The Biomodification and Biomimetic Synthesis of 2D Nanomaterial-Based Nanohybrids for Biosensor Applications: A Review
Abstract
1. Introduction
2. Biomodification of 2DNMs
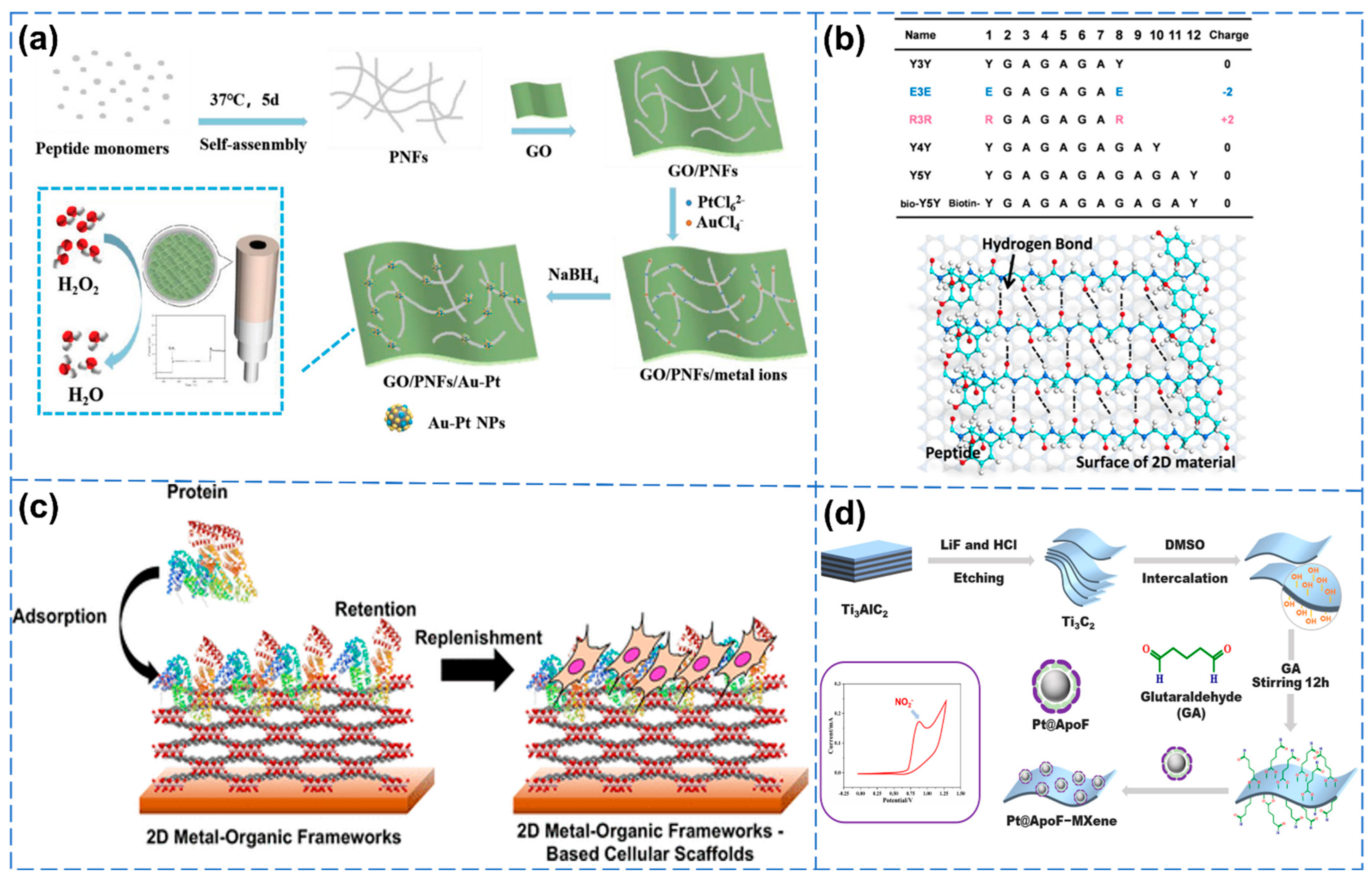
2.1. Peptides
2.2. Proteins
2.3. DNA/RNA
2.4. Bio-Enzymes
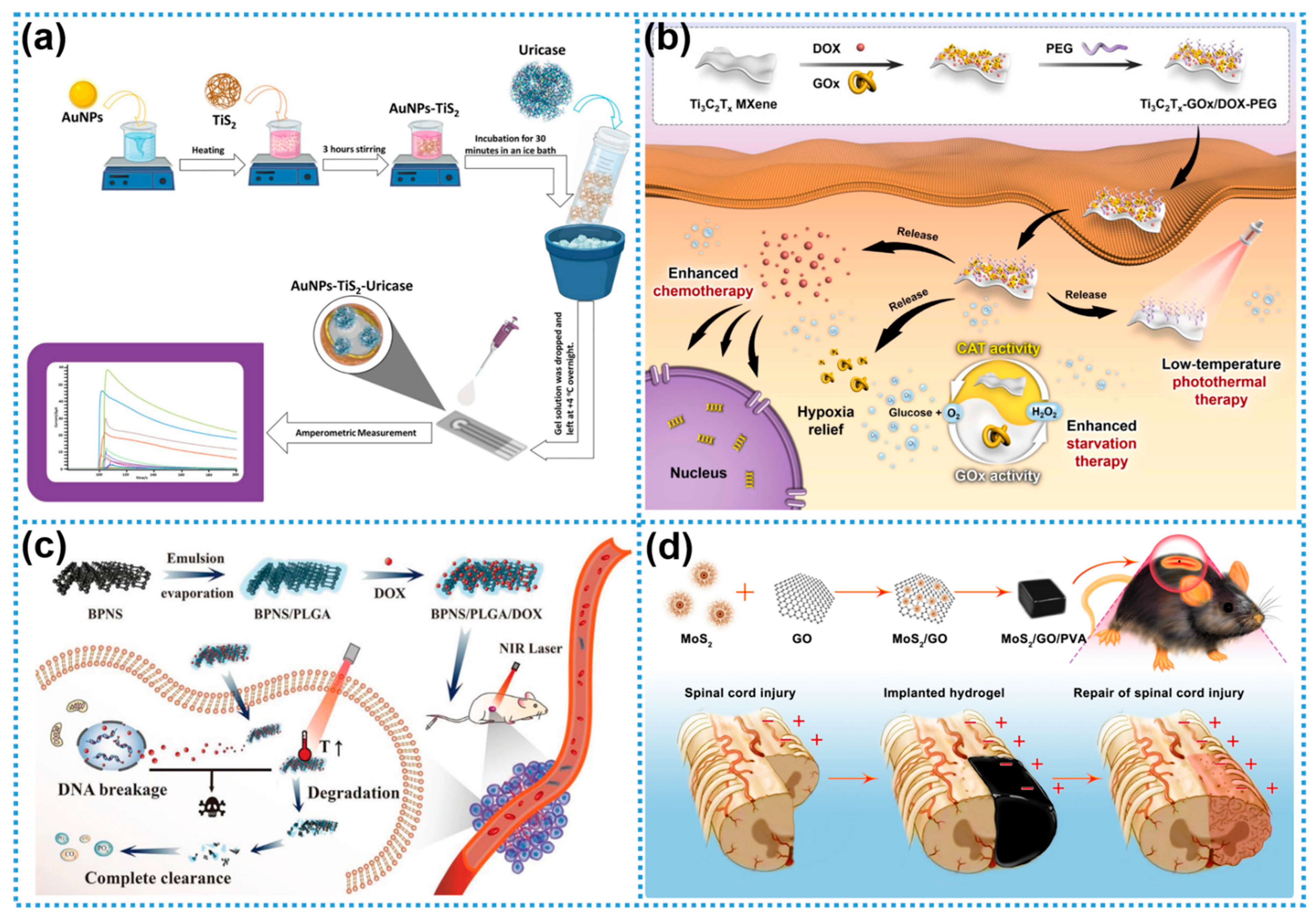
2.5. Biopolymers
2.6. Bioactive Polysaccharides
2.7. Biomolecule Nanointerface Engineering
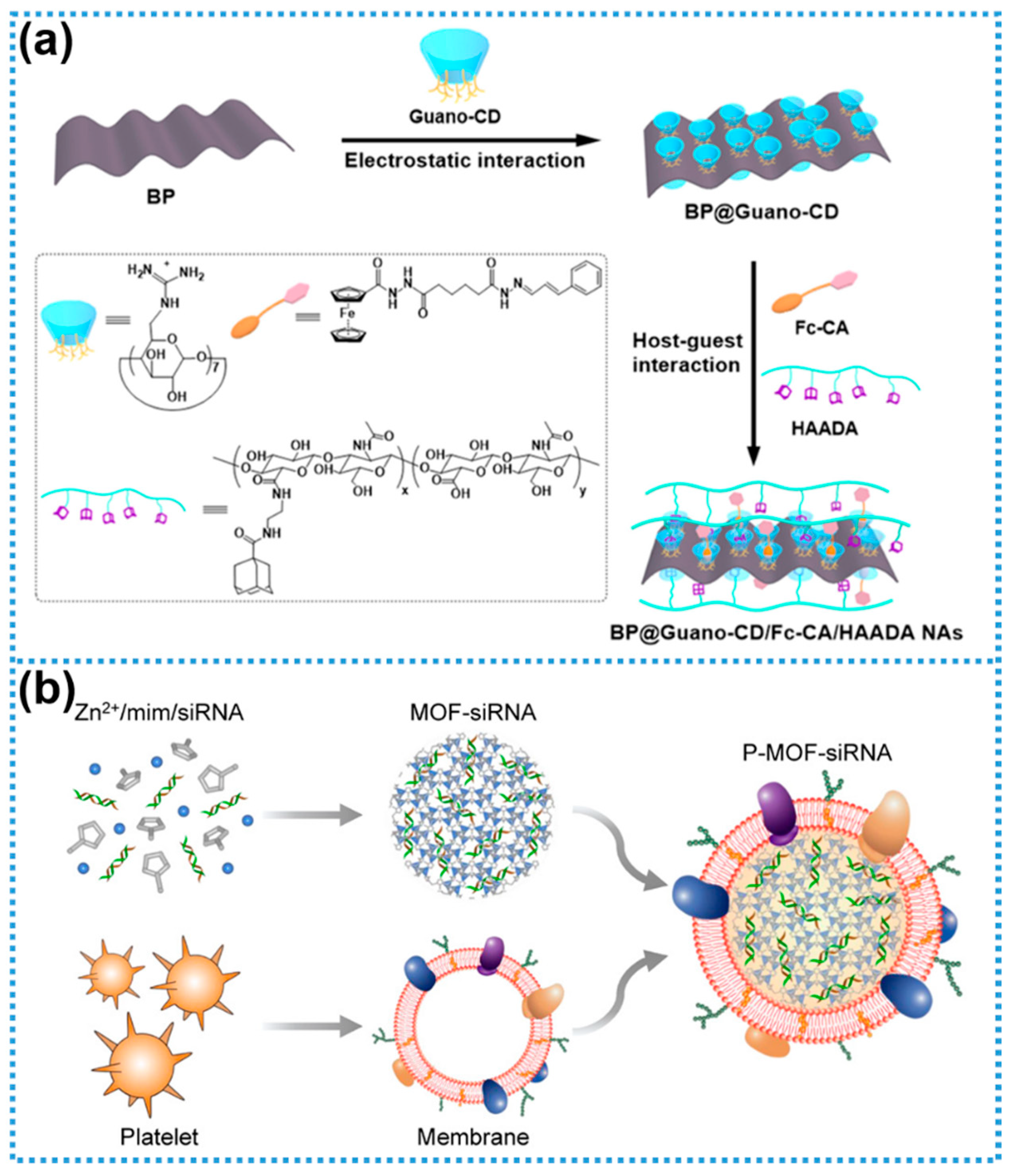
2.8. Summary of Biomodification
3. Biomimetic Synthesis of 2DNMs
3.1. Bio-Templating
3.2. Biomolecule-Directed Self-Assembly
3.3. Biomineralization Strategies
3.4. Biomimetic Functional Integration
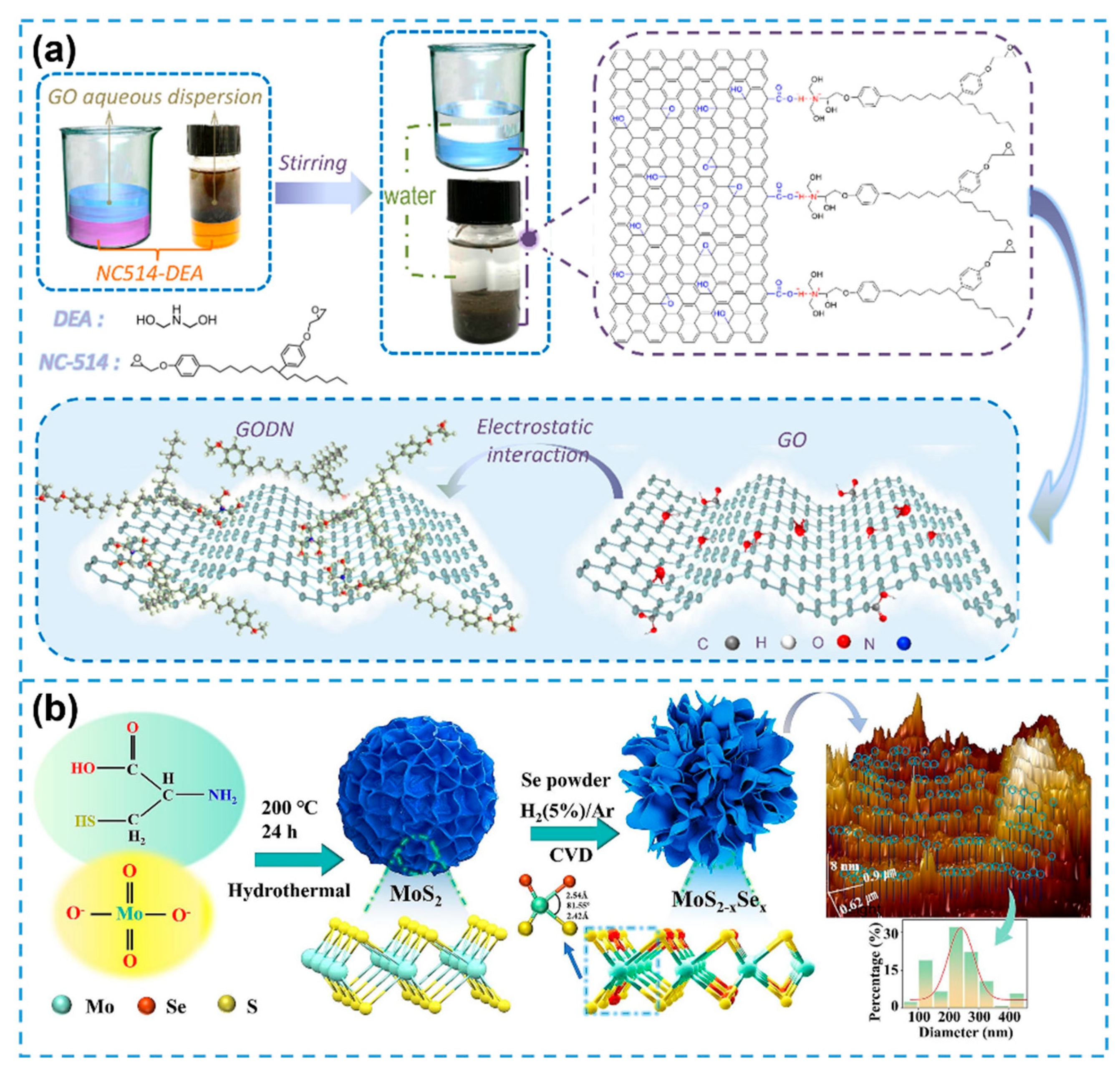
3.5. Summary of Biomimetic Synthesis
4. Biosensor Applications of 2DNMs
4.1. Colorimetric Biosensors
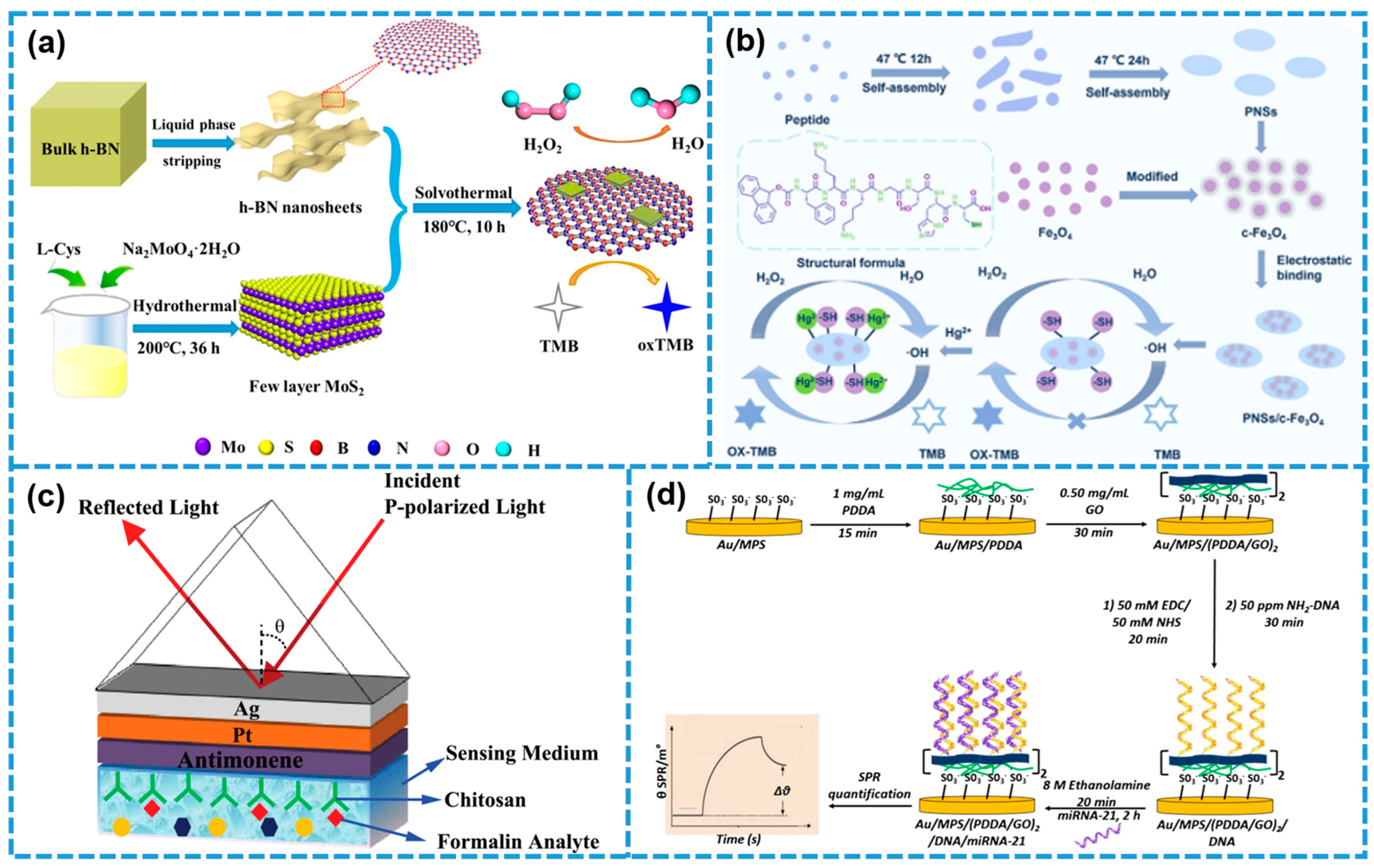
4.2. SPR Biosensors
4.3. Electrochemical Biosensors
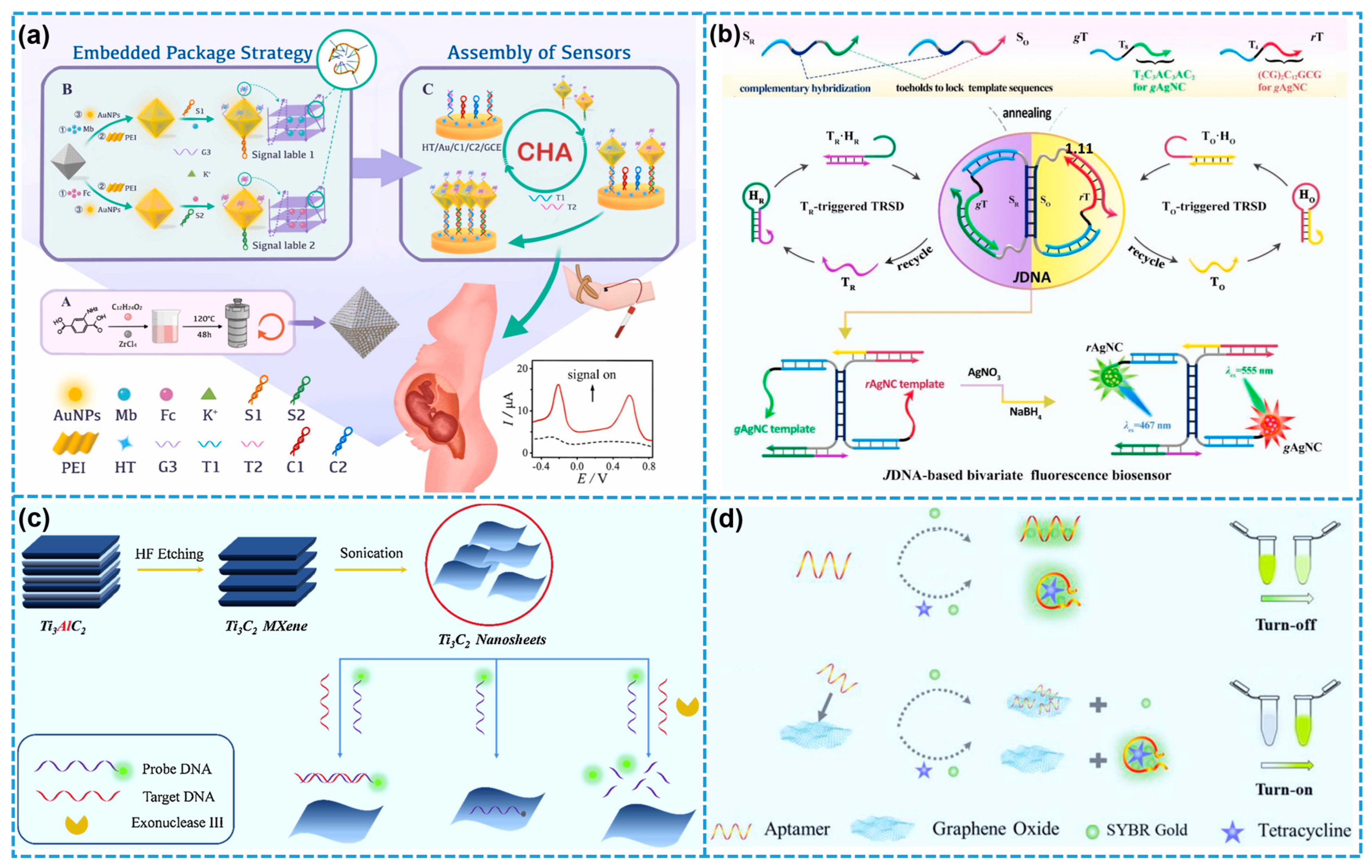
4.4. Fluorescent Biosensors
4.5. Photoelectrochemical (PEC) Biosensors

5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, A.A.; Lotfy, L.A.; Eid, A.M.; Rafat, Y.; Makhlouf, A.H.; Elmotim, N.M.; Al-saudi, N.W.; Algyar, H.S.; El-Samad, S.A.; Zamel, N.Y.; et al. 2D bismuth nanomaterials: From basic knowledge to recently applied energy applications. J. Alloys Compd. 2025, 1020, 179375. [Google Scholar] [CrossRef]
- Yi, M.Y.; Liang, B.; Xiao, H.; Tan, W.; Yang, W.J.; He, X.; Stehle, Y.Y.; Hu, J.H.; Zeng, K.; Yang, G. Tunable 1D–2D carbon nanomaterials for broadband and high-performance microwave absorption via ultrasonic spray ice template. ACS Appl. Mater. Interfaces 2025, 17, 9702–9715. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.P.; Shen, L.Y.; Yu, H.F. Two-dimensional nanomaterials for polymer-based packaging applications: A colloidal perspective. Nanomaterials 2025, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Sikri, N.; Behera, B.; Kumar, A.; Kumar, V.; Pandey, O.P.; Mehta, J.; Kumar, S. Recent advancements on 2D nanomaterials as emerging paradigm for the adsorptive removal of microcontaminants. Adv. Colloid Interface Sci. 2025, 340, 103441. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Wu, J.H.; Yu, L.G.; Bi, J.L.; Wang, Y.D.; Hu, X.Y.; Zhang, Y.J.; Li, W.H. Two-dimensional nanomaterials as lubricant additives: The state-of-the-art and future prospects. J. Mater. Chem. C 2025, 13, 4327–4373. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Zhu, M.J.; Liu, Q.; Xiao, Y.; Cui, Z.R.; Guo, C.C. High-temperature quantum hall effect in graphite-gated graphene heterostructure devices with high carrier mobility. Nanomaterials 2022, 12, 3777. [Google Scholar] [CrossRef]
- Li, B.S.; Lai, C.; Zeng, G.M.; Huang, D.L.; Qin, L.; Zhang, M.M.; Cheng, M.; Liu, X.G.; Yi, H.; Zhou, C.Y.; et al. Black phosphorus, a rising star 2D nanomaterial in the post-graphene era: Synthesis, properties, modifications, and photocatalysis applications. Small 2019, 15, 1804565. [Google Scholar] [CrossRef]
- Swain, K.K.; Pradhan, S.K.; Late, D.J. MXene-based nanomaterials for pollution remediation: A review. Chemistryselect 2025, 10, e202404885. [Google Scholar] [CrossRef]
- Li, W.Q.; Xie, G.Y.; Xu, H.Y. Emerging trends in MXene research: Synthesis, process and hybrid with nanomaterials for biosensing. Coord. Chem. Rev. 2025, 531, 216493. [Google Scholar] [CrossRef]
- Rajarathinam, T.; Jayaraman, S.; Kim, C.-S.; Yoon, J.-H.; Chang, S.-C. Two-dimensional nanozyme nanoarchitectonics customized electrochemical bio diagnostics and lab-on-chip devices for biomarker detection. Adv. Colloid Interface Sci. 2025, 341, 103474. [Google Scholar] [CrossRef]
- Heidarshenas, B.; Yuan, Y.H.; El-Shafay, A.S. Advancements in 2D nanomaterial-enhanced nanofluids: Stability, thermophysical properties, and industrial applications. Powder Technol. 2025, 454, 120687. [Google Scholar] [CrossRef]
- He, J.W.; Huang, P.; Li, B.J.; Xing, Y.Q.; Wu, Z.; Lee, T.C.; Liu, L. Untethered soft robots based on 1D and 2D nanomaterials. Adv. Mater. 2025, 37, 2413648. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtu, M.; Latiff, N.M.; Pumera, M. Two-dimensional materials in biomedical, biosensing and sensing applications. Chem. Soc. Rev. 2021, 50, 619–657. [Google Scholar] [CrossRef] [PubMed]
- Issaka, E.; Wariboko, M.A.; Agyekum, E.A. Synergy and coordination between biomimetic nanoparticles and biological cells/tissues/organs/systems: Applications in nanomedicine and prospect. Biomed. Mater. Devices 2024, 2, 1–33. [Google Scholar] [CrossRef]
- Vural, M.; Demirel, M.C. Biocomposites of 2D layered materials. Nanoscale Horiz. 2025, 10, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zeng, F.; Wang, Y.; Li, Y.; Chen, G.; Jiang, J.; Wang, Q. Assembly and functionality of 2D protein arrays. Adv. Sci. 2025, 12, 2416485. [Google Scholar] [CrossRef]
- Li, H.; Ma, C.; Wang, L.Q.; Li, T.; Li, P.; Hu, Y.; Dai, X.Y.; Wu, D.J.; Chang, M.Q.; Chen, Y.; et al. 2D black ferroelectric perovskite nanocatalysts enable defect modulation-augmented piezocatalytic glioma therapy. Adv. Funct. Mater. 2025, 35, 2412983. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yang, M.; Wu, Q.Y.; Xue, J.J.; Liu, H.Y. Engineering two-dimensional nanomaterials for photothermal therapy. Angew. Chem. Int. Ed. 2025, 64, e202424768. [Google Scholar] [CrossRef]
- Kamboukos, A.; Todorova, N.; Yarovsky, I. Exploring 2D graphene-based nanomaterials for biomedical applications: A theoretical modeling perspective. Small Sci. 2025, 5, 2400505. [Google Scholar] [CrossRef]
