Detection of Cannabinoids in Oral Fluid Specimens as the Preferred Biological Matrix for a Point-of-Care Biosensor Diagnostic Device
Abstract
1. Introduction
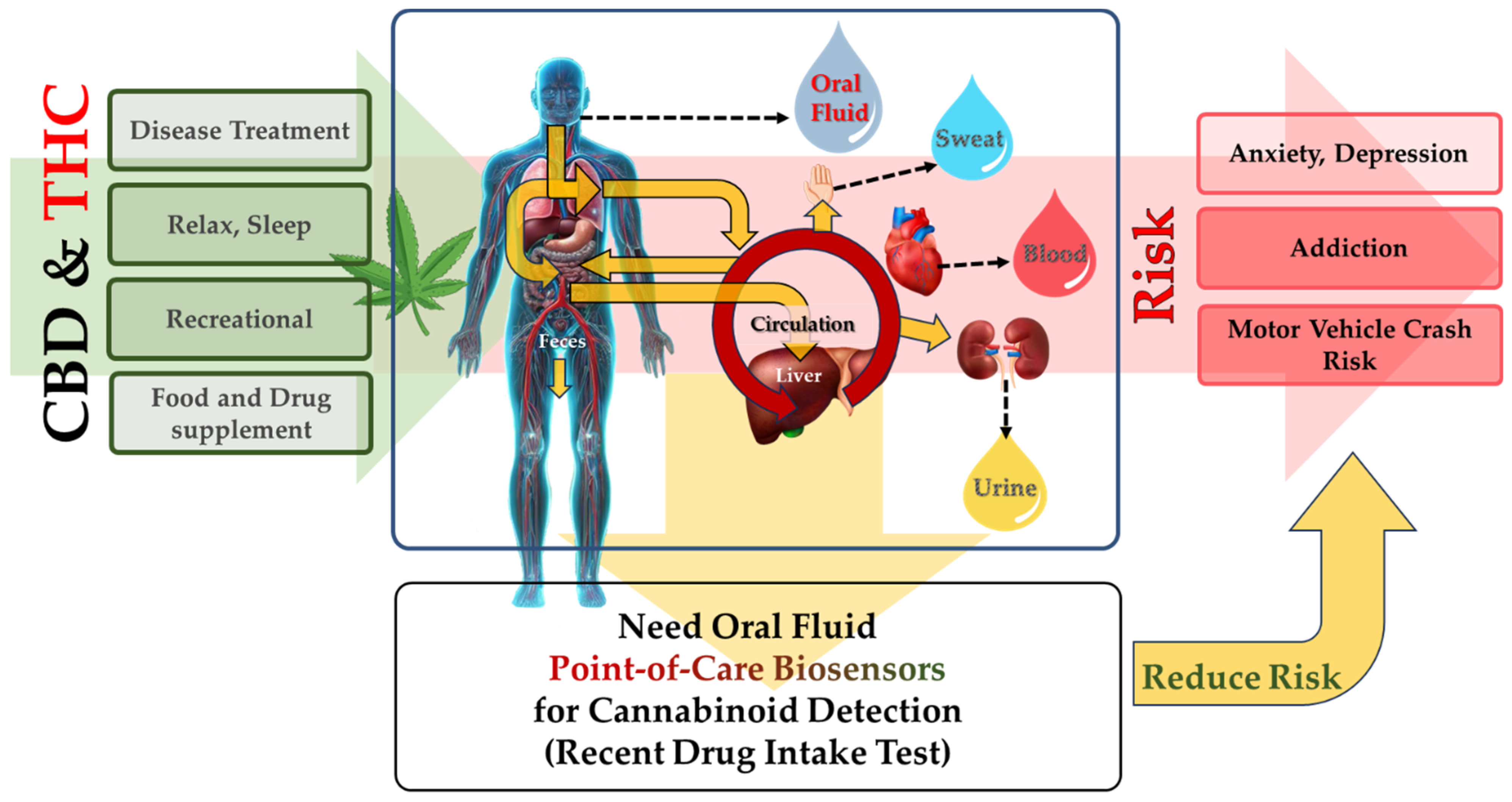
1.1. Why Is It Important to Perform Cannabinoids Detection?
1.2. Different Types of Cannabinoids
1.3. Preferred Cannabinoids as Targets for Detection
1.4. Existing Challenges in the Detection of Cannabinoids
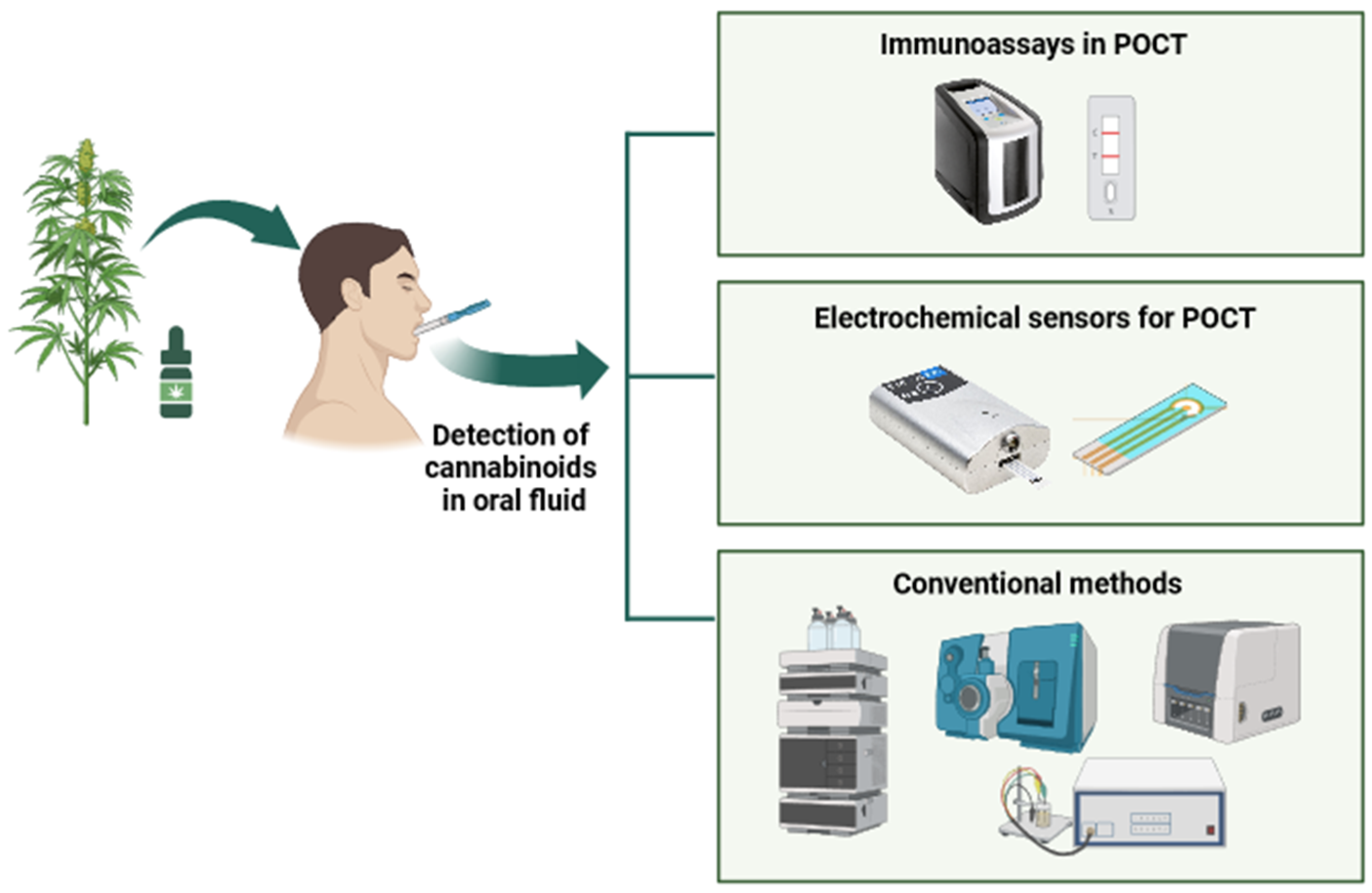
2. Detection of Cannabinoids
2.1. Conventional Detection Procedure
2.2. Immunoassays in Point-of-Care Biosensors
2.3. Electrochemical Sensors for Point-of-Care Testing
3. Biological Matrices for the Detection of Cannabinoids
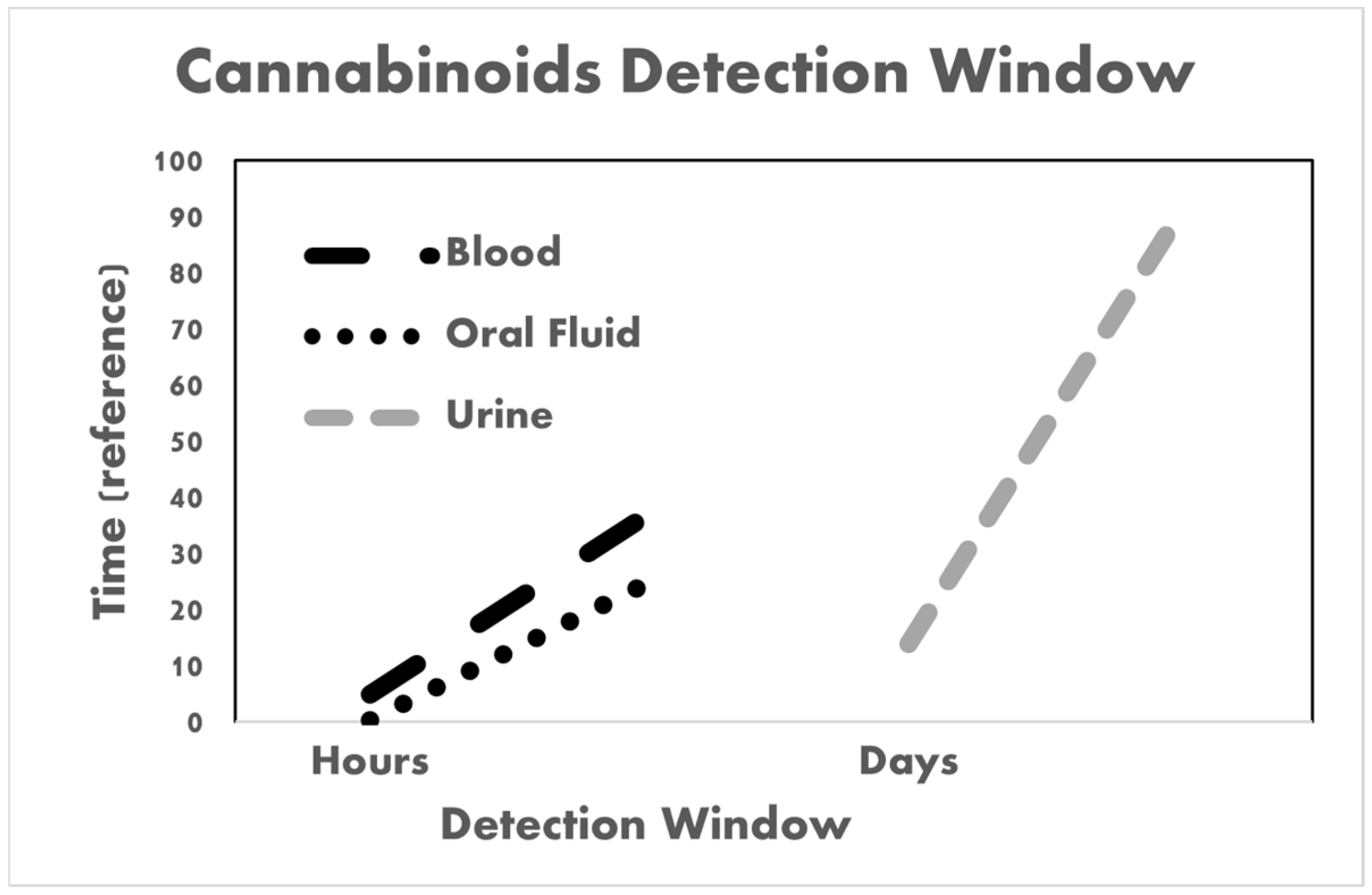
3.1. Various Biological Matrices, including Blood and Urine Specimens
3.2. Oral Fluid as the Preferred Biological Matrix
3.3. Collection of Oral Fluid Specimens for Cannabinoids Recovery
3.4. Stability of Cannabinoids within Oral Fluid Specimens
4. Detection of Cannabinoids in Oral Fluid Specimens in Lab-Based Techniques and on-Site Point-of-Care Biosensors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanson, K.; Garcia, A. State medical marijuana laws. In Proceedings of the National Conference of State Legislators, Denver, CO, USA, 5–8 August 2019. [Google Scholar]
- Bifulco, M.; Pisanti, S. Medicinal Use of Cannabis in Europe; EMBO Reports; EMBO: Heidelberg, Germany, 2015; pp. 130–132. [Google Scholar]
- Available online: https://www.usnews.com/news/best-states/articles/where-is-marijuana-legal-a-guide-to-marijuana-legalization (accessed on 14 December 2023).
- Kokona, A.; Tarricone, I.; Di Forti, M.; Carra, E. Cannabis, Migration, and Psychosis Onset. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–88. [Google Scholar]
- Linares, I.; Crippa, J.; Chagas, M. Beneficial effects of cannabis and related compounds on sleep. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 877–882. [Google Scholar]
- Petrilli, K.; Hines, L.; Adams, S.; Morgan, C.J.; Curran, H.V.; Freeman, T.P. High potency cannabis use, mental health symptoms and cannabis dependence: Triangulating the evidence. Addict. Behav. 2023, 144, 107740. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Reed, K. Marijuana and Breastfeeding. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 527–532. [Google Scholar]
- Norberg, M.M.; Rooke, S.E.; Albertella, L.; Copeland, J.; Kavanagh, D.J.; Lau, A. The first mHealth app for managing cannabis use: Gauging its potential helpfulness. J. Addict. Behav. Ther. Rehabil. 2014, 3, 1. [Google Scholar]
- Huestis, M.A.; Smith, M.L. Cannabinoid Markers in Biological Fluids and Tissues: Revealing Intake. Trends Mol. Med. 2018, 24, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Johnson, O.E.; Miskelly, G.M.; Rindelaub, J.D. Testing for cannabis intoxication: Current issues and latest advancements. Wiley Interdiscip. Rev. Forensic Sci. 2022, 4, e1450. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. In Phytocannabinoids; Springer: Berlin/Heidelberg, Germany, 2017; pp. 103–131. [Google Scholar]
- Radwan, M.M.; Wanas, A.S.; Chandra, S.; ElSohly, M.A. Natural cannabinoids of cannabis and methods of analysis. In Cannabis sativa L.—Botany and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 161–182. [Google Scholar]
- Razdan, R.K. Structure-activity relationships in cannabinoids. Pharmacol. Rev. 1986, 38, 75. [Google Scholar] [PubMed]
- Pertwee, R.G. The central neuropharmcology of psychotropic cannabinoids. Pharmacol. Ther. 1988, 36, 189–261. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacological and therapeutic targets for Δ9 tetrahydrocannabinol and cannabidiol. Euphytica 2004, 140, 73–82. [Google Scholar] [CrossRef]
- Grotenhermen, F.; Russo, E.; Zuardi, A.W. Even high doses of oral cannabidiol do not cause THC-like effects in humans: Comment on Merrick et al. Cannabis Cannabinoid Res. 2016, 1, 102–112, Erratum in Cannabis Cannabinoid Res. 2017, 2, 1–4. [Google Scholar] [CrossRef]
- Borges, R.; da Silva, A. Cannabidiol as an antioxidant. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. e122–e130. [Google Scholar]
- Zuardi, A.; de Souza Crippa, J.; Hallak, J.; Campos, A.; Guimarães, F. The anxiolytic effects of Cannabidiol (CBD). In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. e131–e139. [Google Scholar]
- Coimbra, N.; Mendes-Gomes, J.; Da Silva, J.; Dos Anjos-Garcia, T.; Ullah, F.; Almada, R. New Ethological and Morphological Perspectives for the Investigation of Panicolytic-like Effects of Cannabidiol. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. e140–e149. [Google Scholar]
- Zalman, D.; Bar-Sela, G. Cannabis and Synthetic Cannabinoids for Cancer Patients: Multiple Palliative Indications Together With Promising Laboratory Antineoplastic Effects. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 859–868. [Google Scholar]
- Tellioğlu, T.; Tellioğlu, Z. The use of medical marijuana in the treatment of psychiatric disorders. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 869–876. [Google Scholar]
- Kanaan, A.S.; Müller-Vahl, K. Cannabinoid-based medicines for the treatment of gilles de la tourette syndrome. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 883–892. [Google Scholar]
- Mecha, M.; Feliú, A.; Carrillo-Salinas, F.; Guaza, C. Cannabidiol and Multiple Sclerosis. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 893–904. [Google Scholar]
- Selvarajah, D.; Gandhi, R.; Tesfaye, S. Cannabinoids and Their Effects on Painful Neuropathy. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 905–916. [Google Scholar]
- Abdel-Salam, O. Cannabis for Basal Ganglia Disorders (Parkinson Disease and Huntington Disease). In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 917–930. [Google Scholar]
- Lahat, A. Medical cannabis for the treatment of inflammatory bowel disease. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 931–938. [Google Scholar]
- dos Santos, R.; Hallak, J.; Zuardi, A.; de Souza Crippa, J. Cannabidiol for the treatment of drug use disorders. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 939–946. [Google Scholar]
- Vera, G.; Fichna, J.; Abalo, R. Cannabinoids and effects on the gastrointestinal tract: A focus on motility. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 947–957. [Google Scholar]
- Deiana, S. Potential Medical Uses of Cannabigerol: A Brief Overview. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 958–967. [Google Scholar]
