Development of a Rapid-Response Fluorescent Probe for H2S: Mechanism Elucidation and Biological Applications
Abstract
1. Introduction
2. Experimental
2.1. General
2.2. Synthetic Route and Characterization
2.3. Cell Culture and Imaging
3. Result and Discussion
3.1. Synthesis
3.2. Photophysical Properties of M1 and M2
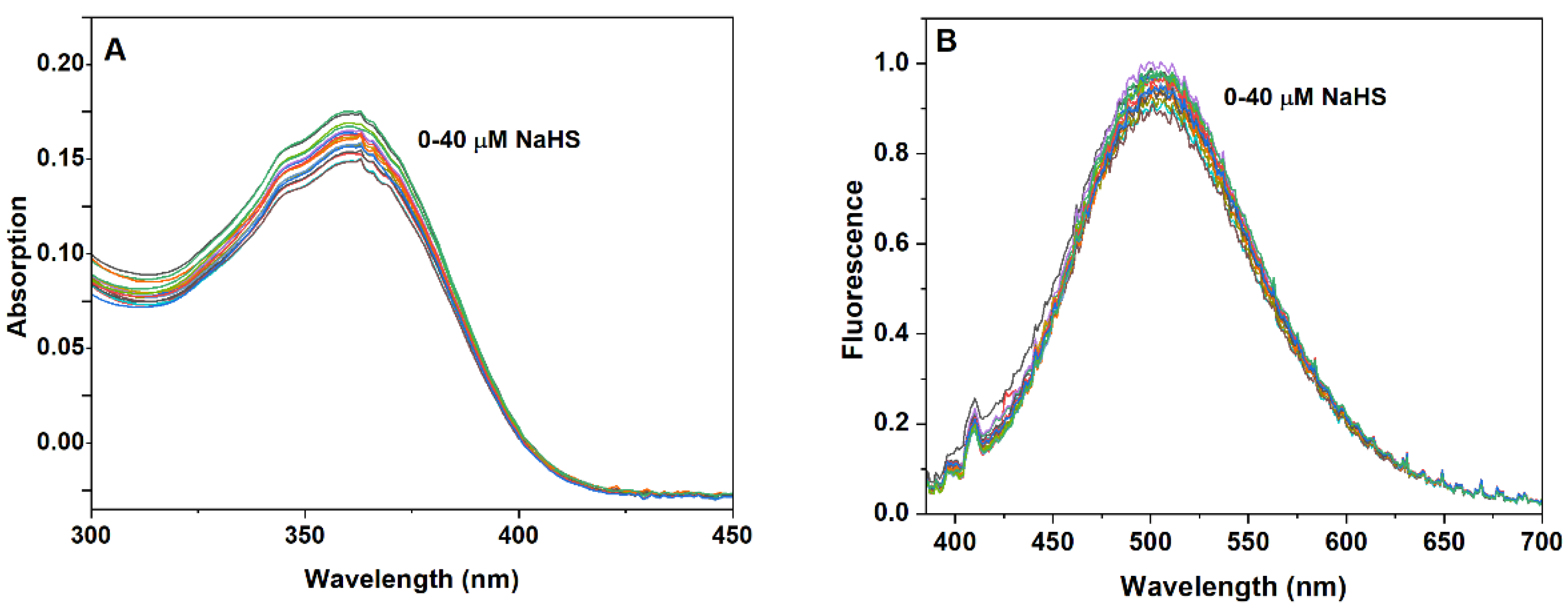
3.3. Quantitative Detection of H2S
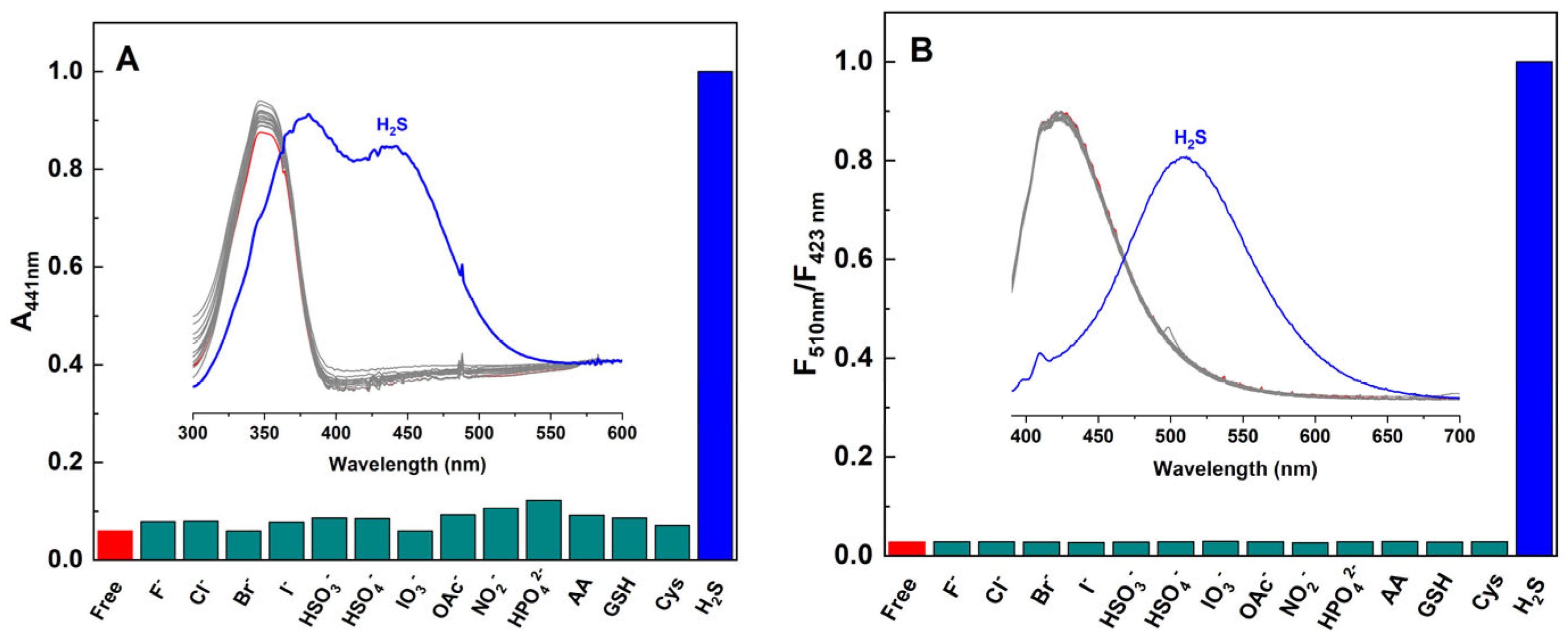
3.4. Selectivity of M1 to H2S
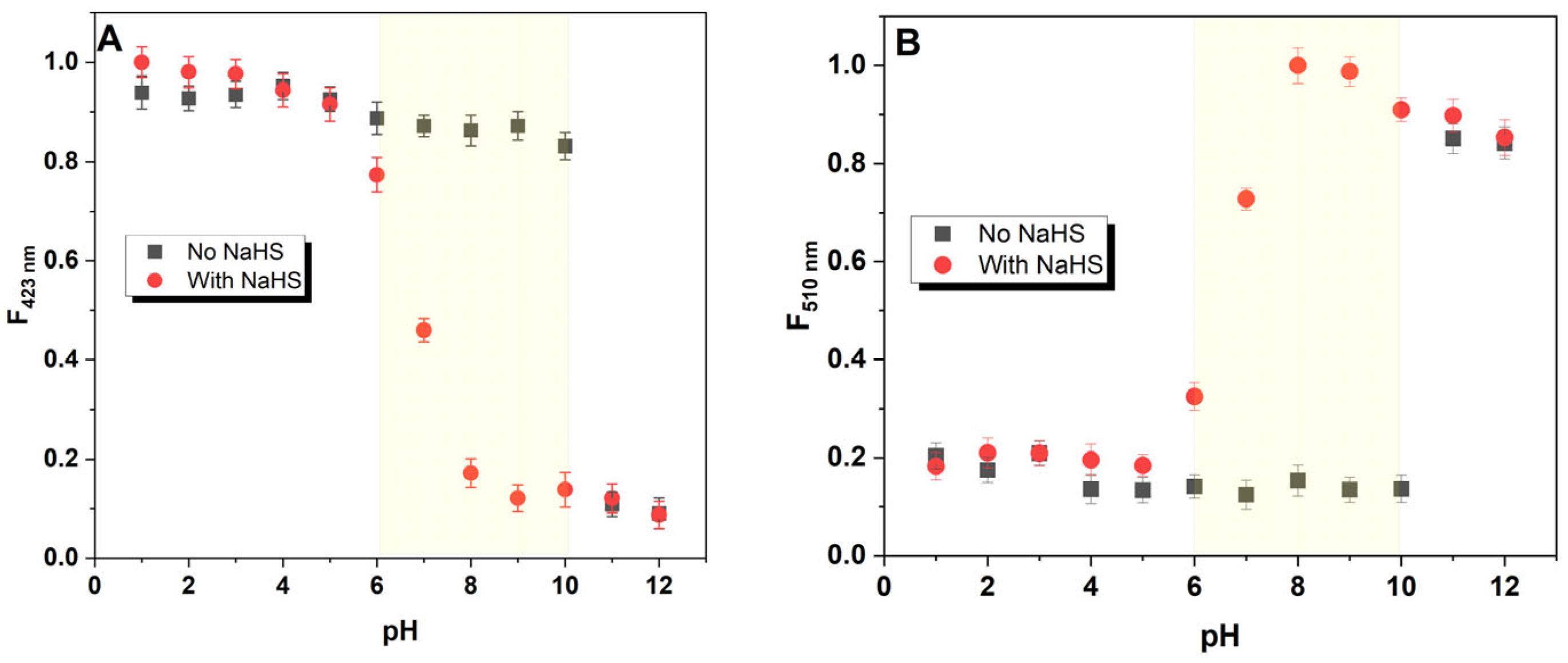
3.5. pH Effect on the Sensing Capability of M1
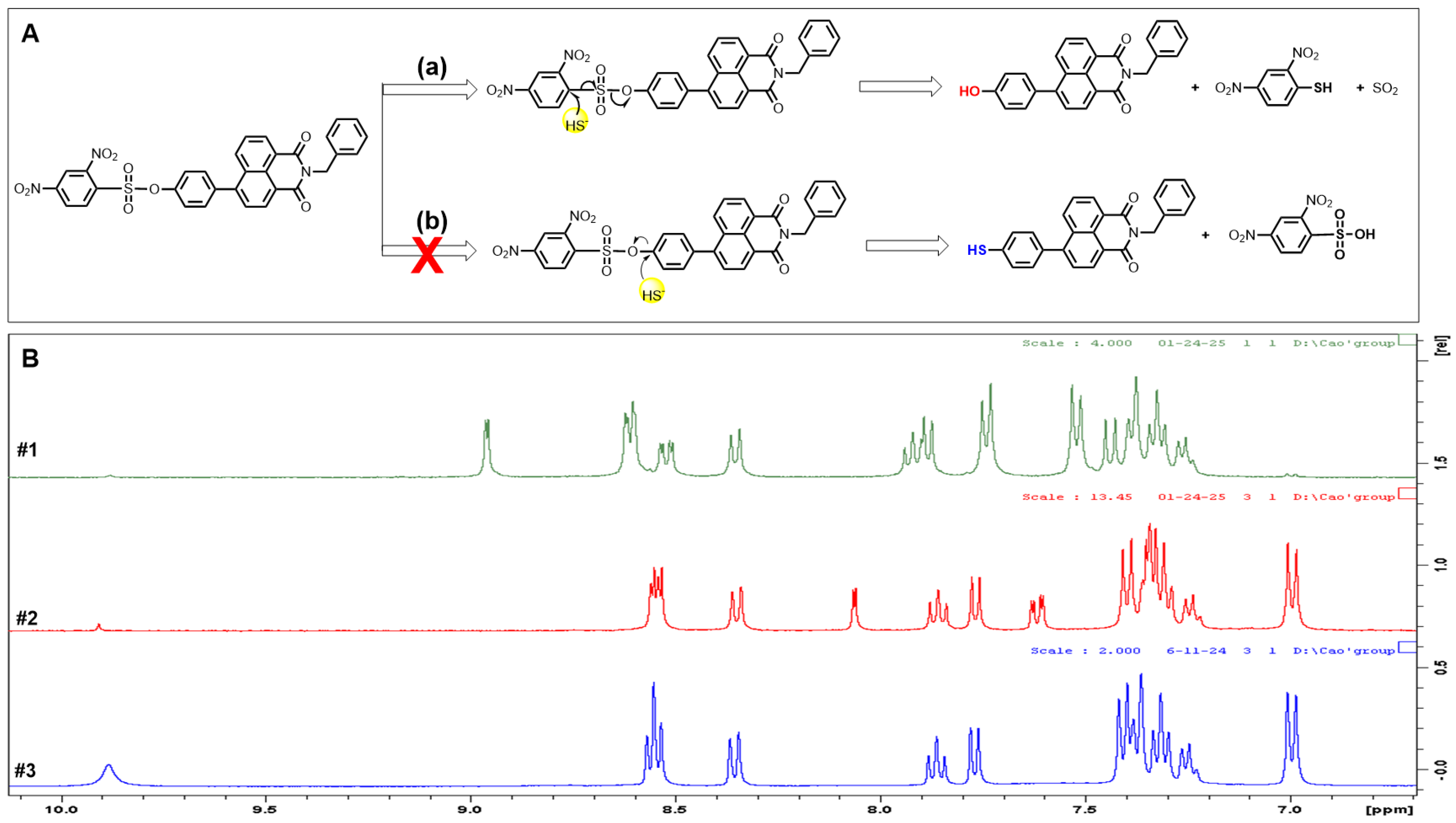
3.6. The Sensing Mechanism for H2S
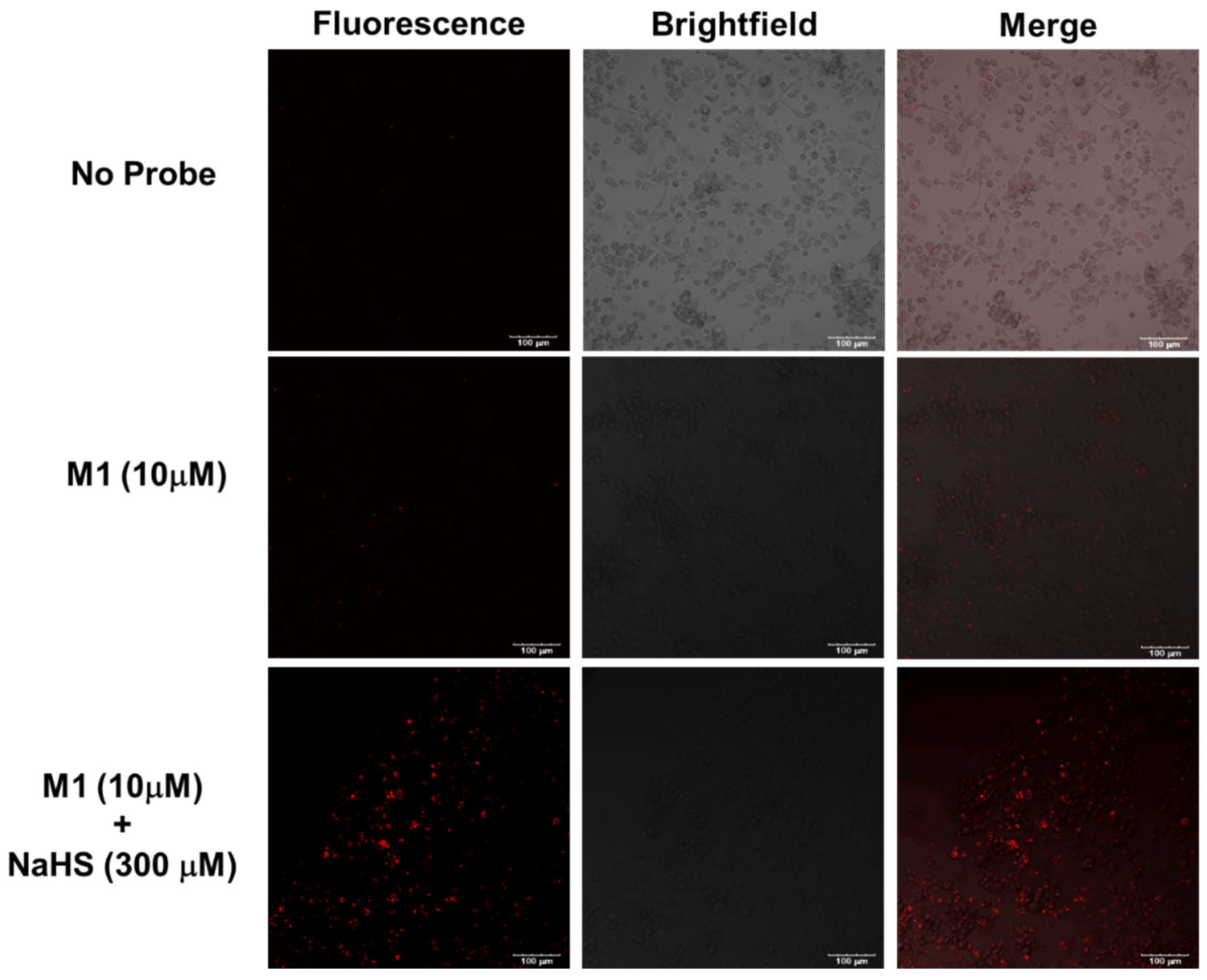
3.7. Detection of Cellular H2S in MDA-MB-231 Cells
3.8. Sensing Capability of M2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolluru, G.K.; Shackelford, R.E.; Shen, X.; Dominic, P.; Kevil, C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2023, 20, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid. Redox Signal. 2014, 20, 818–830. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Nguyen, P.L.; Park, S.H.; Jung, C.H.; Jeon, T.I. Hydrogen Sulfide and Liver Health: Insights into Liver Diseases. Antioxid. Redox Signal. 2024, 40, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J. Physiological role of hydrogen sulfide in the kidney and its therapeutic implications for kidney diseases. Biomed. Pharmacother. 2023, 166, 115396. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, R.; Matsuki, N.; Kimura, H. The Possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef]
