Molecularly Imprinted SERS Plasmonic Sensor for the Detection of Malachite Green
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Construction of the MI-SERS Sensor
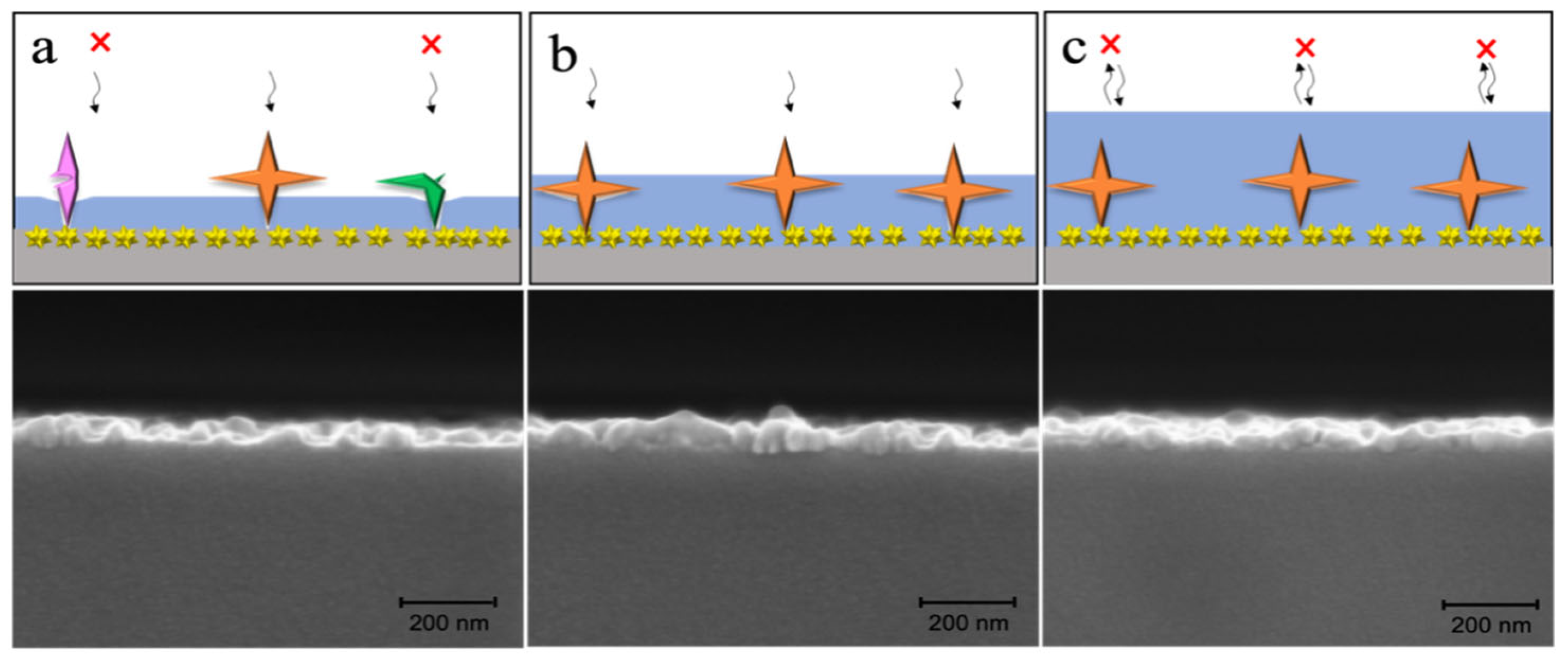
2.3.1. Functionalization of Glass Capillaries
2.3.2. Preparation of the SERS Substrate
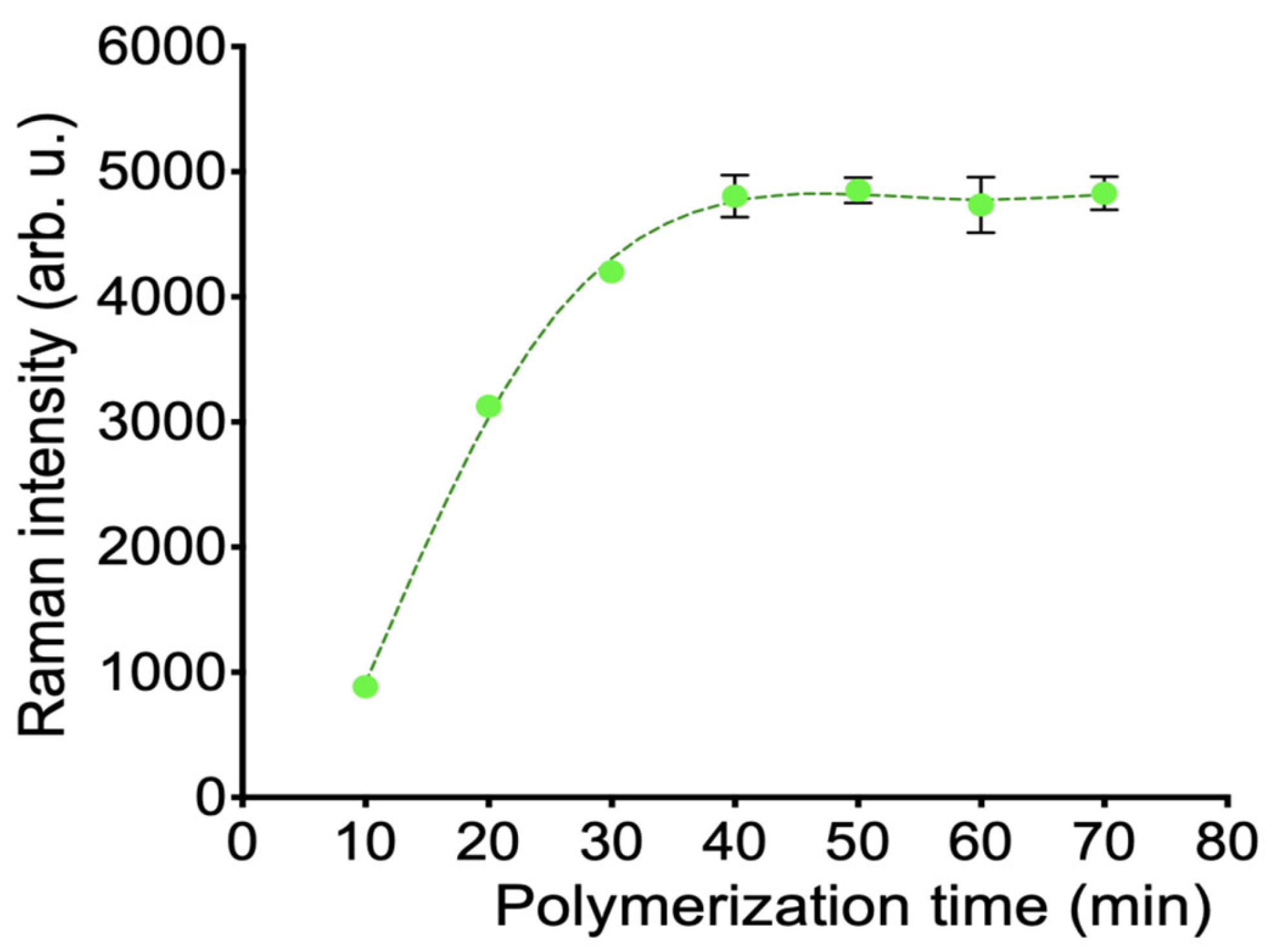
2.3.3. Preparation of the Polydopamine-Imprinted Layer
2.4. Performance Testing of the MI-SERS Sensor
3. Results and Discussion
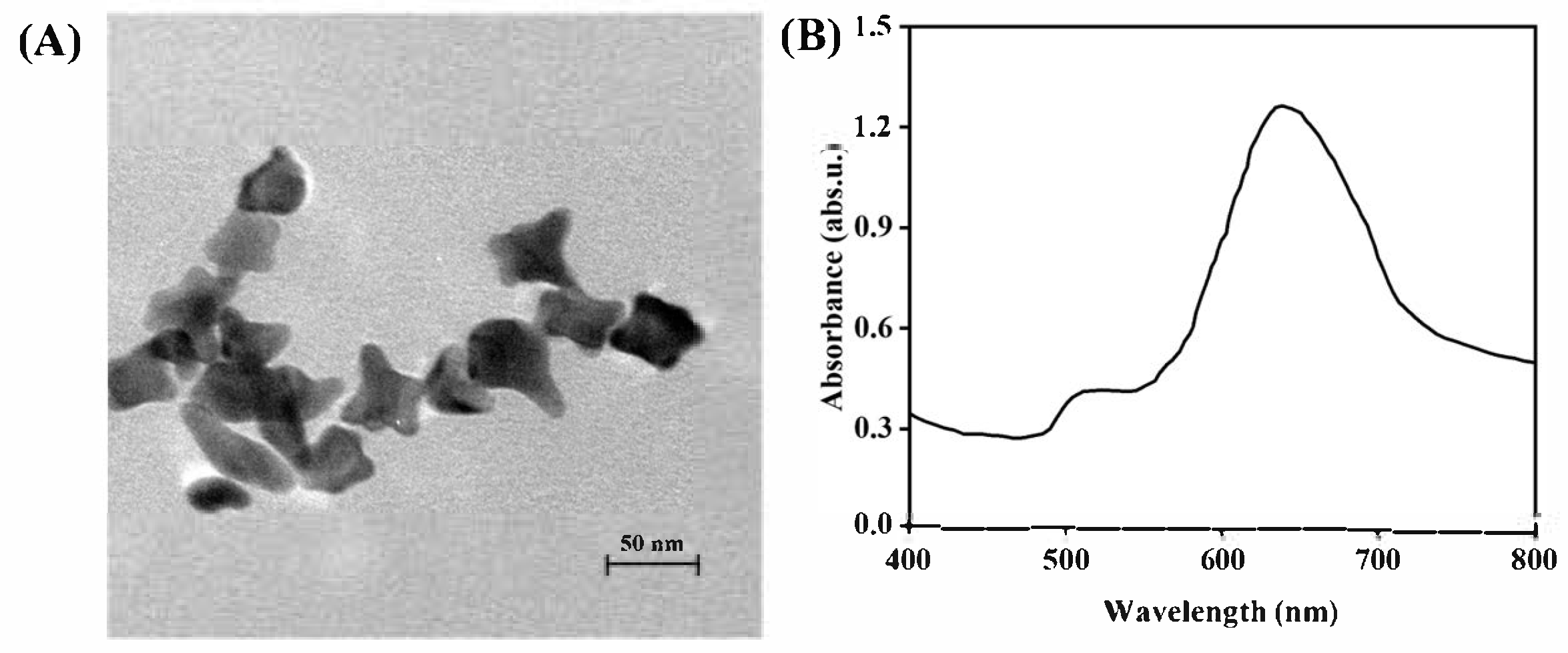
3.1. Construction and Condition Optimization of MI-SERS Sensor
3.2. Selectivity and Anti-Interference Ability of the MI-SERS Sensor
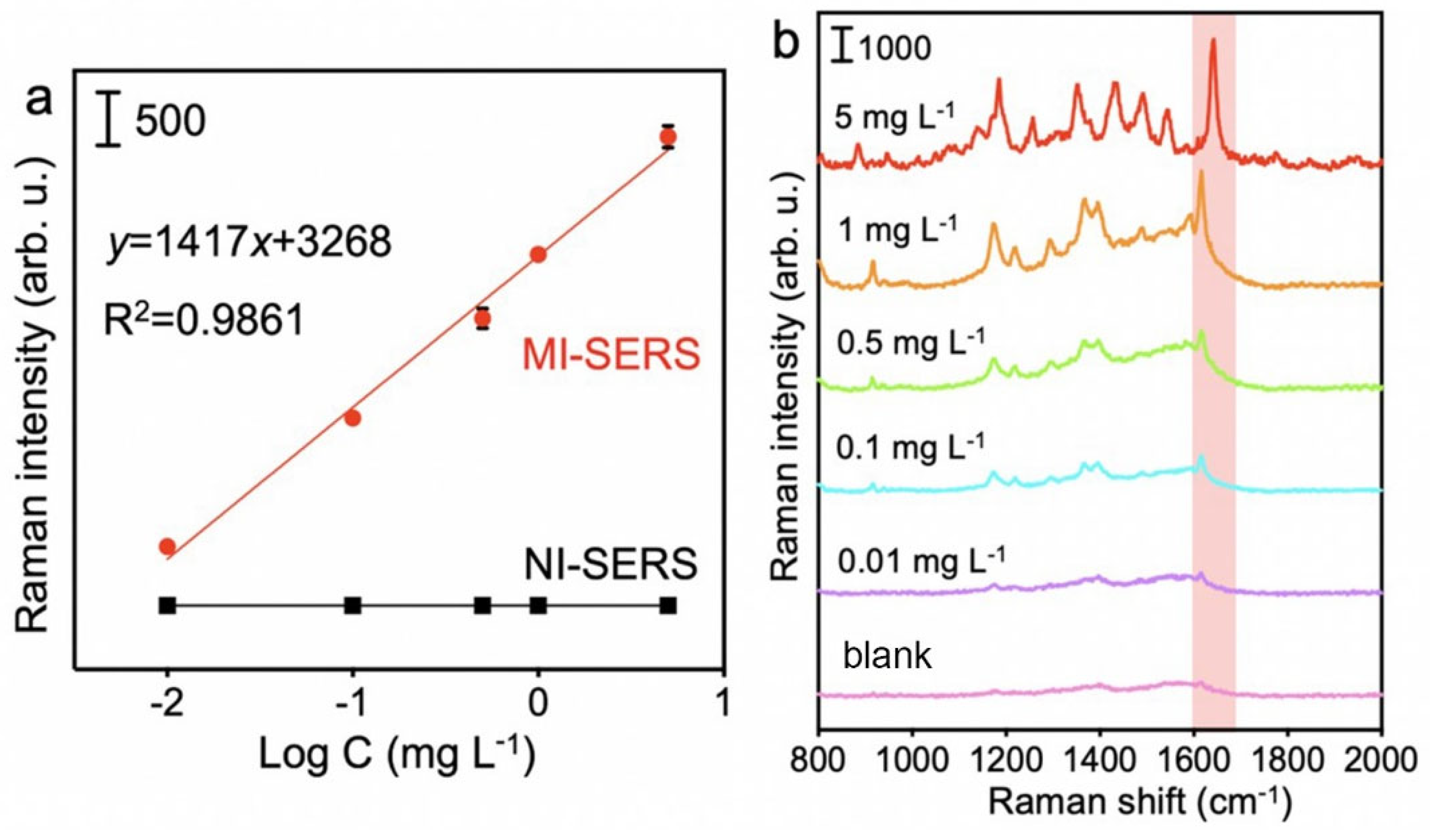
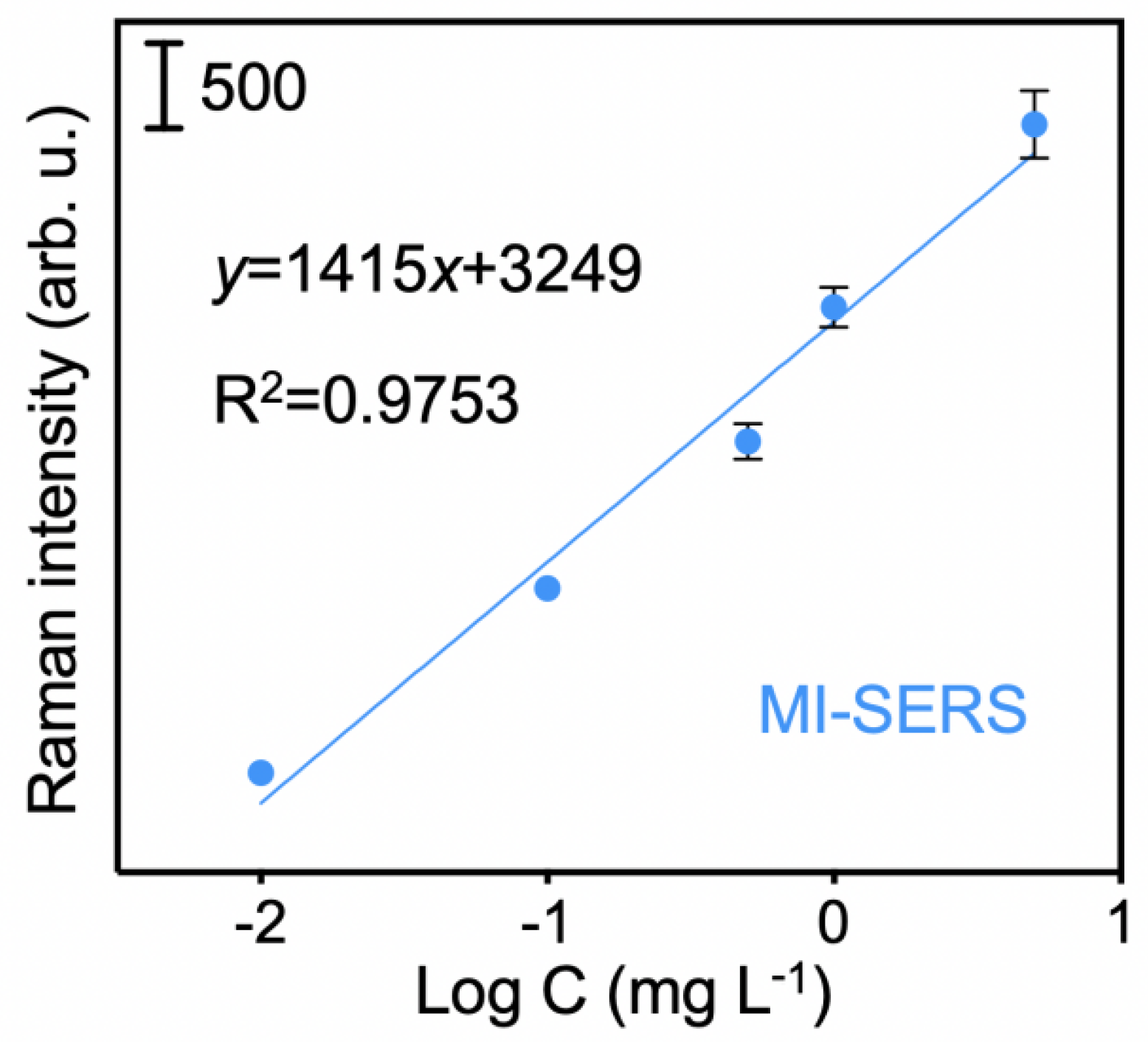
3.3. Sensitivity of the MI-SERS Sensor
3.4. Reproducibility and Stability of the MI-SERS Sensor
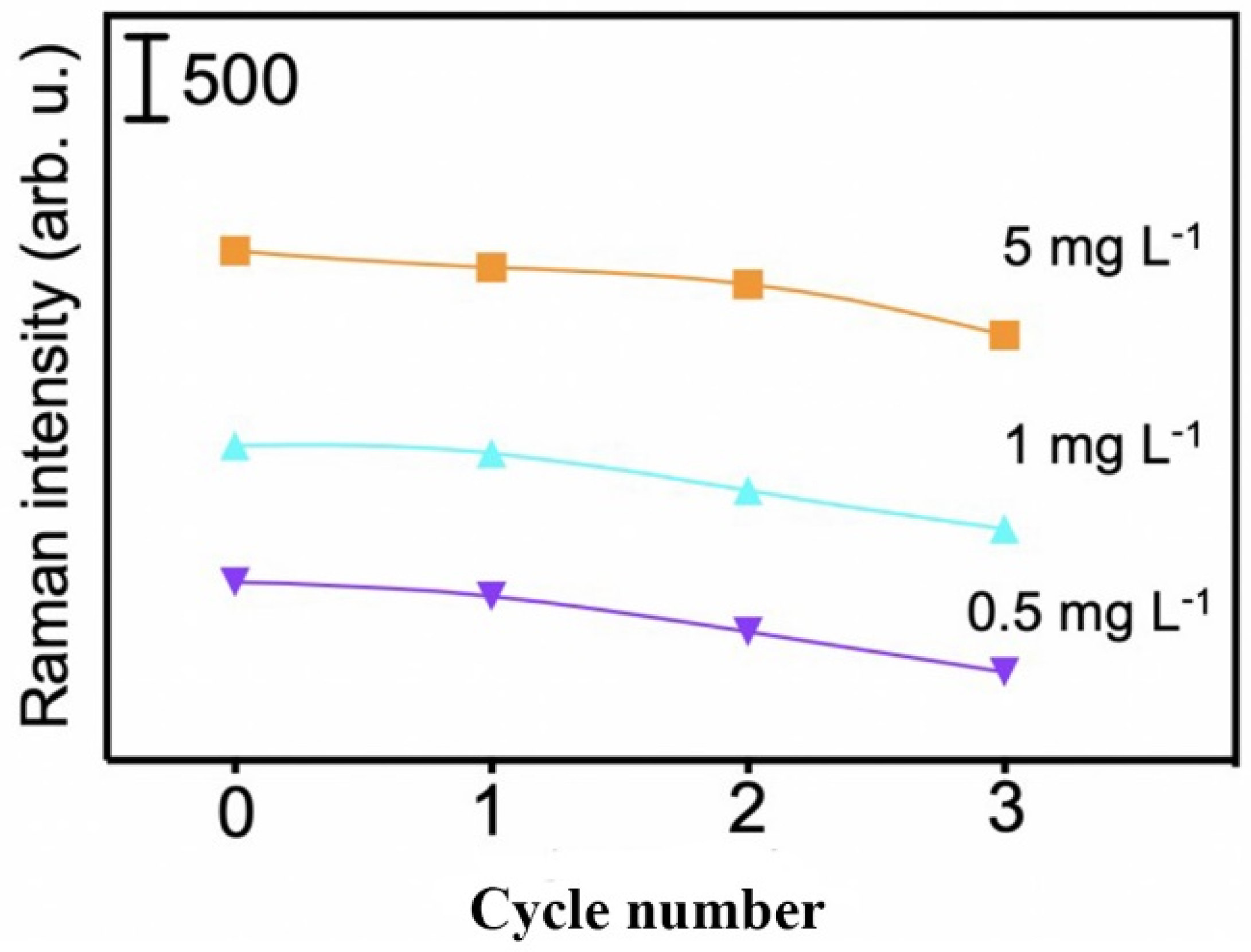
3.5. The Reusability of the MI-SERS Sensor
3.6. Application of the MI-SERS Sensor to Actual Seawater Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.H.; Zhang, J.R.; Pan, Z.L.; Li, D.L. Review of methods for the detection and determination of malachite green and leuco-malachite green in aquaculture. Crit. Rev. Anal. Chem. 2018, 49, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Wang, C.L.; Wei, G.; Wang, X.S.; Liu, W.H.; Yang, G.Q.; Zhang, P.; Li, Q.L.; Geng, X.H.; Chen, L.X.; et al. Facile fluorescence detection of malachite green in fish using molecularly imprinted polymers doped CdTe quantum dots based system. Food Chem. 2024, 442, 138458. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.Y.; Mokhtar, Z.; Hasan, M.; Halim, H.N.A. Integrated adsorption and photocatalytic degradation of malachite green using carbon based nanocomposites (ZnO-AC). IOP Conf. Ser. Earth Environ. Sci. 2023, 1135, 012005. [Google Scholar] [CrossRef]
- Hussain Hakami, A.A.; Ahmed, M.A.; Khan, M.A.; Alothman, Z.A.; Rafatullah, M.; Islam, M.A.; Siddiqui, M.R. Quantitative analysis of malachite green in environmental samples using liquid chromatography-mass spectrometry. Water 2021, 13, 2864. [Google Scholar] [CrossRef]
- Uzcan, F.; Erbas, Z.; Soylak, M. Supramolecular solvent-based liquid phase microextraction of malachite green at trace level from water samples for its UV–vis spectrophotometric detection. Int. J. Environ. Anal. Chem. 2019, 99, 595–605. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, D.Q.; Yang, Y.L. Magnetic solid-phase extraction for determination of the total malachite green, gentian violet and leucomalachite green, leucogentian violet in aquaculture water by high-performance liquid chromatography with fluorescence detection. J. Sep. Sci. 2016, 39, 2347–2355. [Google Scholar] [CrossRef]
