Advancements in Brain Research: The In Vivo/In Vitro Electrochemical Detection of Neurochemicals
Abstract
1. Introduction
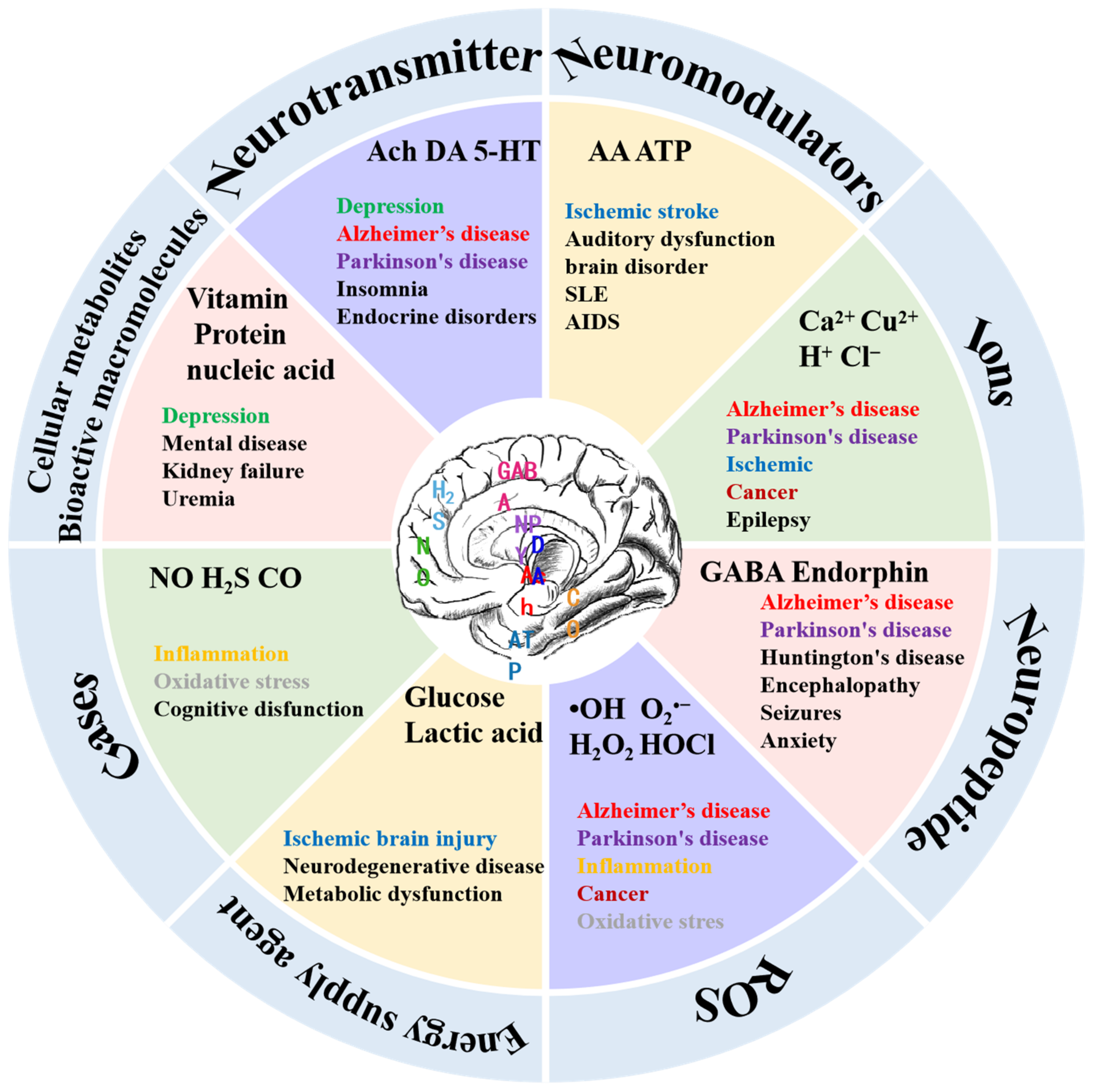
2. Types of Neurochemicals and Associated Diseases
3. In Vivo Electrochemical Measurements of Neurochemicals in the Brain Tissue
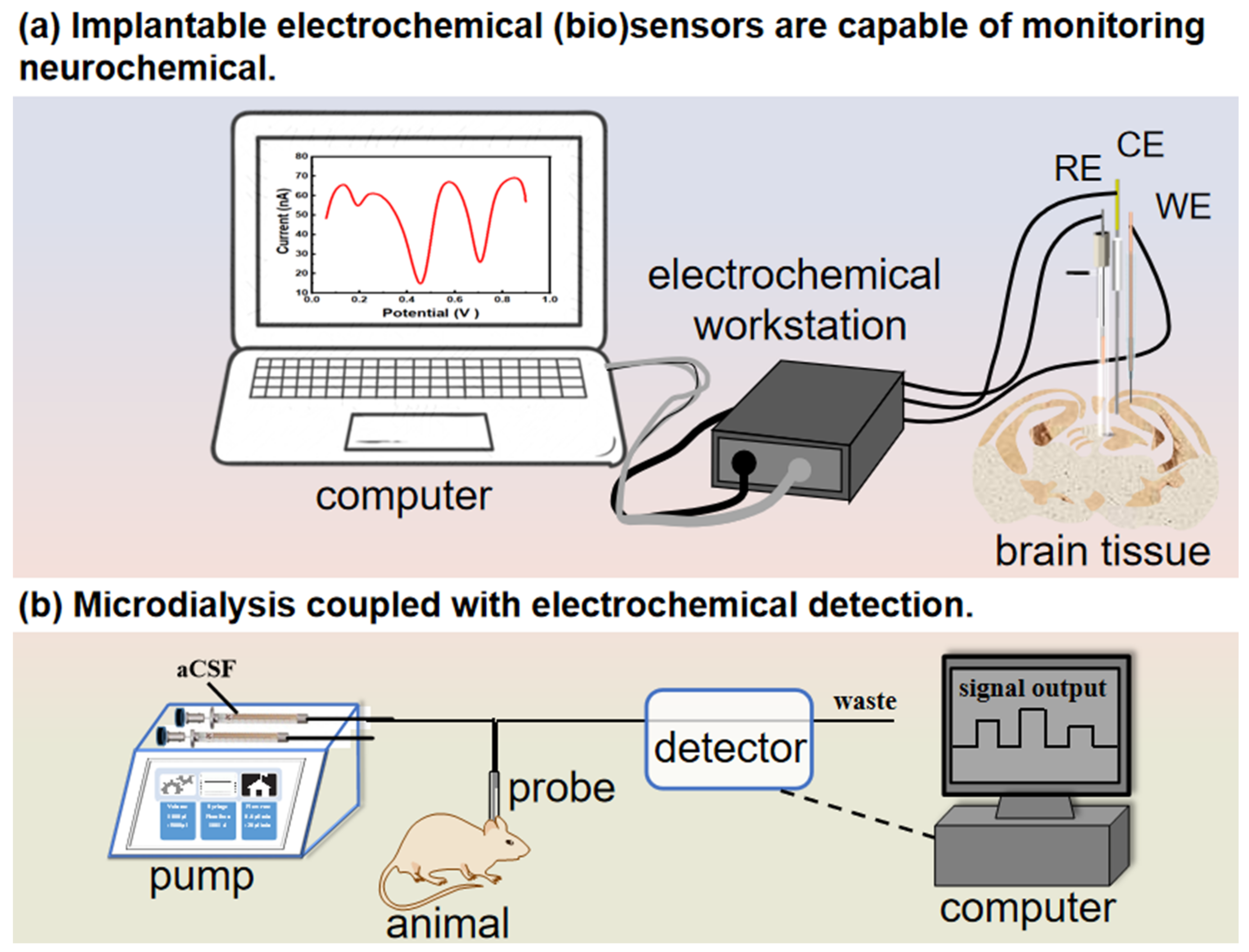
3.1. Implantable Electrochemical Biosensors to Monitor Neurochemicals in the Brain Tissue
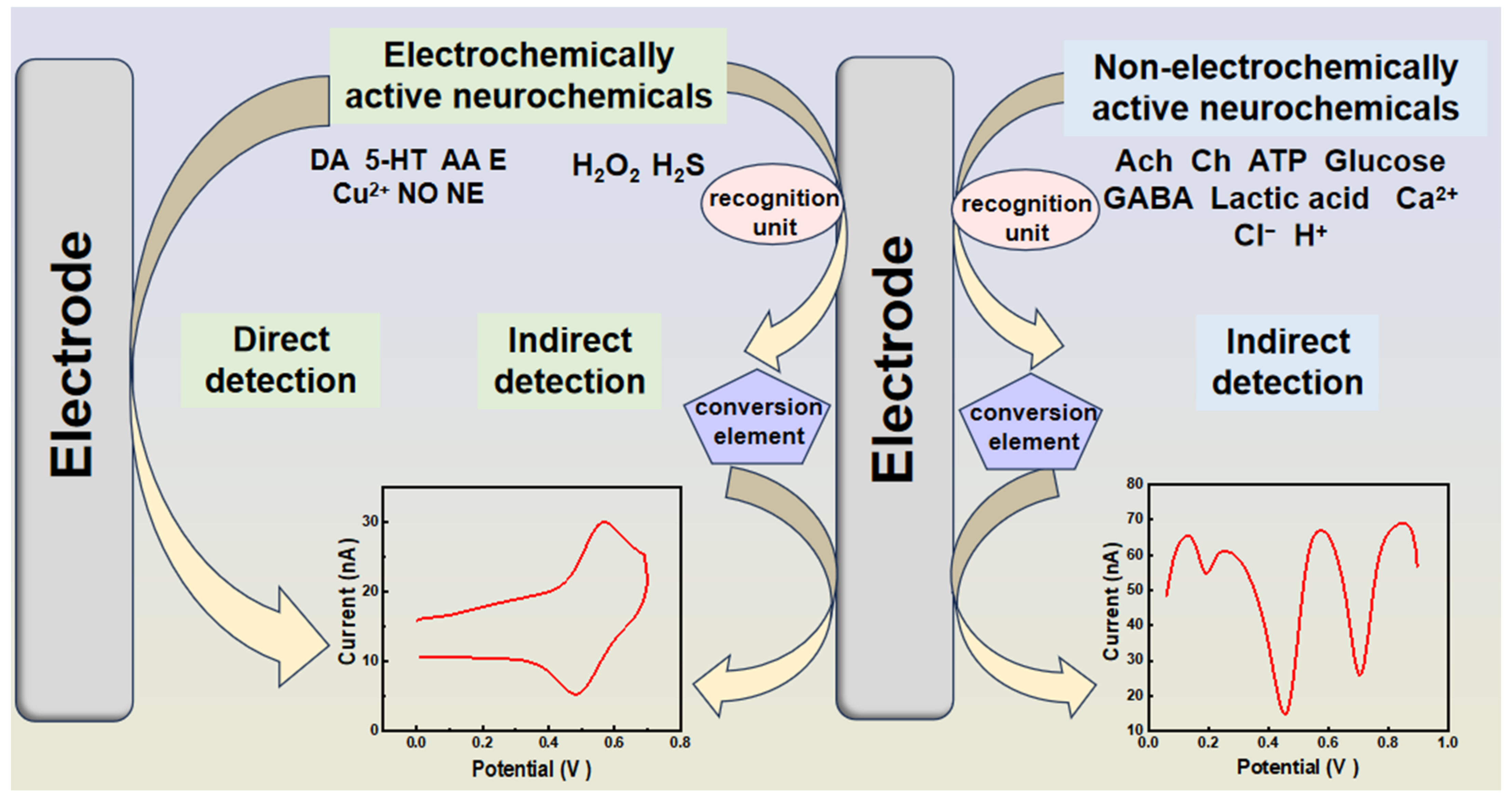
3.1.1. Non-Electroactive Neurochemicals
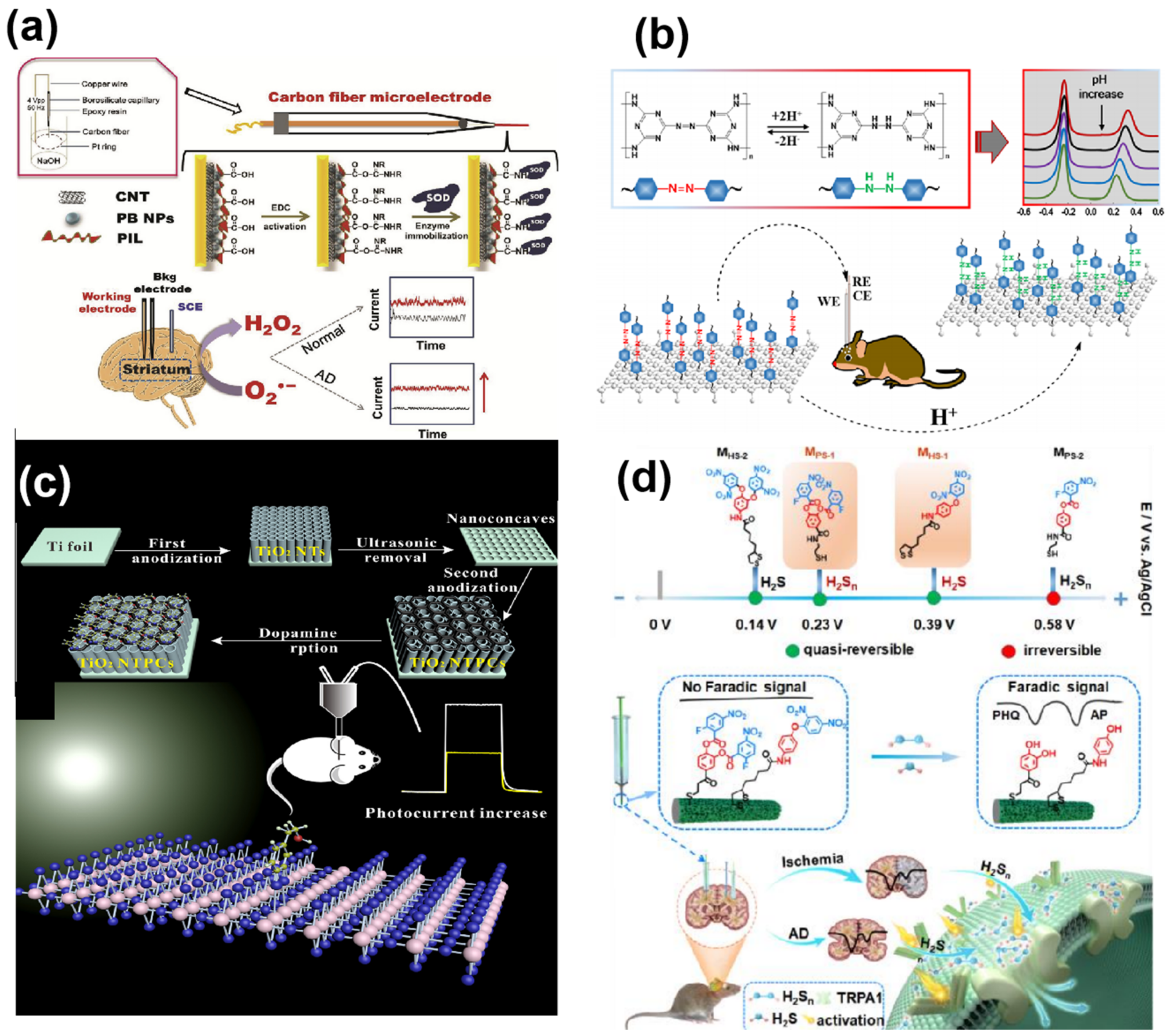
3.1.2. Electroactive Neurochemicals
3.2. Microdialysis Coupled with Electrochemical Detection
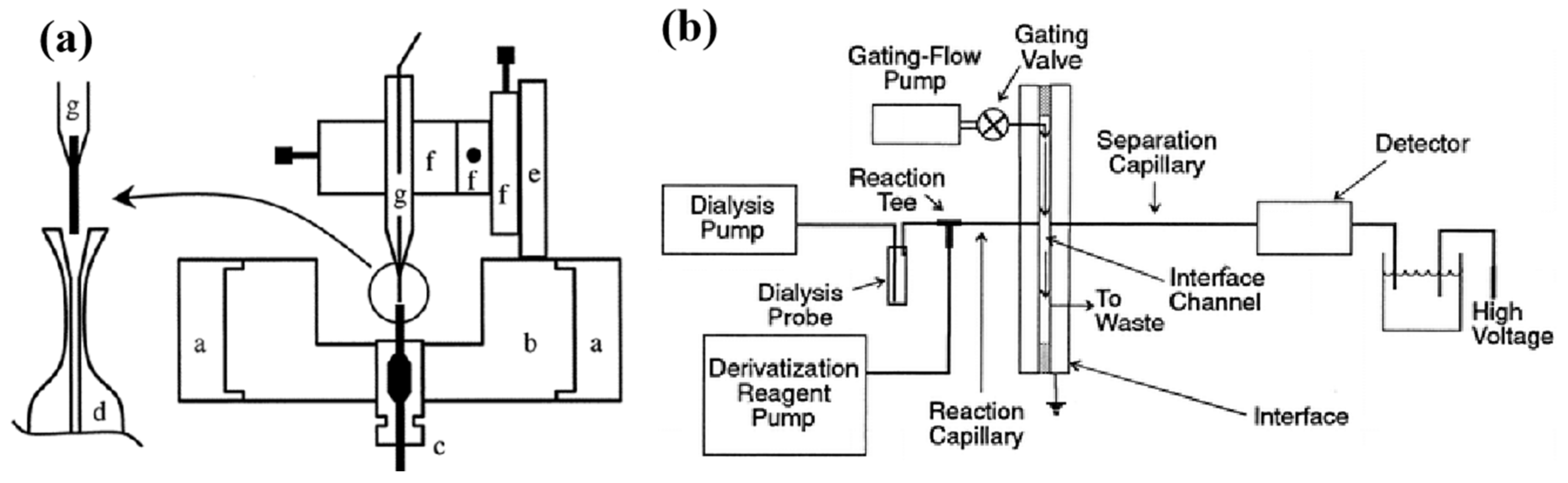
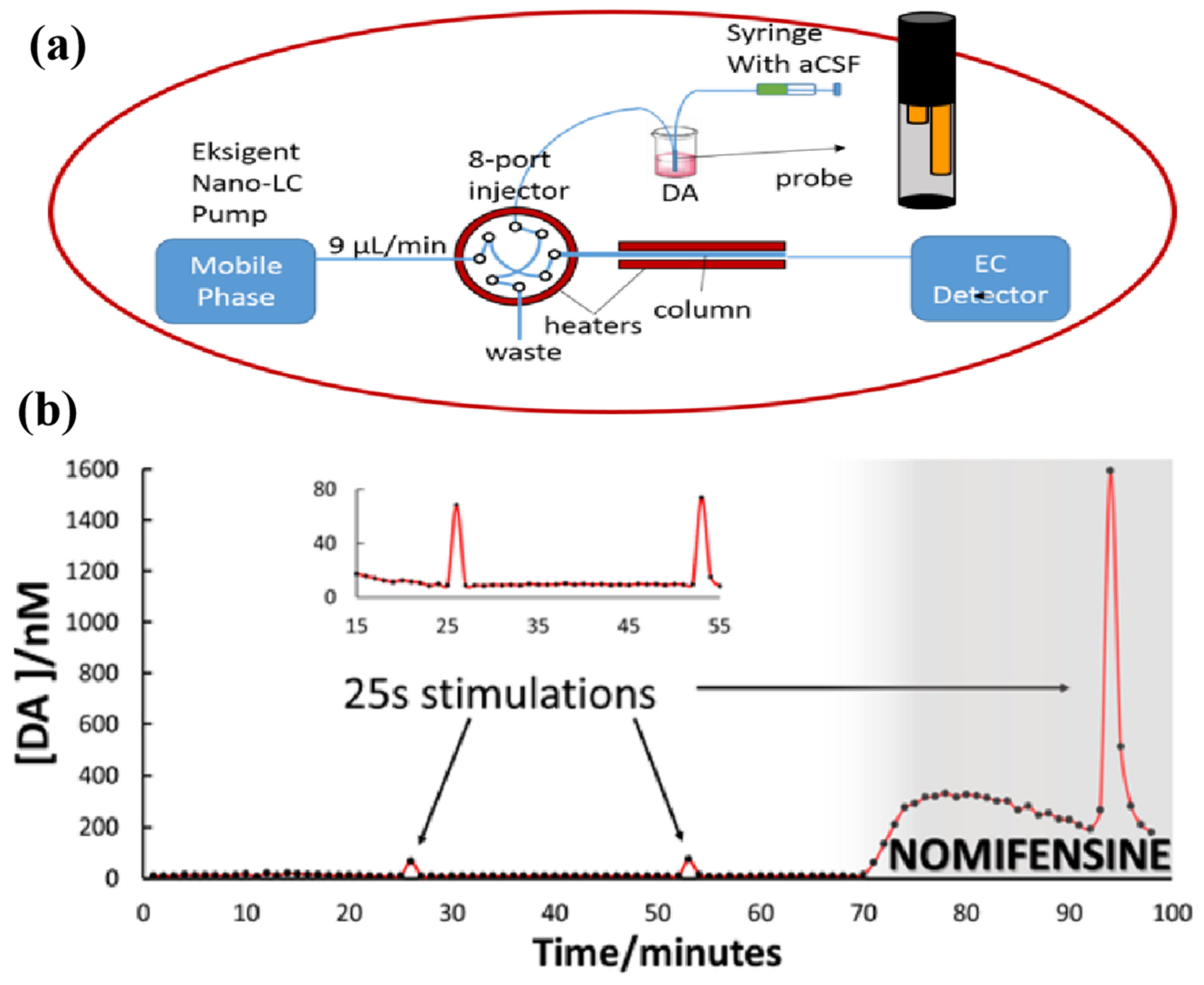
3.2.1. Detection Techniques with Separation Means
| Neurochemical | Technique | Sampling Site | Temporal Resolution | LOD | Ref. |
|---|---|---|---|---|---|
| DA | MD-LC | Striatum | 1 min | - | [180] |
| DA | MD-HPLC | Nucleus accumbens septi | 1 min | - | [181] |
| DA | MD-HPLC | Striatum | 2 min | - | [182,183] |
| DA | MD-HPLC | Striatum, Cortex | 2 min | - | [184] |
| DA | MD-HPLC | Striatum | 1 min | 40 nM | [185] |
| MD-CapUHPLC | Striatum | 1 min | 0.15 nM | [178] | |
| DA | MD-ME | Striatum | 65 | 200 μM | [168] |
| DA | MD-ME | Striatum | 100 s | 1 μM | [169] |
| GABA | MD-HPLC | - | 1 min | - | [186] |
| Glu | MD-CE | Striatum | 25 s | - | [187] |
| Glu | MD-HPLC | Hippocampus | 1 min | - | [186] |
| Glu and AA | MD-HPLC | Auditory cortex | 0.1/1 μM | [179] | |
| Neuroactive amines and AAs | MD-CapLC | Striatum | 10 s | 0.09−0.35 nM | [188] |
| 5-HIAA | MD-CapLC | Hippocampus | 2 min | 3 nM | [189] |
| 5-HT | MD-CapLC | Hippocampus | 1 min | 56 μM | [189] |
| 5-HT | MD-HPLC | Hippocampus | 90 s | - | [186] |
| 5-HT | MD-HPLC | Striatum, Cortex | 2 min | - | [184] |
| 5-HT | MD-HPLC | Hippocampus, Striatum | 3 min | 0.8 fM | [177] |
| 5-HT | MD-CapUHPLC | Hippocampus | 1 min | 70 pM | [176] |
| 5-HT | MD-CapUHPLC | Striatum | 36 s | 0.3 nM | [22] |
| 5-HT | MD-CapUHPLC | Striatum | 30 s | 160 pM | [19] |
| NA, DA, and 5-HT | MD-UHPLC | Prefrontal cortex | 12 min | 32/42/83 pM | [190] |
| NA, DA, and 5-HT | MD-CapUHPLC | Striatum, Amygdala, Hippocampus | 21 min | 0.75/0.75/1.5 nM | [191] |
| NA, DA, and 5-HT | MD-UHPLC | Hippocampus | 8 min | 83/58/60 pM | [192] |
| Monoamines | MD-UHPLC | Hippocampus, Prefrontal cortex, Striatum | 20 min | 100 pM | [193] |
| Ach | MD-HPLC | Striatum | 7 min | 20 fmol | [194] |
| Ach | MD-HPLC | Hippocampus | 6 min | 10 fmol | [195] |
3.2.2. Detection Techniques with Biosensors
| Neurochemical | Technique | Sampling Site | Temporal Resolution | LOD | Ref. |
|---|---|---|---|---|---|
| DA | MD-Biosensors | Striatum | 4 min | 0.31 nM | [199] |
| DA | MD-Biosensors | Striatum | 10 min | - | [200] |
| Glucose and lactate | MD-Biosensors | Cortex | 30 s | - | [201,202,203] |
| Glucose and lactate | MD-Biosensors | Cortex | 1 min | - | [204] |
| Glucose and lactate | MD-Biosensors | Striatum | - | 2.39/2.52 µM | [205] |
| Lactate | MD-Biosensors | Striatum | 1 min | - | [206] |
| Glucose | MD-Biosensors | Cortex | 2 min | 50 nM | [207] |
| Glucose | MD-Biosensors | Auditory cortex | - | 10 µM | [208] |
| Glucose | MD-Biosensors | Striatum | - | 1.8 µM | [209] |
| Glucose | MD-Biosensors | Striatum | - | 0.28 µM | [210] |
| Glucose | MD-Biosensors | Striatum | - | 3.33 µM | [211] |
| Ach | MD-Biosensors | Striatum | - | 1 µM | [24] |
| Hypoxanthine | MD-Biosensors | Striatum | - | 0.40 µM | [23] |
| Cu2+ | MD-Biosensors | Striatum | - | 13 nM | [212] |
| ATP | MD-Biosensors | Cortex | - | 50 pM | [213] |
| ATP | MD-Biosensors | Cortex | 0.5 s | 0.1 fmol | [214] |
| H2O2 | MD-Biosensors | Cortex, Striatum, Hippocampus | - | 1 µM | [79] |

3.3. Emerging Techniques
4. In Vitro Electrochemical Measurements of Biomolecules in the Brain Tissue
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Zhang, X.; Hatamie, A.; Ewing, A.G. Nanoelectrochemical analysis inside a single living cell. Curr. Opin. Electrochem. 2020, 22, 94–101. [Google Scholar] [CrossRef]
- Song, Q.; Li, Q.; Yan, J.; Song, Y. Echem methods and electrode types of the current in vivo electrochemical sensing. RSC Adv. 2022, 12, 17715–17739. [Google Scholar] [CrossRef]
- Hatamie, A.; He, X.; Zhang, X.-W.; Oomen, P.E.; Ewing, A.G. Advances in nano/microscale electrochemical sensors and biosensors for analysis of single vesicles, a key nanoscale organelle in cellular communication. Biosens. Bioelectron. 2023, 220, 114899. [Google Scholar] [CrossRef]
- Hatami, A.; Zhang, X.W.; Pieter, E.O.; Andrew, G.E. Nanoscale Electrochemical Sensors for Intracellular Measurements at the Single Cell. In Handbook of Nanobioelectrochemistry: Application in Devices and Biomolecular Sensing; Springer Nature: Singapore, 2023; pp. 131–152. [Google Scholar]
- Kissinger, P.T.; Hart, J.B.; Adams, R.N. Voltammetry in brain tissue—A new neurophysiological measurement. Brain Res. 1973, 55, 209–213. [Google Scholar] [CrossRef]
- Shin, M.; Venton, B.J. Fast-Scan Cyclic Voltammetry (FSCV) Reveals Behaviorally Evoked Dopamine Release by Sugar Feeding in the Adult Drosophila Mushroom Body. Angew. Chem. Int. Ed. 2022, 61, e202207399. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Shin, M.; Lavrik, N.V.; Venton, B.J. 3D-Printed Carbon Nanoelectrodes for In Vivo Neurotransmitter Sensing. Nano Lett. 2020, 20, 6831–6836. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, Q.; Zhang, L.; Tian, Y. Rational Design of Specific Recognition Molecules for Simultaneously Monitoring of Endogenous Polysulfide and Hydrogen Sulfide in the Mouse Brain. Angew. Chem. Int. Ed. 2019, 58, 13948–13953. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhu, M.; Ye, H.; Zeng, C.; Wang, S.; Niu, Y. Carbon fiber microelectrode array loaded with the diazonium salt-single-walled carbon nanotubes composites for the simultaneous monitoring of dopamine and serotonin in vivo. Anal. Chim. Acta 2021, 1186, 339086. [Google Scholar] [CrossRef]
- Ganesana, M.; Trikantzopoulos, E.; Maniar, Y.; Lee, S.T.; Venton, B.J. Development of a novel micro biosensor for in vivo monitoring of glutamate release in the brain. Biosens. Bioelectron. 2019, 130, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wu, F.; Li, L.; Yang, X.; Xu, C.; Yu, P.; Ma, F.; Mao, L. Natural Leukocyte Membrane-Masked Microelectrodes with an Enhanced Antifouling Ability and Biocompatibility for In Vivo Electrochemical Sensing. Anal. Chem. 2020, 92, 11374–11379. [Google Scholar] [CrossRef]
- Hu, K.; Le Vo, K.L.; Hatamie, A.; Ewing, A.G. Quantifying Intracellular Single Vesicular Catecholamine Concentration with Open Carbon Nanopipettes to Unveil the Effect of L-DOPA on Vesicular Structure. Angew. Chem. Int. Ed. 2022, 61, e202113406. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Gerhardt, G.A. Self-Referencing Ceramic-Based Multisite Microelectrodes for the Detection and Elimination of Interferences from the Measurement of l-Glutamate and Other Analytes. Anal. Chem. 2001, 73, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, G.; Gan, L.; Yuan, B. In Situ Electrochemical Formation of Oxo-Functionalized Graphene on Glassy Carbon Electrode with Chemical Fouling Recovery and Antibiofouling Properties for Electrochemical Sensing of Reduced Glutathione. Antioxidants 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Ji, W.; Tang, Q.; Wei, H.; Zhang, S.; Mao, J.; Zhang, Y.; Mao, L.; Zhang, M. Low-Fouling Nanoporous Conductive Polymer-Coated Microelectrode for In Vivo Monitoring of Dopamine in the Rat Brain. Anal. Chem. 2019, 91, 10786–10791. [Google Scholar] [CrossRef]
- Kalant, H. A microdialysis procedure for extraction and isolation of corticosteroids from peripheral blood plasma. Biochem. J. 1958, 69, 99–103. [Google Scholar] [CrossRef]
