Microwave Antenna Sensing for Glucose Monitoring in a Vein Model Mimicking Human Physiology
Abstract
1. Introduction
2. Materials and Methods
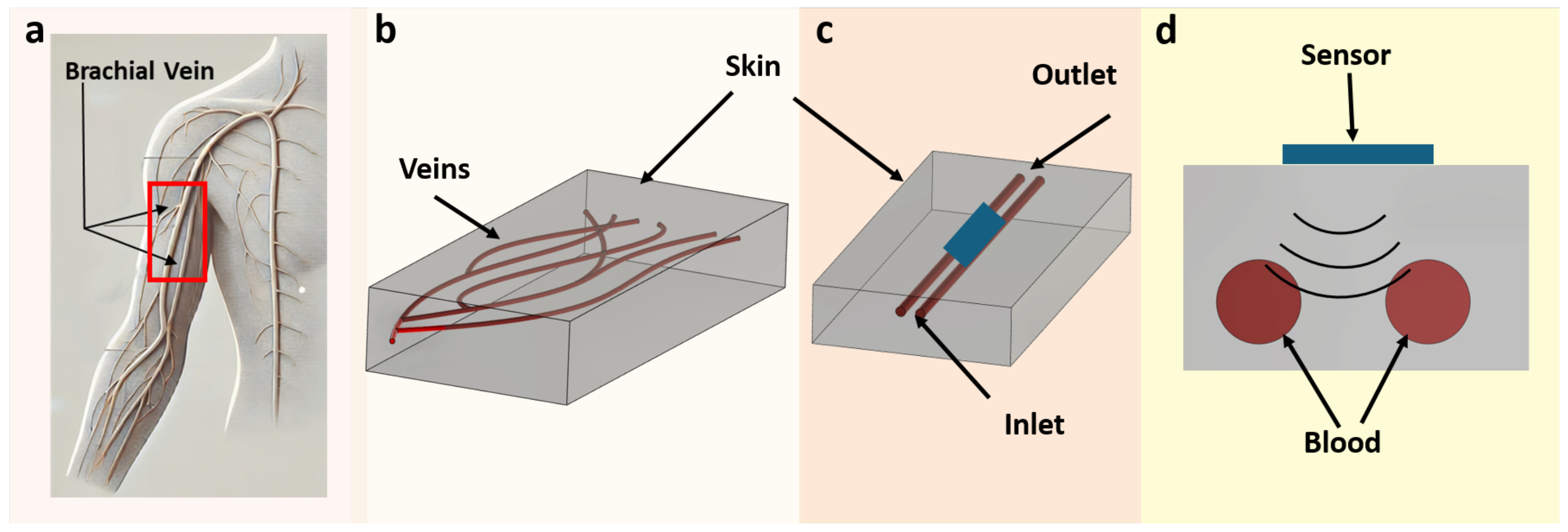
2.1. Phantom Design and Composition
2.2. Construction of the Phantom
2.3. Preparation of Blood-Mimicking Solutions
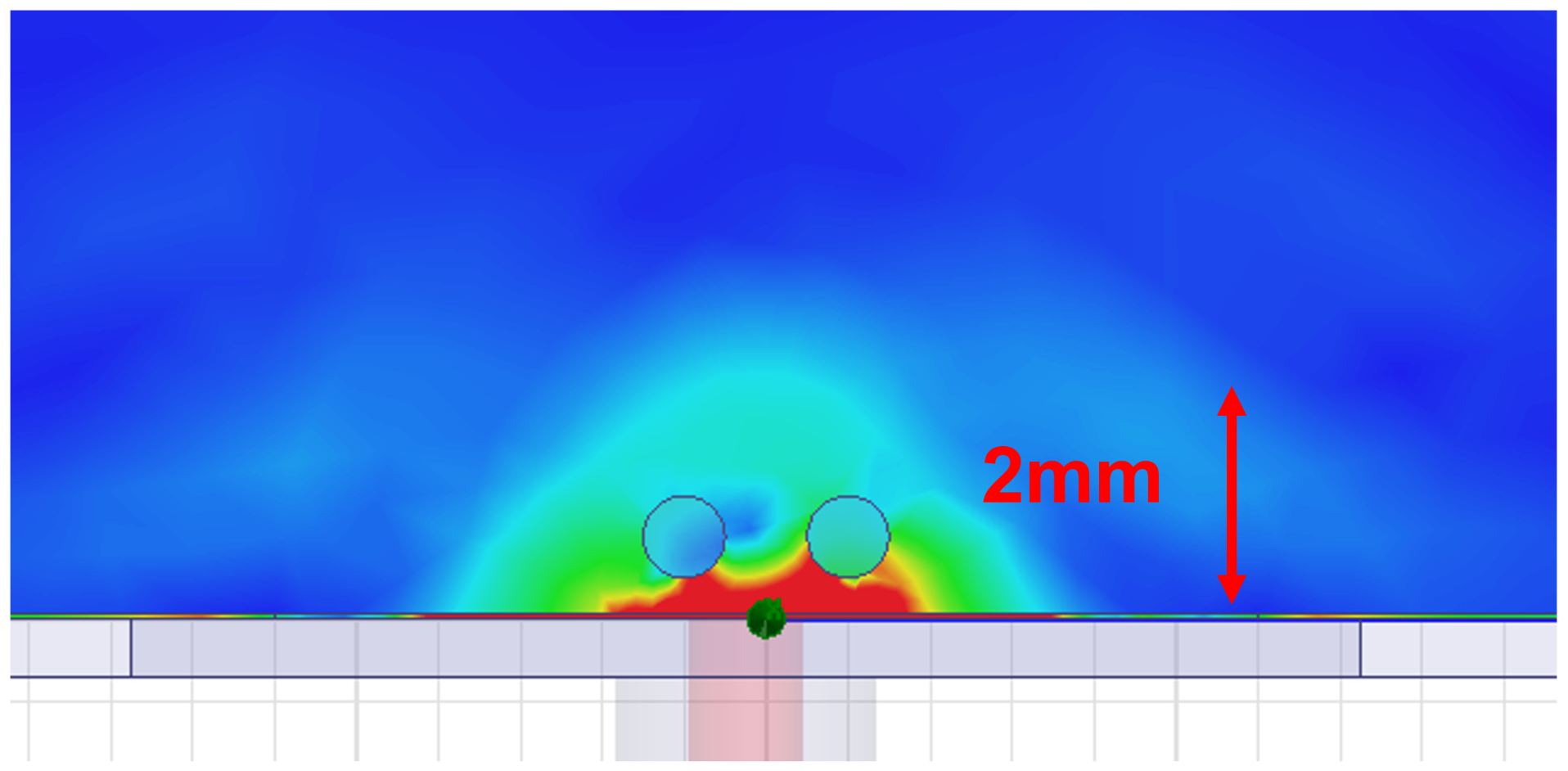
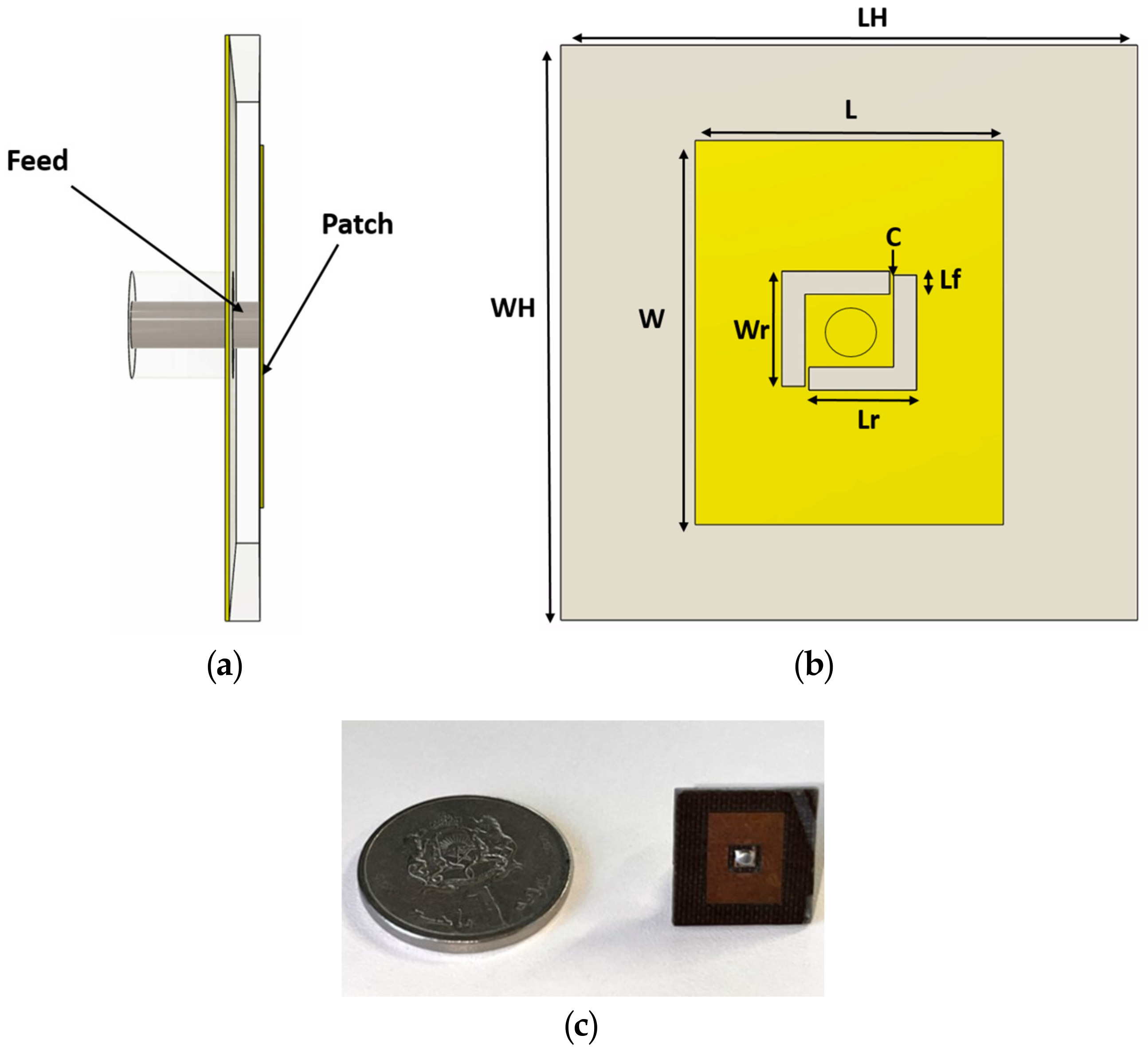
2.4. Antenna Design and Simulation
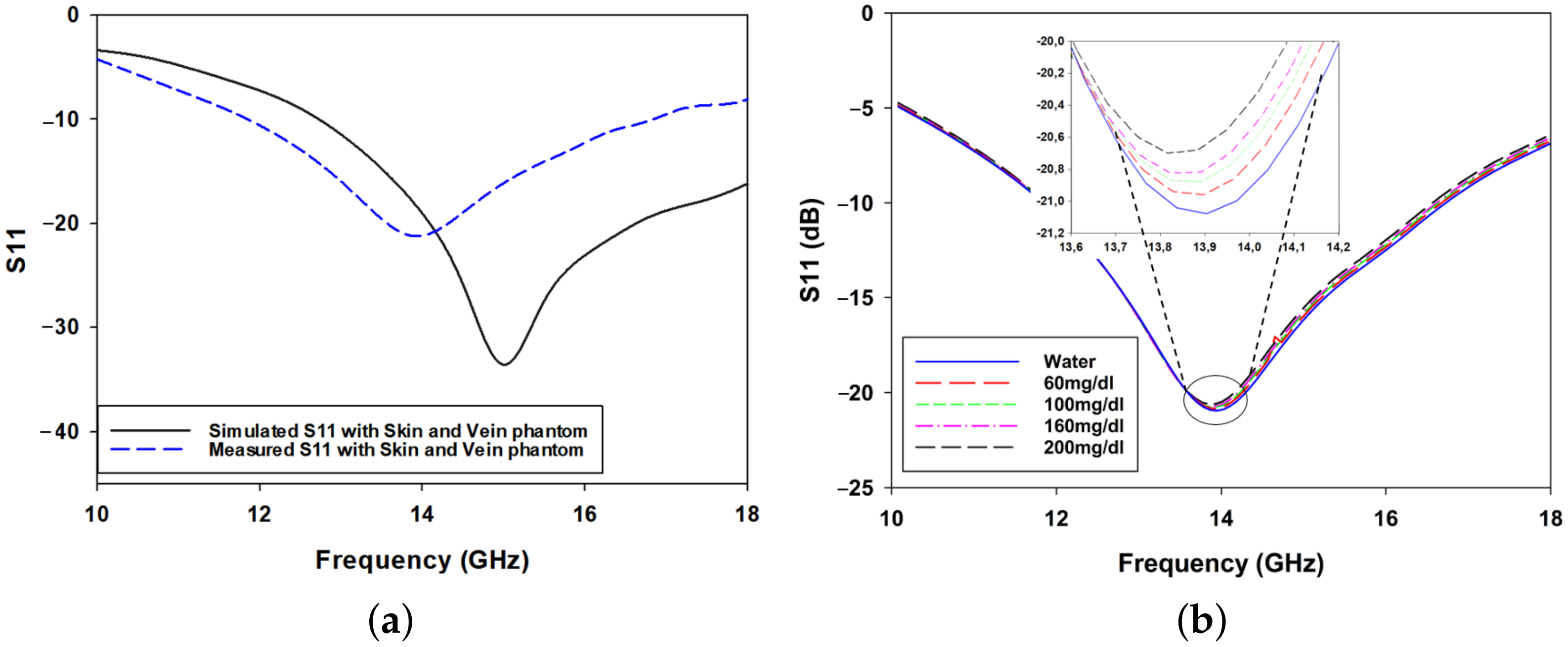
3. Experimental Results
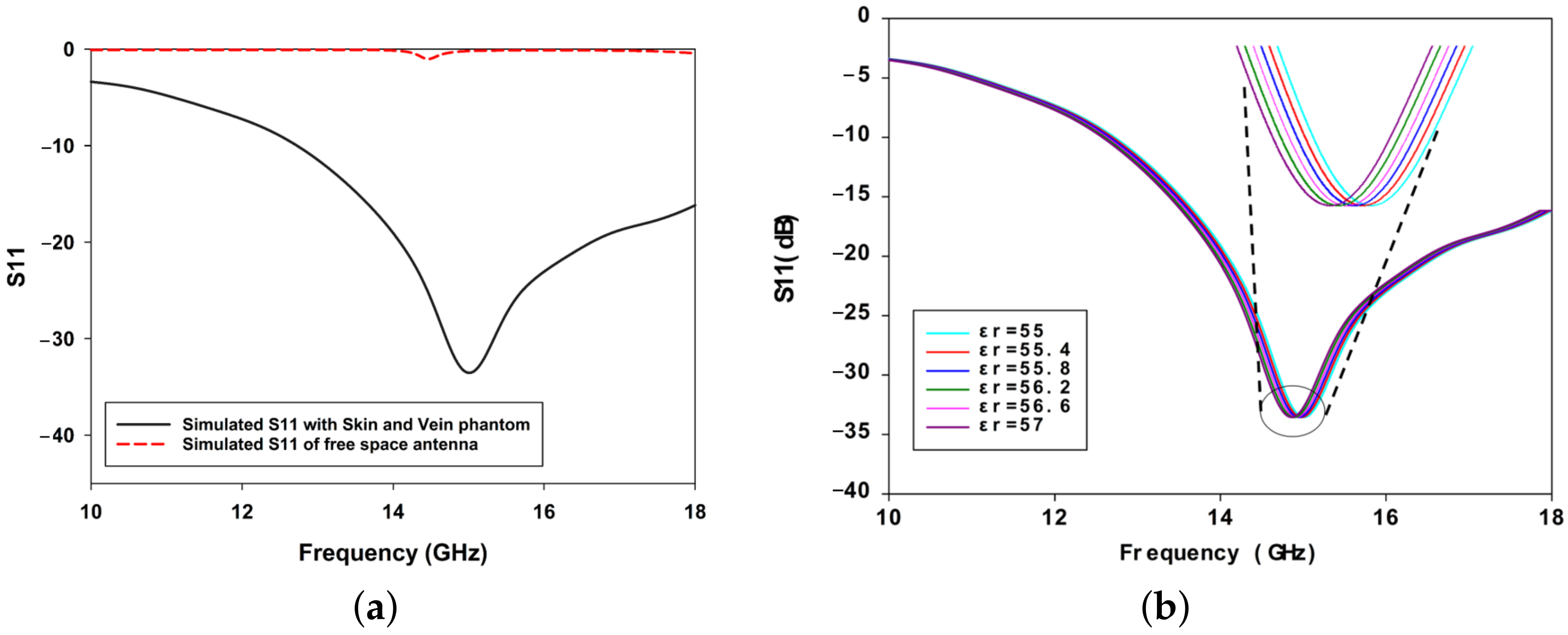
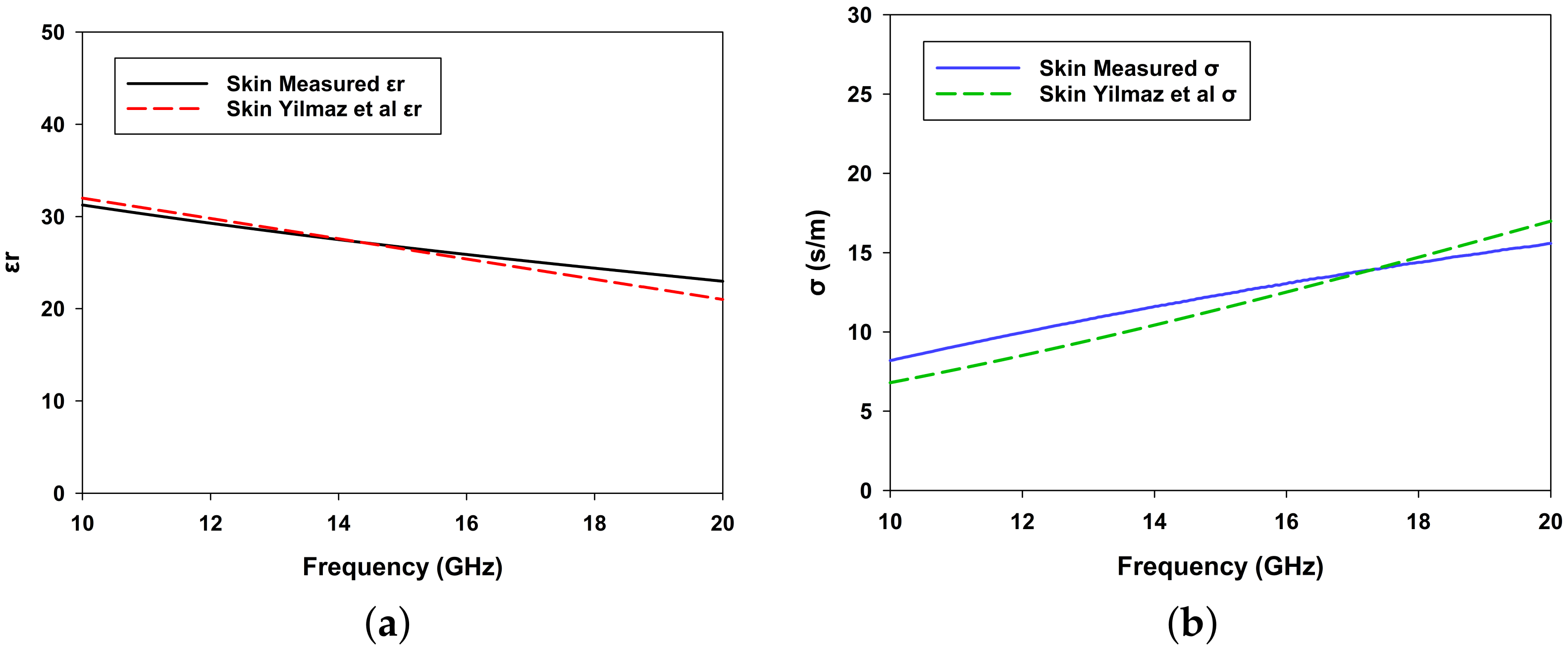
3.1. Dielectric Properties of the Phantom Skin and Blood-Mimicking Glucose Solution
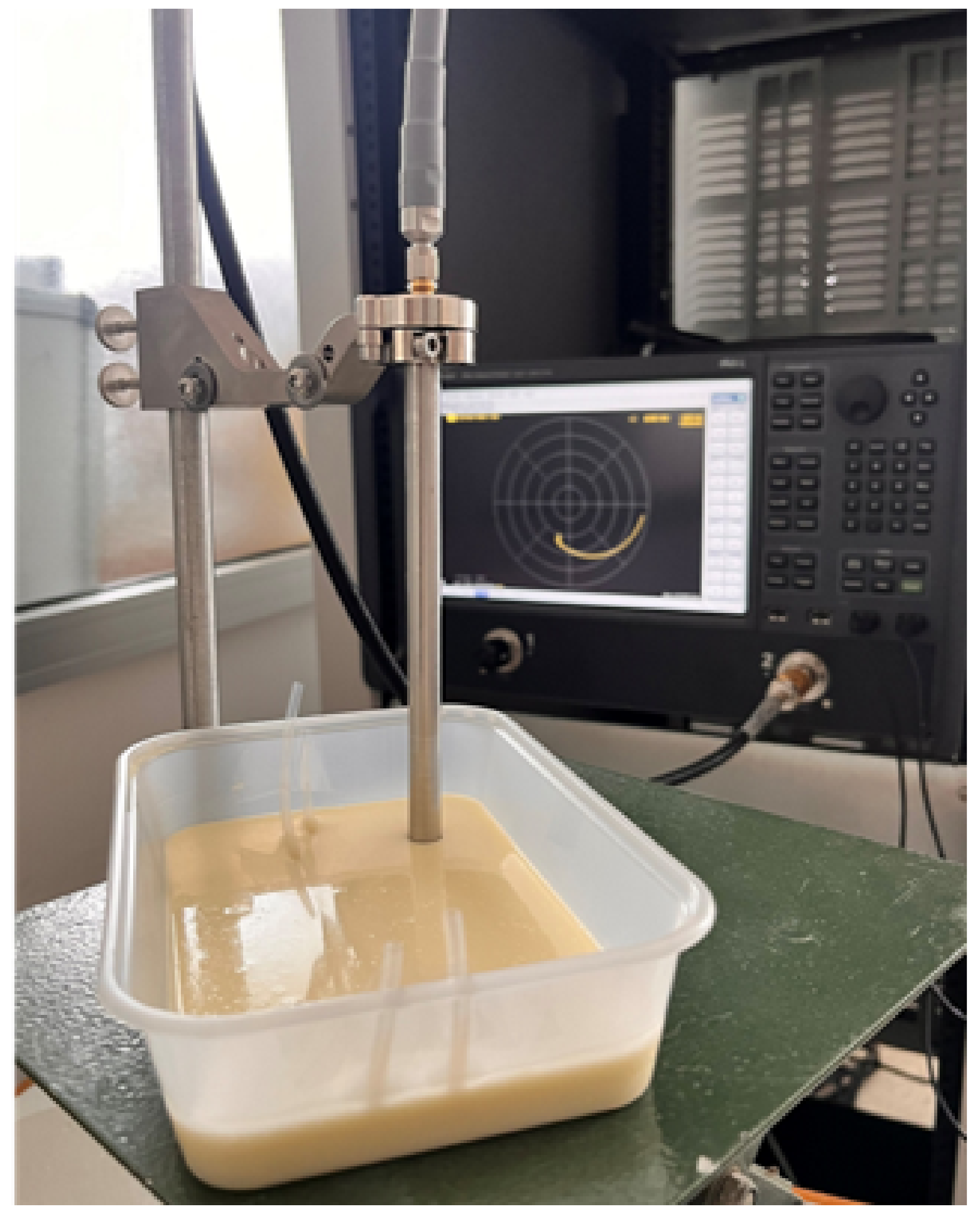
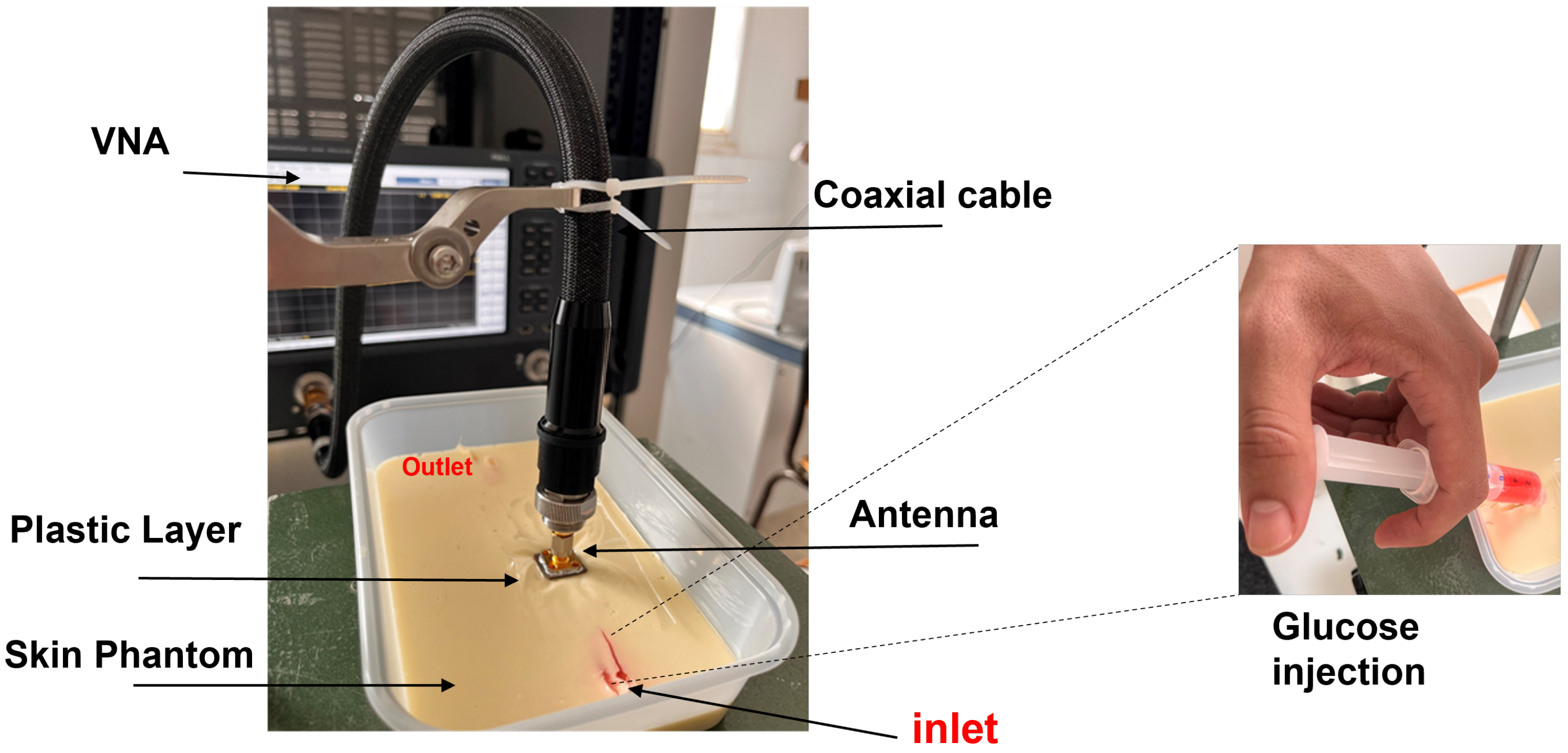
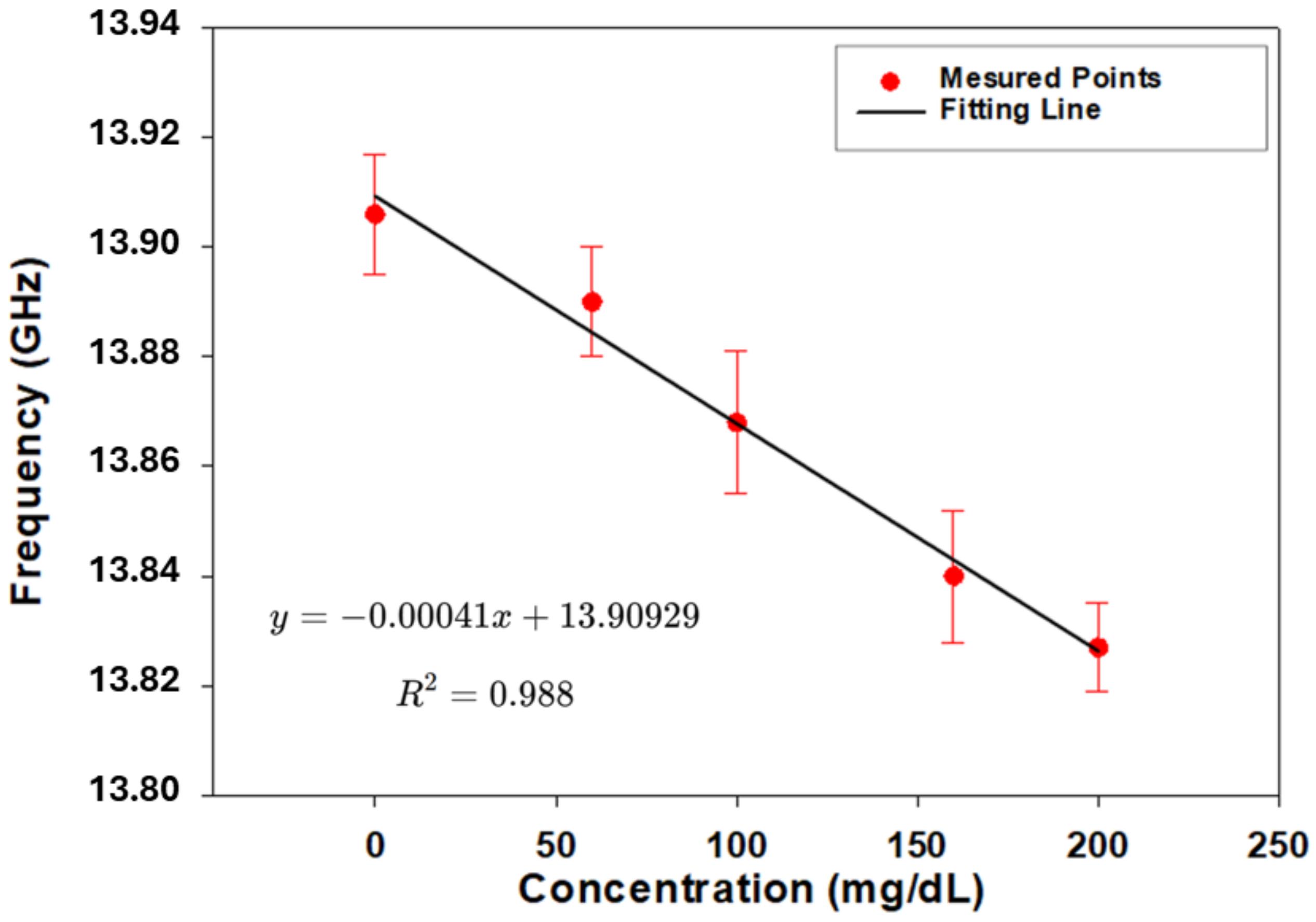
3.2. Measurement Setup: Skin Phantom with Glucose Solutions Mimicking Physiological Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar]
- Kim, S.E.; Han, K.; Cho, W.K.; Suh, B.K. Cardiovascular Complications, Kidney Failure, and Mortality in Young-Onset Type 1 and 2 Diabetes: Data from the Korean National Health Insurance Service. Diabetes Care 2024, 48, 422–429. [Google Scholar]