- Lutomia, D.; Poria, R.; Kala, D.; Garg, P.; Nagraik, R.; Kaushal, A.; Gupta, S.; Kumar, D. 2D nanomaterials in biosensing: Synthesis, characterization, integration in biosensors and their applications. Biosens. Bioelectron. X 2025, 24, 100615. [Google Scholar] [CrossRef]
- Das, P.K.; Adil, O.; Shamsi, M.H. Electrochemical biosensing based on nucleic acid adsorption on two-dimensional nanomaterials: A review. ACS Appl. Nano Mater. 2025, 8, 6797–6817. [Google Scholar] [CrossRef]
- Kizhepat, S.; Rasal, A.S.; Chang, J.-Y.; Wu, H.-F. Development of two-dimensional functional nanomaterials for biosensor applications: Opportunities, challenges, and future prospects. Nanomaterials 2023, 13, 1520. [Google Scholar] [CrossRef]
- El Barghouti, M.; Akjouj, A.; Mir, A. Modeling of surface plasmon resonance biosensor based on Ag/BiFeO3/Ni using 2D nanomaterial perovskite MAPbBr3. Mater. Today Commun. 2022, 33, 104591. [Google Scholar] [CrossRef]
- Manoharan, A.K.; Batcha, M.I.K.; Mahalingam, S.; Raj, B.; Kim, J. Recent advances in two-dimensional nanomaterials for healthcare monitoring. ACS Sens. 2024, 9, 1706–1734. [Google Scholar] [CrossRef]
- Tiwari, M.; Bangruwa, N.; Mishra, D. 0D, 1D, and 2D magnetic nanostructures: Classification and their applications in modern biosensors. Talanta Open 2023, 8, 100257. [Google Scholar] [CrossRef]
- Ruan, S.J.; Luo, D.; Li, M.; Wang, J.T.; Ling, L.C.; Yu, A.P.; Chen, Z.W. Synthesis and functionalization of 2D nanomaterials for application in lithium-based energy storage systems. Energy Storage Mater. 2021, 38, 200–230. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Yang, S.B. Ultrathin two-dimensional metallic nanomaterials. Mater. Chem. Front. 2018, 2, 456–467. [Google Scholar] [CrossRef]
- Yang, F.; Hu, P.; Yang, F.; Hua, X.J.; Chen, B.; Gao, L.L.; Wang, K.S. Photocatalytic applications and modification methods of two-dimensional nanomaterials: A review. Tungsten 2024, 6, 77–113. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, I.-D. Recent developments in 2D nanomaterials for chemiresistive-type gas sensors. Electron. Mater. Lett. 2018, 14, 221–260. [Google Scholar] [CrossRef]
- Chen, F.; Tang, Q.; Ma, T.; Zhu, B.H.; Wang, L.Y.; He, C.; Luo, X.L.; Cao, S.J.; Ma, L.; Cheng, C. Structures, properties, and challenges of emerging 2D materials in bioelectronics and biosensors. Inf. Mat. 2022, 4, e12299. [Google Scholar] [CrossRef]
- Guan, G.; Han, M.-Y. Functionalized hybridization of 2D nanomaterials. Adv. Sci. 2019, 6, 1901837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yin, H.; Ai, S. Applications of two-dimensional layered nanomaterials in photoelectrochemical sensors: A comprehensive review. Coord. Chem. Rev. 2021, 447, 214156. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. 2D nanomaterials as electrochemical (bio)sensing transducers in the post-graphene era. Trends Anal. Chem. 2024, 172, 117610. [Google Scholar] [CrossRef]
- Chakraborty, G.; Padmashree, R.; Prasad, A. Recent advancement of surface modification techniques of 2-D nanomaterials. Mater. Sci. Eng. B 2023, 297, 116817. [Google Scholar] [CrossRef]
- Knappe, G.A.; Wamhoff, E.-C.; Read, B.J.; Irvine, D.J.; Bathe, M. In situ covalent functionalization of DNA origami virus-like particles. ACS Nano 2021, 15, 14316–14322. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, Q.; Xiong, Z.Q.; Zhang, S.Y.; Deng, S.; Liu, D.X.; Zhang, X.J. Surface functionalization of two-dimensional nanomaterials beyond graphene: Applications and ecotoxicity. Adv. Colloid Interface Sci. 2025, 336, 103357. [Google Scholar] [CrossRef]
- Zhang, P.F.; Zhao, L.; Liu, H.; Chen, H.; Wu, Y.M.; Wang, X.Y.; Liu, G.; Zeng, Y. Genetically engineered membrane-coated nanoparticles as versatile platforms with reduced protein corona for targeted siRNA delivery. Adv. Ther. 2023, 6, 2300228. [Google Scholar] [CrossRef]
- Liu, L.; Klausen, L.H.; Dong, M.D. Two-dimensional peptide based functional nanomaterials. Nano Today 2018, 23, 40–58. [Google Scholar] [CrossRef]
- Kaygisiz, K.; Sementa, D.; Athiyarath, V.; Chen, X.; Ulijn, R.V. Context dependence in assembly code for supramolecular peptide materials and systems. Nat. Rev. Mater. 2025, 10, 1–24. [Google Scholar] [CrossRef]
- Mu, R.Q.; Zhu, D.Z.; Abdulmalik, S.; Wijekoon, S.; Wei, G.; Kumbar, S.G. Stimuli-responsive peptide assemblies: Design, self-assembly, modulation, and biomedical applications. Bioact. Mater. 2024, 35, 181–207. [Google Scholar] [CrossRef]
- Qian, Y.; Di, S.; Wang, L.; Li, Z. Recent advances in the synthesis and applications of graphene–polypeptide nanocomposites. J. Mater. Chem. B 2021, 9, 6521–6535. [Google Scholar] [CrossRef]
- Wang, Y.; Di, S.; Yu, J.; Wang, L.; Li, Z. Recent advances of graphene–biomacromolecule nanocomposites in medical applications. J. Mater. Chem. B 2023, 11, 500–518. [Google Scholar] [CrossRef]
- Liu, B.; He, P.; Kong, H.; Zhu, D.Z.; Wei, G. Peptide-induced synthesis of graphene-supported Au/Pt bimetallic nanoparticles for electrochemical biosensor application. Macromol. Mater. Eng. 2022, 307, 2100886. [Google Scholar] [CrossRef]
- Yang, G.; Guo, Q.; Kong, H.; Luan, X.; Wei, G. Structural design, biomimetic synthesis, and environmental sustainability of graphene-supported g-C3N4/TiO2 hetero-aerogels. Environ. Sci. Nano 2023, 10, 1257–1267. [Google Scholar] [CrossRef]