- Bito, L.; Davson, H.; Levin, E.; Murray, M.; Snider, N. The Concentrations of Free Amino acids and Other Electrolytes in Cerebrospinal Fluid, In Vivo Dialysate of Brain, and Blood Plasma of the DOG. J. Neurochem. 1966, 13, 1057–1067. [Google Scholar] [CrossRef]
- Zhang, J.; Jaquins-Gerstl, A.; Nesbitt, K.M.; Rutan, S.C.; Michael, A.C.; Weber, S.G. In Vivo Monitoring of Serotonin in the Striatum of Freely Moving Rats with One Minute Temporal Resolution by Online Microdialysis–Capillary High-Performance Liquid Chromatography at Elevated Temperature and Pressure. Anal. Chem. 2013, 85, 9889–9897. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhang, N.; Maddukuri, N. Flow-gated capillary electrophoresis: A powerful technique for rapid and efficient chemical separation. Anal. Methods 2018, 10, 3131–3143. [Google Scholar] [CrossRef]
- Qian, J.; Wu, Y.; Yang, H.; Michael, A.C. An Integrated Decoupler for Capillary Electrophoresis with Electrochemical Detection: Application to Analysis of Brain Microdialysate. Anal. Chem. 1999, 71, 4486–4492. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Jaquins-Gerstl, A.; Shu, Z.; Michael, A.C.; Weber, S.G. Optimization for speed and sensitivity in capillary high performance liquid chromatography. The importance of column diameter in online monitoring of serotonin by microdialysis. J. Chromatogr. A 2012, 1251, 54–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- König, M.; Thinnes, A.; Klein, J. Microdialysis and its use in behavioural studies: Focus on acetylcholine. J. Neurosci. Methods 2018, 300, 206–215. [Google Scholar] [CrossRef]
- Kho, C.M.; Enche Ab Rahim, S.K.; Ahmad, Z.A.; Abdullah, N.S. A Review on Microdialysis Calibration Methods: The Theory and Current Related Efforts. Mol. Neurobiol. 2016, 54, 3506–3527. [Google Scholar] [CrossRef]
- Saylor, R.A.; Lunte, S.M. A review of microdialysis coupled to microchip electrophoresis for monitoring biological events. J. Chromatogr. A 2015, 1382, 48–64. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, J.; Xiao, T.; Yu, P.; Mao, L. Online electrochemical systems for continuous neurochemical measurements with low-potential mediator-based electrochemical biosensors as selective detectors. Analyst 2015, 140, 5039–5047. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, P.; Mao, L. A multi-enzyme microreactor-based online electrochemical system for selective and continuous monitoring of acetylcholine. Analyst 2015, 140, 3781–3787. [Google Scholar] [CrossRef]
- Zhang, M.; Mao, L. Enzyme-based amperometric biosensors for continuous and on-line monitoring of cerebral extracellular microdialysate. FBL 2005, 10, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Bobin, S.; Popot, M.A.; Bonnaire, Y.; Tabet, J.C. Approach to the determination of insulin-like-growth-factor-I (IGF-I) concentration in plasma by high-performance liquid chromatography-ion trap mass spectrometry: Use of a deconvolution algorithm for the quantification of multiprotonated molecules in electrospray ionization. Analyst 2001, 126, 1996–2001. [Google Scholar]
- Dagher, A.; Robbins, T.W. Personality, addiction, dopamine: Insights from Parkinson’s disease. Neuron 2009, 61, 502–510. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Swanson, J.M. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol. Psychiatry 2004, 9, 557–569. [Google Scholar] [CrossRef]
- Brennan, A.R.; Arnsten, A.F.T. Neuronal mechanisms underlying attention deficit hyperactivity disorder—The influence of arousal on prefrontal cortical function. Ann. N. Y. Acad. Sci. 2008, 1129, 236–245. [Google Scholar] [CrossRef]
- Kurian, M.A.; Gissen, P.; Smith, M.; Heales, S.J.R.; Clayton, P.T. The monoamine neurotransmitter disorders: An expanding range of neurological syndromes. Lancet Neurol. 2011, 10, 721–733. [Google Scholar] [CrossRef]
- Marecos, C.; Ng, J.; Kurian, M.A. What is new for monoamine neurotransmitter disorders? J. Inherit. Metab. Dis. 2014, 37, 619–626. [Google Scholar] [CrossRef]
- Hamdan, S.K.; Mohd Zain, A. In vivo Electrochemical Biosensor for Brain Glutamate Detection: A Mini Review. Malays. J. Med. Sci. 2014, 21, 12–26. [Google Scholar] [PubMed]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.R.W. MR Spectroscopy in Neurodegenerative Disease. Mol. Imaging Biol. 2007, 9, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.M.; Morrison, P.D.; Pilowsky, L.S. Review: Glutamate and dopamine dysregulation in schizophrenia—A synthesis and selective review. J. Psychopharmacol. 2007, 21, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000, 23, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lin, Y.; Xiang, L.; Yu, P.; Su, L.; Mao, L. Comparative study of change in extracellular ascorbic acid in different brain ischemia/reperfusion models with in vivo microdialysis combined with on-line electrochemical detection. Neurochem. Int. 2008, 52, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, J.-x.; Ma, F.-r.; Yu, L.-s.; Lin, Y.-q.; Liu, K.; Mao, L.-q. Change of extracellular ascorbic acid in the brain cortex following ice water vestibular stimulation: An on-line electrochemical detection coupled with in vivo microdialysis sampling for guinea pigs. Chin. Med. J. 2008, 121, 1120–1125. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Hao, J.; Liu, J.; Yu, P.; Ma, F.; Mao, L. Online electrochemical system as an in vivo method to study dynamic changes of ascorbate in rat brain during 3-methylindole-induced olfactory dysfunction. Analyst 2016, 141, 2199–2207. [Google Scholar] [CrossRef]
- Fouani, L.; Menezes, S.V.; Paulson, M.; Richardson, D.R.; Kovacevic, Z. Metals and metastasis: Exploiting the role of metals in cancer metastasis to develop novel anti-metastatic agents. Pharmacol. Res. 2017, 115, 275–287. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef]
- Graefe, A.; Stanca, S.E.; Nietzsche, S.; Kubicova, L.; Beckert, R.; Biskup, C.; Mohr, G.J. Development and Critical Evaluation of Fluorescent Chloride Nanosensors. Anal. Chem. 2008, 80, 6526–6531. [Google Scholar] [CrossRef]
- Ashton, T.D.; Jolliffe, K.A.; Pfeffer, F.M. Luminescent probes for the bioimaging of small anionic species in vitro and in vivo. Chem. Soc. Rev. 2015, 44, 4547–4595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Carney, K.E.; Falgoust, L.; Pan, J.W.; Sun, D.; Zhang, Z. Emerging roles of Na+/H+ exchangers in epilepsy and developmental brain disorders. Prog. Neurobiol. 2016, 138–140, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Brady, J.D.; Mohr, C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007, 10, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, L.L.; Strawn, J.R.; Sah, R. Acid–base dysregulation and chemosensory mechanisms in panic disorder: A translational update. Transl. Psychiatry 2015, 5, e572. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Ando, H.; Tsutsui, K. Chapter 103—Gasotransmitter Family. In Handbook of Hormones; Academic Press: San Diego, CA, USA, 2016; pp. 601–602. [Google Scholar]
- Pałasz, A.; Menezes, I.C.; Worthington, J.J. The role of brain gaseous neurotransmitters in anxiety. Pharmacol. Rep. 2021, 73, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Qin, J.; Chang, X.; Yang, Z.; Bu, D.; Du, J. Modulating effect of hydrogen sulfide on gamma-aminobutyric acid B receptor in recurrent febrile seizures in rats. Neurosci. Res. 2005, 53, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bereguiain, M.A.; Samhan-Arias, A.K.; Martin-Romero, F.J.; Gutierrez-Merino, C. Hydrogen Sulfide Raises Cytosolic Calcium in Neurons Through Activation of L-Type Ca2+ Channels. Antioxid. Redox Signal. 2008, 10, 31–42. [Google Scholar] [CrossRef]
