Recent Advances in Natural-Polymer-Based Hydrogels for Body Movement and Biomedical Monitoring
Abstract
1. Introduction
2. Natural-Polymer-Based Hydrogels
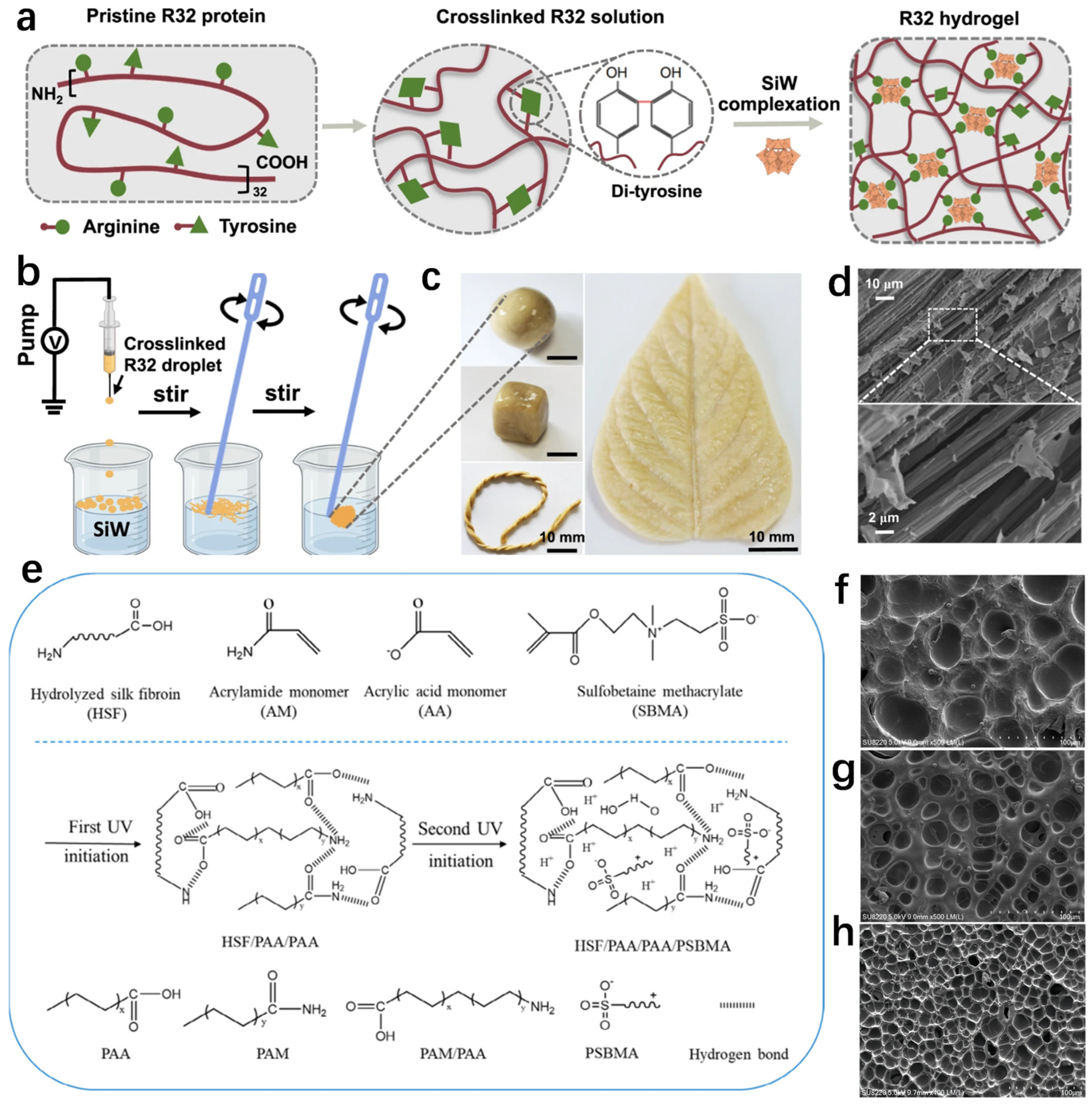
2.1. Protein-Based Hydrogel
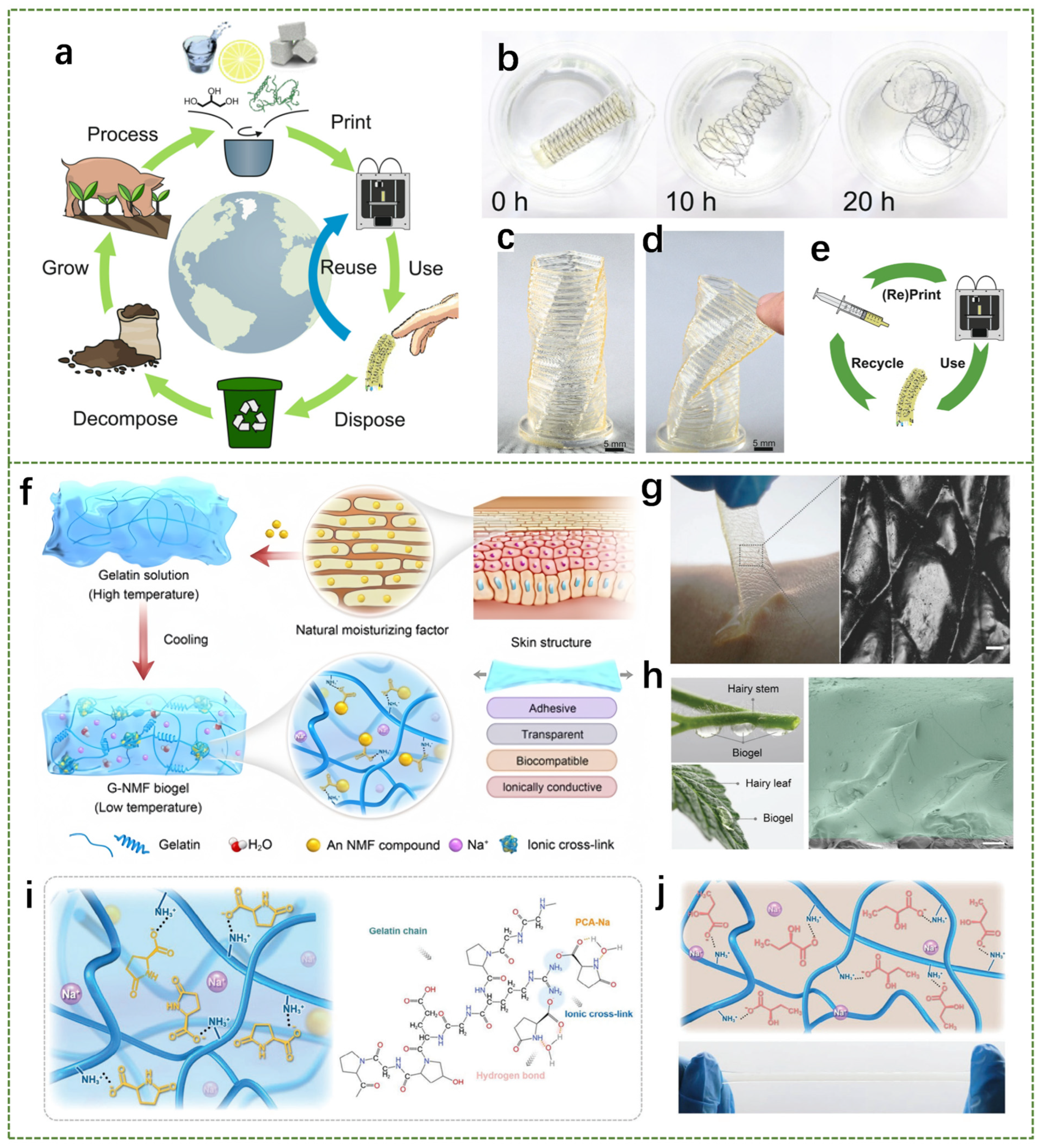
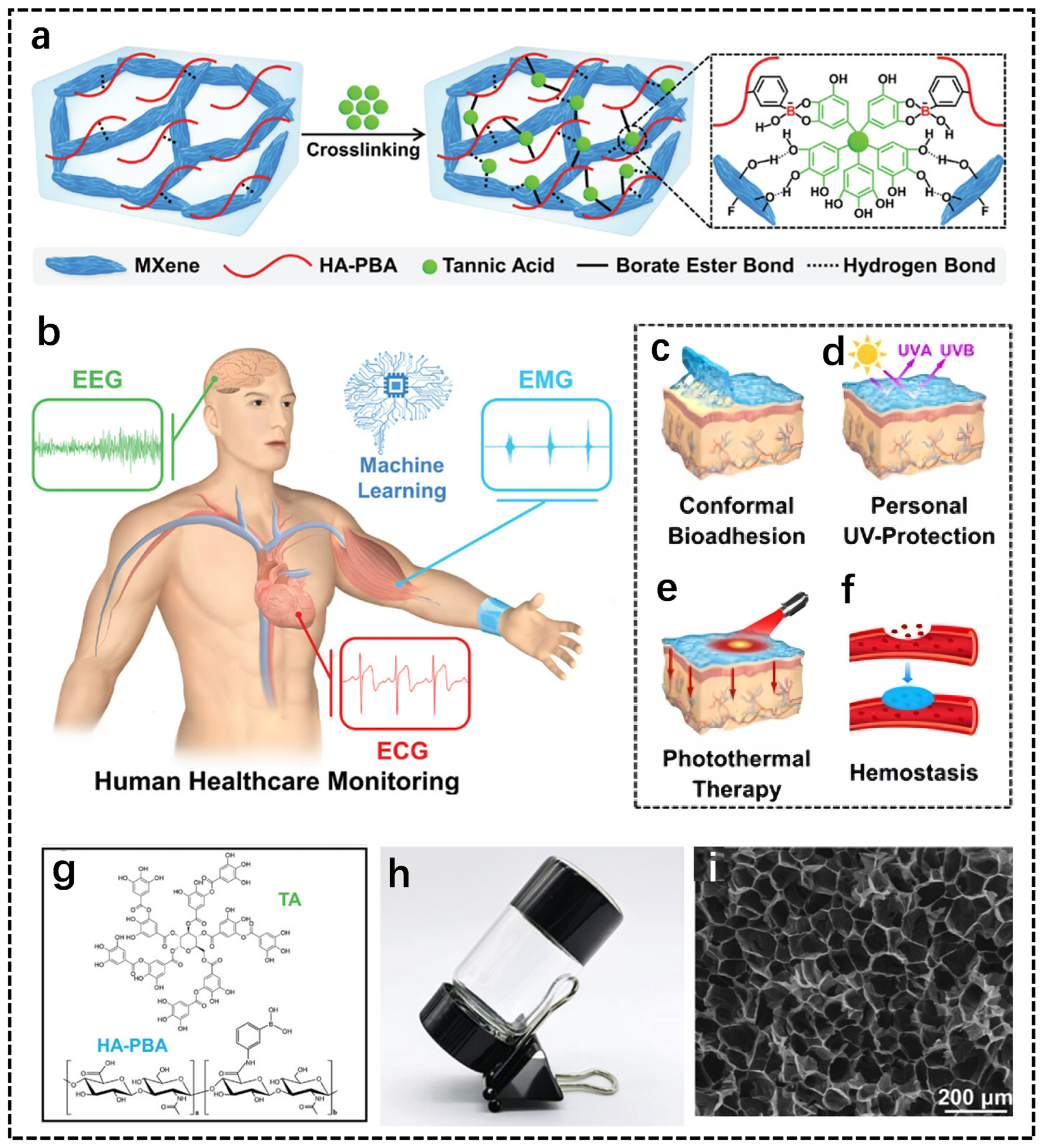
2.2. Polysaccharide-Based Hydrogel

3. Applications
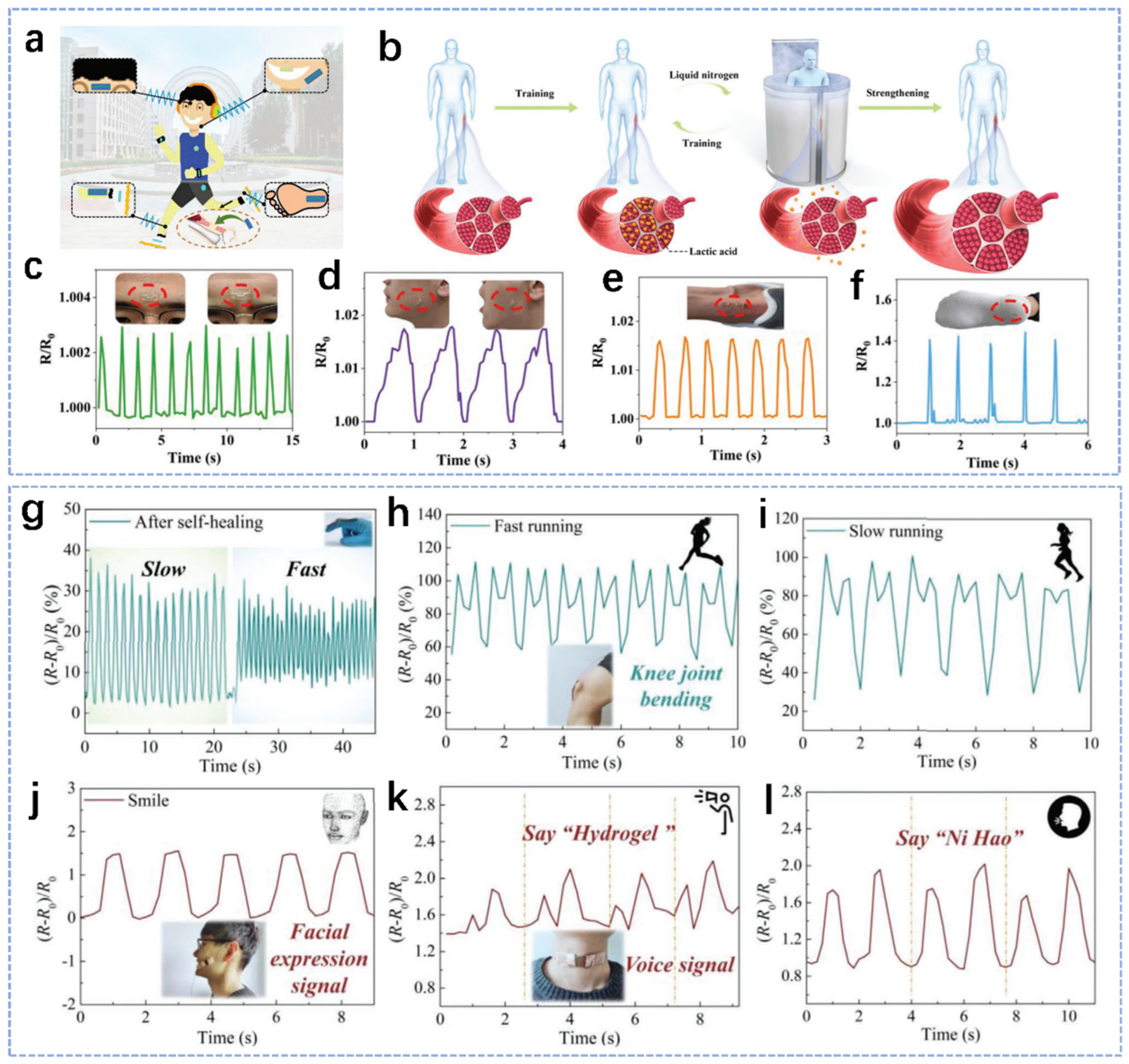
3.1. Body Movement Detection
3.2. Biomedical Monitoring
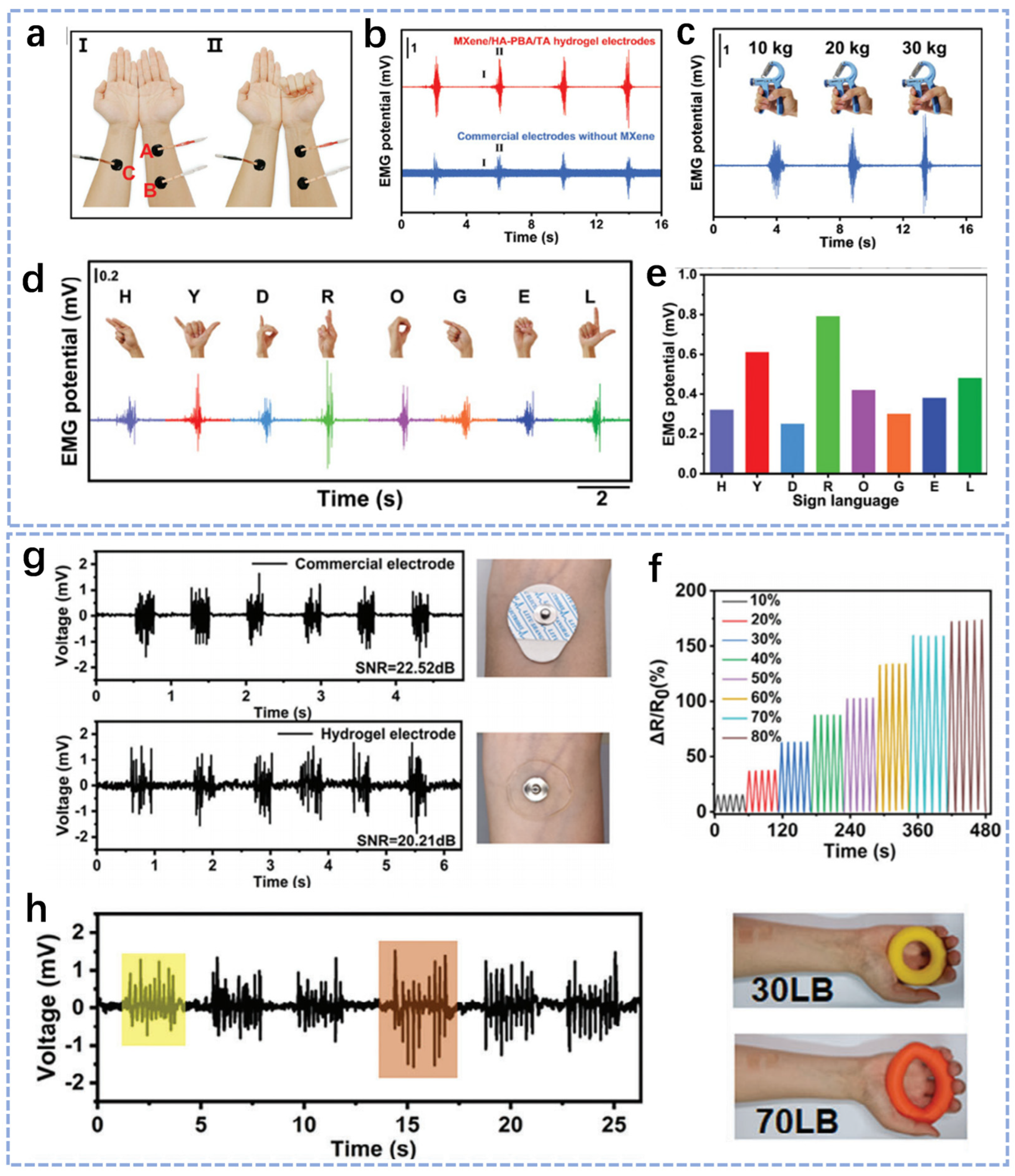
3.2.1. EMG
3.2.2. ECG
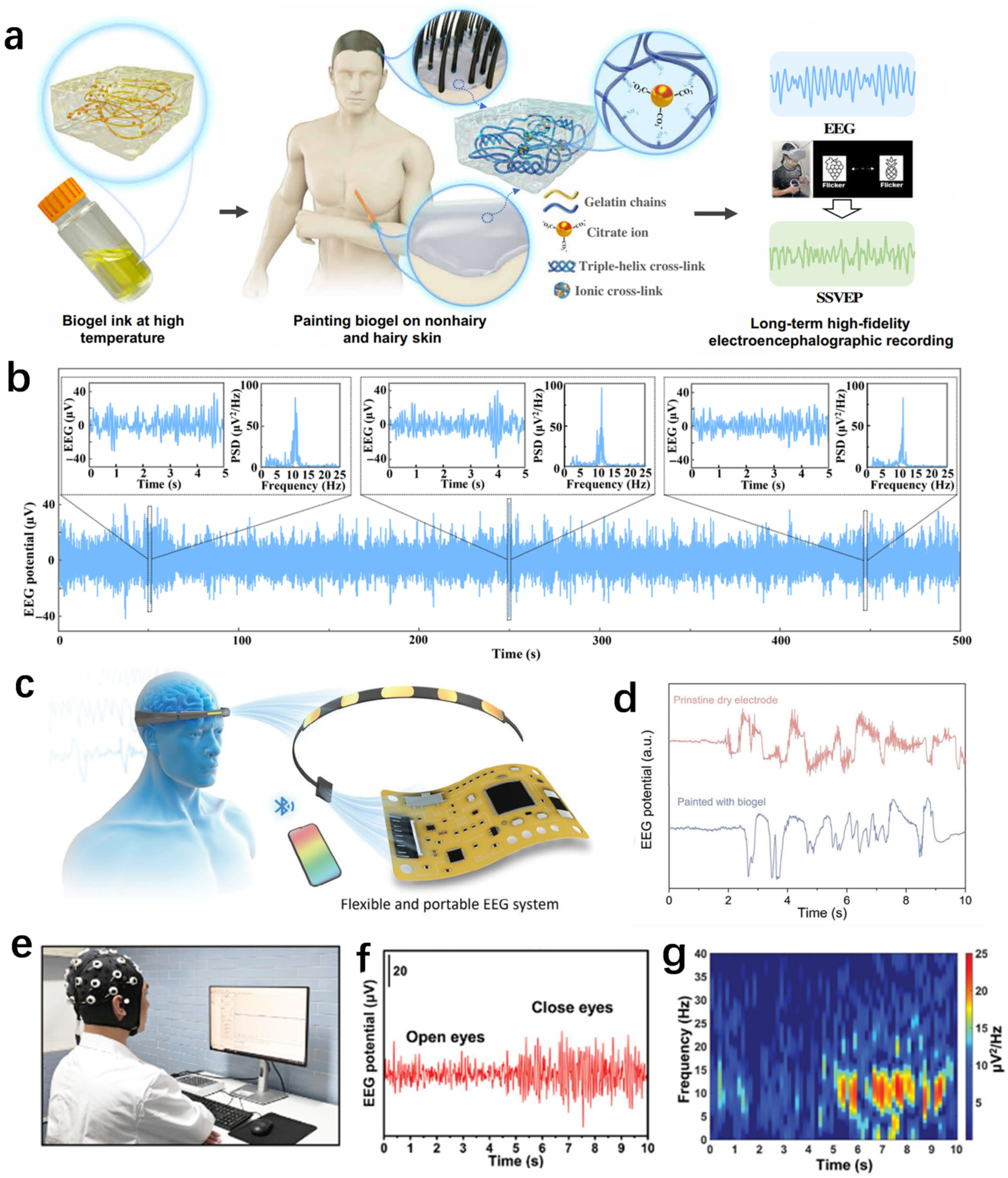
3.2.3. EEG
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| ECG | electrocardiogram |
| EMG | electromyogram |
| EEG | electroencephalogram |
| SEM | scanning electron microscope |
| SF | silk fibroin |
| CNFs | cellulose nanofibrils |
| Gly | glycerol |
| TA@CNF | tannic acid-decorated callulose nanofibrils |
| PTCM-Gly | PAAm-TA@CNF-MXene-Gly |
| TA | tannic acid |
| PAAm | polyacrylamide |
| PAAm SN | single network polyacrylamide hydrogel |
| DN | double network |
| fc-DN | fiber-connected dual networks |
| f-DN | fibrillar dual networks |
| C-DN | connected dual networks |
| DA | degree of acrylation |
| AG | agarose |
| AcAG | acrylated agarose |
| AAm | acrylamide |
| HA | hyaluronic acid |
| Ti3C2Tx | MDlene |
| PBA | phenylboronic acid |
| TA | tannic acid |
| PBS | phosphate buffered saline |
| HMI | human-machine interaction |
| PCA-Na | sodium pyrrolidone carboxylic acid |
References
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D.; et al. Hydrogel-Based Flexible Electronics. Adv. Mater. 2023, 35, 2205326. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, T.Y.; Huang, Z.D.; Yang, J. A New Class of Electronic Devices Based on Flexible Porous Substrates. Adv. Sci. 2022, 9, 2105084. [Google Scholar] [CrossRef]
- Zhao, W.C.; Zhou, H.F.; Li, W.K.; Chen, M.L.; Zhou, M.; Zhao, L. An Environment-Tolerant Ion-Conducting Double-Network Composite Hydrogel for High-Performance Flexible Electronic Devices. Nano-Micro Lett. 2024, 16, 99. [Google Scholar] [CrossRef]
- Zheng, X.R.; Hu, M.S.; Liu, Y.X.; Zhang, J.; Li, X.X.; Li, X.M.; Yang, H. High-resolution flexible electronic devices by electrohydrodynamic jet printing: From materials toward applications. Sci. China-Mater. 2022, 65, 2089–2109. [Google Scholar] [CrossRef]
- Zhu, S.S.; Liu, Z.H.; Li, W.Y.; Zhang, H.W.; Dai, G.L.; Zhou, X. Research progress of self-healing polymer materials for flexible electronic devices. J. Polym. Sci. 2023, 61, 1554–1571. [Google Scholar] [CrossRef]
- Bao, R.R.; Wang, C.F.; Dong, L.; Shen, C.Y.; Zhao, K.; Pan, C.F. CdS nanorods/organic hybrid LED array and the piezo-phototronic effect of the device for pressure mapping. Nanoscale 2016, 8, 8078–8082. [Google Scholar] [CrossRef]
- Xiao, X.; Zheng, Z.Y.; Zhong, X.W.; Gao, R.H.; Piao, Z.H.; Jiao, M.L.; Zhou, G.M. Rational Design of Flexible Zn-Based Batteries for Wearable Electronic Devices. ACS Nano 2023, 17, 1764–1802. [Google Scholar] [CrossRef]
- Li, W.W.; Liu, J.; Wei, J.N.; Yang, Z.Y.; Ren, C.L.; Li, B.X. Recent Progress of Conductive Hydrogel Fibers for Flexible Electronics: Fabrications, Applications, and Perspectives. Adv. Funct. Mater. 2023, 33, 2213485. [Google Scholar] [CrossRef]
- Liu, D.J.; Zhu, P.C.; Zhang, F.K.; Li, P.S.; Huang, W.H.; Li, C.; Han, N.N.; Mu, S.R.; Zhou, H.; Mao, Y.C. Intrinsically stretchable polymer semiconductor based electronic skin for multiple perceptions of force, temperature, and visible light. Nano Res. 2023, 16, 1196–1204. [Google Scholar] [CrossRef]
- Li, J.; Carlos, C.; Zhou, H.; Sui, J.J.; Wang, Y.K.; Silva-Pedraza, Z.; Yang, F.; Dong, Y.T.; Zhang, Z.Y.; Hacker, T.A.; et al. Stretchable piezoelectric biocrystal thin films. Nat. Commun. 2023, 14, 6562. [Google Scholar] [CrossRef]
- Vu, C.; Truong, T.; Kim, J. Fractal structures in flexible electronic devices. Mater. Today Phys. 2022, 27, 100795. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Qu, H.Q.; Diao, S. Flexible Inductance Pressure Sensor for Wearable Electronic Devices. J. Nanoelectron. Optoelectron. 2023, 18, 652–662. [Google Scholar] [CrossRef]
- Xiang, X.; He, Q.; Xia, S.; Deng, Z.J.; Zhang, H.F.; Li, H.L. Study of capacitance type flexible electronic devices based on polyacrylamide and reduced graphene oxide composite hydrogel. Eur. Polym. J. 2022, 171, 111200. [Google Scholar] [CrossRef]
- Feng, T.X.; Ling, D.; Li, C.Y.; Zheng, W.T.; Zhang, S.C.; Li, C.; Emel’yanov, A.; Pozdnyakov, A.S.; Lu, L.J.; Mao, Y.C. Stretchable on-skin touchless screen sensor enabled by ionic hydrogel. Nano Res. 2023, 17, 4462–4470. [Google Scholar] [CrossRef]
- Jiang, J.X.; Chen, X.; Mei, Z.X.; Chen, H.T.; Chen, J.Y.; Wang, X.; Li, S.F.; Zhang, R.Y.; Zheng, G.F.; Li, W.W. Review of Droplet Printing Technologies for Flexible Electronic Devices: Materials, Control, and Applications. Micromachines 2024, 15, 333. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, S.C.; Yin, H.F.; Weisbecker, H.; Tran, H.T.; Guo, Z.H.; Han, T.H.; Wang, Y.H.; Liu, Y.H.; Wu, Y.Z.; et al. Skin-inspired, sensory robots for electronic implants. Nat. Commun. 2024, 15, 4777. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Cao, L.Q.; Cui, J.J.; Fu, G.W.; Jiang, R.Y.; Xu, X.; Guan, C. Flexible Energy Storage Devices to Power the Future. Adv. Mater. 2024, 36, e2306090. [Google Scholar] [CrossRef]
