity. Analyst 2018, 143, 761–767. [Google Scholar] [CrossRef]
- Vuojola, J.; Riuttamaki, T.; Kulta, E.; Arppe, R.; Soukka, T. Fluorescence-quenching-based homogeneous caspase-3 activity assay using photon upconversion. Anal. Chim. Acta 2012, 725, 67–73. [Google Scholar] [CrossRef]
- Valanne, A.; Malmi, P.; Appelblom, H.; Niemelä, P.; Soukka, T. A dual-step fluorescence resonance energy transfer-based quenching assay for screening of caspase-3 inhibitors. Anal. Biochem. 2008, 375, 71–81. [Google Scholar] [CrossRef] [PubMed]







| Quencher | Fluorophore | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|
| AuNPs | 5-TAMRA | 0–1 unit/mL | 0.0079 unit/mL | [55] |
| AuNPs | 5-TAMRA | 0–300 ng/mL | 73 pg/mL | [56] |
| AuNP | FAM | 10–300 ng/mL | 10 ng/mL | [57] |
| AuNPs | CdTe QDs | 27.68–129.15 pM | 18 pM | [58] |
| AuNPs | Ru@SiNPs | 0.05–1 U/mL | 0.05 U/mL | [59] |
| Au NBPs@PDA | FITC | 0–100 ng/mL | 99 pg/mL | [60] |
| AuNPs@Mem | FITC | 3.2–100 pg/mL | 1.3 pg/mL | [61] |
| GO | FAM | 7.25–362 ng/mL | 7.25 ng/mL | [62] |
| GO | FAM | 0.5–6.0 U/mL | 0.5 U/mL | [63] |
| rGO | FAM | 0.4–7 U/mL | 0.33 U/mL | [64] |
| MCN | FITC | 0.01–1 ng/mL | 0.4 pg/mL | [65] |
| MOFs | AuNCs | 0.3–150 ng/mL | 0.12 ng/mL | [66] |
| MoS2@PDA | FAM | 2–360 ng/mL | 0.33 ng/mL | [67] |
| PNPs | FAM | 2–40 nM | 0.09 nM | [68] |
| UCNPs | RB | 0.01–1000 pg/mL | 0.01 pg/mL | [69] |
| Cu-PQQ | FITC | 0.01–5 ng/mL | 7 pg/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Liu, L.; He, C.; Chang, Y.; Wang, W. A Metal–Organic Hybrid Composed of Dual Quenching Cofactors as a Nanoquencher for the Fluorescent Determination of Protease Caspase-3. Biosensors 2025, 15, 354. https://doi.org/10.3390/bios15060354
Gao F, Liu L, He C, Chang Y, Wang W. A Metal–Organic Hybrid Composed of Dual Quenching Cofactors as a Nanoquencher for the Fluorescent Determination of Protease Caspase-3. Biosensors. 2025; 15(6):354. https://doi.org/10.3390/bios15060354
Chicago/Turabian StyleGao, Fengli, Lin Liu, Cancan He, Yong Chang, and Weiqiang Wang. 2025. "A Metal–Organic Hybrid Composed of Dual Quenching Cofactors as a Nanoquencher for the Fluorescent Determination of Protease Caspase-3" Biosensors 15, no. 6: 354. https://doi.org/10.3390/bios15060354
APA StyleGao, F., Liu, L., He, C., Chang, Y., & Wang, W. (2025). A Metal–Organic Hybrid Composed of Dual Quenching Cofactors as a Nanoquencher for the Fluorescent Determination of Protease Caspase-3. Biosensors, 15(6), 354. https://doi.org/10.3390/bios15060354