- Ge, K.; Hu, Y.L.; Li, G.K. Recent progress on solid substrates for surface-enhanced Raman spectroscopy analysis. Biosensors 2022, 12, 941. [Google Scholar] [CrossRef]
- Kumar, P.; Khosla, R.; Soni, M.; Deva, D.; Sharma, S.K. A highly sensitive, flexible SERS sensor for malachite green detection based on Ag decorated microstructured PDMS substrate fabricated from Taro leaf as template. Sens. Actuators B 2017, 246, 477–486. [Google Scholar] [CrossRef]
- Gao, M.; Yao, J.C.; Quan, Y.N.; Yang, J.H.; Huo, P.W.; Dai, J.D.; Yan, Y.S.; Ma, C.C. Neodymium doped zinc oxide for ultersensitive SERS substrate. J. Mater. Sci. Mater. Electron. 2019, 30, 20537–20543. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Stevenson, R.; Stokes, R.J.; Macmillan, D.; Armstrong, D.; Faulds, K.; Wadsworth, R.; Kunuthur, S.; Suckling, C.J.; Graham, D. In situ detection of pterins by SERS. Analyst 2009, 134, 1561. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.B.; Zhou, F.; Chu, R.; Ye, H.; Zhang, C.; Shui, L.L.; Liu, Y. Rapid and sensitive in-situ detection of pesticide residues in real tea soup with optical fiber SERS probes. J. Food Compos. Anal. 2024, 134, 106520. [Google Scholar] [CrossRef]