- Farré, M.; Farré, A.; Fiz, J.; Torrens, M. Cannabis Use in fibromyalgia. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. e158–e167. [Google Scholar]
- Robson, P. Therapeutic potential of cannabinoid medicines. Drug Test. Anal. 2014, 6, 24–30. [Google Scholar] [CrossRef]
- Piomelli, D.; Beltramo, M.; Giuffrida, A.; Stella, N. Endogenous Cannabinoid Signaling. Neurobiol. Dis. 1998, 5, 462–473. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Costa, M.A.; Almada, M.; Correia-da-Silva, G.; Teixeira, N.A. Endogenous cannabinoids revisited: A biochemistry perspective. Prostaglandins Other Lipid Mediat. 2013, 102–103, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Garrett, E.R.; Hunt, C.A. Physicochemical properties, solubility, and protein binding of Δ9 -tetrahydrocannabinol. J. Pharm. Sci. 1974, 63, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Huestis, M.A. Current knowledge on cannabinoids in oral fluid. Drug Test. Anal. 2014, 6, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.; Osselton, D.; Robinson, S. Chapter 10—Drug Testing. In Drugs and the Future; Nutt, D., Robbins, T.W., Stimson, G.V., Ince, M., Jackson, A., Eds.; Academic Press: Burlington, ON, Canada, 2007; pp. 315–336. [Google Scholar]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Melanson, S.E.F. Drug-of-Abuse Testing at the Point of Care. Clin. Lab. Med. 2009, 29, 503–509. [Google Scholar] [CrossRef]
- Scherer, J.N.; Fiorentin, T.R.; Borille, B.T.; Pasa, G.; Sousa, T.R.V.; von Diemen, L.; Limberger, R.P.; Pechansky, F. Reliability of point-of-collection testing devices for drugs of abuse in oral fluid: A systematic review and meta-analysis. J. Pharm. Biomed. Anal. 2017, 143, 77–85. [Google Scholar] [CrossRef]
- Desrosiers, N.A.; Huestis, M.A. Oral Fluid Drug Testing: Analytical Approaches, Issues and Interpretation of Results. J. Anal. Toxicol. 2019, 43, 415–443. [Google Scholar] [CrossRef]
- Mazina, J.; Spiljova, A.; Vaher, M.; Kaljurand, M.; Kulp, M. A rapid capillary electrophoresis method with LED-induced native fluorescence detection for the analysis of cannabinoids in oral fluid. J. Anal. Methods 2015, 7, 7741–7747. [Google Scholar] [CrossRef]
- Stevenson, H.; Bacon, A.; Joseph, K.M.; Gwandaru, W.R.W.; Bhide, A.; Sankhala, D.; Dhamu, V.N.; Prasad, S. A rapid response electrochemical biosensor for detecting THC in saliva. Sci. Rep. 2019, 9, 12701. [Google Scholar] [CrossRef]
- Xu, C.; Wang, W.; Wang, S.; Hou, K.; Li, H. Potential analytical methods for on-site oral drug test: Recent developments and applications. Trends Anal. Chem. 2019, 120, 115649. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Heterogeneous and homogeneous immunoassays for drug analysis. Bioanalysis 2014, 6, 2877–2896. [Google Scholar] [CrossRef] [PubMed]
- Drummer, O.H. Drug testing in oral fluid. Clin. Biochem. Rev. 2006, 27, 147–159. [Google Scholar] [PubMed]
- Yu, H.; Lee, H.; Cheong, J.; Woo, S.W.; Oh, J.; Oh, H.-K.; Lee, J.-H.; Zheng, H.; Castro, C.M.; Yoo, Y.-E.; et al. A rapid assay provides on-site quantification of tetrahydrocannabinol in oral fluid. Sci. Transl. Med. 2021, 13, eabe2352. [Google Scholar] [CrossRef]
- Wang, K.; Qin, W.; Hou, Y.; Xiao, K.; Yan, W. The application of lateral flow immunoassay in point of care testing: A review. Nano Biomed. Eng. 2016, 8, 172–183. [Google Scholar] [CrossRef]
- Schwope, D.M.; Milman, G.; Huestis, M.A. Validation of an enzyme immunoassay for detection and semiquantification of cannabinoids in oral fluid. Clin. Chem. 2010, 56, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Datta, P. Immunoassay Design for Screening of Drugs of Abuse. In Critical Issues in Alcohol and Drugs of Abuse Testing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 121–128. [Google Scholar]
- Vanstechelman, S.; Isalberti, C.; Van der Linden, T.; Pil, K.; Legrand, S.-A.; Verstraete, A.G. Analytical evaluation of four on-site oral fluid drug testing devices. J. Anal. Toxicol. 2012, 36, 136–140. [Google Scholar] [CrossRef]
- Carrio, A.; Sampedro, C.; Sanchez-Lopez, J.L.; Pimienta, M.; Campoy, P. Automated Low-Cost Smartphone-Based Lateral Flow Saliva Test Reader for Drugs-of-Abuse Detection. Sensors 2015, 15, 29569–29593. [Google Scholar] [CrossRef]
- Niedbala, R.S.; Feindt, H.; Kardos, K.; Vail, T.; Burton, J.; Bielska, B.; Li, S.; Milunic, D.; Bourdelle, P.; Vallejo, R. Detection of analytes by immunoassay using up-converting phosphor technology. Anal. Biochem. 2001, 293, 22–30. [Google Scholar] [CrossRef]
- Corstjens, P.L.A.M.; Li, S.; Zuiderwijk, M.; Kardos, K.; Abrams, W.R.; Niedbala, R.S.; Tanke, H.J. Infrared up-converting phosphors for bioassays. IEEE Proc. Nanobiotechnol. 2005, 152, 64–72. [Google Scholar] [CrossRef]
- Swortwood, M.J.; Newmeyer, M.N.; Abulseoud, O.A.; Andersson, M.; Barnes, A.J.; Scheidweiler, K.B.; Huestis, M.A. On-site oral fluid Δ 9-tetrahydrocannabinol (THC) screening after controlled smoked, vaporized, and oral cannabis administration. Forensic Toxicol. 2017, 35, 133–145. [Google Scholar] [CrossRef]
- Crouch, D.J. Oral fluid collection: The neglected variable in oral fluid testing. Forensic Sci. Int. 2005, 150, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Dickson, S.; Park, A.; Nolan, S.; Kenworthy, S.; Nicholson, C.; Midgley, J.; Pinfold, R.; Hampton, S. The recovery of illicit drugs from oral fluid sampling devices. Forensic Sci. Int. 2007, 165, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Amini, K.; Sepehrifard, A.; Valinasabpouri, A.; Safruk, J.; Angelone, D.; de Campos Lourenco, T. Recent advances in electrochemical sensor technologies for THC detection—A narrative review. J. Cannabis Res. 2022, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Klimuntowski, M.; Alam, M.M.; Singh, G.; Howlader, M.M.R. Electrochemical Sensing of Cannabinoids in Biofluids: A Noninvasive Tool for Drug Detection. ACS Sens. 2020, 5, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liu, T.; Wang, Y.; Miao, P. Electrochemical aptasensors for detection of small molecules, macromolecules, and cells. Rev. Anal. Chem. 2016, 35, 201–211. [Google Scholar] [CrossRef]
- Laghlimi, C.; Moutcine, A.; Chtaini, A.; Isaad, J.; Soufi, A.; Ziat, Y.; Amhamdi, H.; Belkhanchi, H. Recent advances in electrochemical sensors and biosensors for monitoring drugs and metabolites in pharmaceutical and biological samples. Admet Dmpk 2023, 11, 151–173. [Google Scholar] [CrossRef]