- Schreier, S.M.; Muellner, M.K.; Steinkellner, H.; Hermann, M.; Esterbauer, H.; Exner, M.; Gmeiner, B.M.K.; Kapiotis, S.; Laggner, H. Hydrogen Sulfide Scavenges the Cytotoxic Lipid Oxidation Product 4-HNE. Neurotox. Res. 2010, 17, 249–256. [Google Scholar] [CrossRef]
- Xie, C.; Luo, K.; Tan, L.; Yang, Q.; Zhao, X.; Zhou, L. A Review for In Vitro and In Vivo Detection and Imaging of Gaseous Signal Molecule Carbon Monoxide by Fluorescent Probes. Molecules 2022, 27, 8842. [Google Scholar] [CrossRef]
- Li, W.; Li, R.; Chen, R.; Liang, X.; Song, W.; Lin, W. Activatable Photoacoustic Probe for In Situ Imaging of Endogenous Carbon Monoxide in the Murine Inflammation Model. Anal. Chem. 2021, 93, 8978–8985. [Google Scholar] [CrossRef]
- Fu, G.-Q.; Xia, Y.-S.; Jiang, W.-L.; Wang, W.-X.; Tan, Z.-K.; Guo, K.-Y.; Mao, G.-J.; Li, C.-Y. A novel precipitating-fluorochrome-based fluorescent probe for monitoring carbon monoxide during drug-induced liver injury. Talanta 2022, 243, 123398. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, S.-Y.; Hong, S.; Chen, Q.-W.; Zeng, X.; Rong, L.; Zhong, Z.-L.; Zhang, X.-Z. Biomimetic carbon monoxide nanogenerator ameliorates streptozotocin induced type 1 diabetes in mice. Biomaterials 2020, 245, 119986. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Tang, Y.; Huang, H.; Song, W.; Lin, W. A fluorogenic probe for detecting CO with the potential integration of diagnosis and therapy (IDT) for cancer. Sens. Actuators B Chem. 2021, 344, 130245. [Google Scholar] [CrossRef]
- Zhang, S.; Lachance, B.B.; Mattson, M.P.; Jia, X. Glucose metabolic crosstalk and regulation in brain function and diseases. Prog. Neurobiol. 2021, 204, 102089. [Google Scholar] [CrossRef]
- Hollyer, T.R.; Bordoni, L.; Kousholt, B.S.; van Luijk, J.; Ritskes-Hoitinga, M.; Østergaard, L. The evidence for the physiological effects of lactate on the cerebral microcirculation: A systematic review. J. Neurochem. 2019, 148, 712–730. [Google Scholar] [CrossRef]
- Xapelli, S.; Agasse, F.; Ferreira, R.; Silva, P.A.; Malva, O.J. Neuropeptide Y as an Endogenous Antiepileptic, Neuroprotective and Pro-Neurogenic Peptide. Recent Pat. CNS Drug Discov. 2006, 1, 315–324. [Google Scholar] [CrossRef]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting Appetite-Regulating Pathways in the Hypothalamic Regulation of Body Weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar]
- O’Loughlin, E.K.; Pakan, J.M.P.; McDermott, K.W.; Yilmazer-Hanke, D. Expression of neuropeptide Y1 receptors in the amygdala and hippocampus and anxiety-like behavior associated with Ammon’s horn sclerosis following intrahippocampal kainate injection in C57BL/6J mice. Epilepsy Behav. 2014, 37, 175–183. [Google Scholar] [CrossRef]
- Loewi, O. Über humorale übertragbarkeit der Herznervenwirkung. Pflüger’s Arch. Gesamte Physiol. Menschen Tiere 1921, 189, 239–242. [Google Scholar] [CrossRef]
- Watson, C.J.; Venton, B.J.; Kennedy, R.T. In Vivo Measurements of Neurotransmitters by Microdialysis Sampling. Anal. Chem. 2006, 78, 1391–1399. [Google Scholar] [CrossRef]
- Khan, A.S.; Michael, A.C. Invasive consequences of using micro-electrodes and microdialysis probes in the brain. TrAC Trends Anal. Chem. 2003, 22, 503–508. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Honarvarfard, E.; Baharifar, H.; Gholami, M.; Faridbod, F.; Larijani, B.; Faridi Majidi, R.; Khorramizadeh, M.R. Nanomaterial based electrochemical sensing of the biomarker serotonin: A comprehensive review. Microchim. Acta 2019, 186, 49. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Direct electron transfer between copper-containing proteins and electrodes. Biosens. Bioelectron. 2005, 20, 2517–2554. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Mao, L.; Okajima, T.; Ohsaka, T. Superoxide Dismutase-Based Third-Generation Biosensor for Superoxide Anion. Anal. Chem. 2002, 74, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Mao, L.; Okajima, T.; Ohsaka, T. Electrochemistry and Electrocatalytic Activities of Superoxide Dismutases at Gold Electrodes Modified with a Self-Assembled Monolayer. Anal. Chem. 2004, 76, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, C.; Yang, K.; Hong, W.; Lu, Y.; Yu, P.; Mao, L. Photoinduced Regeneration of an Aptamer-Based Electrochemical Sensor for Sensitively Detecting Adenosine Triphosphate. Anal. Chem. 2018, 90, 4968–4971. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhuo, Y.; Xiao, X.; Li, S.; Han, K.; Lu, M.; Zhang, J.; Chen, S.; Gu, H. Facile Electrochemical Microbiosensor Based on In Situ Self-Assembly of Ag Nanoparticles Coated on Ti3C2Tx for In Vivo Measurements of Chloride Ions in the PD Mouse Brain. Anal. Chem. 2021, 93, 7647–7656. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, C.; Liu, Y.; Feng, Y.; Han, K.; Xiang, H.; Shi, G.; Gu, H. A ratiometric electrochemical microsensor for monitoring chloride ions in vivo. Analyst 2021, 146, 6202–6210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, M.; Zuo, Y.; Chen, S.; Zhuo, Y.; Lu, M.; Shi, G.; Gu, H. In Vivo Monitoring of pH in Subacute PD Mouse Brains with a Ratiometric Electrochemical Microsensor Based on Poly(melamine) Films. ACS Sens. 2022, 7, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhao, L.; Zhou, Y.; Wei, X.; Xu, C.; Zhang, Y.; Xu, M. Novel Self-Calibrating Amperometric and Ratiometric Electrochemical Nanotip Microsensor for pH Measurement in Rat Brain. Anal. Chem. 2021, 93, 13815–13822. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, F.; Liu, W.; Zhu, T.; Zhang, J.Z.H.; Chen, C.; Dai, Z.; Peng, H.; Huang, J.-L.; Hu, Q.; et al. An Electrochemical Biosensor with Dual Signal Outputs: Toward Simultaneous Quantification of pH and O2 in the Brain upon Ischemia and in a Tumor during Cancer Starvation Therapy. Angew. Chem. Int. Ed. 2017, 56, 10471–10475. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, Y. An Electrochemical Biosensor with Dual Signal Outputs for Ratiometric Monitoring the Levels of H2O2 and pH in the Microdialysates from a Rat Brain. Electroanalysis 2018, 30, 1047–1053. [Google Scholar] [CrossRef]
- Moon, J.; Ha, Y.; Kim, M.; Sim, J.; Lee, Y.; Suh, M. Dual Electrochemical Microsensor for Real-Time Simultaneous Monitoring of Nitric Oxide and Potassium Ion Changes in a Rat Brain during Spontaneous Neocortical Epileptic Seizure. Anal. Chem. 2016, 88, 8942–8948. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Dong, H.; Feng, S.; Shi, G.; Lin, L.; Tian, Y. An Electrochemophysiological Microarray for Real-Time Monitoring and Quantification of Multiple Ions in the Brain of a Freely Moving Rat. Angew. Chem. Int. Ed. 2020, 59, 10426–10430. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, L.; Dai, L.; Wang, Y.; Tian, Y. Nonenzymatic Electrochemical Sensor with Ratiometric Signal Output for Selective Determination of Superoxide Anion in Rat Brain. Anal. Chem. 2021, 93, 5570–5576. [Google Scholar] [CrossRef]
- Li, R.; Guo, D.; Ye, J.; Zhang, M. Stabilization of Prussian blue with polyaniline and carbon nanotubes in neutral media for in vivo determination of glucose in rat brains. Analyst 2015, 140, 3746–3752. [Google Scholar] [CrossRef]
- Forderhase, A.G.; Styers, H.C.; Lee, C.A.; Sombers, L.A. Simultaneous voltammetric detection of glucose and lactate fluctuations in rat striatum evoked by electrical stimulation of the midbrain. Anal. Bioanal. Chem. 2020, 412, 6611–6624. [Google Scholar] [CrossRef]
- Weltin, A.; Kieninger, J.; Enderle, B.; Gellner, A.-K.; Fritsch, B.; Urban, G.A. Polymer-based, flexible glutamate and lactate microsensors for in vivo applications. Biosens. Bioelectron. 2014, 61, 192–199. [Google Scholar] [CrossRef]