- Rossing, P.; Persson, F.; Frimodt-Møller, M.; Hansen, T.W. Linking kidney and cardiovascular complications in diabetes—Impact on prognostication and treatment: The 2019 Edwin Bierman award lecture. Diabetes 2021, 70, 39–50. [Google Scholar] [PubMed]
- Swamy, S.; Noor, S.M.; Mathew, R.O. Cardiovascular disease in diabetes and chronic kidney disease. J. Clin. Med. 2023, 12, 6984. [Google Scholar] [CrossRef]
- Seidu, S.; Kunutsor, S.K.; Ajjan, R.A.; Choudhary, P. Efficacy and safety of continuous glucose monitoring and intermittently scanned continuous glucose monitoring in patients with type 2 diabetes: A systematic review and meta-analysis of interventional evidence. Diabetes Care 2024, 47, 169–179. [Google Scholar]
- Kerr, D.; Duncan, I.; Repetto, E.; Maroun, R.; Wu, A.; Perkins, C.; Bergman, G.; Giorgino, F. Cost analysis of self-monitoring blood glucose in nonintensively managed type 2 diabetes. Am. J. Manag. Care 2023, 29, 670–675. [Google Scholar]
- Shi, L.; Fonseca, V.; Childs, B. Economic burden of diabetes-related hypoglycemia on patients, payors, and employers. J. Diabetes Its Complicat. 2021, 35, 107916. [Google Scholar]
- Rodbard, D. Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technol. Ther. 2016, 18, S2–S3. [Google Scholar]