- Jin, R.N.; Xu, J.J.; Duan, L.J.; Gao, G.H. Chitosan-driven skin-attachable hydrogel sensors toward human motion and physiological signal monitoring. Carbohydr. Polym. 2021, 268, 118240. [Google Scholar] [CrossRef]
- Li, C.; Bu, F.; Wang, Q.Z.; Liu, X.Y. Recent Developments of Inkjet-Printed Flexible Energy Storage Devices. Adv. Mater. Interfaces 2022, 9, 2201051. [Google Scholar] [CrossRef]
- Chen, J.Y.; Liu, F.F.; Abdiryim, T.; Liu, X. An overview of conductive composite hydrogels for flexible electronic devices. Adv. Compos. Hybrid Mater. 2024, 7, 35. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart materials for flexible electronics and devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.Y.; Cai, Z.F.; Ding, Z.Q.; Chen, T.Y.; Zhang, J.C.; Chen, F.B.; Shen, J.Y.; Chen, F.; Li, R.; Zhou, X.C.; et al. Skin-Inspired Surface-Microstructured Tough Hydrogel Electrolytes for Stretchable Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 21895–21903. [Google Scholar] [CrossRef] [PubMed]
- Badawi, M.N.; Kuniyil, M.; Bhatia, M.; Kumar, S.S.A.; Mrutunjaya, B.; Luqman, M.; Adil, S.F. Recent advances in flexible/stretchable hydrogel electrolytes in energy storage devices. J. Energy Storage 2023, 73, 108810. [Google Scholar] [CrossRef]
- Carbone, M. NiO-Based Electronic Flexible Devices. Appl. Sci. 2022, 12, 2839. [Google Scholar] [CrossRef]
- Li, H.L.; Lv, T.; Sun, H.H.; Qian, G.J.; Li, N.; Yao, Y.; Chen, T. Ultrastretchable and superior healable supercapacitors based on a double cross-linked hydrogel electrolyte. Nat. Commun. 2019, 10, 536. [Google Scholar] [CrossRef]
- Pan, Z.; Fu, Q.Q.; Wang, M.H.; Gao, H.L.; Dong, L.; Zhou, P.; Cheng, D.D.; Chen, Y.; Zou, D.H.; He, J.C.; et al. Designing nanohesives for rapid, universal, and robust hydrogel adhesion. Nat. Commun. 2023, 14, 5378. [Google Scholar] [CrossRef]
- Zhou, G.Q.; Li, M.C.; Liu, C.Z.; Chen, W.M.; Yu, G.M.; Zhang, D.T.; Li, Z.L.; Mei, C.T. A flexible Zn-ion capacitor based on wood derived porous carbon and polyacrylamide/cellulose nanofiber hydrogel. Ind. Crops Prod. 2023, 193, 116216. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhang, L.; Lin, X.Y.; Lu, J.; Huang, Z.; Sun, P.H.; Zhang, Y.B.; Xu, X.; Li, Q.T.; Liu, H. Dual-network polyvinyl alcohol/polyacrylamide/xanthan gum ionic conductive hydrogels for flexible electronic devices. Int. J. Biol. Macromol. 2023, 233, 123573. [Google Scholar] [CrossRef]
- Yuan, X.M.; Guo, C.R.; Wang, Z.J.; Jiang, H.W.; He, Y.; Xu, J.; Guo, B. Liquid Metal-Hydrogel Biosensor for Behavior and Sweat Monitoring. ACS Appl. Electron. Mater. 2023, 5, 1420–1428. [Google Scholar] [CrossRef]
- Zhang, N.; Qin, C.; Feng, T.X.; Li, J.; Yang, Z.R.; Sun, X.P.; Liang, E.J.; Mao, Y.C.; Wang, X.D. Non-contact cylindrical rotating triboelectric nanogenerator for harvesting kinetic energy from hydraulics. Nano Res. 2020, 13, 1903–1907. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Cai, X.J.; Lv, Y.; Tang, X.Y.; Shi, N.W.; Zhou, J.Z.; Yan, M.Y.; Li, Y.P. Self-healing, ultra-stretchable, and highly sensitive conductive hydrogel reinforced by sulfate polysaccharide from Enteromorpha prolifera for human motion sensing. Int. J. Biol. Macromol. 2023, 253, 126847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Y.Q.; Liu, Y.H.; Liu, L.X.; Yin, J.L.; Sun, B.Z.; Wang, G.L.; Zhang, Y.Q. A Self-Healing PVA-Linked Phytic Acid Hydrogel-Based Electrolyte for High-Performance Flexible Supercapacitors. Nanomaterials 2023, 13, 380. [Google Scholar] [CrossRef]
- Zheng, A.B.; Qin, Y.X.; Xia, Q.; Chen, Y.X.; Zhang, X.S. Multifunctional starch-based double-network hydrogels as electronic skin. J. Phys. D-Appl. Phys. 2023, 56, 465302. [Google Scholar] [CrossRef]
- Li, Z.L.; Lin, Z.Q. Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Rong, Q.F.; Lei, W.W.; Liu, M.J. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem.-Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Wan, H.X.; Chen, Y.; Tao, Y.Z.; Chen, P.; Wang, S.; Jiang, X.Y.; Lu, A. MXene-Mediated Cellulose Conductive Hydrogel with Ultrastretchability and Self-Healing Ability. ACS Nano 2023, 17, 20699–20710. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.T.; Chen, N.; Lu, B.; Xu, J.L.; Rodriguez, R.D.; Sheremet, E.; Hu, Y.D.; Chen, J.J. Flexible solid-state Zn-Co MOFs@MXene supercapacitors and organic ion hydrogel sensors for self-powered smart sensing applications. Nano Energy 2023, 118, 108936. [Google Scholar] [CrossRef]
- Yang, R.Z.; Guo, Z.P.; Yu, Z.H.; Du, F.Y.; Thyagaraja, V.G.N.; Lin, L.Q.; Yu, D.R.; Xu, P.C.; Armstrong, J.N.; Lin, S.T.; et al. 3D-printed conducting polymer hydrogel-based DC generator for self-powered electromechanical sensing. Nano Energy 2023, 117, 108857. [Google Scholar] [CrossRef]