- Whitfield, N.L.; Kreimier, E.L.; Verdial, F.C.; Skovgaard, N.; Olson, K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potentialsignificance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1930–R1937. [Google Scholar] [CrossRef]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef]
- Pozzi, G.; Gobbi, G.; Masselli, E.; Carubbi, C.; Presta, V.; Ambrosini, L.; Vitale, M.; Mirandola, P. Buffering adaptive immunity by hydrogen sulfide. Cells 2022, 11, 325. [Google Scholar] [CrossRef]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 21, 2943–2948. [Google Scholar] [CrossRef]
- Zhu, L.; Duan, W.; Wu, G.; Zhang, D.; Wang, L.; Chen, D.; Chen, Z.; Yang, B. Protective effect of hydrogen sulfide on endothelial cells through Sirt1-FoxO1-mediated autophagy. Ann. Transl. Med. 2020, 8, 1586. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lu, M.; Wu, Z.; Wong, P.T.; Bian, J. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol. Pharmacol. 2009, 75, 27–34. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 30, 5–20. [Google Scholar] [CrossRef]
- Paul, B.D.; Pieper, A.A. Neuroprotective signaling by hydrogen sulfide and its dysregulation in Alzheimer’s disease. Curr. Opin. Chem. Biol. 2024, 82, 102511. [Google Scholar] [CrossRef]