- Nava, C.; Hedenmalm, A.M.; Borys, F.; Hooft, L.; Bruschettini, M.; Jenniskens, K. Accuracy of continuous glucose monitoring in preterm infants: A systematic review and meta-analysis. BMJ Open 2020, 10, e045335. [Google Scholar]
- Fleming, G.A.; Petrie, J.R.; Bergenstal, R.M.; Holl, R.W.; Peters, A.L.; Heinemann, L. Diabetes digital app technology: Benefits, challenges, and recommendations. A consensus report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) Diabetes Technology Working Group. Diabetes Care 2020, 43, 250–260. [Google Scholar]
- Di Filippo, D.; Sunstrum, F.N.; Khan, J.U.; Welsh, A.W. Non-invasive glucose sensing technologies and products: A comprehensive review for researchers and clinicians. Sensors 2023, 23, 9130. [Google Scholar] [CrossRef] [PubMed]
- Peel, E.; Parry, O.; Douglas, M.; Lawton, J. Blood glucose self-monitoring in non-insulin-treated type 2 diabetes: A qualitative study of patients’ perspectives. Br. J. Gen. Pract. 2004, 54, 183–188. [Google Scholar] [PubMed]
- Gonzales, W.V.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-invasive blood glucose monitoring technology: A review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Yilmaz, T.; Foster, R.; Hao, Y. Radio-frequency and microwave techniques for non-invasive measurement of blood glucose levels. Diagnostics 2019, 9, 6. [Google Scholar] [CrossRef]