- Cao, C.; Ji, S.B.; Jiang, Y.; Su, J.T.; Xia, H.R.; Li, H.C.; Tian, C.H.; Wong, Y.J.; Feng, X.; Chen, X.D. Mitigating the Overheat of Stretchable Electronic Devices Via High-Enthalpy Thermal Dissipation of Hydrogel Encapsulation. Adv. Mater. 2024, 36, e2401875. [Google Scholar] [CrossRef]
- Du, Y.C.; Kim, J.H.; Kong, H.; Li, A.A.; Jin, M.L.; Kim, D.H.; Wang, Y. Biocompatible Electronic Skins for Cardiovascular Health Monitoring. Adv. Healthc. Mater. 2024, 13, e2303461. [Google Scholar] [CrossRef]
- Gao, J.L.; Li, X.M.; Xu, L.A.; Yan, M.Q.; Bi, H.; Wang, Q.Y. Transparent multifunctional cellulose-based conductive hydrogel for wearable strain sensors and arrays. Carbohydr. Polym. 2024, 329, 121784. [Google Scholar] [CrossRef]
- Han, S.J.; Wu, Q.R.; Xu, Y.D.; Zhang, J.Y.; Chen, A.B.; Chen, Y.J.; Huang, J.R.; Yang, X.X.; Guan, L.H. Multi-Functional Eutectic Hydrogel for 3D Printable Flexible Omnidirectional Strain Sensors. Adv. Mater. Technol. 2023, 8, 2301123. [Google Scholar] [CrossRef]
- He, Q.H.; Cheng, Y.; Deng, Y.J.; Wen, F.; Lai, Y.K.; Li, H.Q. Conductive Hydrogel for Flexible Bioelectronic Device: Current Progress and Future Perspective. Adv. Funct. Mater. 2024, 34, 2308974. [Google Scholar] [CrossRef]
- Zhang, S.P.; Guo, F.M.; Gao, X.; Yang, M.D.; Huang, X.G.; Zhang, D.; Li, X.J.; Zhang, Y.J.; Shang, Y.Y. and Cao, A.Y. High-Strength, Antiswelling Directional Layered PVA/MXene Hydrogel for Wearable Devices and Underwater Sensing. Adv. Sci. 2024, 11, 202405880. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, N.; Tang, Y.; Wang, M.; Chao, M.; Liang, E. A paper triboelectric nanogenerator for self-powered electronic systems. Nanoscale 2017, 9, 14499–14505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Dong, L.Z.; Liu, L.Y.; Wu, Z.F.; Pan, D.D.; Liu, L.L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Qiao, Z.; Yang, M.; Wu, K.; Xin, N.N.; Xiao, J.M.; Liu, X.Y.; Wu, C.H.; Wei, D.; Sun, J.; et al. Hydrogen-bonding topological remodeling modulated ultra-fine bacterial cellulose nanofibril-reinforced hydrogels for sustainable bioelectronics. Biosens. Bioelectron. 2023, 231, 115288. [Google Scholar] [CrossRef]
- Zhu, S.H.; Zhou, Q.F.; Yi, J.; Xu, Y.H.; Fan, C.Y.; Lin, C.X.; Wu, J.Y.; Lin, Y.H. Using Wool Keratin as a Structural Biomaterial and Natural Mediator to Fabricate Biocompatible and Robust Bioelectronic Platforms. Adv. Sci. 2023, 10, 2207400. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in Biodegradable Electronic Skin: Material Progress and Recent Applications in Sensing, Robotics, and Human-Machine Interfaces. Adv. Mater. 2023, 35, e2203193. [Google Scholar] [CrossRef]
- Zhang, H.D.; Shi, L.W.E.; Zhou, J.P. Recent developments of polysaccharide-based double-network hydrogels. J. Polym. Sci. 2023, 61, 7–43. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liu, J.; Li, L.; Zheng, X.J.; Tang, K.Y.; Pei, Y. Collagen-Based Flexible Electronic Devices for Electrochemical Energy Storage and Sensing. Macromol. Rapid Commun. 2023, 44, e2200977. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.C.; Jiang, W.K.; Zhang, S.; Tong, L.; Mao, J.H.; Wei, G.; Zuo, M.; Ni, Y.H. Lignin sulfonate induced ultrafast polymerization of double network hydrogels with anti-freezing, high strength and conductivity and their sensing applications at extremely cold conditions. Compos. Part B-Eng. 2021, 217, 108879. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, J.; Xu, H.; Huang, C.H.; Lu, Y.; Cui, H.Y.; Tan, Y.B. Chitosan-driven biocompatible hydrogel based on water-soluble polypyrrole for stable human-machine interfaces. Carbohydr. Polym. 2022, 295, 119890. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Bai, Z.X.; Zheng, M.H.; Yue, O.Y.; Hou, M.D.; Cui, B.Q.; Su, R.R.; Wei, C.; Liu, X.H. Engineered gelatin-based conductive hydrogels for flexible wearable electronic devices: Fundamentals and recent advances. J. Sci. 2022, 7, 100451. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Zarrintaj, P.; Khodadadi, A.; Arefi, A.; Seidi, F.; Shokrani, H.; Saeb, M.R.; Mozafari, M. Polysaccharide-based electroconductive hydrogels: Structure, properties and biomedical applications. Carbohydr. Polym. 2022, 278, 118998. [Google Scholar] [CrossRef] [PubMed]
- You, L.J.; Zheng, Z.J.; Xu, W.J.; Wang, Y.; Xiong, W.J.; Xiong, C.H.; Wang, S.Y. Self-healing and adhesive MXene-polypyrrole/silk fibroin/polyvinyl alcohol conductive hydrogels as wearable sensor. Int. J. Biol. Macromol. 2024, 263, 130439. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.B.; Xing, Y.Z.; Sun, C.A.Y.; Fan, L.Q.; Shi, H.B.; Zhang, Q.H.; Li, Y.G.; Hou, C.Y.; Wang, H.Z. A bio-adhesive ion-conducting organohydrogel as a high-performance non-invasive interface for bioelectronics. Chem. Eng. J. 2022, 427, 130886. [Google Scholar] [CrossRef]
- Min, J.K.; Jung, Y.; Ahn, J.; Lee, J.G.; Lee, J.; Ko, S.H. Recent Advances in Biodegradable Green Electronic Materials and Sensor Applications. Adv. Mater. 2023, 35, e2211273. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.B.; Wang, J.Q.; Dai, X.F.; Shao, Z.Q.; Huang, X.N. Dual physically crosslinked healable polyacrylamide/cellulose nanofibers nanocomposite hydrogels with excellent mechanical properties. Carbohydr. Polym. 2018, 193, 73–81. [Google Scholar] [CrossRef]