- Frey, O.; Holtzman, T.; McNamara, R.M.; Theobald, D.E.H.; van der Wal, P.D.; de Rooij, N.F.; Dalley, J.W.; Koudelka-Hep, M. Enzyme-based choline and l-glutamate biosensor electrodes on silicon microprobe arrays. Biosens. Bioelectron. 2010, 26, 477–484. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Davis, V.A.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Glutaraldehyde Cross-Linked Glutamate Oxidase Coated Microelectrode Arrays: Selectivity and Resting Levels of Glutamate in the CNS. ACS Chem. Neurosci. 2013, 4, 721–728. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Pomerleau, F.; Palmer, M.; Day, B.K.; Huettl, P.; Gerhardt, G.A. Improved ceramic-based multisite microelectrode for rapid measurements of l-glutamate in the CNS. J. Neurosci. Methods 2002, 119, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Day, B.K.; Pomerleau, F.; Burmeister, J.J.; Huettl, P.; Gerhardt, G.A. Microelectrode array studies of basal and potassium-evoked release of l-glutamate in the anesthetized rat brain. J. Neurochem. 2006, 96, 1626–1635. [Google Scholar] [CrossRef]
- Hascup, K.N.; Hascup, E.R.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J. Pharmacol. Exp. Ther. 2008, 324, 725. [Google Scholar] [CrossRef] [PubMed]
- McLamore, E.S.; Mohanty, S.; Shi, J.; Claussen, J.; Jedlicka, S.S.; Rickus, J.L.; Porterfield, D.M. A self-referencing glutamate biosensor for measuring real time neuronal glutamate flux. J. Neurosci. Methods 2010, 189, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, E.C.; Pomerleau, F.; Huettl, P.; Strömberg, I.; Gerhardt, G.A. Chronic second-by-second measures of l-glutamate in the central nervous system of freely moving rats. J. Neurochem. 2007, 102, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.L.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Zhang, Z. Real-time glutamate measurements in the putamen of awake rhesus monkeys using an enzyme-based human microelectrode array prototype. J. Neurosci. Methods 2010, 185, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Konradsson-Geuken, Å.; Gash, C.R.; Alexander, K.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Bruno, J.P. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse 2009, 63, 1069–1082. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Palmer, M.; Gerhardt, G.A. l-lactate measures in brain tissue with ceramic-based multisite microelectrodes. Biosens. Bioelectron. 2005, 20, 1772–1779. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Pomerleau, F.; Huettl, P.; Gash, C.R.; Werner, C.E.; Bruno, J.P.; Gerhardt, G.A. Ceramic-based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosens. Bioelectron. 2008, 23, 1382–1389. [Google Scholar] [CrossRef]
- Ledo, A.; Lourenço, C.F.; Laranjinha, J.; Brett, C.M.A.; Gerhardt, G.A.; Barbosa, R.M. Ceramic-Based Multisite Platinum Microelectrode Arrays: Morphological Characteristics and Electrochemical Performance for Extracellular Oxygen Measurements in Brain Tissue. Anal. Chem. 2017, 89, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Yan, X.; Shi, X.; Ou, S.; Gu, H.; Yin, X.; Shi, G.; Yu, Y. In vivo monitoring of superoxide anion from Alzheimer’s rat brains with functionalized ionic liquid polymer decorated microsensor. Biosens. Bioelectron. 2019, 144, 111665. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Lubin, A.A.; Plaxco, K.W. Folding-Based Electrochemical Biosensors: The Case for Responsive Nucleic Acid Architectures. Acc. Chem. Res. 2010, 43, 496–505. [Google Scholar] [CrossRef]
- Farjami, E.; Campos, R.; Nielsen, J.S.; Gothelf, K.V.; Kjems, J.; Ferapontova, E.E. RNA Aptamer-Based Electrochemical Biosensor for Selective and Label-Free Analysis of Dopamine. Anal. Chem. 2013, 85, 121–128. [Google Scholar] [CrossRef]
- Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N. Fast and Selective Plasmonic Serotonin Detection with Aptamer-Gold Nanoparticle Conjugates. Sensors 2017, 17, 681. [Google Scholar] [CrossRef]
- Santos-Cancel, M.; Simpson, L.W.; Leach, J.B.; White, R.J. Direct, Real-Time Detection of Adenosine Triphosphate Release from Astrocytes in Three-Dimensional Culture Using an Integrated Electrochemical Aptamer-Based Sensor. ACS Chem. Neurosci. 2019, 10, 2070–2079. [Google Scholar] [CrossRef]
- Xiao, Y.; Piorek, B.D.; Plaxco, K.W.; Heeger, A.J. A Reagentless Signal-On Architecture for Electronic, Aptamer-Based Sensors via Target-Induced Strand Displacement. J. Am. Chem. Soc. 2005, 127, 17990–17991. [Google Scholar] [CrossRef]
- Li, H.; Arroyo-Currás, N.; Kang, D.; Ricci, F.; Plaxco, K.W. Dual-Reporter Drift Correction to Enhance the Performance of Electrochemical Aptamer-Based Sensors in Whole Blood. J. Am. Chem. Soc. 2016, 138, 15809–15812. [Google Scholar] [CrossRef]
- Swensen, J.S.; Xiao, Y.; Ferguson, B.S.; Lubin, A.A.; Lai, R.Y.; Heeger, A.J.; Plaxco, K.W.; Soh, H.T. Continuous, Real-Time Monitoring of Cocaine in Undiluted Blood Serum via a Microfluidic, Electrochemical Aptamer-Based Sensor. J. Am. Chem. Soc. 2009, 131, 4262–4266. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.M.; Du, Z.; Bigelow, E.T.; Eles, J.R.; Horner, A.R.; Catt, K.A.; Weber, S.G.; Jamieson, B.G.; Cui, X.T. Aptamer-functionalized neural recording electrodes for the direct measurement of cocaine in vivo. J. Mater. Chem. B 2017, 5, 2445–2458. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX—A (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.; Kundu, N.; Young, B.E.; Vieira, P.A.; Sczepanski, J.T.; Arroyo-Currás, N. Nuclease Hydrolysis Does Not Drive the Rapid Signaling Decay of DNA Aptamer-Based Electrochemical Sensors in Biological Fluids. Langmuir 2021, 37, 5213–5221. [Google Scholar] [CrossRef]
- Leung, K.K.; Downs, A.M.; Ortega, G.; Kurnik, M.; Plaxco, K.W. Elucidating the Mechanisms Underlying the Signal Drift of Electrochemical Aptamer-Based Sensors in Whole Blood. ACS Sens. 2021, 6, 3340–3347. [Google Scholar] [CrossRef]
- Arroyo-Currás, N.; Somerson, J.; Vieira, P.A.; Ploense, K.L.; Kippin, T.E.; Plaxco, K.W. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl. Acad. Sci. USA 2017, 114, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dauphin-Ducharme, P.; Arroyo-Currás, N.; Tran, C.H.; Vieira, P.A.; Li, S.; Shin, C.; Somerson, J.; Kippin, T.E.; Plaxco, K.W. A Biomimetic Phosphatidylcholine-Terminated Monolayer Greatly Improves the In Vivo Performance of Electrochemical Aptamer-Based Sensors. Angew. Chem. Int. Ed. 2017, 56, 7492–7495. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.; Zhu, A.; Shi, G.; Tian, Y. In vivo monitoring of local pH values in a live rat brain based on the design of a specific electroactive molecule for H+. Chem. Commun. 2016, 52, 3717–3720. [Google Scholar] [CrossRef]
- Cui, B.; Liu, P.; Liu, X.; Liu, S.; Zhang, Z. Molecularly imprinted polymers for electrochemical detection and analysis: Progress and perspectives. J. Mater. Res. Technol. 2020, 9, 12568–12584. [Google Scholar] [CrossRef]
- Kröger, S.; Turner, A.P.F.; Mosbach, K.; Haupt, K. Imprinted Polymer-Based Sensor System for Herbicides Using Differential-Pulse Voltammetry on Screen-Printed Electrodes. Anal. Chem. 1999, 71, 3698–3702. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Han, H.-Z.; Cheng, C.-C.; Chen, L.-C.; Chang, H.-C.; Chen, J.-J.J. Modification of platinum microelectrode with molecularly imprinted over-oxidized polypyrrole for dopamine measurement in rat striatum. Sens. Actuators B Chem. 2012, 171–172, 93–101. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Z.; Wu, W.; Fu, B.; Wu, H.; Zhang, Z. Recognition unit-free and self-cleaning photoelectrochemical sensing platform on TiO2 nanotube photonic crystals for sensitive and selective detection of dopamine release from mouse brain. Biosens. Bioelectron. 2017, 87, 396–403. [Google Scholar] [CrossRef]