- Kiani, S.; Rezaei, P.; Fakhr, M. Dual-frequency microwave resonant sensor to detect noninvasive glucose-level changes through the fingertip. IEEE Trans. Instrum. Meas. 2021, 70, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Z.; Yue, W.; Zhang, C.; Gao, S.; Wang, C. Reusable, non-invasive, and ultrafast radio frequency biosensor based on optimized integrated passive device fabrication process for quantitative detection of glucose levels. Sensors 2020, 20, 1565. [Google Scholar] [CrossRef]
- Yunos, M.F.A.M.; Manczak, R.; Guines, C.; Mansor, A.F.M.; Mak, W.C.; Khan, S.; Ramli, N.A.; Pothier, A.; Nordin, A.N. RF remote blood glucose sensor and a microfluidic vascular phantom for sensor validation. Biosensors 2021, 11, 494. [Google Scholar] [CrossRef]
- Adhikari, K.K.; Kim, N.Y. Ultrahigh-sensitivity mediator-free biosensor based on a microfabricated microwave resonator for the detection of micromolar glucose concentrations. IEEE Trans. Microw. Theory Tech. 2015, 64, 319–327. [Google Scholar] [CrossRef]
- Kazemi, N.; Abdolrazzaghi, M.; Light, P.E.; Musilek, P. In–human testing of a non-invasive continuous low–energy microwave glucose sensor with advanced machine learning capabilities. Biosens. Bioelectron. 2023, 241, 115668. [Google Scholar]