- Seo, J.W.; Kim, H.; Kim, K.; Choi, S.Q.; Lee, H.J. Calcium-Modified Silk as a Biocompatible and Strong Adhesive for Epidermal Electronics. Adv. Funct. Mater. 2018, 28, 1800802. [Google Scholar] [CrossRef]
- Chen, M.Z.; Qian, X.Y.; Cai, J.; Zhou, J.P.; Lu, A. Electronic skin based on cellulose/KCl/sorbitol organohydrogel. Carbohydr. Polym. 2022, 292, 119645. [Google Scholar] [CrossRef]
- Han, X.K.; Lu, T.Y.; Zhang, Z.C.; Wang, H.; Lu, S.R. Tremella polysaccharide-based conductive hydrogel with anti-freezing and self-healing ability for motion monitoring and intelligent interaction. Int. J. Biol. Macromol. 2023, 248, 125987. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.Y.; Ping, J.F.; Xiong, J.Q.; Ying, Y.B. Sustainable Natural Bio-Origin Materials for Future Flexible Devices. Adv. Sci. 2022, 9, e2200560. [Google Scholar] [CrossRef]
- Lee, H.; Jang, J.; Lee, J.; Shin, M.; Lee, J.S.; Son, D. Stretchable Gold Nanomembrane Electrode with Ionic Hydrogel Skin-Adhesive Properties. Polymers 2023, 15, 3852. [Google Scholar] [CrossRef]
- Shi, Z.J.; Gao, X.; Ullah, M.W.; Li, S.X.; Wang, Q.; Yang, G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016, 111, 40–54. [Google Scholar] [CrossRef]
- Sun, X.; Mao, Y.M.; Yu, Z.Y.; Yang, P.; Jiang, F. A Biomimetic “Salting Out-Alignment-Locking” Tactic to Design Strong and Tough Hydrogel. Adv. Mater. 2024, 36, 2400084. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.Y.; Qi, R.L.; Yuan, H.X. Recent Advances of Natural-Polymer-Based Hydrogels for Wound Antibacterial Therapeutics. Polymers 2023, 15, 3305. [Google Scholar] [CrossRef]
- Zhu, T.X.; Mao, J.J.; Cheng, Y.; Liu, H.R.; Lv, L.; Ge, M.Z.; Li, S.H.; Huang, J.Y.; Chen, Z.; Li, H.Q.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Cui, C.; Fu, Q.J.; Meng, L.; Hao, S.W.; Dai, R.G.; Yang, J. Recent Progress in Natural Biopolymers Conductive Hydrogels for Flexible Wearable Sensors and Energy Devices: Materials, Structures, and Performance. ACS Appl. Bio Mater. 2021, 4, 85–121. [Google Scholar] [CrossRef]
- Bao, Z.T.; Xian, C.H.; Yuan, Q.J.; Liu, G.T.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Heaysman, C.L.; Willis, S.; Lewis, A.L. Physical hydrogels with self-assembled nanostructures as drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1141–1159. [Google Scholar] [CrossRef]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent trends in peptide and protein-based hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef]
- Leng, Z.W.; Zhu, P.C.; Wang, X.C.; Wang, Y.F.; Li, P.S.; Huang, W.; Li, B.C.; Jin, R.; Han, N.N.; Wu, J.; et al. Sebum-Membrane-Inspired Protein-Based Bioprotonic Hydrogel for Artificial Skin and Human-Machine Merging Interface. Adv. Funct. Mater. 2023, 33, 2211056. [Google Scholar] [CrossRef]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef]
- Huang, S.C.; Zhu, Y.J.; Huang, X.Y.; Xia, X.X.; Qian, Z.G. Programmable adhesion and morphing of protein hydrogels for underwater robots. Nat. Commun. 2024, 15, 195. [Google Scholar] [CrossRef]
- Chen, B.; Cao, Y.D.; Li, Q.Y.; Yan, Z.; Liu, R.; Zhao, Y.J.; Zhang, X.; Wu, M.Y.; Qin, Y.X.; Sun, C.; et al. Liquid metal-tailored gluten network for protein-based e-skin. Nat. Commun. 2022, 13, 1206. [Google Scholar] [CrossRef]
- Jo, M.; Min, K.; Roy, B.; Kim, S.; Lee, S.; Park, J.Y.; Kim, S. Protein-Based Electronic Skin Akin to Biological Tissues. ACS Nano 2018, 12, 5637–5645. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.K.; Yang, Y.H.; Fang, G.Q.; Yao, J.R.; Shao, Z.Z.; Chen, X. Robust Protein Hydrogels from Silkworm Silk. ACS Sustain. Chem. Eng. 2016, 4, 1500–1506. [Google Scholar] [CrossRef]
- Liu, J.W.; Ge, X.D.; Liu, L.; Xu, W.; Shao, R. Challenges and opportunities of silk protein hydrogels in biomedical applications. Mater. Adv. 2022, 3, 2291–2308. [Google Scholar] [CrossRef]
- Liu, J.W.; Sun, H.W.; Peng, Y.W.; Chen, L.G.; Xu, W.; Shao, R. Preparation and Characterization of Natural Silk Fibroin Hydrogel for Protein Drug Delivery. Molecules 2022, 27, 3418. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, J.; Wu, H.; Zeng, L.H.; Xu, S.P. Silk fibroin-based conductive and antibacterial hydrogels with a dual network for flexible sensors. J. Appl. Polym. Sci. 2023, 140, e54499. [Google Scholar] [CrossRef]
- Han, D.; Farino, C.; Yang, C.; Scott, T.; Browe, D.; Choi, W.; Freeman, J.W.; Lee, H. Soft Robotic Manipulation and Locomotion with a 3D Printed Electroactive Hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 17512–17518. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Li, D.F.; Ye, Y.X.; Li, D.R.; Li, X.Y.; Mu, C.D. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-PEG composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2016, 137, 508–514. [Google Scholar] [CrossRef]
- Heiden, A.; Preninger, D.; Lehner, L.; Baumgartner, M.; Drack, M.; Woritzka, E.; Schiller, D.; Gerstmayr, R.; Hartmann, F.; Kaltenbrunner, M. 3D printing of resilient biogels for omnidirectional and exteroceptive soft actuators. Sci. Robot. 2022, 7, eabk2119. [Google Scholar] [CrossRef]