- Si, B.; Song, E. Molecularly imprinted polymers for the selective detection of multi-analyte neurotransmitters. Microelectron. Eng. 2018, 187–188, 58–65. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Zhang, L.; Zuo, P.; Ye, B.-c.; Yao, J.; Chen, W. Supportless electrochemical sensor based on molecularly imprinted polymer modified nanoporous microrod for determination of dopamine at trace level. Biosens. Bioelectron. 2016, 78, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Martinov, M.; Vitvitsky, V.; Seravalli, J.; Wedmann, R.; Filipovic, M.R.; Banerjee, R. Biosynthesis and Reactivity of Cysteine Persulfides in Signaling. J. Am. Chem. Soc. 2016, 138, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Wong, P.T.H.; Bian, J.-S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.S.; Wightman, R.M. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Adamah-Biassi, E.B.; Almonte, A.G.; Blagovechtchenski, E.; Grinevich, V.P.; Weiner, J.L.; Bonin, K.D.; Budygin, E.A. Real time adenosine fluctuations detected with fast-scan cyclic voltammetry in the rat striatum and motor cortex. J. Neurosci. Methods 2015, 256, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Venton, B.J. Sawhorse Waveform Voltammetry for Selective Detection of Adenosine, ATP, and Hydrogen Peroxide. Anal. Chem. 2014, 86, 7486–7493. [Google Scholar] [CrossRef]
- Van Gompel, J.J.; Bower, M.R.; Worrell, G.A.; Stead, M.; Chang, S.-Y.; Goerss, S.J.; Kim, I.; Bennet, K.E.; Meyer, F.B.; Marsh, W.R.; et al. Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia 2014, 55, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liu, Y.; Ma, C.; Zhang, J.; Zhu, A.; Shi, G. Development of Glass-sealed Gold Nanoelectrodes for in vivo Detection of Dopamine in Rat Brain. Electroanalysis 2018, 30, 1041–1046. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Wolfbeis, O.S.; Hirsch, T.; Swami, N.S. Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim. Acta 2015, 182, 1–41. [Google Scholar] [CrossRef]
- Thomas, T.; Mascarenhas, R.J.; Nethravathi, C.; Rajamathi, M.; Kumara Swamy, B.E. Graphite oxide bulk modified carbon paste electrode for the selective detection of dopamine: A voltammetric study. J. Electroanal. Chem. 2011, 659, 113–119. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Dong, H.; Yu, Q.; Zhang, S.; Chen, H. Sensitive detection of dopamine using a platinum microelectrode modified by reduced graphene oxide and gold nanoparticles. J. Electroanal. Chem. 2019, 848, 113244. [Google Scholar] [CrossRef]
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct in Vivo Electrochemical Detection of Resting Dopamine Using Poly(3,4-ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Anal. Chem. 2019, 91, 12917–12927. [Google Scholar] [CrossRef]
- He, E.; Xu, S.; Dai, Y.; Wang, Y.; Xiao, G.; Xie, J.; Xu, S.; Fan, P.; Mo, F.; Wang, M.; et al. SWCNTs/PEDOT:PSS-Modified Microelectrode Arrays for Dual-Mode Detection of Electrophysiological Signals and Dopamine Concentration in the Striatum under Isoflurane Anesthesia. ACS Sens. 2021, 6, 3377–3386. [Google Scholar] [CrossRef]
- Hou, H.; Jin, Y.; Wei, H.; Ji, W.; Xue, Y.; Hu, J.; Zhang, M.; Jiang, Y.; Mao, L. A Generalizable and Noncovalent Strategy for Interfacing Aptamers with a Microelectrode for the Selective Sensing of Neurotransmitters In Vivo. Angew. Chem. Int. Ed. 2020, 59, 18996–19000. [Google Scholar] [CrossRef]
- Li, X.; Jin, Y.; Zhu, F.; Liu, R.; Jiang, Y.; Jiang, Y.; Mao, L. Electrochemical Conjugation of Aptamers on a Carbon Fiber Microelectrode Enables Highly Stable and Selective In Vivo Neurosensing. Angew. Chem. Int. Ed. 2022, 61, e202208121. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, R.; Zuo, Y.; Zhang, Z.; Zhuo, Y.; Lu, M.; Chen, S.; Gu, H. Efficient Electrochemical Microsensor for In Vivo Monitoring of H2O2 in PD Mouse Brain: Rational Design and Synthesis of Recognition Molecules. Anal. Chem. 2022, 94, 9130–9139. [Google Scholar]
- Zhang, S.; Feng, T.-T.; Zhang, L.; Zhang, M.-N. In Vivo Electrochemical Detection of Hydrogen Peroxide and Dopamine. Chin. J. Anal. Chem. 2019, 47, 1664–1670. [Google Scholar] [CrossRef]
- Taylor, I.M.; Robbins, E.M.; Catt, K.A.; Cody, P.A.; Happe, C.L.; Cui, X.T. Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosens. Bioelectron. 2017, 89, 400–410. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Q.; Zhang, X.; Li, M.; Zhu, A.; Shi, G. Development of gold nanoparticle-sheathed glass capillary nanoelectrodes for sensitive detection of cerebral dopamine. Biosens. Bioelectron. 2015, 63, 262–268. [Google Scholar]
- Zhu, M.; Zeng, C.; Ye, J.; Sun, Y. Simultaneous in vivo voltammetric determination of dopamine and 5-Hydroxytryptamine in the mouse brain. Appl. Surf. Sci. 2018, 455, 646–652. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Krahe, D.D.; Wu, B.; Pwint, M.Y.; Cao, Q.; Cui, X.T. Stable in-vivo electrochemical sensing of tonic serotonin levels using PEDOT/CNT-coated glassy carbon flexible microelectrode arrays. Biosens. Bioelectron. 2023, 230, 115242. [Google Scholar] [CrossRef]
- Wu, F.; Cheng, H.; Wei, H.; Xiong, T.; Yu, P.; Mao, L. Galvanic Redox Potentiometry for Self-Driven in Vivo Measurement of Neurochemical Dynamics at Open-Circuit Potential. Anal. Chem. 2018, 90, 13021–13029. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.-b.; Jiang, Y.; Zhang, J.; Chen, S.; Zeng, R.; Zhuo, Y.; Lu, M.; Shi, G.; Gu, H. Tailoring Oxygen-Containing Groups on Graphene for Ratiometric Electrochemical Measurements of Ascorbic Acid in Living Subacute Parkinson’s Disease Mouse Brains. Anal. Chem. 2021, 93, 16598–16607. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, H.; Zhang, L.; Tian, Y. Development of an Efficient Biosensor for the In Vivo Monitoring of Cu+ and pH in the Brain: Rational Design and Synthesis of Recognition Molecules. Angew. Chem. Int. Ed. 2017, 56, 16328–16332. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Zhou, X.; Zhu, A.; Zhang, L.; Qin, Y.; Shi, G.; Tian, Y. A Two-Channel Ratiometric Electrochemical Biosensor for In Vivo Monitoring of Copper Ions in a Rat Brain Using Gold Truncated Octahedral Microcages. Angew. Chem. Int. Ed. 2013, 52, 8129–8133. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Qiu, W.; Zhang, M. In Vivo Monitoring of H2O2 with Polydopamine and Prussian Blue-coated Microelectrode. Anal. Chem. 2016, 88, 7769–7776. [Google Scholar] [CrossRef]
- O’Riordan, S.L.; Lowry, J.P. In vivo characterisation of a catalase-based biosensor for real-time electrochemical monitoring of brain hydrogen peroxide in freely-moving animals. Anal. Methods 2017, 9, 1253–1264. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, L.; Tian, Y. Highly Stable Electrochemical Probe with Bidentate Thiols for Ratiometric Monitoring of Endogenous Polysulfide in Living Mouse Brains. Anal. Chem. 2022, 94, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Meiller, A.; Sequeira, E.; Marinesco, S. Electrochemical Nitric Oxide Microsensors Based on a Fluorinated Xerogel Screening Layer for in Vivo Brain Monitoring. Anal. Chem. 2020, 92, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Plock, N.; Kloft, C. Microdialysis—Theoretical background and recent implementation in applied life-sciences. Eur. J. Pharm. Sci. 2005, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bito, L.Z.; Davson, H. Local variations in cerebrospinal fluid composition and its relationship to the composition of the extracellular fluid of the cortex. Exp. Neurol. 1966, 14, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, K.; Gong, K.; Su, L.; Chen, Y.; Mao, L. Continuous On-Line Monitoring of Extracellular Ascorbate Depletion in the Rat Striatum Induced by Global Ischemia with Carbon Nanotube-Modified Glassy Carbon Electrode Integrated into a Thin-Layer Radial Flow Cell. Anal. Chem. 2005, 77, 6234–6242. [Google Scholar] [CrossRef]