- Martins, A.J.; Velásquez, R.J.; Gaillac, D.B.; Santos, V.N.; Tami, D.C.; Souza, R.N.; Osorio, F.C.; Fogli, G.A.; Soares, B.S.; do Rego, C.G.; et al. A comprehensive review of non-invasive optical and microwave biosensors for glucose monitoring. Biosens. Bioelectron. 2024, 271, 117081. [Google Scholar]
- Costanzo, S.; Cioffi, V.; Qureshi, A.M.; Borgia, A. Gel-like human mimicking phantoms: Realization procedure, dielectric characterization and experimental validations on microwave wearable body sensors. Biosensors 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Matrone, G.; Magenes, G.; Pasian, M. On the Low-Cost Production of Tissue-Mimicking Skin Phantoms Up to 40 GHz. IEEE J. Electromagn. RF Microwaves Med. Biol. 2024, 8, 51–58. [Google Scholar]
- Yilmaz, T.; Foster, R.; Hao, Y. Broadband tissue mimicking phantoms and a patch resonator for evaluating noninvasive monitoring of blood glucose levels. IEEE Trans. Antennas Propag. 2014, 62, 3064–3075. [Google Scholar]
- Särestöniemi, M.; Singh, D.; Dessai, R.; Heredia, C.; Myllymäki, S.; Myllylä, T. Realistic 3d phantoms for validation of microwave sensing in health monitoring applications. Sensors 2024, 24, 1975. [Google Scholar] [CrossRef]