- Lan, L.Y.; Ping, J.F.; Li, H.Y.; Wang, C.J.; Li, G.; Song, J.Z.; Ying, Y.B. Skin-Inspired All-Natural Biogel for Bioadhesive Interface. Adv. Mater. 2024, 36, 2401151. [Google Scholar] [CrossRef]
- Ma, Y.H.; Liu, K.; Lao, L.; Li, X.; Zhang, Z.C.; Lu, S.R.; Li, Y.Q.; Li, Z.W. A stretchable, self-healing, okra polysaccharide-based hydrogel for fast-response and ultra-sensitive strain sensors. Int. J. Biol. Macromol. 2022, 205, 491–499. [Google Scholar] [CrossRef]
- Wan, S.; Wu, N.; Ye, Y.Z.; Li, S.B.; Huang, H.Z.; Chen, L.; Bi, H.C.; Sun, L.T. Highly Stretchable Starch Hydrogel Wearable Patch for Electrooculographic Signal Detection and Human-Machine Interaction. Small Struct. 2021, 2, 2100105. [Google Scholar] [CrossRef]
- Wang, Q.H.; Pan, X.F.; Guo, J.J.; Huang, L.L.; Chen, L.H.; Ma, X.J.; Cao, S.L.; Ni, Y.H. Lignin and cellulose derivatives-induced hydrogel with asymmetrical adhesion, strength, and electriferous properties for wearable bioelectrodes and self-powered sensors. Chem. Eng. J. 2021, 414, 128903. [Google Scholar] [CrossRef]
- Lin, J.N.; Jiao, G.L.; Kermanshahi-pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef]
- Shi, H.D.; Deng, Y.X.; Shi, Y. Cellulose-Based Stimuli-Responsive Anisotropic Hydrogel for Sensor Applications. ACS Appl. Nano Mater. 2023, 6, 11524–11530. [Google Scholar] [CrossRef]
- Kimura, M.; Shinohara, Y.; Takizawa, J.; Ren, S.; Sagisaka, K.; Lin, Y.D.; Hattori, Y.; Hinestroza, J.P. Versatile Molding Process for Tough Cellulose Hydrogel Materials. Sci. Rep. 2015, 5, 16266. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiang, L.; Ou, H.; Li, F.; Zhang, Y.; Qian, Y.; Hao, L.; Diao, J.; Zhang, M.; Zhu, P.; et al. MXene-Based Conductive Organohydrogels with Long-Term Environmental Stability and Multifunctionality. Adv. Funct. Mater. 2020, 30, 2005135. [Google Scholar] [CrossRef]
- Zhang, L.M.; Wu, C.X.; Huang, J.Y.; Peng, X.H.; Chen, P.; Tang, S.Q. Synthesis and characterization of a degradable composite agarose/HA hydrogel. Carbohydr. Polym. 2012, 88, 1445–1452. [Google Scholar] [CrossRef]
- Lee, Y.; So, J.H.; Koo, H.J. A Transparent Hydrogel-Ionic Conductor with High Water Retention and Self-Healing Ability. Materials 2024, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Chen, Z.H.; Chen, R.; Wei, J. A self-healing and conductive ionic hydrogel based on polysaccharides for flexible sensors. Chin. J. Chem. Eng. 2023, 53, 73–82. [Google Scholar] [CrossRef]
- Lei, K.; Li, Z.; Zhu, D.D.; Sun, C.Y.; Sun, Y.L.; Yang, C.C.; Zheng, Z.; Wang, X.L. Polysaccharide-based recoverable double-network hydrogel with high strength and self-healing properties. J. Mater. Chem. B 2020, 8, 794–802. [Google Scholar] [CrossRef]
- Fang, Y.H.; Liang, C.; Liljeström, V.; Lv, Z.P.; Ikkala, O.; Zhang, H. Toughening Hydrogels with Fibrillar Connected Double Networks. Adv. Mater. 2024, 36, 2402282. [Google Scholar] [CrossRef]
- Yasin, A.; Ren, Y.; Li, J.A.; Sheng, Y.L.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.L.; Xu, Z.S.; Li, Z.H.; Zhang, L.Q.; Wan, P.B. Flexible Conformally Bioadhesive MXene Hydrogel Electronics for Machine Learning-Facilitated Human-Interactive Sensing. Adv. Mater. 2024, 36, e2401035. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Culebras, M.; Oliveira, J.M.; Koffler, J.; Collins, M.N. 3D printable electroconductive gelatin-hyaluronic acid materials containing polypyrrole nanoparticles for electroactive tissue engineering. Adv. Compos. Hybrid Mater. 2023, 6, 109. [Google Scholar] [CrossRef]
- Chang, S.L.; Deng, Y.; Li, N.; Wang, L.J.; Shan, C.X.; Dong, L. Continuous synthesis of ultra-fine fiber for wearable mechanoluminescent textile. Nano Res. 2023, 16, 9379–9386. [Google Scholar] [CrossRef]
- Zhu, P.C.; Zhang, B.S.; Wang, H.Y.; Wu, Y.H.; Cao, H.J.; He, L.B.; Li, C.Y.; Luo, X.P.; Li, X.; Mao, Y.C. 3D printed triboelectric nanogenerator as self-powered human-machine interactive sensor for breathing-based language expression. Nano Res. 2022, 15, 7460–7467. [Google Scholar] [CrossRef]
- Yuan, X.M.; Zhu, Z.; Xia, P.C.; Wang, Z.J.; Zhao, X.; Jiang, X.; Wang, T.M.; Gao, Q.; Xu, J.; Shan, D.B.; et al. Tough Gelatin Hydrogel for Tissue Engineering. Adv. Sci. 2023, 10, e2301665. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Gao, Y.; Ren, X.Y.; Gao, G.H. Polysaccharide-tackified composite hydrogel for skin-attached sensors. J. Mater. Chem. C 2021, 9, 3343–3351. [Google Scholar] [CrossRef]
- Zhu, P.C.; Mu, S.R.; Huang, W.H.; Sun, Z.Y.; Lin, Y.Y.; Chen, K.; Pan, Z.F.; Haghighi, M.G.; Sedghi, R.; Wang, J.L.; et al. Soft multifunctional neurological electronic skin through intrinsically stretchable synaptic transistor. Nano Res. 2024, 17, 6550–6559. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.X.; Yang, X.G.; Lv, C.F.; Huang, W.T.; Li, F.K.; Zhang, C.; Wu, Y.R.; Dong, L.; Shan, C.X. Highly sensitive humidity sensors based on hexagonal boron nitride nanosheets for contactless sensing. Nano Res. 2023, 16, 10279–10286. [Google Scholar] [CrossRef]