- Lu, Y.D.; Wu, C.J.; Wu, Y.; You, R.Y.; Lin, G.; Chen, Y.Q.; Feng, S.Y. Ag-coated cellulose fibers as surface-enhanced Raman scattering substrates for adsorptive detection of malachite green. Materials 2018, 11, 1197. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, W.S.; Pei, L.; Lai, K.Q.; Rasco, B.A.; Huang, Y.Q. Rapid analysis of malachite green and leucomalachite green in fish muscles with surface-enhanced resonance Raman scattering. Food Chem. 2015, 169, 80–84. [Google Scholar] [CrossRef]
- Sun, H.B.; Liu, H.; Wu, Y.Y. A flexible and highly sensitive surface-enhanced Raman scattering film in-situ detection of malachite green on fish skin. Mater. Lett. 2017, 207, 125–128. [Google Scholar] [CrossRef]
- Dykstra, H.M.; Hall, S.B.; Waterland, M.R. A spectroelectrochemical investigation of nanoparticle and molecular resonances in surface enhanced Raman scattering from crystal violet and malachite green. J. Raman Spectrosc. 2016, 48, 405–412. [Google Scholar] [CrossRef]
- Wang, C.Q.; Xiao, L.F.; Dai, C.; Nguyen, A.H.; Littlepage, L.E.; Schultz, Z.D.; Li, J. A statistical approach of background removal and spectrum identification for SERS data. Sci. Rep. 2020, 10, 1460. [Google Scholar] [CrossRef]