- Li, P.Y.; Sakuma, K.; Tsuchiya, S.; Sun, L.H.; Hayamizu, Y. Fibroin-like peptides self-assembling on two-dimensional materials as a molecular scaffold for potential biosensing. ACS Appl. Mater. Interfaces 2019, 11, 20670–20677. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Tanaka, S.; Tsuruoka, T.; Nagahama, K. Two-dimensional metal–organic framework-based cellular scaffolds with high protein adsorption, retention, and replenishment capabilities. ACS Appl. Mater. Interfaces 2022, 14, 34443–34454. [Google Scholar] [CrossRef]
- Mu, R.Q.; Zhu, D.Z.; Wei, G. Ti3C2 nanosheets functionalized with ferritin–biomimetic platinum nanoparticles for electrochemical biosensors of nitrite. Biosensors 2024, 14, 258. [Google Scholar] [CrossRef]
- Wei, G.; Xu, F.G.; Li, Z.; Jandt, K.D. Protein-promoted synthesis of Pt nanoparticles on carbon nanotubes for electrocatalytic nanohybrids with enhanced glucose sensing. J. Phys. Chem. C 2011, 115, 11453–11460. [Google Scholar] [CrossRef]
- Chen, M.; Wang, M.; Niu, W.; Cheng, W.; Guo, Y.; Wang, Y.D.; Luo, M.; Xie, C.X.; Leng, T.T.; Zhang, X.X.; et al. Multifunctional protein-decorated bioactive glass nanoparticles for tumor-specific therapy and bioimaging in vitro and in vivo. ACS Appl. Mater. Interfaces 2021, 13, 14985–14994. [Google Scholar] [CrossRef]
- Gaur, M.; Maurya, S.; Akhtar, M.S.; Yadav, A.B. Synthesis and evaluation of BSA-loaded PLGA-chitosan composite nanoparticles for the protein-based drug delivery system. ACS Omega 2023, 8, 18751–18759. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, J.; Cai, Y.; Zhong, Y.; Li, Y.; Zhou, B. Evaluation of stability and antitumor activity of two-dimensional black phosphorus modified with positively charged protein. Nanotechnology 2023, 34, 335701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xie, Z.-X.; Liang, L.; Zhang, P.; Ma, X.; Kong, Z.; Shen, J.-W.; Hu, W. Theoretical investigation on the adsorption orientation of DNA on two-dimensional MoSe2. Chem. Phys. 2021, 551, 111329. [Google Scholar] [CrossRef]
- Wu, J.F.; Gao, X.; Ge, L.; Zhao, G.C.; Wang, G.F. A fluorescence sensing platform of theophylline based on the interaction of RNA aptamer with graphene oxide. RSC Adv. 2019, 9, 19813–19818. [Google Scholar] [CrossRef]
- Lu, J.Y.; Wang, M.H.; Han, Y.W.; Deng, Y.; Zeng, Y.J.; Li, C.; Yang, J.; Li, G.X. Functionalization of covalent organic frameworks with DNA via covalent modification and the application to exosomes detection. Anal. Chem. 2022, 94, 5055–5061. [Google Scholar] [CrossRef]
- Lee, C.Y.; Park, K.S.; Jung, Y.K.; Park, H.G. A label-free fluorescent assay for deoxyribonuclease I activity based on DNA-templated silver nanocluster/graphene oxide nanocomposite. Biosens. Bioelectron. 2017, 93, 293–297. [Google Scholar] [CrossRef]
- Öndeş, B.; Evli, S.; Şahin, Y.; Uygun, M.; Uygun, D.A. Uricase based amperometric biosensor improved by AuNPs-TiS2 nanocomposites for uric acid determination. Microchem. J. 2022, 181, 107725. [Google Scholar] [CrossRef]
- Qiao, Q.Q.; Wang, J.Y.; Long, K.; Li, L.W.; Chen, J.H.; Guo, Y.H.; Xu, Z.Q.; Kuang, Y.; Ji, T.J.; Li, C. A cascaded enzyme system based on the catalase-like activity of Ti3C2Tx MXene nanosheets for the efficient combination cancer therapy. Nano Today 2024, 54, 102059. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Putzer, D.; Stuendl, N.; Lohberger, B.; Awaja, F. Enhanced osteogenic differentiation of human primary mesenchymal stem and progenitor cultures on graphene oxide/poly(methyl methacrylate) composite scaffolds. Materials 2020, 13, 2991. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Fang, Q.; Sun, J.; Du, Y.; Aisa, H.A. A cascade nanoreactor based on metal azolate framework integrated natural enzyme for α-glucosidase activity assay and inhibitor screening. J. Colloid Interface Sci. 2025, 695, 137764. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, Y.F.; Wang, M.; Wang, H.M.; Lu, C.H.; Yang, H.H. Biodegradable black-phosphorus-nanosheet-based nanoagent for enhanced chemo-photothermal therapy. Part. Part. Syst. Charact. 2020, 37, 2000243. [Google Scholar] [CrossRef]
- Chen, L.L.; Wang, W.S.; Lin, Z.F.; Lu, Y.; Chen, H.; Li, B.L.; Li, Z.; Xia, H.; Li, L.H.; Zhang, T. Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J. Nanobiotechnol. 2022, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Stojanović, G.M.; Hassan, R.; Anand, T.J.S.; Al-Ejji, M.; Hasan, A. Role of graphene oxide in bacterial cellulose-gelatin hydrogels for wound dressing applications. ACS Omega 2023, 8, 15909–15919. [Google Scholar] [CrossRef]
- Sharma, S.; Bhende, M.; Mulwani, P.; Patil, V.; Verma, H.R.; Kumar, S. Mechanically improved chitosan/graphene oxide nanocomposite hydrogel for sustained release of levofloxacin. Int. J. Biol. Macromol. 2025, 289, 139481. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhou, G.; Wen, Y.; Ye, J.; Li, X.; Wang, X. Recent advances on stimuli-responsive biopolymer-based nanocomposites for drug delivery. Compos. Part B Eng. 2023, 266, 111018. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold degradation in bone tissue engineering: An overview. Int. Biodeterior. Biodegrad. 2023, 180, 105599. [Google Scholar] [CrossRef]
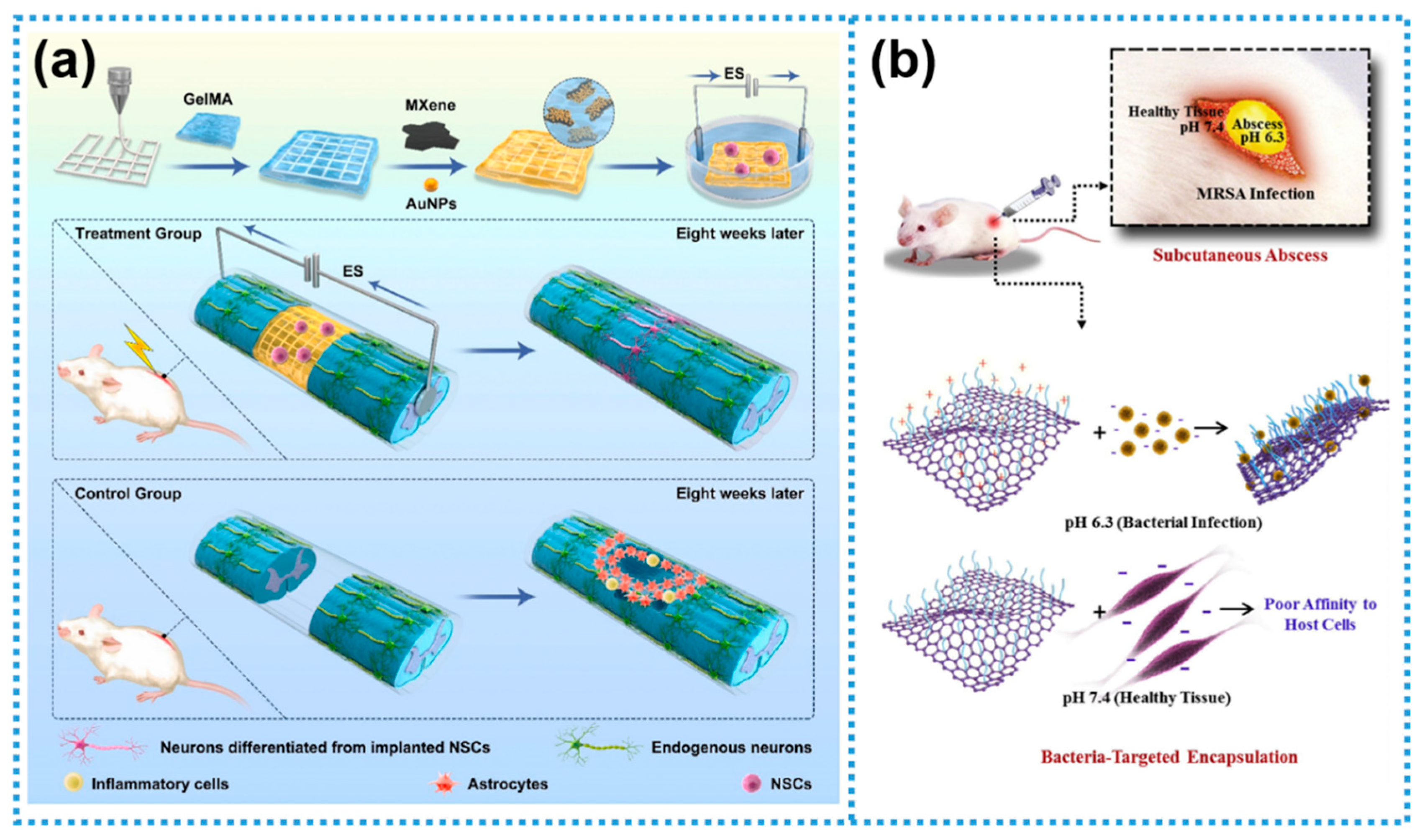