- Shao, C.Y.; Meng, L.; Cui, C.; Yang, J. An integrated self-healable and robust conductive hydrogel for dynamically self-adhesive and highly conformable electronic skin. J. Mater. Chem. C 2019, 7, 15208–15218. [Google Scholar] [CrossRef]
- Sun, J.; Wu, X.Y.; Xiao, J.M.; Zhang, Y.S.; Ding, J.; Jiang, J.; Chen, Z.H.; Liu, X.Y.; Wei, D.; Zhou, L.X.; et al. Hydrogel-Integrated Multimodal Response as a Wearable and Implantable Bidirectional Interface for Biosensor and Therapeutic Electrostimulation. ACS Appl. Mater. Interfaces 2023, 15, 5897–5909. [Google Scholar] [CrossRef]
- Wang, Y.L.; Huang, H.L.; Wu, J.L.; Han, L.; Yang, Z.L.; Jiang, Z.C.; Wang, R.; Huang, Z.J.; Xu, M. Ultrafast Self-Healing, Reusable, and Conductive Polysaccharide-Based Hydrogels for Sensitive Ionic Sensors. ACS Sustain. Chem. Eng. 2020, 8, 18506–18518. [Google Scholar] [CrossRef]
- Xu, H.Y.; Xie, Y.Y.; Zhu, E.W.; Liu, Y.; Shi, Z.Q.; Xiong, C.X.; Yang, Q.L. Supertough and ultrasensitive flexible electronic skin based on nanocellulose/sulfonated carbon nanotube hydrogel films. J. Mater. Chem. A 2020, 8, 6311–6318. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, C.; Huang, W.Y.; Liu, C.L.; Zhang, Y. Wearable Sensor Based on a Tough Conductive Gel for Real-Time and Remote Human Motion Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 11957–11972. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Y.; Ren, X.Y.; Gao, G.H. Alginate fiber toughened gels similar to skin intelligence as ionic sensors. Carbohydr. Polym. 2020, 235, 116018. [Google Scholar] [CrossRef]
- Dai, S.P.; Wang, S.; Yan, H.; Xu, J.; Hu, H.W.; Ding, J.N.; Yuan, N.Y. Stretchable and self-healable hydrogel-based capacitance pressure and strain sensor for electronic skin systems. Mater. Res. Express. 2019, 6, 0850b9. [Google Scholar] [CrossRef]
- Duan, J.F.; Wen, H.K.; Zong, S.Y.; Li, T.; Lv, H.; Liu, L.J. Soft/Hard Controllable Conversion Galactomannan Ionic Conductive Hydrogel as a Flexible Sensor. ACS Appl. Electron. Mater. 2021, 3, 5000–5014. [Google Scholar] [CrossRef]
- Hussain, I.; Ma, X.F.; Luo, Y.L.; Luo, Z.Y. Fabrication and characterization of glycogen-based elastic, self-healable, and conductive hydrogels as a wearable strain-sensor for flexible e-skin. Polymer 2020, 210, 122961. [Google Scholar] [CrossRef]
- Ling, Q.J.; Liu, W.T.; Liu, J.C.; Zhao, L.; Ren, Z.J.; Gu, H.B. Highly Sensitive and Robust Polysaccharide-Based Composite Hydrogel Sensor Integrated with Underwater Repeatable Self-Adhesion and Rapid Self-Healing for Human Motion Detection. ACS Appl. Mater. Interfaces 2022, 14, 24741–24754. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, J.; Sun, Y.G.; Wang, H.L.; Du, X.S.; Cheng, X.; Du, Z.L.; Wang, H.B. Skin-inspired nanofibrillated cellulose-reinforced hydrogels with high mechanical strength, long-term antibacterial, and self-recovery ability for wearable strain/pressure sensors. Carbohydr. Polym. 2021, 261, 117894. [Google Scholar]
- Yang, M.; Cheng, Y.F.; Yue, Y.; Chen, Y.; Gao, H.; Li, L.; Cai, B.; Liu, W.J.; Wang, Z.Y.; Guo, H.Z.; et al. High-Performance Flexible Pressure Sensor with a Self-Healing Function for Tactile Feedback. Adv. Sci. 2022, 9, e2200507. [Google Scholar]
- Hao, F.Y.; Maimaitiyiming, X.; Sun, S. 3D Printed Multifunctional Self-Adhesive and Conductive Polyacrylamide/Chitosan/Sodium Carboxymethyl Cellulose/CNT Hydrogels as Flexible Sensors. Macromol. Chem. Phys. 2023, 224, 2200272. [Google Scholar] [CrossRef]
- Ye, Y.H.; Zhang, Y.F.; Chen, Y.; Han, X.S.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.D.; Shen, B.; Mo, J.Y.; Tang, F.Y.; Feng, J. Highly stretchable and self-healing double network hydrogel based on polysaccharide and polyzwitterion for wearable electric skin. Polymer 2020, 194, 122381. [Google Scholar] [CrossRef]
- Wang, Y.P.; Qu, Z.J.; Wang, W.; Yu, D. PVA/CMC/PEDOT:PSS mixture hydrogels with high response and low impedance electronic signals for ECG monitoring. Colloid Surf. B-Biointerfaces 2021, 208, 112088. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Tang, Q.H.; Zhou, J.Y.; Zhao, C.H.; Li, J.P.; Wang, H.T. Conductive and Eco-friendly Biomaterials-based Hydrogels for Noninvasive Epidermal Sensors: A Review. ACS Biomater. Sci. Eng. 2023, 10, 191–218. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, R.A.; Liu, H.; Li, D.L.; Fu, S.M.; Jin, K.M.; Cheng, Y.G.; Fu, Z.W.; Xing, F.; Tian, Y. Tough, high conductivity pectin polysaccharide-based hydrogel for strain sensing and real-time information transmission. Int. J. Biol. Macromol. 2024, 257, 128757. [Google Scholar] [CrossRef]
- Lu, L.J.; Hu, G.S.; Liu, J.Q.; Yang, B. 5G NB-IoT System Integrated with High-Performance Fiber Sensor Inspired by Cirrus and Spider Structures. Adv. Sci. 2024, 11, 2309894. [Google Scholar] [CrossRef]
- Ma, F.X.; Wu, Y.J.; Dai, S.G.; Lin, P.; Sun, J.L.; Dong, L. A soft-contact hybrid electromagnetic-triboelectric nanogenerator for self-powered water splitting towards hydrogen production. Nano Res. 2024, 17, 6567–6574. [Google Scholar] [CrossRef]