- Pholsiri, T.; Khamcharoen, W.; Vimolmangkang, S.; Siangproh, W.; Chailapakul, O. Paper-based electrochemical sensor for simultaneous detection of salivary Δ9-tetrahydrocannabinol and thiocyanate to differentiate illegal cannabis smokers. Sens. Actuators B Chem. 2023, 383, 133571. [Google Scholar] [CrossRef]
- Joosten, F.; Parrilla, M.; van Nuijs, A.L.N.; Ozoemena, K.I.; De Wael, K. Electrochemical detection of illicit drugs in oral fluid: Potential for forensic drug testing. Electrochim. Acta 2022, 436, 141309. [Google Scholar] [CrossRef]
- Moore, C.; Coulter, C.; Rana, S.; Vincent, M.; Snares, J. Analytical Procedure for the Determination of the Marijuana Metabolite 11-nor-Δ9-Tetrahydrocannabinol-9-carboxylic Acid in Oral Fluid Specimens. J. Anal. Toxicol. 2006, 30, 409–412. [Google Scholar] [CrossRef][Green Version]
- Verstraete, A.G. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther. Drug Monit. 2004, 26, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Bosker, W.M.; Huestis, M.A. Oral Fluid Testing for Drugs of Abuse. Clin. Chem. 2009, 55, 1910–1931. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, J.G.; Moeller, M.; van Ruitenbeek, P.; Theunissen, E.L.; Schneider, E.; Kauert, G. Cognition and motor control as a function of Δ9-THC concentration in serum and oral fluid: Limits of impairment. Drug Alcohol Depend. 2006, 85, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, B.; Abuse, A. Strengths and limitations of two cannabis-impaired driving detection methods: A review of the literature. Am. J. Drug Alcohol Abus. 2019, 45, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Agius, R.; Nadulski, T.; Moore, C. Validation of LUCIO®-Direct-ELISA kits for the detection of drugs of abuse in urine: Application to the new German driving licence re-granting guidelines. Forensic Sci. Int. 2012, 215, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Zarandona, I.; Ortiz, L.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Simultaneous quantification of major cannabinoids and metabolites in human urine and plasma by HPLC-MS/MS and enzyme-alkaline hydrolysis. Drug Test. Anal. 2017, 9, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, N.A.; Lee, D.; Concheiro-Guisan, M.; Scheidweiler, K.B.; Gorelick, D.A.; Huestis, M.A. Urinary cannabinoid disposition in occasional and frequent smokers: Is THC-glucuronide in sequential urine samples a marker of recent use in frequent smokers? Clin. Chem. 2014, 60, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Guidet, C.; Gregoire, M.; Le Dreau, A.; Vrignaud, B.; Deslandes, G.; Monteil-Ganière, C. Cannabis intoxication after accidental ingestion in infants: Urine and plasma concentrations of Δ-9-tetrahydrocannabinol (THC), THC-COOH and 11-OH-THC in 10 patients. Clin. Toxicol. 2019, 58, 421–423. [Google Scholar] [CrossRef]
- Hayley, A.C.; Downey, L.A.; Hansen, G.; Dowell, A.; Savins, D.; Buchta, R.; Catubig, R.; Houlden, R.; Stough, C. Detection of delta-9-tetrahydrocannabinol (THC) in oral fluid, blood and urine following oral consumption of low-content THC hemp oil. Forensic Sci. Int. 2018, 284, 101–106. [Google Scholar] [CrossRef]
- Heltsley, R.; DePriest, A.; Black, D.L.; Crouch, D.J.; Robert, T.; Marshall, L.; Meadors, V.M.; Caplan, Y.H.; Cone, E.J. Oral fluid drug testing of chronic pain patients. II. Comparison of paired oral fluid and urine specimens. J. Anal. Toxicol. 2012, 36, 75–80. [Google Scholar] [CrossRef]
- Khidr, H.I.; Tegin, C. Strategies for preventing and detecting false-negatives in urine drug screens. Curr. Psychiatry 2017, 16, e1–e3. [Google Scholar]
- Kim, J.Y.; Kwon, W.; Kim, H.S.; Suh, S.I.; In, M.K. Estimation of measurement uncertainty for the quantification of 11-nor-delta 9-tetrahydrocannabinol-9-carboxylic acid and its glucuronide in urine using liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2014, 38, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Kim, J.Y.; Suh, S.; In, M.K. Direct quantification of 11-nor-Δ9-tetrahydrocannabinol-9- carboxylic acid and its glucuronide in urine using liquid chromatography-tandem mass spectrometry. Anal. Methods 2013, 5, 3028–3034. [Google Scholar] [CrossRef]
- Lillsunde, P.; Korte, T. Comprehensive drug screening in urine using solid-phase extraction and combined TLC and GC/MS identification. J. Anal. Toxicol. 1991, 15, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Nelson, G.J.; McMillin, G.A. Evaluation of the nexscreen and drugcheck waive RT urine drug detection cups. J. Anal. Toxicol. 2013, 37, 30–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maharjan, A.S.; Johnson-Davis, K.L. Issues of Interferences With Immunoassays Used for Screening of Drugs of Abuse in Urine, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 129–139. [Google Scholar]
- Manno, B.R.; Manno, B.S.; Kemp, P.M.; Alford, D.D.; Abukhalaf, I.K.; McWilliams, M.E.; Hagaman, B.S.; Fitzgerald, B.S. Temporal indication of marijuana use can be estimated from plasma and urine concentrations of Δ9-tetrahydrocannabinol, 11-hydroxy-Δ9-tetrahydrocannabinol, and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid. J. Anal. Toxicol. 2001, 25, 538–549. [Google Scholar] [CrossRef]
- Meier, U.; Dussy, F.; Scheurer, E.; Mercer-Chalmers-Bender, K.; Hangartner, S. Cannabinoid concentrations in blood and urine after smoking cannabidiol joints. Forensic Sci. Int. 2018, 291, 62–67. [Google Scholar] [CrossRef]
- Mordal, J.; Holm, B.; Mørland, J.; Bramness, J.G. Recent substance intake among patients admitted to acute psychiatric wards: Physician’s assessment and on-site urine testing compared with comprehensive laboratory analyses. J. Clin. Psychopharmacol. 2010, 30, 455–459. [Google Scholar] [CrossRef]
- Niedbala, R.S.; Kardos, K.W.; Fritch, D.F.; Kardos, S.; Fries, T.; Waga, J.; Robb, J.; Cone, E.J. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J. Anal. Toxicol. 2001, 25, 289–303. [Google Scholar] [CrossRef]
- Pacifici, R.; Pichini, S.; Pellegrini, M.; Rotolo, M.C.; Giorgetti, R.; Tagliabracci, A.; Busardò, F.P.; Huestis, M.A. THC and CBD concentrations in blood, oral fluid and urine following a single and repeated administration of “light cannabis”. Anal. Clin. Chem. 2020, 58, 682–689. [Google Scholar] [CrossRef]