- Kong, W.J.; Zhao, Y.L.; Xiao Yu, Y.; Chen, J.; Chen, Y.H.; Zhao, Z.Y.; Chen, X.N.; Wang, F.; Fu, C. Combined treatment using novel multifunctional MAu-GelMA hydrogel loaded with neural stem cells and electrical stimulation promotes functional recovery from spinal cord injury. Ceram. Int. 2023, 49, 20623–20636. [Google Scholar] [CrossRef]
- Qian, W.; Yan, C.; He, D.F.; Yu, X.Z.; Yuan, L.; Liu, M.L.; Luo, G.X.; Deng, J. pH-triggered charge-reversible of glycol chitosan conjugated carboxyl graphene for enhancing photothermal ablation of focal infection. Acta Biomater. 2018, 69, 256–264. [Google Scholar] [CrossRef]
- Lee, Z.J.; Xie, C.; Ng, K.; Suleria, H.A.R. Unraveling the bioactive interplay: Seaweed polysaccharide, polyphenol and their gut modulation effect. Crit. Rev. Food Sci. Nutr. 2023, 65, 382–405. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; van Teijlingen, A.; Tuttle, T.; Ulijn, R.V. Integrating computation, experiment, and machine learning in the design of peptide-based supramolecular materials and systems. Angew. Chem. Int. Ed. 2023, 62, e202218067. [Google Scholar] [CrossRef]
- Lokhande, G.; Carrow, J.K.; Thakur, T.; Xavier, J.R.; Parani, M.; Bayless, K.J.; Gaharwar, A.K. Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018, 70, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Spiridonov, V.; Lukmanova, A.; Pozdyshev, D.; Antonova, Y.; Kusaja, V.; Muronetz, V.; Yaroslavov, A. Enzyme-induced degradation of natural and artificial linear polyanions. Carbohydr. Res. 2024, 546, 109310. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Liang, K. Nano-bio-interface engineering of metal-organic frameworks. Nano Today 2021, 40, 101256. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Y.Y.; Cui, H.B.; Yao, X.; Guan, G.J.; Han, M.Y. Nano-bio-interface: Unleashing the potential of noble nanometals. Small Sci. 2024, 4, 2300227. [Google Scholar] [CrossRef] [PubMed]
- Srikrajang, S.; Kabir, L.; Sagadevan, S.; Wijaya, K.; Oh, W.-C. Representative modeling of biocompatible MXene nanocomposites for next-generation biomedical technologies and healthcare. J. Mater. Chem. B 2025, 13, 2912–2951. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Tian, Y.; Sheng, X.L.; Liu, C.S.; Wei, L.Q.; Wang, J.; Chen, Y. Construction of a black phosphorous-based noncovalent multiple nanosupramolecular assembly for synergistic targeted photothermal and chemodynamic therapy. Chin. Chem. Lett. 2025, 36, 110193. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.K.; Nuzzo, M.; Yang, X.C.; Yang, Y.P.; Zhang, X.P. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials 2018, 182, 279–288. [Google Scholar] [CrossRef]
- Li, J.X.; Zheng, L.L.; Zeng, L.; Zhang, Y.; Jiang, L.; Song, J.L. RGD peptide-grafted graphene oxide as a new biomimetic nanointerface for impedance-monitoring cell behaviors. J. Nanomater. 2016, 2016, 2828512. [Google Scholar] [CrossRef]
- Zhuang, J.; Gong, H.; Zhou, J.R.; Zhang, Q.Z.; Gao, W.W.; Fang, R.H.; Zhang, L.F. Targeted gene silencing in vivo by platelet membrane–coated metal-organic framework nanoparticles. Sci. Adv. 2020, 6, eaaz6108. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Luan, X.; He, P.; Zhu, D.Z.; Mu, R.Q.; Wang, Y.; Wei, G. Fabrication and functional regulation of biomimetic interfaces and their antifouling and antibacterial applications: A review. Small 2024, 20, 2308091. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.A.; Cai, M.X.; Niu, P.P.; Zhang, H.; Zhang, S.Q.; Sun, Y. Ultrashort peptides induce biomineralization. Compos. B Eng. 2022, 244, 110196. [Google Scholar] [CrossRef]
- Boskey, A.L.; Villarreal-Ramirez, E. Intrinsically disordered proteins and biomineralization. Matrix Biol. 2016, 52–54, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, D.; Carneiro, K.M.M. DNA nanostructures as templates for biomineralization. Nat. Rev. Chem. 2021, 5, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.C.; Guo, J.H.; Li, Q.C.; Xie, H. Lipopolysaccharide-stabilized ionic colloids induce biomineralization. Colloids Surf. B 2022, 211, 112331. [Google Scholar] [CrossRef]
- Munyemana, J.C.; He, H.X.; Ding, S.L.; Yin, J.; Xi, P.X.; Xiao, J.X. Synthesis of manganese phosphate hybrid nanoflowers by collagen-templated biomineralization. RSC Adv. 2018, 8, 2708–2713. [Google Scholar] [CrossRef]
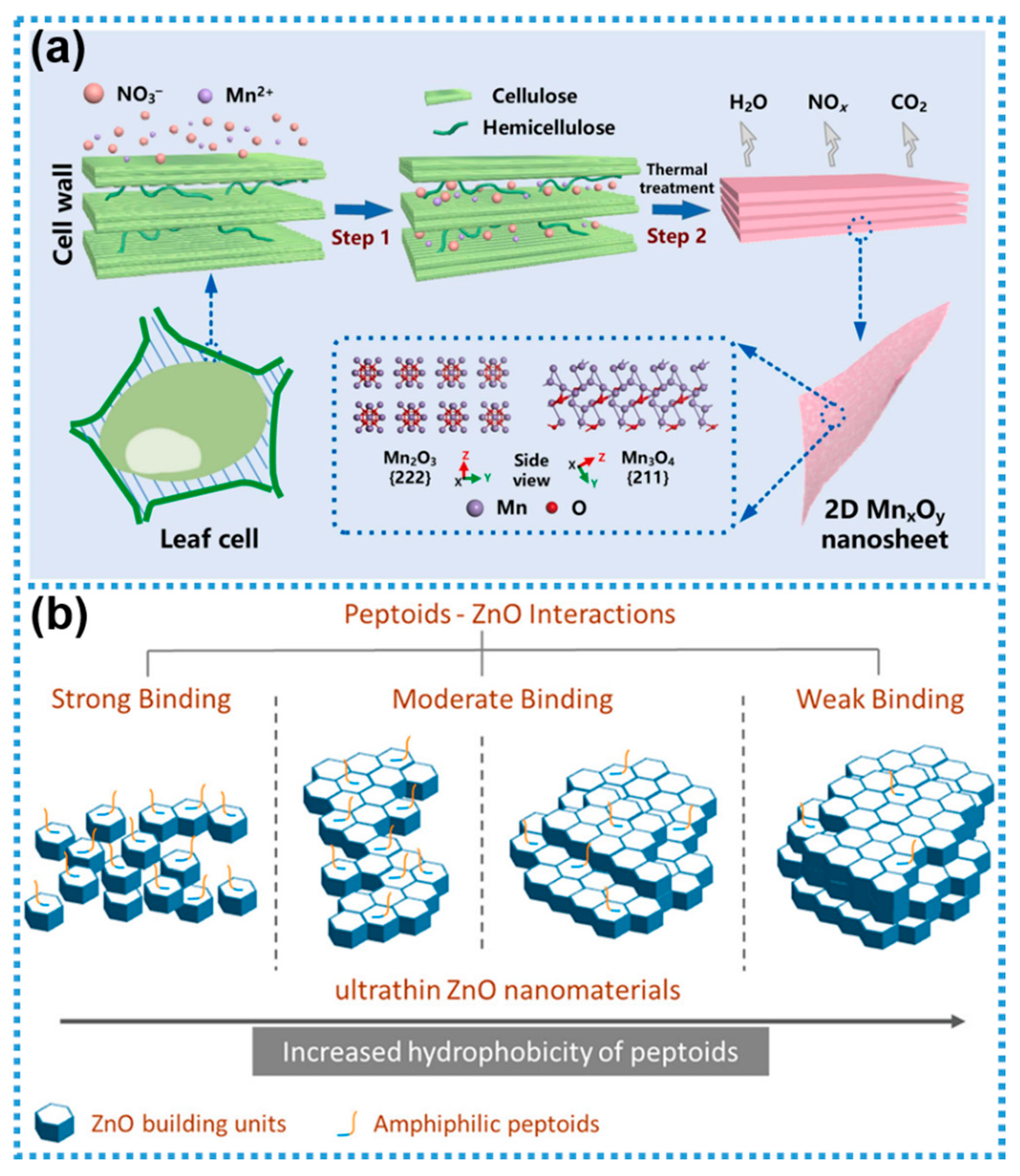
- Zhao, Y.Y.; Yu, M.H.; Cui, R.Y.; Lin, Y.; Chen, Z.G.; Wu, Z.Y. Space-confined growth of two-dimensional manganese oxide nanosheets in plant-cell structures for efficient tetracycline degradation. Mater. Today Commun. 2024, 40, 109496. [Google Scholar] [CrossRef]
- Yang, W.C.; Cai, B.; Lachowski, K.J.; Yin, Q.X.; De Yoreo, J.J.; Pozzo, L.D.; Chen, C.L. Insights into the biomimetic synthesis of 2D ZnO nanomaterials through peptoid engineering. J. Phys. Chem. Lett. 2023, 14, 9732–9739. [Google Scholar] [CrossRef]
- Kong, H.; Han, J.R.; Yang, M.; Lai, L.X.; Sun, Y.B.; Luan, X.; Ren, W.Z.; Wu, A.G.; Wei, G. Two-dimensional peptide nanosheets functionalized with gold nanorods for photothermal therapy of tumors. J. Mater. Chem. B 2023, 11, 3445–3452. [Google Scholar] [CrossRef]
- Ding, T.; Xing, Y.X.; Wang, Z.Q.; Guan, H.D.; Wang, L.C.; Zhang, J.X.; Cai, K.Y. Structural complementarity from DNA for directing two-dimensional polydopamine nanomaterials with biomedical applications. Nanoscale Horiz. 2019, 4, 652–657. [Google Scholar] [CrossRef]