- Szabo, C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2012, 17, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.Q.; Chen, H.J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.Y.; et al. Hydrogen sulfide biology and its role in cancer. Molecules 2022, 25, 3389. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, Y.; Wang, K.; Luo, C.; Xie, M.; Shi, X.; Lin, X. Review of Chemical Sensors for Hydrogen Sulfide Detection in Organisms and Living Cells. Sensors 2023, 23, 3316. [Google Scholar] [CrossRef]
- Wang, K.; Bi, C.; Zelenkov, L.; Liu, X.; Song, M.; Wang, W.; Makarov, S.; Yin, W. Fluorescent sensing for the detection and quantification of sulfur-containing gases. ACS Sens. 2024, 9, 5708–5727. [Google Scholar] [CrossRef]
- Fosnacht, K.G.; Pluth, M.D. Activity-based fluorescent probes for hydrogen sulfide and related reactive sulfur species. Chem. Rev. 2024, 124, 4124–4257. [Google Scholar] [CrossRef]
- Ranjana, M.; Kulkarni, R.M.; Sunil, D. Small molecule optical probes for detection of H2S in water samples: A review. ACS Omega 2024, 21, 14672–14691. [Google Scholar]
- May, P.M.; Batka, D.; Hefter, G.; Königsberger, E.; Rowland, D. Goodbye to S2- in Aqueous Solution. Chem. Commun. 2018, 54, 1980–1983. [Google Scholar] [CrossRef]
- Peng, H.; Cheng, Y.; Dai, C.; King, A.L.; Predmore, B.L.; Lefer, D.J.; Wang, B. A fluorescent probe for fast and quantitative detection of hydrogen sulfide in blood. Angew. Chem. Int. Ed. 2011, 50, 9672–9675. [Google Scholar] [CrossRef] [PubMed]