- Pacifici, R.; Pichini, S.; Pellegrini, M.; Tittarelli, R.; Pantano, F.; Mannocchi, G.; Rotolo, M.C.; Busardò, F.P. Determination of cannabinoids in oral fluid and urine of “light cannabis” consumers: A pilot study. Clin. Chem. Lab. Med. 2019, 57, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Raes, E.; Verstraete, A.G. Usefulness of roadside urine drug screening in drivers suspected of driving under the influence of drugs (DUID). J. Anal. Toxicol. 2005, 29, 632–636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Röhrich, J.; Schimmel, I.; Zörntlein, S.; Becker, J.; Drobnik, S.; Kaufmann, T.; Kuntz, V.; Urban, R. Concentrations of Δ9-tetrahydrocannabinol and 11-nor-9-carboxytetrahydrocannabinol in blood and urine after passive exposure to cannabis smoke in a coffee shop. J. Anal. Toxicol. 2010, 34, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.; Verstraete, A.; Proença, P.; Corte-Real, F.; Monsanto, P.; Vieira, D.N. Validated method for the simultaneous determination of Δ9-THC and Δ9-THC-COOH in oral fluid, urine and whole blood using solid-phase extraction and liquid chromatography-mass spectrometry with electrospray ionization. Forensic Sci. Int. 2007, 170, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Toennes, S.W.; Kauert, G.F.; Steinmeyer, S.; Moeller, M.R. Driving under the influence of drugs—Evaluation of analytical data of drugs in oral fluid, serum and urine, and correlation with impairment symptoms. Forensic Sci. Int. 2005, 152, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, J.A. Drug abuse urine tests: False-positive results. Pharm. Lett. Prescr. Lett. 2005, 21, 1–5. [Google Scholar]
- Allen, K.R. Screening for drugs of abuse: Which matrix, oral fluid or urine? Ann. Clin. Biochem. 2011, 48, 531–541. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Shaari, K.; Verpoorte, R. Biotransformation of Tetrahydrocannabinol. Phytochem. Rev. 2016, 15, 921–934. [Google Scholar] [CrossRef]
- Myers, R.E.; Sprague, J.M.; Meikle, H.; Anderson, V.; Symmes, D.; Schneider, G.E. Delta-9-Tetrahydrocannabinol: Metabolism and Disposition. Clin. Pharmacol. Ther. 1971, 272, 1965–1967. [Google Scholar]
- Kabir, A.; Locatelli, M.; Ulusoy, H.I. Recent trends in microextraction techniques employed in analytical and bioanalytical sample preparation. Separations 2017, 4, 36. [Google Scholar] [CrossRef]
- Wiencek, J.R.; Colby, J.M.; Nichols, J.H. Rapid Assessment of Drugs of Abuse. Adv. Clin. Chem. 2017, 80, 193–225. [Google Scholar] [PubMed]
- Anizan, S.; Huestis, M.A. The potential role of oral fluid in antidoping testing. Clin. Chem. 2014, 60, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Cone, E.J.; Bigelow, G.E.; Herrmann, E.S.; Mitchell, J.M.; LoDico, C.; Flegel, R.; Vandrey, R. Nonsmoker exposure to secondhand cannabis smoke. III. Oral fluid and blood drug concentrations and corresponding subjective effects. J. Anal. Toxicol. 2015, 39, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Schwope, D.M.; Milman, G.; Barnes, A.J.; Gorelick, D.A.; Huestis, M.A. Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clin. Chem. 2012, 58, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Newmeyer, M.N.; Desrosiers, N.A.; Lee, D.; Mendu, D.R.; Barnes, A.J.; Gorelick, D.A.; Huestis, M.A. Cannabinoid disposition in oral fluid after controlled cannabis smoking in frequent and occasional smokers. Drug Test. Anal. 2014, 6, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Sobolesky, P.M.; Smith, B.E.; Hubbard, J.A.; Stone, J.; Marcotte, T.D.; Grelotti, D.J.; Grant, I.; Fitzgerald, R.L. Validation of a liquid chromatography-tandem mass spectrometry method for analyzing cannabinoids in oral fluid. Clin. Chim. Acta 2019, 491, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, N.A.; Lee, D.; Schwope, D.M.; Milman, G.; Barnes, A.J.; Gorelick, D.A.; Huestis, M. On-site test for cannabinoids in oral fluid. Clin. Chem. 2012, 58, 1418–1425. [Google Scholar] [CrossRef]
- Dobri, S.C.D.; Moslehi, A.H.; Davies, T.C. Are oral fluid testing devices effective for the roadside detection of recent cannabis use? A systematic review. Public Health 2019, 171, 57–65. [Google Scholar] [CrossRef]
- White, R.M.; Moore, C.M. Detection of Drugs and Their Metabolites in Oral Fluid; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Saar-Reismaa, P.; Erme, E.; Vaher, M.; Kulp, M.; Kaljurand, M.; Mazina-Šinkar, J. In Situ Determination of Illegal Drugs in Oral Fluid by Portable Capillary Electrophoresis with Deep UV Excited Fluorescence Detection. Anal. Chem. 2018, 90, 6253–6258. [Google Scholar] [CrossRef]
- Antunes, M.; Barroso, M.; Gallardo, E. Analysis of Cannabinoids in Biological Specimens: An Update. Int. J. Environ. Res. Public Health 2023, 20, 2312. [Google Scholar] [CrossRef]
- Huestis, M.A.; Cone, E. Relationship of Δ9-tetrahydrocannabinol concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J. Anal. Toxicol. 2004, 28, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Forde, M.D.; Koka, S.; Eckert, S.E.; Carr, A.B.; Wong, D.T. Systemic assessments utilizing saliva: Part 1—General considerations and current assessments. Int. J. Prosthodont. 2006, 19, 43–52. [Google Scholar] [PubMed]
- Gjerde, H.; Langel, K.; Favretto, D.; Verstraete, A.G. Estimation of equivalent cutoff thresholds in blood and oral fluid for drug prevalence studies. J. Anal. Toxicol. 2014, 38, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Spindle, T.R.; Cone, E.J.; Herrmann, E.S.; Mitchell, J.M.; Flegel, R.; LoDico, C.; Bigelow, G.E.; Vandrey, R. Pharmacokinetics of Cannabis Brownies: A Controlled Examination of Δ9-Tetrahydrocannabinol and Metabolites in Blood and Oral Fluid of Healthy Adult Males and Females. J. Anal. Toxicol. 2020, 44, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Vincent, M.; Rana, S.; Coulter, C.; Agrawal, A.; Soares, J. Stability of Delta(9)-tetrahydrocannabinol (THC) in oral fluid using the Quantisal collection device. Forensic Sci. Int. 2006, 164, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Cohier, C.; Mégarbane, B.; Roussel, O. Illicit Drugs in Oral Fluid: Evaluation of Two Collection Devices. J. Anal. Toxicol. 2017, 41, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wille, S.M.R.; Di Fazio, V.; Ramírez-Fernandez, M.d.M.; Kummer, N.; Samyn, N. Driving Under the Influence of Cannabis: Pitfalls, Validation, and Quality Control of a UPLC-MS/MS Method for the Quantification of Tetrahydrocannabinol in Oral Fluid Collected With StatSure, Quantisal, or Certus Collector. Ther. Drug Monit. 2013, 35, 101–111. [Google Scholar] [CrossRef]
- Jaffe, A.; Molnar, S.; Williams, N.; Wong, E.; Todd, T.; Caputo, C.; Tolentino, J.; Ye, S. Review and Recommendations for Drug Testing in Substance Use Treatment Contexts. J. Reward Defic. Syndr. Addict. Sci. 2016, 2, 28–45. [Google Scholar] [CrossRef]