- Ding, L.J.; Liu, B.; Peil, A.; Fan, S.; Chao, J.; Liu, N. DNA-directed assembly of photonic nanomaterials for diagnostic and therapeutic applications. Adv. Mater. 2025, 37, 2500086. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Hu, H.Q.; Sun, Z.G.; He, P.; Zhu, D.Z.; Xu, Y.Y.; Liu, B.; Wei, G. Assembling Ag2S quantum dots onto peptide nanosheet as a biomimetic two-dimensional nanoplatform for synergistic near infrared-II fluorescent imaging and photothermal therapy of tumor. J. Colloid Interface Sci. 2024, 663, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; He, P.; Gu, G.H.; Zhu, D.Z.; Luan, X.; Mu, R.Q.; Wei, G. Gold nanoparticles-modified 2D self-assembled amphiphilic peptide nanosheets with high biocompatibility and photothermal therapy efficiency. Macromol. Rapid Commun. 2024, 45, 2400386. [Google Scholar] [CrossRef]
- Xu, M.J.; Xu, Y.Y.; Du, C.X.; Gu, G.H.; Wei, G. Biomimetic CuCoO2 nanosheets reinforced with self-assembling peptide nanofibers for tumor photothermal therapy. RSC Adv. 2024, 14, 39163–39172. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, C.; Dong, Y.C.; Wang, D.M.; Cao, T.Y.; Wang, S.; Liu, D.S. Fold 2D woven DNA origami to origami+ structures. Adv. Funct. Mater. 2019, 29, 1809097. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, P. Amyloid-mediated fabrication of organic–inorganic hybrid materials and their biomedical applications. Adv. Mater. Interfaces 2020, 7, 2001060. [Google Scholar] [CrossRef]
- Dauphin, Y. A brief history of biomineralization studies. ACS Biomater. Sci. Eng. 2023, 9, 1774–1790. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Feng, Y.M.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.W.; Zhang, D.Y.; Feng, L. Biomineralization forming process and bio-inspired nanomaterials for biomedical application: A review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef]
- Vigil, T.N.; Spangler, L.C. Understanding biomineralization mechanisms to produce size-controlled, tailored nanocrystals for optoelectronic and catalytic applications: A review. ACS Appl. Nano Mater. 2024, 7, 18626–18654. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.Z.; Liu, B.; Kong, H.; Xiong, Z.; Guo, L.; Wei, G. Biomineralization of ZrO2 nanoparticles on graphene oxide-supported peptide/cellulose binary nanofibrous membranes for high-performance removal of fluoride ions. Chem. Eng. J. 2022, 430, 132721. [Google Scholar] [CrossRef]
- Fan, Z.C.; Chen, Y.S.; Li, Q.; Gadora, K.; Ji, Z.S.; Wu, D.; Zhou, J.P.; Ding, Y.; Cheng, H. Graphene oxide-templated biomineralization nanosystem enables multi-drug loading and controllable release. J. Nanopart. Res. 2024, 26, 125. [Google Scholar] [CrossRef]
- Awasthi, G.P.; Kaliannagounder, V.K.; Maharjan, B.; Lee, J.Y.; Park, C.H.; Kim, C.S. Albumin-induced exfoliation of molybdenum disulfide nanosheets incorporated polycaprolactone/zein composite nanofibers for bone tissue regeneration. Mater. Sci. Eng. C 2020, 116, 111162. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Mei, J.; Yan, C.; Liao, T.; Bell, J.; Sun, Z.Q. Bioinspired 2D nanomaterials for sustainable applications. Adv. Mater. 2020, 32, 1902806. [Google Scholar] [CrossRef]
- Yi, H.; Yan, M.; Huang, D.L.; Zeng, G.M.; Lai, C.; Li, M.F.; Huo, X.Q.; Qin, L.; Liu, S.Y.; Liu, X.G.; et al. Synergistic effect of artificial enzyme and 2D nano-structured Bi2WO6 for eco-friendly and efficient biomimetic photocatalysis. Appl. Catal. B 2019, 250, 52–62. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, L.; Liu, C.B.; Lan, X.J.; Zhao, H.C. Engineering the interface in graphene oxide/epoxy composites using bio-based epoxy-graphene oxide nanomaterial to achieve superior anticorrosion performance. J. Colloid Interface Sci. 2021, 587, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, J.J.; Yang, G.Y.; Li, C.B.; Li, Y.J. Bioinspired wood-like coaxial fibers based on MXene@graphene oxide with superior mechanical and electrical properties. Nanoscale 2020, 12, 21325–21333. [Google Scholar] [CrossRef]
- Wu, H.; Qi, L.; Song, B.; Tong, Y.; Li, L.; Ikram, M.; Shi, K. MoS2−xSex lamellae assembled with lotus-leaf-like structures for sensitive NO2 gas sensors at room temperature. Chem. Eng. J. 2024, 502, 157906. [Google Scholar] [CrossRef]
- Garg, M.; Gupta, A.; Sharma, A.L.; Singh, S. Advancements in 2D materials based biosensors for oxidative stress biomarkers. ACS Appl. Bio Mater. 2021, 4, 5944–5960. [Google Scholar] [CrossRef]
- Zhu, D.Z.; Liu, B.; Wei, G. Two-dimensional material-based colorimetric biosensors: A review. Biosensors 2021, 11, 259. [Google Scholar] [CrossRef]
- Chen, S.; Xu, S.; Fan, X.; Xiao, X.; Duan, Z.; Zhao, X.; Chen, G.; Zhou, Y.; Chen, J. Advances in 2D materials for wearable biomonitoring. Mater. Sci. Eng. R Rep. 2025, 164, 100971. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Wang, J.; Zhang, X.; Jiao, R.; Ren, Y.; Zhang, D.; Ling, N.; Ye, Y. Recent advances and future prospects of wearable sensors based on nanomaterial sensing mechanisms for biosafety monitoring. Chem. Eng. J. 2025, 512, 162695. [Google Scholar] [CrossRef]
- Zhu, D.Z.; He, P.; Kong, H.; Yang, G.Z.; Luan, X.; Wei, G. Biomimetic graphene-supported ultrafine platinum nanowires for colorimetric and electrochemical detection of hydrogen peroxide. J. Mater. Chem. B 2022, 10, 9216–9225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Wang, L.X.; An, F.P.; Xu, H.; Yin, Z.J.; Tang, S.R.; Yang, H.H.; Song, H.B. Graphitic carbon nitride supported platinum nanocomposites for rapid and sensitive colorimetric detection of mercury ions. Anal. Chim. Acta 2017, 980, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Yagüe, J.C.; Paniagua-González, G.; Garcinuño, R.M.; García-Mayor, A.; Fernández-Hernando, P. Colorimetric molecularly imprinted polymer-based sensors for rapid detection of organic compounds: A review. Chemosensors 2025, 13, 163. [Google Scholar] [CrossRef]