- Coles-Black, J.; Bolton, D.; Chuen, J. Accessing 3D printed vascular phantoms for procedural simulation. Front. Surg. 2021, 7, 626212. [Google Scholar] [CrossRef]
- Pacioni, A.; Carbone, M.; Freschi, C.; Viglialoro, R.; Ferrari, V.; Ferrari, M. Patient-specific ultrasound liver phantom: Materials and fabrication method. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1065–1075. [Google Scholar]
- Ionita, C.N.; Mokin, M.; Varble, N.; Bednarek, D.R.; Xiang, J.; Snyder, K.V.; Siddiqui, A.H.; Levy, E.I.; Meng, H.; Rudin, S. Challenges and limitations of patient-specific vascular phantom fabrication using 3D Polyjet printing. In Proceedings of SPIE–The International Society for Optical Engineering; NIH Public Access: Bethesda, MD, USA, 2014; Volume 9038. [Google Scholar]
- Spivack, D.E.; Kelly, P.; Gaughan, J.P.; van Bemmelen, P.S. Mapping of superficial extremity veins: Normal diameters and trends in a vascular patient-population. Ultrasound Med. Biol. 2012, 38, 190–194. [Google Scholar]
- El Arroud, F.Z.; El Fakhouri, K.; Zaarour, Y.; Ramdani, C.; Griguer, H.; Aznabet, M.; Alami, R.E.; El Bouhssini, M. Thermal effect of microwave radiation on Dactylopius opuntiae in Morocco and coaxial probe for permittivity measurements. IEEE Access 2024, 12, 80910–80921. [Google Scholar]