- Han, X.K.; Lu, T.Y.; Wang, H.; Zhang, Z.C.; Lu, S.R. Self-Healing and Freeze-Resistant Boat-Fruited Sterculia Seed Polysaccharide/Silk Fiber Hydrogel for Wearable Strain Sensors. ACS Sustain. Chem. Eng. 2023, 11, 13756–13764. [Google Scholar] [CrossRef]
- Kong, L.S.; Gao, Z.J.; Li, X.Y.; Gao, G.H. An amylopectin-enabled skin-mounted hydrogel wearable sensor. J. Mater. Chem. B 2021, 9, 1082–1088. [Google Scholar] [CrossRef]
- Lee, Y.; Yim, S.G.; Lee, G.W.; Kim, S.; Kim, H.S.; Hwang, D.Y.; An, B.S.; Lee, J.H.; Seo, S.; Yang, S.Y. Self-Adherent Biodegradable Gelatin-Based Hydrogel Electrodes for Electrocardiography Monitoring. Sensors 2020, 20, 5737. [Google Scholar] [CrossRef]
- Liu, W.; Xie, R.J.; Zhu, J.Y.; Wu, J.S.; Hui, J.F.; Zheng, X.Y.; Huo, F.W.; Fan, D.D. A temperature responsive adhesive hydrogel for fabrication of flexible electronic sensors. NPJ Flex. Electron. 2022, 6, 68. [Google Scholar] [CrossRef]
- Chen, G.; Matsuhisa, N.; Liu, Z.Y.; Qi, D.P.; Cai, P.Q.; Jiang, Y.; Wan, C.J.; Cui, Y.J.; Leow, W.R.; Liu, Z.J.; et al. Plasticizing Silk Protein for On-Skin Stretchable Electrodes. Adv. Mater. 2018, 30, e1800129. [Google Scholar] [CrossRef] [PubMed]
- Farago, E.; MacIsaac, D.; Suk, M.; Chan, A.D.C. A Review of Techniques for Surface Electromyography Signal Quality Analysis. IEEE Rev. Biomed. Eng. 2023, 16, 472–486. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, R.R.; Kang, B.B.; Zhao, Z.D.; Song, S.S. High-performance ionic conductive double-network hydrogel enabling a long-term flexible strain sensor. Colloid Surf. A-Physicochem. Eng. Asp. 2023, 663, 131051. [Google Scholar] [CrossRef]
- Ni, Q.C.; Lou, Q.; Shen, C.L.; Zheng, G.S.; Song, R.W.; Hao, J.N.; Liu, J.L.; Zhu, J.Y.; Zang, J.H.; Dong, L.; et al. Sensitive humidity sensor based on moisture-driven energy generation. Nano Res. 2024, 17, 5578–5586. [Google Scholar] [CrossRef]
- Wang, G.; Liu, M.J.; Zhang, C.P.; Xia, S.; Gao, G.H.; Shi, Y.F. Amylopectin- assisted hydrogel conductors for multi-modal physiological signal acquisition. Eur. Polym. J. 2024, 207, 112843. [Google Scholar] [CrossRef]
- Yan, L.W.; Zhou, T.; Han, L.; Zhu, M.Y.; Cheng, Z.; Li, D.; Ren, F.Z.; Wang, K.F.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 2010465. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.L.; Liu, Y.; Li, Y.F.; Li, J.S.; Qin, Z.H.; Jiao, T.F. Ultrastretchable, Self-Adhesive and conductive MXene nanocomposite hydrogel for body-surface temperature distinguishing and electrophysiological signal monitoring. Chem. Eng. J. 2024, 483, 149303. [Google Scholar] [CrossRef]
- Guo, X.J.; Qin, W.J.; Gu, C.S.; Li, X.X.; Chen, M.Y.; Zhai, H.L.; Zhao, X.C.; Liu, H.C.; Zhao, B.X.; Zhang, Y.; et al. High-Adhesion, Weather Resistance, Reusable PAM/Gly/Gel/TA/Fe3+ Biopolymer Dual-Network Conductive Hydrogel for Flexible Bioelectrode. Adv. Mater. Technol. 2024, 9, 2302072. [Google Scholar] [CrossRef]
- Han, K.; Bai, Q.; Wu, W.D.; Sun, N.; Cui, N.; Lu, T.L. Gelatin-based adhesive hydrogel with self-healing, hemostasis, and electrical conductivity. Int. J. Biol. Macromol. 2021, 183, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; You, L.J.; Cai, X.X.; Yang, J.H.; Chen, H.M.; Shi, X.M.; Wu, J.J.; Wang, J.H.; Xiong, C.H.; Wang, S.Y. Fermentation-Inspired Gelatin Hydrogels with a Controllable Supermacroporous Structure and High Ductility for Wearable Flexible Sensors. ACS Appl. Mater. Interfaces 2022, 14, 26338–26349. [Google Scholar] [CrossRef]
- Choi, Y.; Park, K.; Choi, H.; Son, D.; Shin, M. Self-Healing, Stretchable, Biocompatible, and Conductive Alginate Hydrogels through Dynamic Covalent Bonds for Implantable Electronics. Polymers 2021, 13, 1133. [Google Scholar] [CrossRef]
- Xu, J.X.; Zhang, H.Y.; Guo, Z.Y.; Zhang, C.Y.; Tan, H.H.; Gong, G.; Yu, M.L.; Xu, L.J. Fully physical crosslinked BSA-based conductive hydrogels with high strength and fast self-recovery for human motion and wireless electrocardiogram sensing. Int. J. Biol. Macromol. 2023, 230, 123195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Yang, J.W.; Wang, H.Y.; Wang, C.Y.; Gu, Y.H.; Xu, Y.M.; Lee, S.; Yokota, T.; Haick, H.; Someya, T.; et al. A 10-micrometer-thick nanomesh-reinforced gas-permeable hydrogel skin sensor for long-term electrophysiological monitoring. Sci. Adv. 2024, 10, eadj5389. [Google Scholar] [CrossRef]
- Shi, Y.F.; Fu, X.L.; Wang, W.; Yu, D. Stretchable, adhesive and low impedance hydrogel prepared by one-pot method used as ECG electrodes. Colloid Surf. A-Physicochem. Eng. Asp. 2023, 662, 130998. [Google Scholar] [CrossRef]
- Nandi, R.; Agam, Y.; Amdursky, N. A Protein-Based Free-Standing Proton-Conducting Transparent Elastomer for Large-Scale Sensing Applications. Adv. Mater. 2021, 33, 2101208. [Google Scholar] [CrossRef]