- Adil, O.; Shamsi, M.H. Transformative biomedical devices to overcome biomatrix effects. Biosens. Bioelectron. 2025, 279, 117373. [Google Scholar] [CrossRef]
- Masson, J.-F. Consideration of sample matrix effects and “biological” noise in optimizing the limit of detection of biosensors. ACS Sens. 2020, 5, 3290–3292. [Google Scholar] [CrossRef]
- Lutomia, D.; Poria, R.; Kala, D.; Singh, A.K.; Gupta, M.K.; Kumar, D.; Kaushal, A.; Gupta, S.J.M.A. Unlocking the potential of 2D nanomaterial-based biosensors in biomarker-based detection of Helicobacter pylori. Mater. Adv. 2025, 6, 117–142. [Google Scholar] [CrossRef]
- Mphuthi, N.; Sikhwivhilu, L.; Ray, S.S. Functionalization of 2D MoS2 nanosheets with various metal and metal oxide nanostructures: Their properties and application in electrochemical sensors. Biosensors 2022, 12, 386. [Google Scholar] [CrossRef]
- Li, Z.H.; Liu, X.Y.; Liang, X.H.; Zhong, J.H.; Guo, L.Q.; Fu, F.F. Colorimetric determination of xanthine in urine based on peroxidase-like activity of WO3 nanosheets. Talanta 2019, 204, 278–284. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.F. 2D/2D h-BN/N-doped MoS2 heterostructure catalyst with enhanced peroxidase-like performance for visual colorimetric determination of H2O2. Chem. Asian J. 2020, 15, 1315–1323. [Google Scholar] [CrossRef]
- Luan, X.; Zhu, D.Z.; He, P.; Han, P.; Wei, G. Self-assembled peptide nanosheets functionalized with Fe3O4 nanoparticles with enhanced nanozymatic activity for sensitive colorimetric detection and selective adsorption of Hg2+. Environ. Sci. Nano 2023, 10, 2767–2777. [Google Scholar] [CrossRef]
- Singh, M.K.; Pal, S.; Prajapati, Y.K. Design and analysis of an SPR sensor based on antimonene and platinum for the detection of formalin. IEEE Trans. Nanobiosci. 2023, 22, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mujica, M.L.; Zhang, Y.Y.; Bédioui, F.; Gutiérrez, F.; Rivas, G. Label-free graphene oxide–based SPR genosensor for the quantification of microRNA21. Anal. Bioanal. Chem. 2020, 412, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Yesudasu, V.; Neelaveni, P.; Bhuvaneswari, B.; Mukesh, S.; Ramkumar, G.; Kannagi, L.; Karpagam, M.; Subha, T.D.; Rashed, A.N.Z.; Ferdous, A.H.M.I.; et al. Silver dielectric material black phosphorus–based surface plasmon resonance biosensor for alcohol detection. Plasmonics 2024, 19, 2129–2139. [Google Scholar] [CrossRef]
- Topor, C.V.; Puiu, M.; Bala, C. Strategies for surface design in surface plasmon resonance (SPR) sensing. Biosensors 2023, 13, 465. [Google Scholar] [CrossRef]
- Ruan, J.F.; Tu, J.Y.; Wang, D.L.; Tao, Z.; Yuan, Y.; Ji, S.W. Perfect absorption based on Ti3C2Tx surface plasmon resonance. Opt. Mater. 2023, 137, 113604. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Lu, G.-Z.; Li, S.-E.; Chen, Y.-T.; Tsao, Y.-C.; Chao, Y.-C.; Shen, J.-L.; Chen, Y.-F. Wrinkled graphene structure and localized surface plasmon resonance induced stretchable white random lasers based on GLN-functionalized 2D WS2 quantum dots. Adv. Opt. Mater. 2024, 12, 2302055. [Google Scholar] [CrossRef]
- Kong, L.; Lv, J.; Gu, Q.; Ying, Y.; Jiang, X.; Si, G. Sensitivity-enhanced SPR sensor based on graphene and subwavelength silver gratings. Nanomaterials 2020, 10, 2125. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, Y.; Haque, M.F.; Leem, J.; Hsieh, E.Y.; Nam, S. Plasmonic sensors based on graphene and graphene hybrid materials. Nano Converg. 2022, 9, 28. [Google Scholar] [CrossRef]
- Mulpur, P.; Yadavilli, S.; Rao, A.M.; Kamisetti, V.; Podila, R. MoS2/WS2/BN-silver thin-film hybrid architectures displaying enhanced fluorescence via surface plasmon coupled emission for sensing applications. ACS Sens. 2016, 1, 826–833. [Google Scholar] [CrossRef]
- Baig, N. Two-dimensional nanomaterials: A critical review of recent progress, properties, applications, and future directions. Compos. Part A 2023, 165, 107362. [Google Scholar] [CrossRef]
- Lawal, A.T. Recent developments in electrochemical sensors based on graphene for bioanalytical applications. Sens. Bio-Sens. Res. 2023, 41, 100571. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Imran, A.B.; Foyez, T. Voltammetric sensors modified with nanomaterials: Applications in rapid detection of bioactive compounds for health and safety monitoring. Discov. Electrochem. 2025, 2, 14. [Google Scholar] [CrossRef]
- Li, J.; Wijaya, L.N.A.; Jang, D.W.; Hu, Y.; You, J.; Cai, Y.; Gao, Z.; Mi, Y.; Luo, Z. 2D materials-based field-effect transistor biosensors for healthcare. Small 2025, 21, 2408961. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.Q. Electrochemical sensors dancing with the brain. ACS Sens. 2020, 5, 2659–2660. [Google Scholar] [CrossRef]