- Bert, L.; Robert, F.; Denoroy, L.; Stoppini, L.; Renaud, B. Enhanced temporal resolution for the microdialysis monitoring of catecholamines and excitatory amino acids using capillary electrophoresis with laser-induced fluorescence detection Analytical developments and in vitro validations. J. Chromatogr. A 1996, 755, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Escalona, J.; Verdeguer, P.; Guzman, N.A. In Vivo Monitoring of Brain Glutamate by Microdialysis Coupled to Capillary Electrophoresis and Laser Induced Fluorescence Detection. J. Liq. Chromatogr. 1993, 16, 2149–2160. [Google Scholar] [CrossRef]
- Hernandez, L.; Tucci, S.; Guzman, N.; Paez, X. In vivo monitoring of glutamate in the brain by microdialysis and capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. A 1993, 652, 393–398. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Weber, P.L.; Bammel, B.P.; Lunte, C.E.; Lunte, S.M.; Smyth, M.R. Monitoring excitatory amino acid release in vivo by microdialysis with capillary electrophoresis- electrochemistry. J. Chromatogr. A 1992, 608, 189–195. [Google Scholar] [CrossRef]
- Robert, F.; Bert, L.; Lambás-Señas, L.; Denoroy, L.; Renaud, B. In vivo monitoring of extracellular noradrenaline and glutamate from rat brain cortex with 2-min microdialysis sampling using capillary electrophoresis with laser-induced fluorescence detection. J. Neurosci. Methods 1996, 70, 153–162. [Google Scholar] [CrossRef]
- Zhou, J.; Heckert, D.M.; Zuo, H.; Lunte, C.E.; Lunte, S.M. On-line coupling of in vivo microdialysis with capillary electrophoresis/electrochemistry. Anal. Chim. Acta 1999, 379, 307–317. [Google Scholar] [CrossRef]
- Tao, L.; Kennedy, R.T. Measurement of antibody-antigen dissociation constants using fast capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 1997, 18, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Lada, M.W.; Vickroy, T.W.; Kennedy, R.T. High Temporal Resolution Monitoring of Glutamate and Aspartate in Vivo Using Microdialysis On-Line with Capillary Electrophoresis with Laser-Induced Fluorescence Detection. Anal. Chem. 1997, 69, 4560–4565. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Greenhagen, R.D.; Lunte, S.M.; Lunte, C.E.; Smyth, M.R.; Radzik, D.M.; Watanabe, N. Capillary electrophoresis with electrochemical detection employing an on-column Nafion joint. J. Chromatogr. A 1992, 593, 305–312. [Google Scholar] [CrossRef]
- Wallingford, R.A.; Ewing, A.G. Separation of serotonin from catechols by capillary zone electrophoresis with electrochemical detection. Anal. Chem. 1989, 61, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Curry, P.D., Jr.; Engstro-Silverman, C.E.; Ewing, A.G. Electrochemical detection for capillary electrophoresis. Electroanalysis 1991, 3, 587–596. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Telting-Diaz, M.W.; Lunte, S.M.; Lunte, C.E.; Smyth, M.R. Capillary electrophoresis—Electrochemistry of microdialysis samples for pharmacokinetic studies. Electroanalysis 1992, 4, 463–468. [Google Scholar] [CrossRef]
- Woolley, A.T.; Lao, K.; Glazer, A.N.; Mathies, R.A. Capillary Electrophoresis Chips with Integrated Electrochemical Detection. Anal. Chem. 1998, 70, 684–688. [Google Scholar] [CrossRef]
- Herzog, G.; Damien, W.M.A. Electrochemical Strategies in Detection Science; Royal Society of Chemistry: London, UK, 2017; Volume 80, p. 1483. [Google Scholar]
- Nandi, P.; Lunte, S.M. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: A review. Anal. Chim. Acta 2009, 651, 1–14. [Google Scholar] [CrossRef]
- Nuchtavorn, N.; Suntornsuk, W.; Lunte, S.M.; Suntornsuk, L. Recent applications of microchip electrophoresis to biomedical analysis. J. Pharm. Biomed. Anal. 2015, 113, 72–96. [Google Scholar] [CrossRef]
- Oborny, N.J.; Costa, E.E.M.; Suntornsuk, L.; Abreu, F.C.; Lunte, S.M. Evaluation of a Portable Microchip Electrophoresis Fluorescence Detection System for the Analysis of Amino Acid Neurotransmitters in Brain Dialysis Samples. Anal. Sci. 2016, 32, 35–40. [Google Scholar] [CrossRef]
- Saylor, R.A.; Lunte, S.M. PDMS/glass hybrid device with a reusable carbon electrode for on-line monitoring of catecholamines using microdialysis sampling coupled to microchip electrophoresis with electrochemical detection. Electrophoresis 2018, 39, 462–469. [Google Scholar] [CrossRef]
- Gunawardhana, S.M.; Bulgakova, G.A.; Barybin, A.M.; Thomas, S.R.; Lunte, S.M. Progress toward the development of a microchip electrophoresis separation-based sensor with electrochemical detection for on-line in vivo monitoring of catecholamines. Analyst 2020, 145, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Lada, M.W.; Kennedy, R.T. Quantitative in Vivo Monitoring of Primary Amines in Rat Caudate Nucleus Using Microdialysis Coupled by a Flow-Gated Interface to Capillary Electrophoresis with Laser-Induced Fluorescence Detection. Anal. Chem. 1996, 68, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Shou, M.; Smith, A.D.; Shackman, J.G.; Peris, J.; Kennedy, R.T. In vivo monitoring of amino acids by microdialysis sampling with on-line derivatization by naphthalene-2,3-dicarboxyaldehyde and rapid micellar electrokinetic capillary chromatography. J. Neurosci. Methods 2004, 138, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ciriacks Klinker, C.; Bowser, M.T. 4-Fluoro-7-nitro-2,1,3-benzoxadiazole as a Fluorogenic Labeling Reagent for the in Vivo Analysis of Amino Acid Neurotransmitters Using Online Microdialysis−Capillary Electrophoresis. Anal. Chem. 2007, 79, 8747–8754. [Google Scholar] [CrossRef] [PubMed]
- McNeff, C.V.; Yan, B.; Stoll, D.R.; Henry, R.A. Practice and theory of high temperature liquid chromatography. J. Sep. Sci. 2007, 30, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Clausen, A.M. Fundamental and practical aspects of ultrahigh pressure liquid chromatography for fast separations. J. Sep. Sci. 2007, 30, 1167–1182. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Y.; Lee, M.L. Ultrahigh pressure liquid chromatography using elevated temperature. J. Chromatogr. A 2006, 1104, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Xu, X.; Zhao, M.K.; Andrews, A.M.; Weber, S.G. Capillary Ultrahigh Performance Liquid Chromatography with Elevated Temperature for Sub-One Minute Separations of Basal Serotonin in Submicroliter Brain Microdialysate Samples. Anal. Chem. 2010, 82, 9611–9616. [Google Scholar] [CrossRef]
- Yang, H.; Thompson, A.B.; McIntosh, B.J.; Altieri, S.C.; Andrews, A.M. Physiologically Relevant Changes in Serotonin Resolved by Fast Microdialysis. ACS Chem. Neurosci. 2013, 4, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Varner, E.L.; Groskreutz, S.R.; Michael, A.C.; Weber, S.G. In Vivo Monitoring of Dopamine by Microdialysis with 1 min Temporal Resolution Using Online Capillary Liquid Chromatography with Electrochemical Detection. Anal. Chem. 2015, 87, 6088–6094. [Google Scholar] [CrossRef]
- Xiong, S.; Song, Y.; Liu, J.; Du, Y.; Ding, Y.; Wei, H.; Bryan, K.; Ma, F.; Mao, L. Neuroprotective effects of MK-801 on auditory cortex in salicylate-induced tinnitus: Involvement of neural activity, glutamate and ascorbate. Hear. Res. 2019, 375, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ngo, K.T.; Varner, E.L.; Michael, A.C.; Weber, S.G. Monitoring Dopamine Responses to Potassium Ion and Nomifensine by in Vivo Microdialysis with Online Liquid Chromatography at One-Minute Resolution. ACS Chem. Neurosci. 2017, 8, 329–338. [Google Scholar] [CrossRef]
- Wise, R.A.; Leeb, K.; Pocock, D.; Newton, P.; Burnette, B.; Justice, J.B. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology 1995, 120, 10–20. [Google Scholar] [CrossRef]