- Shu, Y.Y.; Li, J.J.; Bai, H.Y.; Liang, A.H.; Wen, G.Q.; Jiang, Z.L. A new SERS quantitative analysis method for trace malathion with recognition and catalytic amplification difunctional MOFTb@Au@MIP nanoprobe. Talanta 2024, 267, 125166. [Google Scholar] [CrossRef]
- Chen, X.; Ostovan, A.; Arabi, M.; Wang, Y.; Chen, L.; Li, J. Molecular imprinting-based SERS detection strategy for the large-size protein quantitation and curbing non-specific recognition. Anal. Chem. 2024, 9616, 6417–6425. [Google Scholar] [CrossRef]
- Zare, E.N.; Fallah, Z.; Le, V.T.; Doan, V.-D.; Mudhoo, A.; Joo, S.-W.; Vasseghian, Y.; Tajbakhsh, M.; Moradi, O.; Sillanpää, M.; et al. Remediation of pharmaceuticals from contaminated water by molecularly imprinted polymers: A review. Environ. Chem. Lett. 2022, 20, 2629–2664. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, H.; Lian, Z. Sensitive detection of okadaic acid in marine sediment using magnetic imprinted fluorescent nanoprobe. Microchem. J. 2024, 207, 111952. [Google Scholar] [CrossRef]
- Zhao, J.L.; He, H.; Guo, Z.C.; Liu, Z. Molecularly imprinted and cladded nanoparticles provide better phosphorylation recognition. Anal. Chem. 2021, 93, 16194–16202. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.L.; Wang, H.W.; Huang, C.; Bo, C.M.; Gong, B.L.; Ou, J.J. Progress of molecular imprinting technique for enantioseparation of chiral drugs in recent ten years. J. Chromatogr. A 2022, 1668, 462914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Li, S. Molecularly imprinted polymers-surface-enhanced Raman spectroscopy: State of the art and prospects. Int. J. Environ. Anal. Chem. 2020, 102, 1385–1415. [Google Scholar] [CrossRef]
- Ma, X.; Knowles, J.C.; Poma, A. Biodegradable and sustainable synthetic antibodies—A perspective. Pharmaceutics 2023, 15, 1440. [Google Scholar] [CrossRef]
- Hrelescu, C.; Sau, T.K.; Rogach, A.L.; JäCkel, F.; Laurent, G.; Douillard, L.; Charra, F. Selective excitation of individual plasmonic hotspots at the tips of single gold nanostars. Nano Lett. 2011, 11, 402–407. [Google Scholar] [CrossRef]
- Ma, T.; Yang, W.S.; Liu, S.M.; Zhang, H.J.; Liang, F. A comparison reduction of 4-nitrophenol by gold nanospheres and gold nanostars. Catalysts 2017, 7, 38. [Google Scholar] [CrossRef]
- Palladino, P.; Bettazzi, F.; Scarano, S. Polydopamine: Surface coating, molecular imprinting, and electrochemistry—Successful applications and future perspectives in (bio)analysis. Anal. Bioanal. Chem. 2019, 411, 4327–4338. [Google Scholar] [CrossRef]