- Langel, K.; Engblom, C.; Pehrsson, A.; Gunnar, T.; Ariniemi, K.; Lillsunde, P. Drug Testing in Oral Fluid—Evaluation of Sample Collection Devices. J. Anal. Toxicol. 2008, 32, 393–401. [Google Scholar] [CrossRef]
- Ventura, M.; Pichini, S.; Ventura, R.; Leal, S.; Zuccaro, P.; Pacifici, R.; de la Torre, R. Stability of drugs of abuse in oral fluid collection devices with purpose of external quality assessment schemes. Ther. Drug Monit. 2009, 31, 277–280. [Google Scholar] [CrossRef]
- Drummer, O.H.; Gerostamoulos, D.; Chu, M.; Swann, P.; Boorman, M.; Cairns, I. Drugs in oral fluid in randomly selected drivers. Forensic Sci. Int. 2007, 170, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Speedy, T.; Baldwin, D.; Jowett, G.; Gallina, M.; Jehanli, A. Development and validation of the Cozart® DDS oral fluid collection device. Forensic Sci. Int. 2007, 170, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Pechansky, F.; Scherer, J.N.; Schuch, J.B.; Roglio, V.; Telles, Y.M.; Silvestrin, R.; Pasa, G.; Sousa, T. User experience and operational feasibility of four point-of-collection oral fluid drug-testing devices according to Brazilian traffic agents. Traffic Inj. Prev. 2019, 20, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.-J.; Warner, J.V.; Henman, M.G.; Ferguson, W.E. Recovery of drugs of abuse from Dräger DCD5000 oral fluid collection device in Australia. J. Anal. Toxicol. 2015, 39, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Richeval, C.; Dumestre-Toulet, V.; Wiart, J.-F.; Vanhoye, X.; Humbert, L.; Nachon-Phanithavong, M.; Allorge, D.; Gaulier, J.-m. New psychoactive substances in oral fluid of drivers around a music festival in south-west France in 2017. Forensic Sci. Int. 2019, 297, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Engblom, C.; Gunnar, T.; Rantanen, A.; Lillsunde, P. Driving Under the Influence of Drugs-Amphetamine Concentrations in Oral Fluid and Whole Blood Samples. J. Anal. Toxicol. 2007, 31, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, N.A.; Scheidweiler, K.B.; Huestis, M.A. Quantification of six cannabinoids and metabolites in oral fluid by liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2015, 7, 684–694. [Google Scholar] [CrossRef]
- Kauert, G.F.; Iwersen-Bergmann, S.; Toennes, S.W. Assay of Δ9-Tetrahydrocannabinol (THC) in Oral Fluid—Evaluation of the OraSure Oral Specimen Collection Device. J. Anal. Toxicol. 2006, 30, 274–277. [Google Scholar] [CrossRef]
- Quintela, O.; Crouch, D.J.; Andrenyak, D. Recovery of drugs of abuse from the Immunalysis Quantisal™ oral fluid collection device. J. Anal. Toxicol. 2006, 30, 614–616. [Google Scholar] [CrossRef]
- Beckham, J.C.; Adkisson, K.A.; Hertzberg, J.; Kimbrel, N.A.; Budney, A.J.; Stephens, R.S.; Moore, S.D.; Calhoun, P.S. Mobile contingency management as an adjunctive treatment for co-morbid cannabis use disorder and cigarette smoking. Addict. Behav. 2018, 79, 86–92. [Google Scholar] [CrossRef]
- Desrosiers, N.A.; Milman, G.; Mendu, D.R.; Lee, D.; Barnes, A.J.; Gorelick, D.A.; Huestis, M.A. Cannabinoids in oral fluid by on-site immunoassay and by GC-MS using two different oral fluid collection devices. Anal. Bioanal. Chem. 2014, 406, 4117–4128. [Google Scholar] [CrossRef] [PubMed]
- Saar-Reismaa, P.; Tretjakova, A.; Mazina-Šinkar, J.; Vaher, M.; Kaljurand, M.; Kulp, M. Rapid and sensitive capillary electrophoresis method for the analysis of Ecstasy in an oral fluid. Talanta 2019, 197, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.C.; Tran, M.; Tung, J.K. Oral fluid drug tests: Effects of adulterants and foodstuffs. Forensic Sci. Int. 2005, 150, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Milman, G.; Schwope, D.M.; Barnes, A.J.; Gorelick, D.A.; Huestis, M.A. Cannabinoid Stability in Authentic Oral Fluid after Controlled Cannabis Smoking. Clin. Chem. 2012, 58, 1101–1109. [Google Scholar] [CrossRef]
- UltraSal-2 Oral Fluid Collection Device. In Datasheet; Neogen Corporation: Lansing, MI, USA, 2016; Available online: https://toxicology.neogen.com (accessed on 14 December 2023).
- Tom Blencowe, A.P.a.P.L. (Ed.) DRUID—Driving under the Influence of Drugs, Alcohol and Medicines in Analytical Evaluation of Oral Fluid Screening Devices and Preceding Selection Procedures; Technical University of Denmark: Kongens Lyngby, Denmark, 2010. [Google Scholar]
- Concheiro, M.; De Castro, A.; Quintela, O.; Cruz, A.; Lopez-Rivadulla, M. Development and validation of a method for the quantitation of Δ9tetrahydrocannabinol in oral fluid by liquid chromatography electrospray–mass-spectrometry. J. Chromatogr. B 2004, 810, 319–324. [Google Scholar] [CrossRef]
- Teixeira, H.; Proença, P.; Verstraete, A.; Corte-Real, F.; Vieira, D.N. Analysis of Δ9-tetrahydrocannabinol in oral fluid samples using solid-phase extraction and high-performance liquid chromatography–electrospray ionization mass spectrometry. Forensic Sci. Int. 2005, 150, 205–211. [Google Scholar] [CrossRef]
- Laloup, M.; Fernandez, M.d.M.R.; Wood, M.; Boeck, G.D.; Henquet, C.; Maes, V.; Samyn, N. Quantitative analysis of Δ9-tetrahydrocannabinol in preserved oral fluid by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2005, 1082, 15–24. [Google Scholar] [CrossRef]
- Day, D.; Kuntz, D.J.; Feldman, M.; Presley, L. Detection of THCA in Oral Fluid by GC-MS-MS. J. Anal. Toxicol. 2006, 30, 645–650. [Google Scholar] [CrossRef]
- Moore, C.; Rana, S.; Coulter, C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J. Chromatogr. B 2007, 852, 459–464. [Google Scholar] [CrossRef]
- Quintela, O.; Andrenyak, D.M.; Hoggan, A.M.; Crouch, D.J. A Validated Method for the Detection of Δ9-Tetrahydrocannabinol and 11-nor-9-Carboxy-Δ9-Tetrahydrocannabinol in Oral Fluid Samples by Liquid Chromatography Coupled with Quadrupole-Time-of-Flight Mass Spectrometry. J. Anal. Toxicol. 2007, 31, 157–164. [Google Scholar] [CrossRef][Green Version]
- Milman, G.; Barnes, A.J.; Lowe, R.H.; Huestis, M.A. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J. Chromatogr. A 2010, 1217, 1513–1521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bylda, C.; Leinenbach, A.; Thiele, R.; Kobold, U.; Volmer, D.A. Development of an electrospray LC-MS/MS method for quantification of Δ9-tetrahydrocannabinol and its main metabolite in oral fluid. Drug Test. Anal. 2012, 4, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Coulter, C.; Garnier, M.; Moore, C. Analysis of Tetrahydrocannabinol and its Metabolite, 11-Nor-Δ9-Tetrahydrocannabinol-9-Carboxylic Acid, in Oral Fluid using Liquid Chromatography with Tandem Mass Spectrometry. J. Anal. Toxicol. 2012, 36, 413–417. [Google Scholar] [CrossRef]