- Montoya, L.A.; Pluth, M.D. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun. 2012, 48, 4767–4769. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Ghosh, A.; Jose, D.A. Recent progress in hydrogen sulphide (H2S) sensors by metal displacement approach. Coord. Chem. Rev. 2017, 347, 141–157. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.; Hou, P. A novel imidazo [1,5-A] pyridine-based fluorescent probe with a large stokes shift for imaging hydrogen sulfide. Sens. Actuators B 2018, 256, 1086–1092. [Google Scholar] [CrossRef]
- Henthorn, H.A.; Pluth, M.D. Mechanistic insights into the H2S-mediated reduction of aryl azides commonly used in H2S detection. J. Am. Chem. Soc. 2015, 137, 15330–15336. [Google Scholar] [CrossRef]
- Zhu, T.; Ren, N.; Liu, X.; Dong, Y.; Wang, R.; Gao, J.; Sun, J.; Zhu, Y.; Wang, L.; Fan, C.; et al. Probing the intracellular dynamics of nitric oxide and hydrogen sulfide using an activatable NIR II fluorescence reporter. Angew. Chem. Int. Ed. 2021, 60, 8450–8454. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, P.; Wang, H.; Yu, M.; Gao, Y.; Chen, J. Photoswitchable AIE nanoprobe for lysosomal hydrogen sulfide detection and reversible dual-color Imaging. Sens. Actuators B 2018, 272, 340–347. [Google Scholar] [CrossRef]