- Hu, M.H.; Huang, P.C.; Suo, L.L.; Wu, F.Y. Polydopamine-based molecularly imprinting polymers on magnetic nanoparticles for recognition and enrichment of ochratoxins prior to their determination by HPLC. Microchim. Acta 2018, 185, 300. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Wang, Y.Q.; Mei, R.C.; Fu, L.W.; Li, J.H.; Wang, X.Y.; Chen, L.X. Chiral molecular imprinting-based SERS detection strategy for absolute enantiomeric discrimination. Nat. Commun. 2022, 13, 5757. [Google Scholar] [CrossRef]
- Xia, Y.-F.; Yuan, H.-Q.; Qiao, C.; Li, W.; Wang, R.; Chen, P.Y.; Li, Y.-X.; Bao, G.-M. Multifunctional Eu3+-MOF for simultaneous quantification of malachite green and leuco-malachite green and efficient adsorption of malachite green. J. Hazard. Mater. 2024, 465, 133386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Hou, Z.H.; Lian, Z.R.; Qin, D.; Ge, C.Z. Direct extraction and detection of malachite green from marine sediments by magnetic nano-sized imprinted polymer coupled with spectrophotometric analysis. Mar. Pollut. Bull. 2020, 158, 111363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luan, L.; Huang, Y.; Yao, M.; Li, P.; Xu, W. Mussel-inspired PDA-based MIP-SERS sensor for the detection of trace MG in environmental water. Analyst 2022, 147, 5701–5709. [Google Scholar] [CrossRef] [PubMed]