- Molnar, A.; Lewis, J.; Doble, P.; Hansen, G.; Prolov, T.; Fu, S. A rapid and sensitive method for the identification of delta-9-tetrahydrocannabinol in oral fluid by liquid chromatography–tandem mass spectrometry. Forensic Sci. Int. 2012, 215, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-D.; Chang, Y.-J.; Lin, K.-L.; Chang, Y. Simultaneous determination of Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in oral fluid using isotope dilution liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 402, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, M.; Giroud, C. Letter to the editor regarding “Simultaneous determination of ∆9-tetrahydrocannabinol and 11-nor-9-carboxy-∆9-tetrahydrocannabinol in oral fluid using isotope dilution liquid chromatography tandem mass spectrometry”. Anal. Bioanal. Chem. 2013, 405, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Marta Concheiro, D.L.; Elena, L.; Marilyn, A. Huestis. Simultaneous quantification of Δ9-tetrahydrocannabinol, 11-nor-9-carboxy-tetrahydrocannabinol, cannabidiol and cannabinol in oral fluid by microflow-liquid chromatography–high resolution mass spectrometry. J. Chromatogr. A 2013, 1297, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Scheidweiler, K.B.; Himes, S.K.; Chen, X.; Liu, H.-F.; Huestis, M.A. 11-Nor-9-carboxy-∆ 9-tetrahydrocannabinol quantification in human oral fluid by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 6019–6027. [Google Scholar] [CrossRef]
- Sergi, M.; Montesano, C.; Odoardi, S.; Rocca, L.M.; Fabrizi, G.; Compagnone, D.; Curini, R. Micro extraction by packed sorbent coupled to liquid chromatography tandem mass spectrometry for the rapid and sensitive determination of cannabinoids in oral fluids. J. Chromatogr. A 2013, 1301, 139–146. [Google Scholar] [CrossRef]
- Fabritius, M.; Staub, C.; Mangin, P.; Giroud, C. Analysis of cannabinoids in oral fluid by liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2013, 31, 151–163. [Google Scholar] [CrossRef]
- Barnes, A.J.; Scheidweiler, K.B.; Huestis, M.A. Quantification of 11-Nor-9-Carboxy-Δ9-tetrahydrocannabinol in human oral fluid by gas chromatography–tandem mass spectrometry. Ther. Drug Monit. 2014, 36, 225–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kintz, P.; Brunet, B.; Muller, J.-F.; Serra, W.; Villain, M.; Cirimele, V.; Mura, P. Evaluation of the Cozart DDSV test for cannabis in oral fluid. Ther. Drug Monit. 2009, 31, 131–134. [Google Scholar] [CrossRef]
- Blencowe, T.; Pehrsson, A.; Lillsunde, P.; Vimpari, K.; Houwing, S.; Smink, B.; Mathijssen, R.; Van der Linden, T.; Legrand, S.-A.; Pil, K.; et al. An analytical evaluation of eight on-site oral fluid drug screening devices using laboratory confirmation results from oral fluid. Forensic Sci. Int. 2011, 208, 173–179. [Google Scholar] [CrossRef]
- Strano-Rossi, S.; Castrignanò, E.; Anzillotti, L.; Serpelloni, G.; Mollica, R.; Tagliaro, F.; Pascali, J.P.; Di Stefano, D.; Sgalla, R.; Chiarotti, M. Evaluation of four oral fluid devices (DDS®, Drugtest 5000®, Drugwipe 5+® and RapidSTAT®) for on-site monitoring drugged driving in comparison with UHPLC–MS/MS analysis. Forensic Sci. Int. 2012, 221, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, J.; Zörntlein, S.; Becker, J.; Urban, R. Detection of Δ9-tetrahydrocannabinol and amphetamine-type stimulants in oral fluid using the Rapid Stat™ point-of-collection drug-testing device. J. Anal. Toxicol. 2010, 34, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wille, S.M.; Ramírez-Fernández, M.d.M.; Samyn, N.; De Boeck, G. Conventional and alternative matrices for driving under the influence of cannabis: Recent progress and remaining challenges. Bioanalysis 2010, 2, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Wille, S.M.; Samyn, N.; del Mar Ramírez-Fernández, M.; De Boeck, G. Evaluation of on-site oral fluid screening using Drugwipe-5+®, RapidSTAT® and Drug Test 5000® for the detection of drugs of abuse in drivers. Forensic Sci. Int. 2010, 198, 2–6. [Google Scholar] [CrossRef]
- Cirimele, V.; Villain, M.; Mura, P.; Bernard, M.; Kintz, P. Oral fluid testing for cannabis: On-site Oraline® IV sat device versus GC/MS. Forensic Sci. Int. 2006, 161, 180–184. [Google Scholar] [CrossRef]
- Concheiro, M.; de Castro, A.; Quintela, O.; Cruz, A.; López-Rivadulla, M. Confirmation by LC–MS of drugs in oral fluid obtained from roadside testing. Forensic Sci. Int. 2007, 170, 156–162. [Google Scholar] [CrossRef]
- Pehrsson, A.; Gunnar, T.; Engblom, C.; Seppä, H.; Jama, A.; Lillsunde, P. Roadside oral fluid testing: Comparison of the results of drugwipe 5 and drugwipe benzodiazepines on-site tests with laboratory confirmation results of oral fluid and whole blood. Forensic Sci. Int. 2008, 175, 140–148. [Google Scholar] [CrossRef]
- Pehrsson, A.; Blencowe, T.; Vimpari, K.; Langel, K.; Engblom, C.; Lillsunde, P. An evaluation of on-site oral fluid drug screening devices DrugWipe® 5+ and rapid STAT® using oral fluid for confirmation analysis. J. Anal. Toxicol. 2011, 35, 211–218. [Google Scholar] [CrossRef]
- Gentili, S.; Solimini, R.; Tittarelli, R.; Mannocchi, G.; Busardò, F.P. A study on the reliability of an on-site oral fluid drug test in a recreational context. J. Anal. Methods Chem. 2016, 2016, 1234581. [Google Scholar] [CrossRef]
- Toennes, S.W.; Schneider, K.; Wunder, C.; Kauert, G.F.; Moeller, M.R.; Theunissen, E.L.; Ramaekers, J.G. Influence of ethanol on the pharmacokinetic properties of Δ9-tetrahydrocannabinol in oral fluid. J. Anal. Toxicol. 2013, 37, 152–158. [Google Scholar] [CrossRef]
- Laloup, M.; del Mar Ramirez Fernandez, M.; Wood, M.; De Boeck, G.; Maes, V.; Samyn, N. Correlation of Δ9-tetrahydrocannabinol concentrations determined by LC–MS–MS in oral fluid and plasma from impaired drivers and evaluation of the on-site Dräger DrugTest®. Forensic Sci. Int. 2006, 161, 175–179. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Mohr, A.L.A.; Friscia, M.; Logan, B.K. Field Detection of Drugs of Abuse in Oral Fluid Using the Alere™ DDS®2 Mobile Test System with Confirmation by Liquid Chromatography Tandem Mass Spectrometry (LC–MS/MS). J. Anal. Toxicol. 2017, 42, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.startengine.com/offering/eclipsedx (accessed on 14 December 2023).