- Bradberry, C.W.; Rubino, S.R. Dopaminergic responses to self-administered cocaine in Rhesus monkeys do not sensitize following high cumulative intake. Eur. J. Neurosci. 2006, 23, 2773–2778. [Google Scholar] [CrossRef]
- Bradberry, C.W. Acute and Chronic Dopamine Dynamics in a Nonhuman Primate Model of Recreational Cocaine Use. J. Neurosci. 2000, 20, 7109–7115. [Google Scholar] [CrossRef]
- Bradberry, C.W.; Rubino, S.R. Phasic Alterations in Dopamine and Serotonin Release in Striatum and Prefrontal Cortex in Response to Cocaine Predictive Cues in Behaving Rhesus Macaques. Neuropsychopharmacology 2004, 29, 676–685. [Google Scholar] [CrossRef][Green Version]
- Newton, A.P.; Justice, J.B. Temporal Response of Microdialysis Probes to Local Perfusion of Dopamine and Cocaine Followed with One-Minute Sampling. Anal. Chem. 1994, 66, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.W.; Schmidt-Garcon, P.; Pierrefiche, O.; Bischoff, A.M.; Lalley, P.M. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J. Physiol. 1999, 514, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Sandlin, Z.D.; Shou, M.; Shackman, J.G.; Kennedy, R.T. Microfluidic Electrophoresis Chip Coupled to Microdialysis for in Vivo Monitoring of Amino Acid Neurotransmitters. Anal. Chem. 2005, 77, 7702–7708. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.W.; Witowski, S.R.; Kennedy, R.T. Trace-Level Amino Acid Analysis by Capillary Liquid Chromatography and Application to in Vivo Microdialysis Sampling with 10-s Temporal Resolution. Anal. Chem. 2000, 72, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Parrot, S.; Lambás-Señas, L.; Sentenac, S.; Denoroy, L.; Renaud, B. Highly sensitive assay for the measurement of serotonin in microdialysates using capillary high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B 2007, 850, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Reinhoud, N.J.; Brouwer, H.-J.; van Heerwaarden, L.M.; Korte-Bouws, G.A.H. Analysis of Glutamate, GABA, Noradrenaline, Dopamine, Serotonin, and Metabolites Using Microbore UHPLC with Electrochemical Detection. ACS Chem. Neurosci. 2013, 4, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Ferry, B.; Gifu, E.-P.; Sandu, I.; Denoroy, L.; Parrot, S. Analysis of microdialysate monoamines, including noradrenaline, dopamine and serotonin, using capillary ultra-high performance liquid chromatography and electrochemical detection. J. Chromatogr. B 2014, 951–952, 52–57. [Google Scholar] [CrossRef]
- Van Schoors, J.; Lens, C.; Maes, K.; Michotte, Y.; Smolders, I.; Van Eeckhaut, A. Reassessment of the antioxidative mixture for the challenging electrochemical determination of dopamine, noradrenaline and serotonin in microdialysis samples. J. Chromatogr. B 2015, 998–999, 63–71. [Google Scholar] [CrossRef]
- Van Schoors, J.; Viaene, J.; Van Wanseele, Y.; Smolders, I.; Dejaegher, B.; Vander Heyden, Y.; Van Eeckhaut, A. An improved microbore UHPLC method with electrochemical detection for the simultaneous determination of low monoamine levels in in vivo brain microdialysis samples. J. Pharm. Biomed. Anal. 2016, 127, 136–146. [Google Scholar] [CrossRef]
- Greaney, M.D.; Marshall, D.L.; Bailey, B.A.; Acworth, I.N. Improved method for the routine analysis of acetylcholine release in vivo: Quantitation in the presence and absence of esterase inhibitor. J. Chromatogr. B Biomed. Sci. Appl. 1993, 622, 125–135. [Google Scholar] [CrossRef]
- Roland, J.J.; Stewart, A.L.; Janke, K.L.; Gielow, M.R.; Kostek, J.A.; Savage, L.M.; Servatius, R.J.; Pang, K.C.H. Medial Septum-Diagonal Band of Broca (MSDB) GABAergic Regulation of Hippocampal Acetylcholine Efflux Is Dependent on Cognitive Demands. J. Neurosci. 2014, 34, 506–514. [Google Scholar] [CrossRef]
- Castro, H.C.V.; Valenzuela, L.L.C.; Sanchez, C.S.J.; Pena, P.K.; Perez, J.L.S.; Ibarra, O.J.; Villagran, M.A. An Update of the Classical and Novel Methods Used for Measuring Fast Neurotransmitters During Normal and Brain Altered Function. Curr. Neuropharmacol. 2014, 12, 490–508. [Google Scholar] [CrossRef]
- Wilson, G.S.; Gifford, R. Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 2005, 20, 2388–2403. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Tóth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Gu, H.; Liu, Y.; Ren, T.; Xia, W.; Guo, Y.; Shi, G. An electrochemical biosensor based on double molecular recognition for selective monitoring of cerebral dopamine dynamics at 4 min interval. Sens. Actuators B Chem. 2019, 287, 356–363. [Google Scholar] [CrossRef]
- Wood, E.R.; Coury, A.; Blaha, C.D.; Phillips, A.G. Extracellular dopamine in the rat striatum during ischemia and reperfusion as measured by in vivo electrochemistry and in vivo microdialysis. Brain Res. 1992, 591, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, S.E.; Parkin, M.C.; Bezzina, E.L.; Boutelle, M.G.; Strong, A.J. Transient Changes in Cortical Glucose and Lactate Levels Associated with Peri-Infarct Depolarisations, Studied with Rapid-Sampling Microdialysis. J. Cereb. Blood Flow Metab. 2005, 25, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Parkin, M.; Hopwood, S.; Jones, D.A.; Hashemi, P.; Landolt, H.; Fabricius, M.; Lauritzen, M.; Boutelle, M.G.; Strong, A.J. Dynamic Changes in Brain Glucose and Lactate in Pericontusional Areas of the Human Cerebral Cortex, Monitored with Rapid Sampling On-Line Microdialysis: Relationship with Depolarisation-Like Events. J. Cereb. Blood Flow Metab. 2005, 25, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Hashemi, P.; Razzaq, A.; Parkin, M.C.; Hopwood, S.E.; Boutelle, M.G.; Strong, A.J. Application of Rapid-Sampling, Online Microdialysis to The Monitoring of Brain Metabolism During Aneurysm Surgery. Oper. Neurosurg. 2006, 58, ONS-313–ONS-321. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, W.A.; Zwaagstra, J.J.; Venema, K.; Korf, J. Continuous Ultraslow Microdialysis and Ultrafiltration for Subcutaneous Sampling as Demonstrated by Glucose and Lactate Measurements in Rats. Anal. Chem. 1998, 70, 4696–4700. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yang, Y.; Zhou, X.; Zhou, T.; Shi, G. Online electrochemical method for continuous and simultaneous monitoring of glucose and l-lactate in vivo with graphene hybrids as the electrocatalyst. J. Electroanal. Chem. 2014, 730, 41–47. [Google Scholar] [CrossRef]
- Demestre, M.; Boutelle, M.; Fillenz, M. Stimulated release of lactate in freely moving rats is dependent on the uptake of glutamate. J. Physiol. 1997, 499, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Rhemrev-Boom, R.M.; Tiessen, R.G.; Jonker, A.A.; Venema, K.; Vadgama, P.; Korf, J. A lightweight measuring device for the continuous in vivo monitoring of glucose by means of ultraslow microdialysis in combination with a miniaturised flow-through biosensor. Clin. Chim. Acta 2002, 316, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Xu, J.; Wu, S.; Xiao, T.; Hao, J.; Yu, P.; Mao, L. Rational Design of Bioelectrochemically Multifunctional Film with Oxidase, Ferrocene, and Graphene Oxide for Development of in Vivo Electrochemical Biosensors. Anal. Chem. 2016, 88, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Xiong, P.; Tang, H.; Chen, S.; Long, Y.; Shi, G. In vivo monitoring of cerebral glucose with an updated on-line electroanalytical system. Anal. Bioanal. Chem. 2019, 411, 5929–5935. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Cai, D.; Ren, T.; Xiong, P.; Liu, Y.; Gu, H.; Shi, G. Fabrication of a low background signal glucose biosensor with 3D network materials as the electrocatalyst. Anal. Biochem. 2019, 567, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yu, Y.; Liu, X.; Ni, B.; Zhou, T.; Shi, G. Layer-by-layer self-assembly of functionalized graphene nanoplates for glucose sensing in vivo integrated with on-line microdialysis system. Biosens. Bioelectron. 2012, 32, 118–126. [Google Scholar] [CrossRef]