| Sampling Site | Spike (µg L−1) | Detection (µg L−1) ± SD | Recovery Rate (%) | RSD |
|---|---|---|---|---|
| Yantai University Beach | 5 | 5.57 ± 0.67 | 111.4 | 12.03% |
| 1 | 1.14 ± 0.18 | 114.2 | 15.79% | |
| 0.1 | 0.09 ± 0.01 | 90.2 | 11.11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Sun, D.; Wen, Y.; Wang, M.; Huang, J.; Lian, Z.; Li, J. Molecularly Imprinted SERS Plasmonic Sensor for the Detection of Malachite Green. Biosensors 2025, 15, 329. https://doi.org/10.3390/bios15050329
Zhang H, Sun D, Wen Y, Wang M, Huang J, Lian Z, Li J. Molecularly Imprinted SERS Plasmonic Sensor for the Detection of Malachite Green. Biosensors. 2025; 15(5):329. https://doi.org/10.3390/bios15050329
Chicago/Turabian StyleZhang, Hao, Dani Sun, Yuhao Wen, Mengyuan Wang, Jingying Huang, Ziru Lian, and Jinhua Li. 2025. "Molecularly Imprinted SERS Plasmonic Sensor for the Detection of Malachite Green" Biosensors 15, no. 5: 329. https://doi.org/10.3390/bios15050329
APA StyleZhang, H., Sun, D., Wen, Y., Wang, M., Huang, J., Lian, Z., & Li, J. (2025). Molecularly Imprinted SERS Plasmonic Sensor for the Detection of Malachite Green. Biosensors, 15(5), 329. https://doi.org/10.3390/bios15050329