- Rauf, S.; Bilal, R.M.; Li, J.J.; Vaseem, M.; Ahmad, A.N.; Shamim, A. Fully Screen-Printed and Gentle-to-Skin Wet ECG Electrodes with Compact Wireless Readout for Cardiac Diagnosis and Remote Monitoring. ACS Nano 2024, 18, 10074–10087. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, Y.Z.; Li, Y.Q.; Li, D.; Guo, T.L.; Deng, X.; Zhang, H.P.; Xie, C.M.; Lu, X. Tuning Water-Resistant Networks in Mussel-Inspired Hydrogels for Robust Wet Tissue and Bioelectronic Adhesion. ACS Nano 2023, 17, 2745–2760. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Feng, T.; An, B.L.; Ren, S.S.; Meng, J.F.; Li, K.; Liu, S.Y.; Wu, H.Y.; Zhang, H.; Zhong, C. A Bi-Layer Hydrogel Cardiac Patch Made of Recombinant Functional Proteins. Adv. Mater. 2022, 34, e2201411. [Google Scholar] [CrossRef]
- Alsaafeen, N.B.; Bawazir, S.S.; Jena, K.K.; Seitak, A.; Fatma, B.; Pitsalidis, C.; Khandoker, A.; Pappa, A.M. One-Pot Synthesis of a Robust Crosslinker-Free Thermo-Reversible Conducting Hydrogel Electrode for Epidermal Electronics. ACS Appl. Mater. Interfaces 2024, 16, 11. [Google Scholar] [CrossRef]
- Ding, J.W.; Tang, Y.; Chang, R.H.; Li, Y.; Zhang, L.M.; Yan, F. Reduction in the Motion Artifacts in Noncontact ECG Measurements Using a Novel Designed Electrode Structure. Sensors 2023, 23, 956. [Google Scholar] [CrossRef]
- Xu, Y.D.; Su, Y.J.; Xu, X.C.; Arends, B.; Zhao, G.G.; Ackerman, D.N.; Huang, H.Y.; Reid, S.P.; Santarpia, J.L.; Kim, C.; et al. Porous liquid metal-elastomer composites with high leakage resistance and antimicrobial property for skin-interfaced bioelectronics. Sci. Adv. 2023, 9, eadf0575. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Tie, J.F.; Wang, Y.M.; Mao, Z.P.; Zhang, L.P.; Zhong, Y.; Sui, X.F.; Feng, X.L.; Xu, H. Mussel-inspired chitosan-based hydrogel sensor with pH-responsive and adjustable adhesion, toughness and self-healing capability. Polym. Adv. Technol. 2022, 33, 1867–1880. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, H.Y.; Wang, B.H.; Miyata, H.; Wang, Y.; Nayeem, M.O.G.; Kim, J.J.; Lee, S.; Yokota, T.; Onodera, H.; et al. On-skin paintable biogel for long-term high-fidelity electroencephalogram recording. Sci. Adv. 2022, 8, eabo1396. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Z.W.; Zhang, H.; Huang, Y.J.; Wang, Z.B.; Zhou, Y.; Xu, B.B.; Halila, S.; Chen, J. Ultrastretchable, Highly Transparent, Self-Adhesive, and 3D-Printable Ionic Hydrogels for Multimode Tactical Sensing. Chem. Mater. 2021, 33, 6731–6742. [Google Scholar] [CrossRef]
- Xu, M.; Ding, R.; Zheng, C.; Cai, Y.; Li, H.; Ming, D. Research on High Spatial Resolution SSVEP Signal Acquisition and Analysis Based on Scalp Laplace EEG Electrodes. J. Signal Process. 2023, 39, 1455–1464. [Google Scholar]
- Tomic, N.Z.; Ghodhbane, M.; Matouk, Z.; AlShehhi, N.; Busa, C. Enhancement of Self-Healing Efficacy of Conductive Nanocomposite Hydrogels by Polysaccharide Modifiers. Polymers 2023, 15, 516. [Google Scholar] [CrossRef]
- Li, S.N.; Cong, Y.; Fu, J. Tissue adhesive hydrogel bioelectronics. J. Mater. Chem. B 2021, 9, 4423–4443. [Google Scholar] [CrossRef]
- Liang, H.Y.; Wang, Y.X.; Li, H.H.; Wang, Y.Y.; Liu, P.X.; Liu, R. Development and Characterization of a Dry Ear-EEG Sensor with a Generic Flexible Earpiece. IEEE Trans. Instrum. Meas. 2023, 72, 4006212. [Google Scholar] [CrossRef]
- Manouchehri, S.; Bagheri, B.; Rad, S.H.; Nezhad, M.N.; Kim, Y.C.; Park, O.O.; Farokhi, M.; Jouyandeh, M.; Ganjali, M.R.; Yazdi, M.K.; et al. Electroactive bio-epoxy incorporated chitosan-oligoaniline as an advanced hydrogel coating for neural interfaces. Prog. Org. Coat. 2019, 131, 389–396. [Google Scholar] [CrossRef]
- Shen, G.C.; Zheng, K.Y.; Jiang, C.P.; Shao, S.H.; Zhao, N.; Liu, J.Q. A Gelatin-Based Hydrogel Electrode With High Moisturizing Ability for Wearable EEG Recording. IEEE Sens. J. 2023, 23, 25689–25697. [Google Scholar] [CrossRef]
- Fernández-García, L.; Marí-Buyé, N.; Barios, J.A.; Madurga, R.; Elices, M.; Pérez-Rigueiro, J.; Ramos, M.; Guinea, G.V.; González-Nieto, D. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. Acta Biomater. 2016, 45, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Kaongoen, N.; Choi, J.; Choi, J.W.; Kwon, H.; Hwang, C.; Hwang, G.; Kim, B.H.; Jo, S. The future of wearable EEG: A review of ear-EEG technology and its applications. J. Neural Eng. 2023, 20, 051002. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, S.; Li, S.; Tian, J.; Li, H.; Pan, Z.; Lu, L.; Mao, Y. Recent Advances in Natural-Polymer-Based Hydrogels for Body Movement and Biomedical Monitoring. Biosensors 2024, 14, 415. https://doi.org/10.3390/bios14090415
Liu J, Li S, Li S, Tian J, Li H, Pan Z, Lu L, Mao Y. Recent Advances in Natural-Polymer-Based Hydrogels for Body Movement and Biomedical Monitoring. Biosensors. 2024; 14(9):415. https://doi.org/10.3390/bios14090415
Chicago/Turabian StyleLiu, Jing, Saisai Li, Shuoze Li, Jinyue Tian, Hang Li, Zhifeng Pan, Lijun Lu, and Yanchao Mao. 2024. "Recent Advances in Natural-Polymer-Based Hydrogels for Body Movement and Biomedical Monitoring" Biosensors 14, no. 9: 415. https://doi.org/10.3390/bios14090415
APA StyleLiu, J., Li, S., Li, S., Tian, J., Li, H., Pan, Z., Lu, L., & Mao, Y. (2024). Recent Advances in Natural-Polymer-Based Hydrogels for Body Movement and Biomedical Monitoring. Biosensors, 14(9), 415. https://doi.org/10.3390/bios14090415