- Zaarour, Y.; Cano, J.L.; Fernandez, T.; El Arroud, F.; Faik, A.; El Alami, R.; Griguer, H. Engine Oil Quality Monitoring Using an Additively Manufactured X-Band Microwave Waveguide Sensor. IEEE Access 2024, 12, 181341–181350. [Google Scholar]
- Zaarour, Y.; El Arroud, F.; El Mrabet, O.; Faik, A.; El Alami, R.; Griguer, H. Real-time characterization of phosphate powder with microwave sensor: Investigating the impact of temperature. IEEE Sens. J. 2024, 24, 27847–27858. [Google Scholar]
- Juan, C.G.; Potelon, B.; Quendo, C.; Bronchalo, E. Microwave planar resonant solutions for glucose concentration sensing: A systematic review. Appl. Sci. 2021, 11, 7018. [Google Scholar] [CrossRef]


| Material | Amount (g) | Percentage (%) |
|---|---|---|
| Water | 34 | 55 |
| Gelatine | 6 | 10 |
| Oil | 19 | 30 |
| Soap | 1.8 | 2.8 |
| Salt | 1.4 | 2.2 |
| Parameter | Description | Value (mm) |
|---|---|---|
| W | Width of the patch | 8 |
| L | Length of the patch | 10 |
| Width of the resonant arm | 2.8 | |
| Length of the resonant arm | 3 | |
| C | Feed gap spacing | 0.1 |
| Substrate width | 15 | |