- Hasan, M.R.; Ahommed, M.S.; Daizy, M.; Bacchu, M.S.; Ali, M.R.; Al-Mamun, M.R.; Saad Aly, M.A.; Khan, M.Z.H.; Hossain, S.I. Recent development in electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. X 2021, 8, 100075. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Guo, Z.X.; Wang, Z.Q.; Peng, Q.Y.; Huang, J.J.; Qing, T.; Li, N.; Ruan, J.; Su, H.L. A MOF-embedded/G4-packaged electrochemical labeling strategy-based biosensor for the simultaneous detection of β-thalassemia mutations. Sens. Actuators B Chem. 2025, 432, 137443. [Google Scholar] [CrossRef]
- Wu, L.X.; Gao, J.; Lu, X.B.; Huang, C.S.; Dhanjai; Chen, J.P. Graphdiyne: A new promising member of 2D all-carbon nanomaterial as robust electrochemical enzyme biosensor platform. Carbon 2020, 156, 568–575. [Google Scholar] [CrossRef]
- Lu, J.; Hu, Y.H.; Wang, P.X.; Liu, P.Q.; Chen, Z.G.; Sun, D.P. Electrochemical biosensor based on gold nanoflowers-encapsulated magnetic metal-organic framework nanozymes for drug evaluation with in-situ monitoring of H2O2 released from H9C2 cardiac cells. Sens. Actuators B Chem. 2020, 311, 127909. [Google Scholar] [CrossRef]
- Yang, C.L.; Shi, Y.N.; Zhang, Y.Q.; He, J.Y.; Zhang, Z.H.; Jia, X.Y.; Yuan, R.; Xu, W.J. A bivariate fluorescence biosensor based on Janus DNA nanoarchitecture-loaded dual-emissive Ag nanoclusters as bi-responsive signaling reporters. Biosens. Bioelectron. 2024, 263, 116621. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Lu, D.; Guo, Y.; Guo, S. Ultrathin Ti3C2 nanosheets based “off-on” fluorescent nanoprobe for rapid and sensitive detection of HPV infection. Sens. Actuators B Chem. 2019, 286, 222–229. [Google Scholar] [CrossRef]
- Xu, J.; Qing, T.; Jiang, Z.; Zhang, P.; Feng, B. Graphene oxide-regulated low-background aptasensor for the “turn on” detection of tetracycline. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 260, 119898. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Park, S.; Cho, E.; Chueng, S.T.D.; Yoon, J.S.; Lee, T.; Lee, J.H. Aptameric fluorescent biosensors for liver cancer diagnosis. Biosensors 2023, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Wang, F.Y.; Wang, J.R.; Liu, Y.Q.; Gao, C.M.; Ge, S.G.; Yu, J.H. Catalytic hairpin assembly-assisted CRISPR/Cas12a mediated photoelectrochemical biosensor for sensitive detection of miRNA-122. Sens. Actuators B Chem. 2022, 370, 132480. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, N.; Du, Y.; Ren, X.; Ma, H.M.; Wu, D.; Fan, D.W.; Wei, Q.; Ju, H.X. Nanoarrays-propped in situ photoelectrochemical system for microRNA detection. Biosens. Bioelectron. 2022, 210, 114291. [Google Scholar] [CrossRef]
- Jiang, L.; Du, J.L.; Xu, H.L.; Zhuo, X.H.; Ai, J.L.; Zeng, J.Y.; Yang, R.H.; Xiong, E.R. Ultrasensitive CRISPR/Cas13a-mediated photoelectrochemical biosensors for specific and direct assay of miRNA-21. Anal. Chem. 2023, 95, 1193–1200. [Google Scholar] [CrossRef]
- Liu, S.T.; Chen, J.S.; Liu, X.P.; Mao, C.J.; Jin, B.K. A photoelectrochemical biosensor based on b-TiO/CdS:Eu/Ti3C2 heterojunction for the ultrasensitive detection of miRNA-21. Talanta 2023, 253, 123601. [Google Scholar] [CrossRef]
- Gong, H.X.; Hu, X.H.; Zeng, R.J.; Li, Y.X.; Xu, J.H.; Li, M.J.; Tang, D.P. CRISPR/Cas12a-based photoelectrochemical sensing of microRNA on reduced graphene oxide-anchored Bi2WO6 coupling with catalytic hairpin assembly. Sens. Actuators B Chem. 2022, 369, 132307. [Google Scholar] [CrossRef]
- Huang, M.L.; Deng, H.M.; Cheng, M.L.; Li, H.L.; Yuan, R.; Wei, S.P. A photoelectrochemical biosensor based on Ti3C2Tx MXene/Ag2S as a novel photoelectric material for ultrasensitive detection of microRNA-141. Sens. Actuators B Chem. 2023, 394, 134343. [Google Scholar] [CrossRef]
- Qu, J.; Wang, P.; Wang, Y.; Li, Z.; Yang, F.; Han, C.; Wang, L.; Yu, D. Determination of phospholipids in soybean oil using a phospholipase-choline oxidase biosensor based on g-C3N4-TiO2 nanocomposite material. J. Food Compos. Anal. 2023, 124, 105717. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Q.; Wu, L.; Wang, L.; Sun, L.; Zhou, Z.; Li, G. Silicon-based field-effect glucose biosensor based on reduced graphene oxide-carboxymethyl chitosan-platinum nanocomposite material modified LAPS. Sens. Actuator A Phys. 2024, 366, 114937. [Google Scholar] [CrossRef]
- Shang, H.Y.; Zhang, X.F.; Ding, M.L.; Zhang, A.P. Dual-mode biosensor platform based on synergistic effects of dual-functional hybrid nanomaterials. Talanta 2023, 260, 124584. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.F.; Wang, X.Y.; Luo, X.L. Dual-mode electrochemical assay of prostate-specific antigen based on antifouling peptides functionalized with electrochemical probes and internal references. Anal. Chem. 2019, 91, 15846–15852. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Xie, B.; Gao, H.; Zhang, J.; Fu, H.; Liao, F.; Liao, Y. Self-powered DNAzyme walker enables dual-mode biosensor construction for electrochemiluminescence and electrochemical detection of MicroRNA. Anal. Chem. 2023, 95, 7006–7013. [Google Scholar] [CrossRef]
- Zhai, J.; Li, X.; Zhang, J.; Pan, H.; Peng, Q.; Gan, H.; Su, S.; Yuwen, L.; Song, C. SERS/electrochemical dual-mode biosensor based on multi-functionalized molybdenum disulfide nanosheet probes and SERS-active Ag nanorods array electrodes for reliable detection of cancer-related miRNA. Sens. Actuators B Chem. 2022, 368, 132245. [Google Scholar] [CrossRef]
- Ferreira, J.L.A.; de Almeida, L.F.; da Silva Simões, S.; Diniz, P.H.G.D.; de Sousa Fernandes, D.D. Raman spectroscopy-based authentication of powder goat milk adulteration with cow milk. Food Control 2025, 167, 110800. [Google Scholar] [CrossRef]