- Ismail, I.; Chen, Z.; Ji, X.; Sun, L.; Yi, L.; Xi, Z. A fast-response red shifted fluorescent probe for detection of H2S in living cells. Molecules 2020, 25, 437. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, W.; Li, J.; Zhang, J.; Du, L.; Li, M. Naphthalimide-based fluorescent off/on probes for the detection of thiols. Tetrahedron 2012, 68, 5363–5367. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, J.; Wang, Z.; Tao, F.; Yang, L.; Yuan, G.; Sun, W.; Zhang, X. A color turn-on fluorescent probe for real-time detection of hydrogen sulfide and identification of food spoilage. Chem. Commun. 2021, 57, 5012–5015. [Google Scholar] [CrossRef]
- Echizen, H.; Hanaoka, K. Recent advances in probe design to detect reactive sulfur species and in the chemical reactions employed for fluorescence switching. J. Clin. Biochem. Nutr. 2021, 68, 9–17. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Zhao, Q.; Tang, C.; Zhang, H.; Qin, Y.; Feng, X.; Zhang, J. Fluorescent probes based on nucleophilic aromatic substitution reactions for reactive sulfur and selenium species: Recent progress, applications, and design strategies. Coord. Chem. Rev. 2021, 427, 213601. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ma, X.; Zhou, H.; Chen, J.; Li, M.; Yang, J.; Bai, H.; She, M. Construction of Dcm-Based NIR fluorescent probe for visualization detection of H2S in solution and nanofibrous film. Spectrochim. Acta Part A 2021, 257, 119764. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef]