| Analytical Technique | Sensitivity | Blood | Oral Fluid | Urine | Sweat | Breath | Hair | Point-of-Care |
|---|---|---|---|---|---|---|---|---|
| Immunoassay | High | Yes | Yes | Yes | Yes | No | Yes | Yes |
| General spectrophotometry (ultraviolet, infrared, fluorescence, visible) | Low | No | Yes | No | No | No | No | Yes |
| Raman | Moderate | No | Yes | No | Yes | No | No | Yes |
| Nuclear magnetic resonance | Moderate | Yes | No | Yes | No | No | No | No |
| Gas chromatography-mass spectrometry | High | Yes | Yes | Yes | Yes | Yes | Yes | Not yet |
| Liquid chromatography-mass spectrometry | High | Yes | Yes | Yes | Yes | No | Yes | Not yet |
| High-performance liquid chromatography-mass spectrometry | High | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Isotope ratio mass spectrometry | Low | No | No | Yes | No | No | No | No |
| Thin-layer chromatography | Low | Yes | Yes | Yes | No | No | No | No |
| Gas chromatography–nitrogen phosphorous detector | Moderate | Yes | Yes | Yes | Yes | No | No | No |
| Gas chromatography–flame ionization detector | Low | Yes | Yes | Yes | Yes | Yes | No | No |
| Liquid chromatography–ultraviolet detector | Low | Yes | No | Yes | No | No | No | No |
| Chemiluminescence | High | Yes | Yes | Yes | Yes | Yes | Yes | Not yet |
| Electrochemical detector | High | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Capillary electrophoresis | High | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Supercritical fluid chromatography | Moderate | Yes | Yes | Yes | Yes | No | No | No |
| Biological Matrix | Advantages | Disadvantage | Cannabinoids Detection |
|---|---|---|---|
| Blood |
|
| Show recent drug intake |
| Urine |
|
| It does not show recent drug intake but offers a view of drug usage over the last month |
| Oral fluid |
|
| If there is no direct contamination of the buccal cavity, it depends on the transfer of analytes from the blood into the oral fluid |
| Sweat |
|
| Sample can be easily contaminated from the environment |
| Hair |
|
| Does not show recent intake |
| Matrix | Analyte | Cutoff (ng/mL) | Use | Detection Times | Reference |
|---|---|---|---|---|---|
| Urine | THC-COOH | 15 | Single-use | <3 days | [37,65,96] |
| THC-COOH | Moderate use—four times a week | <4 days | |||
| THC-COOH | Chronic use | 14–90 days | |||
| Blood | THC | 10 | Single-use | <5 h | [65,96] |
| THC THC-COOH | Chronic use | <14 days | [96] | ||
| THC-COOH | Single-use | <36 h | [65] | ||
| Oral fluid | THC CBD CBN THC-COOH | 0.5 | Single and chronic use | THC: 12–34 h CBD: 1–22 h CBN: 1–13.5 h | [96,97,98,99,100,101,102] |
| CBG | 1 | Single-use | 15 min | [101] | |
| THCV | 0.4 | Single-use | 15 min | ||
| THCA-A | 1 | Single-use | <90 min |
| Method of Collection | Advantage | Disadvantage |
|---|---|---|
| Passive drool |
|
|
| Expectoration |
|
|
| Salivary stimulation |
|
|
| Collection device |
|
|
| Device (Manufacturer, City and Country) | Components | Collection Method | Volume Indicator | Oral Fluid Volume (mL) | Extraction Technique | Tetrahydrocannabinol (THC) Recovery (%) | Refs. |
|---|---|---|---|---|---|---|---|
| Certus (Concateno, Corston, UK) | Pad, container, buffer (3 mL), volume adequacy indicator | Absorbent pad is inserted into the buffer | Yes | 1 | Pad placed in the buffer for 24 h at 4 °C | 54 37–44 (71–85) | [112,113] |
| Cozart (Cozart Bioscience, Abingdon, UK) | Pad, container, buffer (2 mL), volume adequacy indicator | Absorbent pad is inserted into the buffer | Yes | 1 | Elute with a proprietary buffer | 96 75.9 (6.2) 94.5 (0.02) 67.4 | [114,115,116,117,118] |
| DCD 5000 (Dräger, Lübeck, Germany) | Cassette: tap, pad, container, buffer, volume adequacy indicator Device: reader, printer (electronic and printed results) | Absorbent pad is part of a device; buffer is added after collection | Yes | 0.38 | Placed in isopropanol for 1 h and centrifuged | 89.8–93.8 | [119,120] |
| DrugWipe 5, 5S, 6, 6S (Securetec, Neubiberg, Germany) | Cassette: collection pad, buffer, LFI strip | Sweep the tongue, saliva was collected by a change of color, not quantitative | No | - | - | - | [121] |
| Greiner (Greiner Bio-One GmbH, Greinerstraße, Austria) | Rinsing solution (6 mL), OF extraction solution (4 mL), collection beaker, 2 OF vacuum transfer tubes | Collection by thoroughly rinsing out the oral cavity (2 min), expectoration into collection beaker, transfer to Saliva Transfer Tubes, add stabilizers | No | Determined spectrophotometrically w/dye in the extraction solution | Determined spectrophotometrically w/dye in the extraction solution | 73.6 (4.3) | [36,115] |
| Intercept (OraSure Technologies, Bethlehem, PA, USA) | Cotton fiber pad, plastic container, buffer (0.8 mL) | Absorbent pad is inserted into the buffer | No | 1 mL max | Centrifuged to recover the buffer-oral fluid mixture Centrifuged to recover the buffer-oral fluid mixture Centrifuge, add 2 mL methanol to stabilization buffer and pad, incubate and shake 15 min, centrifuge | 37.6 31.2–57.2 Additional 19.2–34.4 37.6 39.2 | [115,116,122,123,124] |
| Quantisal (Immunalysis, Pomona, CA, USA) | Cellulose pad, plastic container, buffer (3 mL), volume adequacy indicator | Absorbent pad is inserted into the buffer | Yes | 1 ± 0.1 (10%) | Buffer-oral fluid mixture separated with serum separator tube Pad placed in the buffer for 24 h at 4 °C | 81.3–91.4 94 55.8 55.8 (12.0) 81.3–94.4 (4.8–12.1) 74–80 (12–16) | [44,57,112,113,115,125] |
| OraCol and OraCol Plus (Malvern Medical Developments, Worcester, UK) | Foam swab, microtube, centrifuge tube | Saliva is collected by rubbing the sponge swab firmly along the gum until the sponge is wet | No | 1 | Centrifugation with a tube inserted | <12.5 | [36,115] |
| OraTect III (Branan Medical Corporation, Irvine, CA, USA) | Cassette: collection pad, LFI strip | Directly applied to the mouth | No | - | - | - | [126] |
| OraTube (Varian, Palo Alto, CA, USA) | Pad, plastic container, expresser | Absorbent pad | No | 1.979 mL (in vitro) | - | - | [115] |
| Salicule (Acro Biotech, Montclair, NJ, USA) | Expectoration straw, container marker w/scale | Expectoration | Yes | - | - | - | [36] |
| Saliva-Sampler (StatSure Diagnostic System, Sterling, VA, USA) | Cellulose pad, plastic container, buffer (1 mL), volume adequacy indicator | Absorbent pad, buffer | Yes | 1 | Buffer-oral fluid mixture extracted from the pad with filter | 85.4 65.5–68.1 85.4 (7.0) 100–106 (5–6) | [36,113,115,127] |
| Salivette (Sarstedt AG & Co., Nümbrecht, Germany) | Cotton swab, plastic container | Cotton swab is chewed, placed back into the container then centrifuged | No | Unknown | Centrifugation with a tube inserted | <12.5 | [115,128] |
| Analytical Method | Collection Device | Oral Fluid (OF) Sample Volume | Extraction Method | Derivatization | Analytes Detected (µg/L) | Detection Range (µg/L) | Refs. |
|---|---|---|---|---|---|---|---|
| LC-MS | Plastic tube | 200 µL of expectorated OF | Liquid-liquid extraction | None | THC: 2 | THC: 2–250 | [133] |
| LC-MS | Salivette | 500 µL of Salivette OF | SPE | None | THC: 2 | THC: 2–100 | [134] |
| LC-MS/MS | Intercept | 100 µL OF or 500 µL of Intercept OF | Liquid-liquid extraction | None | THC: 0.5 for 100 µL sample | THC: 0.5–100 | [135] |
| GS-MS/MS | Intercept | 100 µL of Intercept OF | SPE | HFIP and PFAA | THC-COOH: 10 | THC-COOH: 10–240 | [136] |
| 2D-GC-MS | Quantisal | 1 mL of Quantisal OF | SPE | HFIP and TFAA | THC-COOH: 2 | THC-COOH: 2–160 | [64] |
| GS-MS | Quantisal | Unspecified vol. of quantisal OF | SPE | BSTFA | THC: 0.5 CBD: 0.5 CBN: 1 THCAA | THC: 1–16 CBD: 1–16 CBN: 1–16 | [137] |
| LC-QTOF-MS | Plastic tube | 500 µL of synthetic OF | Liquid-liquid extraction | None | THC: 0.05, 0.1 THC-COOH: 0.2, 0.1 | THC: 0.1–100 THC-COOH: 0.1–100 | [138] |