- Alieva, R.; Sokolova, S.; Zhemchuzhina, N.; Pankin, D.; Povolotckaia, A.; Novikov, V.; Kuznetsov, S.; Gulyaev, A.; Moskovskiy, M.; Zavyalova, E. A surface-enhanced Raman spectroscopy-based aptasensor for the detection of deoxynivalenol and T-2 mycotoxins. Int. J. Mol. Sci. 2024, 25, 9534. [Google Scholar] [CrossRef]
- Fan, M.; Weng, Y.; Liu, Y.; Lu, Y.; Xu, L.; Ye, J.; Lin, D.; Qiu, S.; Feng, S. Surface-enhanced Raman spectroscopy-based optical biosensor for liquid biopsy: Toward precision medicine. Laser Photonics Rev. 2024, 18, 2301072. [Google Scholar] [CrossRef]
- Jin, S.; Lednev, I.K.; Jung, Y.M. Recent trends in surface-enhanced Raman spectroscopy-based biosensors: Label-free early disease diagnosis. J. Phys. Chem. C 2024, 128, 8861–8873. [Google Scholar] [CrossRef]
- Chauhan, N.; Saxena, K.; Rawal, R.; Yadav, L.; Jain, U. Advances in surface-enhanced Raman spectroscopy-based sensors for detection of various biomarkers. Prog. Biophys. Mol. Biol. 2023, 184, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Youden, B.; Carrier, A.; Yu, N.; Oakes, K.; Servos, M.; Zhang, X. Nanomaterials for surface-enhanced Raman spectroscopy-based metal detection: A review. Environ. Chem. Lett. 2024, 22, 2425–2465. [Google Scholar] [CrossRef]
- Allegretto, J.A.; Dostalek, J. Metal–organic frameworks in surface enhanced Raman spectroscopy–based analysis of volatile organic compounds. Adv. Sci. 2024, 11, 2401437. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Kim, S.M.; Kato, T.; Yoon, J.; Noh, S.; Park, E.Y.; Park, C.; Lee, T.; Choi, J.-W. Fabrication of MERS-nanovesicle biosensor composed of multi-functional DNA aptamer/graphene-MoS2 nanocomposite based on electrochemical and surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2022, 352, 131060. [Google Scholar] [CrossRef]




| Biomaterials | 2DNMs | Interaction | Ref |
|---|---|---|---|
| Peptides | GO | Non-covalent interactions | [45] |
| Peptides | g-C3N4 | Non-covalent interactions | [46] |
| Peptides | GO/MoS2 | Non-covalent interactions | [47] |
| Peptides | GO | Covalent interactions | [79] |
| Proteins | MOF [MIL-53 (Al)] | Non-covalent interactions | [48] |
| Proteins | Ti3C2 MXene | Covalent interactions | [49] |
| Proteins | BP | Non-covalent interactions | [53] |
| Proteins | Composite nanofibrous sheets | Non-covalent interactions | [78] |
| RNA | GO | Non-covalent interactions | [55] |
| RNA | MOF | Non-covalent interactions | [80] |
| DNA | COF | Covalent interactions | [56] |
| DNA | GO | Non-covalent interactions | [57] |
| Bio-enzymes | TiS2 | Non-covalent interactions | [58] |
| Bio-enzymes | Ti3C2 MXene | Non-covalent interactions | [59] |
| Bio-enzymes | GO | Non-covalent interactions | [60] |
| Bio-enzymes | MOF | Non-covalent interactions | [61] |
| Biopolymers | BP | Non-covalent interactions | [62] |
| Biopolymers | GO/MoS2 | Non-covalent interactions | [63] |
| Biopolymers | MXene | Non-covalent interactions | [68] |
| Bioactive polysaccharides | GO | Covalent interactions | [69] |
| Bioactive polysaccharides | BP | Non-covalent interactions | [77] |
| Strategy | Template/Mediator | Material/Function | Ref |
|---|---|---|---|
| Bio-templating | Collagen | Protein–manganese phosphate nanoflowers | [86] |
| Bio-templating | Cabbage leaf cells | 2D manganese oxide nanosheets | [87] |
| Bio-templating | Hydrophobic peptides | 2D ZnO nanosheets | [88] |
| Biomolecule-directed self-assembly | Fmoc-FKKGSH sequence peptides | 2D PNS/PEG-Ag2S QDs | [92] |
| Biomolecule-directed self-assembly | Fmoc-FKKGSHC sequence peptides | AuNPs/PNS | [93] |
| Biomolecule-directed self-assembly | KIIIIKYWYAF sequence peptides | BMNS-PNF | [94] |
| Biomolecule-directed self-assembly | DNA | DNA origami structures | [95] |
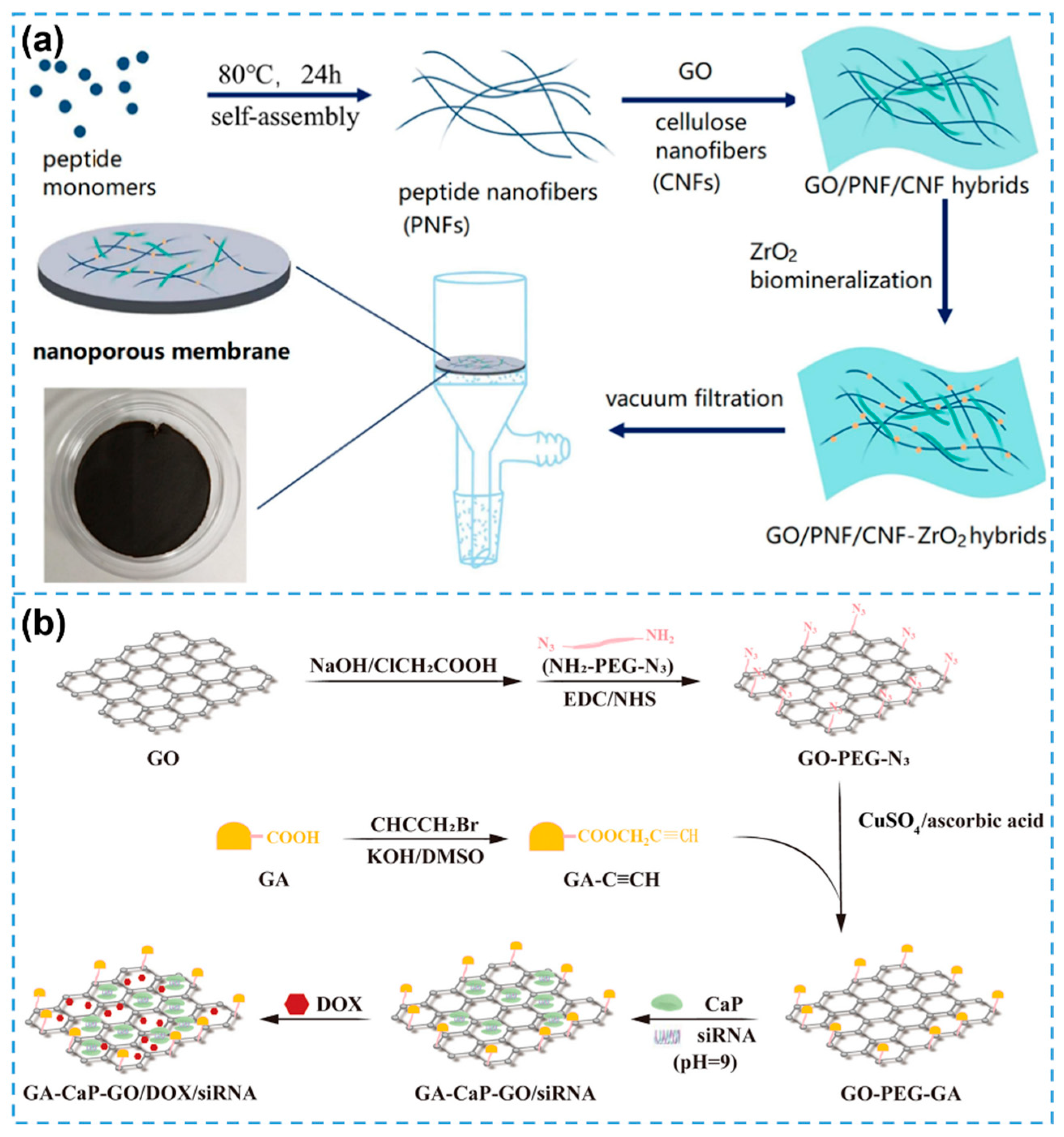
| Biomineralization strategies | PNFs and CNFs | GO/PNF/CNF-ZrO2 composite | [100] |
| Biomineralization strategies | CaP | GA-CaP-GO/DOX/siRNA intelligent drug delivery system | [101] |
| Biomineralization strategies | Albumin | PZM composite scaffold | [102] |
| Biomimetic functional integration | Hemin molecules | 2DNM HBW | [104] |
| Biomimetic functional integration | Cardanol | Bio-based nanocomposites | [105] |
| Biomimetic functional integration | GO/MXene | Biomimetic wood-like coaxial fibers | [106] |
| Biomimetic functional integration | Hierarchical micro-/nanostructures in lotus leaves | MoS2−xSex alloy nanocomposites | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, X.; Wang, Y.; Wei, G. The Biomodification and Biomimetic Synthesis of 2D Nanomaterial-Based Nanohybrids for Biosensor Applications: A Review. Biosensors 2025, 15, 328. https://doi.org/10.3390/bios15050328
Wang R, Wang X, Wang Y, Wei G. The Biomodification and Biomimetic Synthesis of 2D Nanomaterial-Based Nanohybrids for Biosensor Applications: A Review. Biosensors. 2025; 15(5):328. https://doi.org/10.3390/bios15050328
Chicago/Turabian StyleWang, Ranran, Xinyue Wang, Yan Wang, and Gang Wei. 2025. "The Biomodification and Biomimetic Synthesis of 2D Nanomaterial-Based Nanohybrids for Biosensor Applications: A Review" Biosensors 15, no. 5: 328. https://doi.org/10.3390/bios15050328
APA StyleWang, R., Wang, X., Wang, Y., & Wei, G. (2025). The Biomodification and Biomimetic Synthesis of 2D Nanomaterial-Based Nanohybrids for Biosensor Applications: A Review. Biosensors, 15(5), 328. https://doi.org/10.3390/bios15050328