- Wan, D.; Pan, T.; Ou, P.; Zhou, R.; Ouyang, Z.; Luo, L.; Xiao, Z.; Peng, Y. Construct a lysosome-targeting and highly selective fluorescent probe for imaging of hydrogen sulfide in living cells and inflamed tissues. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119311. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Yang, S.; Tian, H.; Liang, S.; Sun, B. Dual-function fluorescent probe for detection of hydrogen sulfide and water content in dimethyl sulfoxide. ACS Omega 2019, 4, 10695–10701. [Google Scholar] [CrossRef]
- Shang, Z.; Meng, Q.; Tian, D.; Wang, Y.; Zhang, Z.; Zhang, Z.; Zhang, R. Red-emitting fluorescent probe for hydrogen sulfide detection and its applications in food freshness determination and in vivo bioimaging. Food Chem. 2023, 427, 136701. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, X.; Zhang, K.; Liu, K.; Li, Z.; Zhao, L. Hydrogen sulfide detection and zebrafish imaging by a designed sensitive and selective fluorescent probe based on resorufin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120265. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xiao, Q.; Wang, L.; Yang, Y. A new fluorescent probe for hydrogen sulfide detection in solution and living cells. Molecules 2023, 28, 6195. [Google Scholar] [CrossRef] [PubMed]


| M1 | M2 | |||||||
|---|---|---|---|---|---|---|---|---|
| λab | ε | λem | Φ | λab | ε | λem | Φ | |
| (nm) | (M−1 cm−1) | (nm) | (nm) | (M−1 cm−1) | (nm) | |||
| DMSO | 352 | 14,220 | 422 | 0.007 | 363 | 18,720 | 483 | 0.008 |
| MeCN | 345 | 19,440 | 423 | 0.027 | 361 | 23,220 | 478 | 0.010 |
| Acetone | 345 | 19,260 | 492 | 0.042 | 357 | 24,180 | 425 | 0.002 |