| LC-MS | Plastic tube | 500 µL of expectorated OF | SPE | None | THC: 2, 5 | THC: 5–2000 | [89] |
| 2D-GC-MS (NICI for THC-COOH) | Quantisal | 1 mL of Quantisal OF | SPE | BSTFA TFAA (for THC-COOH) | THC: 0.5 11-OH-THC: 0.4, 0.5 THC-COOH: 6, 7.5 CBD: 0.5 CBN: 1 | THC: 0.5–50 11-OH-THC: 0.5–50 THC-COOH: 7.5–500 CBD: 0.5–50 CBN: 1–50 | [139] |
| LC-MS/MS | Intercept | 400 µL of Intercept OF | SPE | None | THC: 0.2 THC-COOH: 0.2 | THC: 0.25–8 THC-COOH: 0.25–8 | [140] |
| LC-MS/MS | Quantisal | 1 mL of Quantisal OF | SPE | Triphenylphosphine, 2-picolylamine and 2,2′-dypyridyl disulfide | THC: 0.6, 1 THC-COOH: 6, 10 | THC: 1–100 THC-COOH: 10–1000 | [141] |
| LC-MS/MS (quadrupole/orbital) | Plastic tube | 400 µL of OF in preservation buffer | Liquid-liquid extraction and SPE | None | THC: 2 (1 point calibration) THC-COOH: 7.5 | THC-COOH: 7.5–300 | [102] |
| LC-MS/MS | Plastic tube | 200 µL of expectorated OF | Liquid-liquid extraction | None | THC: 1 | THC: 1–500 | [142] |
| LC-MS/MS | Plastic tube | 250 µL of expectorated OF | Dilute and shoot | Dansyl chloride | THC: 0.005, 0.025 THC-COOH: 2.5 | THC: 0.2–20 | [143] |
| LC-MS/MS | Plastic tube | 250 µL of expectorated OF | SPE | None | THC: 0.1 11-OH-THC: 0.1 THC-COOH: 0.1 CBD: 0.1 CBN: 0.1 | THC: 0.1–50 11-OH-THC: 0.1–50 THC-COOH: 0.1–50 CBD: 0.1–50 CBN: 0.1–50 | [144] |
| LC-HRMS | Oral-Eze, Quantisal | 250 µL of Oral-Eze and 500 µL of Quantisal OF | SPE | None | THC: 0.5 THC-COOH: 9, 12 CBD: 0.5 CBN: 0.5 | THC: 0.5–50 THC-COOH: 12–1020 CBD: 0.5–50 CBN: 0.5–50 | [145] |
| LC-MS/MS | Quantisal | 1 mL of Quantisal OF | SPE | None | THC-COOH: 9, 12 | THC-COOH: 12–1020 | [146] |
| LC-MS/MS | Plastic tube | 225 µL of expectorated OF | MEPS | None | THC: 0.08, 0.25 11-OH-THC: 0.12, 0.4 THC-COOH: 8, 20 CBD: 0.1, 0.3 CBN: 0.12, 0.3 | THC: 0.25–250 11-OH-THC: 0.4–250 THC-COOH: 20–1000 CBD: 0.3–250 CBN: 0.3–250 | [147] |
| LC-MS/MS | StatSure, Quantisal | 100 µL of StatSure 200 µL of Quantisal or Certus OF | Liquid-liquid extraction | None | THC: 5 | THC: 5–320 | [113] |
| LC-MS/MS | Quantisal | 1.5 µL of combined Quantisal sample/methanol extract | SPE | None | THC: 0.3, 0.5 11-OH-THC: 0.2, 0.5 THC-COOH: 50, 80 CBD: 0.3, 0.5 CBN: 0.3, 0.5 THCAA | THC: 0.5–75 11-OH-THC: 0.5–75 THC-COOH: 50–500 CBD: 0.5–75 CBN: 0.5–75 | [148] |
| GC-MS/MS | Quantisal, Oral-Eze | 1 mL of Quantisal or 750 µL of Oral-Eze OF | SPE | HFIP and TFAA | THC-COOH: 7.5, 10 | THC-COOH: 10–1000 | [149] |
| LC-MS/MS | Quantisal | 1 mL of Quantisal OF | SPE | None | THC: 0.1, 0.2 11-OH-THC: 0.1, 0.2 THC-COOH: 15 CBD: 0.1, 0.2 THCV CBG | THC: 0.2–100 11-OH-THC: 0.2–50 THC-COOH: 15–3750 CBD: 0.2–50 | [123] |
| Manufacturer (City, Country) | Device | Year | Interpretation of Result | Device Cutoff (THC ng/mL) | Laboratory Cutoff (ng/mL) | Oral Fluid Confirmation | Sensitivity (%) | Specificity (%) | Accuracy (%) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| Cozart (Abingdon, UK) | RapiScan | 2007 | Instrumental | 600 | - | HPLC/GC-MS | - | 100 | 100 | [102] |
| Cozart DDSV | 2009 | Visual | - | 0.5 | GC-MS | 41.2 | 100 | 60 | [150] | |
| Cozart DDS 806 | 2011 | Instrumental | 31 | 1 | UPLC-MSMS GC-MS | 22 | 100 | 71 | [151] | |
| 2012 | Instrumental | 31 | 10 | UPLC-MSMS | 28.2 | 100 | 78.7 | [51] | ||
| Cozart DDS | 2012 | Instrumental | 31 | 1 | UPLC-MSMS GC-MS | 37.8 | 100 | 94.3 | [152] | |
| Mavand (Eschweiler, Germany) | RapidSTAT | 2010 | Visual/Instrumental | 15 | 1.6 | GC-MS | 85 | 87 | 86.7 | [153] |
| 2011 | Visual | 15 | 1 | GC-MS UPLC-MSMS | 68 56 | 89 90 | 86 78 | [154] [151] | ||
| 2012 | Visual | 15 | 1 2 * 10 | GC-MS GC-MS UPLC-MSMS | 72 71 43.3 | 97 55 88.3 | 93 66 78.2 | [152] [155] [51] | ||
| Biosensor (München, Germany) | BIOSENSE Dynamic | 2011 | Instrumental | Unknown | 1 | UPLC-MSMS GC-MS | 50 | Not reported | 51 | [151] |
| Sun Biomedical (Blackwood, NJ, USA) | OraLine | 2006 | Visual | 4 | 1 | HPLC/GC-MS | 69 | 92 | 74 | [156] |
| OraLine IV | 2007 | Visual | 100 | 1 | HPLC/GC-MS | 100 | 36 | 54.3 | [102] | |
| Varian (Palo Alto, CA, USA) | OraLab | 2007 | Visual | 100 | 1 | HPLC/GC-MS | 40 | 100 | 76 | [102] |
| OraLab | 2007 | Visual | 1 | 2 | LC-MS | 93.3 | 98.6 | 98.1 | [157] | |
| OraLab 6 | 2011 | Visual | 50 | 1 | UPLC-MSMS GC-MS | 16 | 99 | 61 | [151] | |
| Innovacon (San Diego, CA, USA) | OrAlert | 2011 | Visual | 100 | 1 | UPLC-MSMS GC-MS | 11 | 100 | 78 | [151] |
| OrAlert | 2012 | Visual | 100 | 10 | UPLC-MSMS | 23.1 | 100 | 90.9 | [51] | |
| Branan (Irvine, CA, USA) | Oratect | 2007 | Visual | 100 | 1 | HPLC/GC-MS | 0 | 100 | 77.8 | [102] |
| Oratect III | 2011 | Visual | 40 | 1 | UPLC-MSMS GC-MS | 32 | 100 | 41 | [151] | |
| American Bio Medica (Kinderhook, NY, USA) | OralStat | 2007 | Visual | 25 | 1 | HPLC/GC-MS | 70 | 100 | 91.4 | [102] |
| LifePoint (Ontario, CA, USA) | Impact | 2007 | Instrumental | 15 | 1 | HPLC/GC-MS | 100 | 33.3 | 71.4 | [102] |
| Ulti-Med (Ahrensburg, Germany) | SalivaScreen | 2007 | Visual | >100 | 1 | HPLC/GC-MS | - | 100 | 100 | [102] |
| OraSure Technologies (Bethlehem, PA, USA) | Uplink | 2007 | Instrumental | 25 | 1 | HPLC/GC-MS | 100 | 92 | 95.6 | [102] |
| Securetec (Neubiberg, Germany) | DrugWipe | 2007 | Visual | 30 | 1 | HPLC/GC-MS | 80 | 100 | 82.9 | [102] |
| DrugWipe 5 | 2008 | Visual | 30 | 2 | GC-MS | 52 | 91 | 85 | [158] | |
| DrugWipe | 2011 | Visual | 30 | 1 | GC-MS | 43 | 96 | 88 | [154] | |
| DrugWipe | 2012 | Visual | 30 | 1 | GC-MS | 47 | 99 | 93 | [152] | |
| DrugWipe5/5+ | 2011 | Visual | 30 | 1 | GC-MS | 43 | 87 | 82 | [159] | |
| DrugWipe 5A | 2016 | Visual | 30 | 0.6 ng/pad | HS-SPME/GC-MS | 29 | 88 | 53 | [160] | |
| DrugWipe 5 | 2010 | Visual | 30 | 2 * | GC-MS | 71 | 50 | 63 | [155] | |
| DrugWipe 5+ | 2013 | Visual | 30 | Unknown | GC-MS | 88 | 94 | 88 | [161] | |
| Dräger (Lübeck, Germany | DrugTest 5000 | 2006 | Instrumental | 20 | 0.5 | LC–MS–MS | 53 | 94 | 55.5 | [162] |
| DrugTest 5000 | 2010 | Instrumental | 20 | 2 * | GC-MS | 82.5 | 60.5 | 79 | [155] | |
| DrugTest 5000 | 2011 | Instrumental | 5 | 1 | UPLC-MSMS GC-MS | 59 | 96 | 82 | [151] | |
| DrugTest 5000 | 2012 | Instrumental | 5 | Unknown | GC–MS | 91 | 43 | 85.5 | [161] | |
| DrugTest 5000 | 2012 | Instrumental | 5 | 0.5 | 2D-GC-MS GC-MS | 87.7 | 81.2 | 85.5 | [102] | |
| DrugTest 5000 | 2012 | Instrumental | 5 | 1 | GC-MS | 92 | 97 | 97 | [152] | |
| DrugTest 5000 | 2012 | Instrumental | 5 | 10 | UPLC-MSMS | 81 | 96 | 92 | [51] | |
| Alere (North Chicago, IL, USA) | DDS 2 | 2017 | Instrumental | 25 | 1 | LC–MS/MS | 90 | 100 | 97.5 | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trif, C.; Harpaz, D.; Eltzov, E.; Parcharoen, Y.; Pechyen, C.; Marks, R.S. Detection of Cannabinoids in Oral Fluid Specimens as the Preferred Biological Matrix for a Point-of-Care Biosensor Diagnostic Device. Biosensors 2024, 14, 126. https://doi.org/10.3390/bios14030126
Trif C, Harpaz D, Eltzov E, Parcharoen Y, Pechyen C, Marks RS. Detection of Cannabinoids in Oral Fluid Specimens as the Preferred Biological Matrix for a Point-of-Care Biosensor Diagnostic Device. Biosensors. 2024; 14(3):126. https://doi.org/10.3390/bios14030126
Chicago/Turabian StyleTrif, Călin, Dorin Harpaz, Evgeni Eltzov, Yardnapar Parcharoen, Chiravoot Pechyen, and Robert S. Marks. 2024. "Detection of Cannabinoids in Oral Fluid Specimens as the Preferred Biological Matrix for a Point-of-Care Biosensor Diagnostic Device" Biosensors 14, no. 3: 126. https://doi.org/10.3390/bios14030126
APA StyleTrif, C., Harpaz, D., Eltzov, E., Parcharoen, Y., Pechyen, C., & Marks, R. S. (2024). Detection of Cannabinoids in Oral Fluid Specimens as the Preferred Biological Matrix for a Point-of-Care Biosensor Diagnostic Device. Biosensors, 14(3), 126. https://doi.org/10.3390/bios14030126