| Substrate length | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaarour, Y.; El Arroud, F.; Fernandez, T.; Cano, J.L.; El Alami, R.; El Mrabet, O.; Benani, A.; Faik, A.; Griguer, H. Microwave Antenna Sensing for Glucose Monitoring in a Vein Model Mimicking Human Physiology. Biosensors 2025, 15, 282. https://doi.org/10.3390/bios15050282
Zaarour Y, El Arroud F, Fernandez T, Cano JL, El Alami R, El Mrabet O, Benani A, Faik A, Griguer H. Microwave Antenna Sensing for Glucose Monitoring in a Vein Model Mimicking Human Physiology. Biosensors. 2025; 15(5):282. https://doi.org/10.3390/bios15050282
Chicago/Turabian StyleZaarour, Youness, Fatimazahrae El Arroud, Tomas Fernandez, Juan Luis Cano, Rafiq El Alami, Otman El Mrabet, Abdelouheb Benani, Abdessamad Faik, and Hafid Griguer. 2025. "Microwave Antenna Sensing for Glucose Monitoring in a Vein Model Mimicking Human Physiology" Biosensors 15, no. 5: 282. https://doi.org/10.3390/bios15050282
APA StyleZaarour, Y., El Arroud, F., Fernandez, T., Cano, J. L., El Alami, R., El Mrabet, O., Benani, A., Faik, A., & Griguer, H. (2025). Microwave Antenna Sensing for Glucose Monitoring in a Vein Model Mimicking Human Physiology. Biosensors, 15(5), 282. https://doi.org/10.3390/bios15050282