| MeOH | 348 | 21,180 | 431 | 0.028 | 359 | 11,820 | 436 | 0.037 |
| THF | 346 | 20,520 | 474 | 0.077 | 355 | 25,020 | 421 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvorak, T.; Hernandez-Sandoval, H.; Cheku, S.; Mora Valencia González, M.; Borer, L.; Grieser, R.; Carlson, K.A.; Cao, H. Development of a Rapid-Response Fluorescent Probe for H2S: Mechanism Elucidation and Biological Applications. Biosensors 2025, 15, 174. https://doi.org/10.3390/bios15030174
Dvorak T, Hernandez-Sandoval H, Cheku S, Mora Valencia González M, Borer L, Grieser R, Carlson KA, Cao H. Development of a Rapid-Response Fluorescent Probe for H2S: Mechanism Elucidation and Biological Applications. Biosensors. 2025; 15(3):174. https://doi.org/10.3390/bios15030174
Chicago/Turabian StyleDvorak, Trevor, Haley Hernandez-Sandoval, Sunayn Cheku, Marijose Mora Valencia González, Linus Borer, Riley Grieser, Kimberly A. Carlson, and Haishi Cao. 2025. "Development of a Rapid-Response Fluorescent Probe for H2S: Mechanism Elucidation and Biological Applications" Biosensors 15, no. 3: 174. https://doi.org/10.3390/bios15030174
APA StyleDvorak, T., Hernandez-Sandoval, H., Cheku, S., Mora Valencia González, M., Borer, L., Grieser, R., Carlson, K. A., & Cao, H. (2025). Development of a Rapid-Response Fluorescent Probe for H2S: Mechanism Elucidation and Biological Applications. Biosensors, 15(3), 174. https://doi.org/10.3390/bios15030174