- Gu, H.; Hou, Q.; Liu, Y.; Cai, Y.; Guo, Y.; Xiang, H.; Chen, S. On-line regeneration of electrochemical biosensor for in vivo repetitive measurements of striatum Cu2+ under global cerebral ischemia/reperfusion events. Biosens. Bioelectron. 2019, 135, 111–119. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, W.; Ji, W.; Wei, H.; Mao, L. Aptamer superstructure-based electrochemical biosensor for sensitive detection of ATP in rat brain with in vivo microdialysis. Analyst 2019, 144, 1711–1717. [Google Scholar] [CrossRef]
- Yu, P.; He, X.; Zhang, L.; Mao, L. Dual Recognition Unit Strategy Improves the Specificity of the Adenosine Triphosphate (ATP) Aptamer Biosensor for Cerebral ATP Assay. Anal. Chem. 2015, 87, 1373–1380. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, X.; Jiang, D.; Sun, Q.; Zhou, T.; Zhu, M.; Jin, L.; Shi, G. [C3(OH)2mim][BF4]-Au/Pt biosensor for glutamate sensing in vivo integrated with on-line microdialysis system. Biosens. Bioelectron. 2011, 26, 3227–3232. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dong, H.; Tian, Y. Real-time monitoring of peroxynitrite (ONOO−) in the rat brain by developing a ratiometric electrochemical biosensor. Analyst 2019, 144, 2150–2157. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiao, X.; Li, C.; Luo, Y.; Chen, S.; Shi, G.; Han, K.; Gu, H. Facile Ratiometric Electrochemical Sensor for In Vivo/Online Repetitive Measurements of Cerebral Ascorbic Acid in Brain Microdiaysate. Anal. Chem. 2020, 92, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
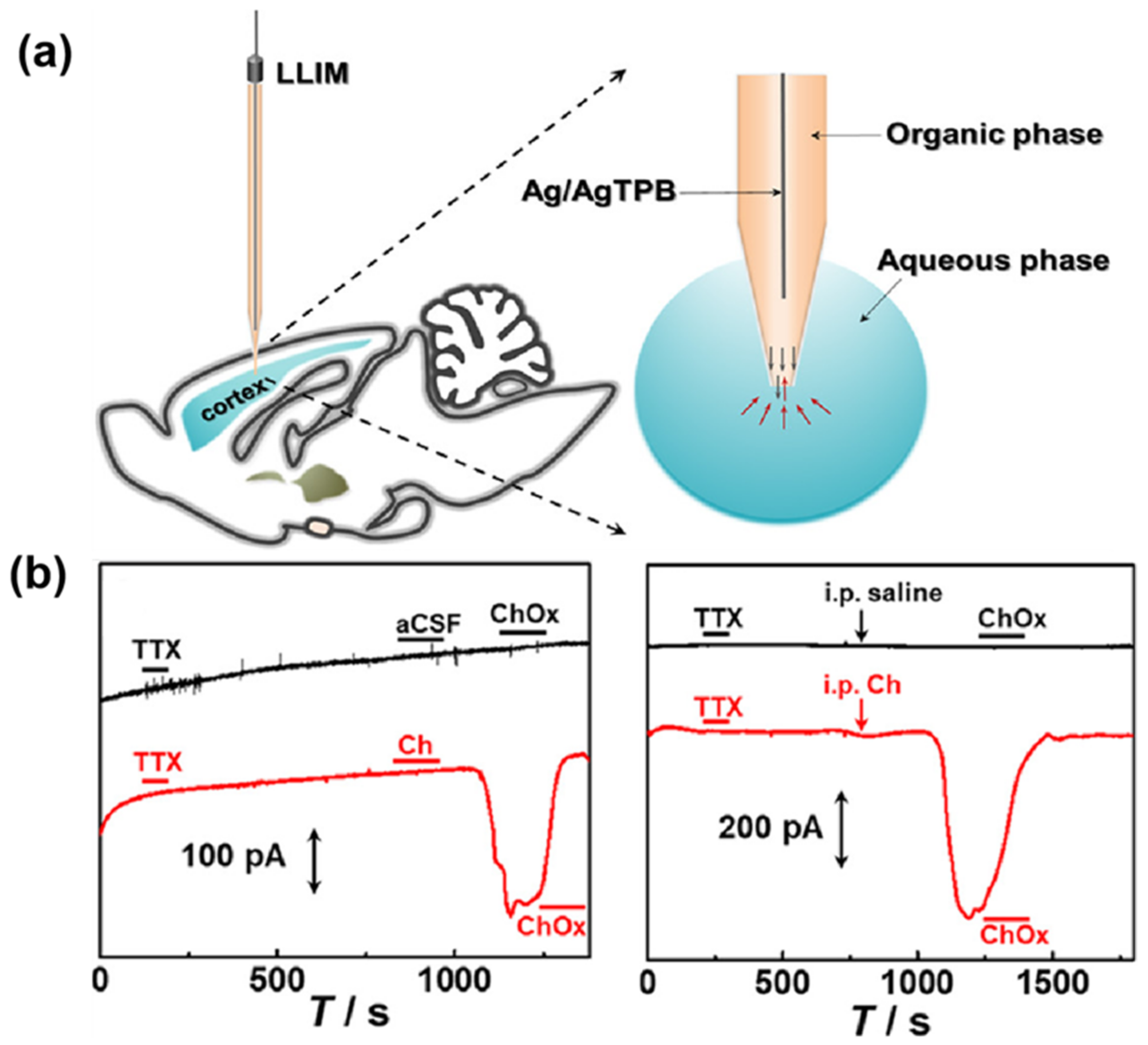
- Wang, X.; Xu, T.; Zhang, Y.; Gao, N.; Feng, T.; Wang, S.; Zhang, M. In Vivo Detection of Redox-Inactive Neurochemicals in the Rat Brain with an Ion Transfer Microsensor. ACS Sens. 2021, 6, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, K.; Li, T.; Jiang, Y.; Yu, P.; Mao, L. Micrometer-Scale Ion Current Rectification at Polyelectrolyte Brush-Modified Micropipets. J. Am. Chem. Soc. 2017, 139, 1396–1399. [Google Scholar] [CrossRef]
- Zhang, K.; He, X.; Liu, Y.; Yu, P.; Fei, J.; Mao, L. Highly Selective Cerebral ATP Assay Based on Micrometer Scale Ion Current Rectification at Polyimidazolium-Modified Micropipettes. Anal. Chem. 2017, 89, 6794–6799. [Google Scholar] [CrossRef]
- Chae, M.-S.; Yoo, Y.K.; Kim, J.; Kim, T.G.; Hwang, K.S. Graphene-based enzyme-modified field-effect transistor biosensor for monitoring drug effects in Alzheimer’s disease treatment. Sens. Actuators B Chem. 2018, 272, 448–458. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Marmisollé, W.A.; Azzaroni, O.; Knoll, W. Acetylcholine biosensor based on the electrochemical functionalization of graphene field-effect transistors. Biosens. Bioelectron. 2020, 148, 111796. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yang, K.-A.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Yang, Y.; et al. Aptamer–field-effect transistors overcome Debye length limitations for small-molecule sensing. Science 2018, 362, 319–324. [Google Scholar] [CrossRef]
- Tan, C.; Robbins, E.M.; Wu, B.; Cui, X.T. Recent Advances in In Vivo Neurochemical Monitoring. Micromachines 2021, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Roham, M.; Daberkow, D.P.; Ramsson, E.S.; Covey, D.P.; Pakdeeronachit, S.; Garris, P.A.; Mohseni, P. A Wireless IC for Wide-Range Neurochemical Monitoring Using Amperometry and Fast-Scan Cyclic Voltammetry. IEEE Trans. Biomed. Circuits Syst. 2008, 2, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; Abate, G.; Borghetti, M.; Marziano, M.; Serpelloni, M.; Uberti, D.L.; Lopomo, N.F.; Memo, M.; Sardini, E. Wireless Point-of-Care Platform with Screen-Printed Sensors for Biomarkers Detection. IEEE Trans. Instrum. Meas. 2017, 66, 2448–2455. [Google Scholar] [CrossRef]
- Tageldeen, M.K.; Gowers, S.A.N.; Leong, C.L.; Boutelle, M.G.; Drakakis, E.M. Traumatic brain injury neuroelectrochemical monitoring: Behind-the-ear micro-instrument and cloud application. J. NeuroEngineering Rehabil. 2020, 17, 114. [Google Scholar] [CrossRef]
- Wu, H.; Meng, Z.; Wang, J.; Yao, G.; Yang, L.; Zeng, Z.; She, K.; Zhao, S.; Wang, G.; Zhang, Y.; et al. Aptamer functionalized cell membrane for brain and nerve cell sensing with high sensitivity and stability. Biosens. Bioelectron. 2023, 227, 115149. [Google Scholar] [CrossRef]
- Hossain, I.; Tan, C.; Doughty, P.T.; Dutta, G.; Murray, T.A.; Siddiqui, S.; Iasemidis, L.; Arumugam, P.U. A Novel Microbiosensor Microarray for Continuous ex Vivo Monitoring of Gamma-Aminobutyric Acid in Real-Time. Front. Neurosci. 2018, 12, 500. [Google Scholar] [CrossRef]
- Asri, R.; O’Neill, B.; Patel, J.C.; Siletti, K.A.; Rice, M.E. Detection of evoked acetylcholine release in mouse brain slices. Analyst 2016, 141, 6416–6421. [Google Scholar]

| Neurochemicals | Sensor Structure | Linear Range | LOD (μM) | Detection Area | Ref. |
|---|---|---|---|---|---|
| ATP | Au/Apt/3-MPA | 0.001–100 µM | 0.5 | Cortex | [73] |
| Cl− | CFME/OxGO/Ti3C2Tx/Ag | 1–700 mM | 10 | Hippocampus | [74] |
| Cl− | CFME/GO/TNWs/Ag/MB | 1–300 mM | 10 | Hippocampus | [75] |
| H+ | CFME/EOGO/PMe | 0.5–600 μM | 0.036 | Hippocampus | [76] |
| H+ | CFNE/CNTs/PoPD | 4.5–8.2 pH | - | Hippocampus | [77] |
| H+ | Hemin-Fc/CNF | 5.5–8.0 pH | - | Striatum, Cortex | [78] |
| H+ | Cat + Fc/SWNT/CFME | 5.91–7.81 pH | - | Striatum | [79] |
| K+ | NO/K+ dual microsensor | 0.01–100 mM | - | Cortex | [80] |
| H+, K+, Ca2+, and Na+ | Mesoporous SiO2/carbon/Co(II) phthalocyanine | 0.1–70.79 μM | - | Hippocampus | [81] |
| 50 μM–140 mM | |||||
| 1 μM–160 mM | |||||
| 130 μM–200 mM | |||||
| O2•− | CFME/SWCNT/MB + ND | 2–200 μM | 0.52 | Striatum, Cortex, Hippocampus | [82] |
| Glucose | Gox/PB/PANI/MWNT/CFE | 50−4000 μM | 40 | Cortex | [83] |
| Lactate | Pt-ceria biosensors | 100 pM−15.5 mM | 0.1 | Hippocampus | [84] |
| K+ | NO/K+ dual microsensor | 10 μM−100 mM | - | Cortex | [80] |
| Glutamate | Pt-Ir/PPD/GlutOx/AsOx/BSA | 5–150 µM | 0.044 | Subthalamic nucleus | [11] |
| Glutamate | GluOx/pDAB/polyimide | Up to 150 µM | 0.22 | Cortex | [85] |
| Neurochemicals | Sensor Structure | Linear Range | LOD | Detection Area | Ref. |
|---|---|---|---|---|---|
| DA | PTA-PANI-coated/CFE | 5–30 µM | - | Striatum | [16] |
| DA | PB/PEDOT/CFdisk | 0.5–10 mM | 0.18 µM | Striatum | [135] |
| DA | PEDOT/GO/CFE | 6.25–212.5 µM | - | Dorsal Striatum | [136] |
| DA | Nafion-Au/GCNE | 0.02–5.6 µM | 0.01 µM | Striatum | [137] |
| DA | AuNPs-rGO/Pt | 0.05–3 µM | 0.01675 µM | Striatum | [129] |
| 5-HT | CFMEA/DS-SWCNT | 0.10–3.40 µM | 5.1 µM | Striatum | [10] |
| 5-HT | CFEA/GR-FeTSPc | 0.05–60 µM | 0.02 µM | Hippocampus | [138] |
| 5-HT | PEDOT/CNT-coated | 0.01–1 µM | - | Hippocampus | [139] |
| AA | SWCNT/CFE | 10–1000 µM | 1000 µM | Cortex | [140] |
| AA | PEDOT/EOGO/CFE | 20–1000 µM | 500 µM | Striatum, Cortex, Hippocampus | [141] |
| Cu2+ | CFME/SWNT + AQ + NS4-C1 + ABTS | 0.5–9.5 µM | 500 nM | Striatum, Cortex, Hippocampus | [142] |
| Cu2+ | CFME/Au/E2Zn2SOD/Ni-NTA | 0.01–35 µM | 3 nM | Striatum | [143] |
| H2O2 | PDA/PB/CNT/CFE | 0−2775 μM | 0.12 μM | Cortex | [144] |
| H2O2 | Cat/ Nafion-PPD/Pt | 25−1000 μM | 1.0 μM | Striatum | [145] |
| H2O2 | Cat + Fc/SWNT/CFME | 1.0–230 mM | - | Striatum | [79] |
| H2S | CFE/mAu/MPS-1 + MHS-1 | 0.2–40 μM | 47 ± 4 μM | Cortex, Striatum, Hippocampus | [9] |
| H2Sn | CFE/Au/FP2 + FcBT | 0.25–20 μM | 50 μM | Cortex, Striatum, Hippocampus | [146] |
| NO | NO/K+ dual microsensor | 0–3.13 μM | - | Cortex | [80] |
| NO | CFE/Ni-P/17-FTMS | 1–3 μM | 12.1 ± 3.4 nM | Cortex | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zuo, Y.; Chen, S.; Hatami, A.; Gu, H. Advancements in Brain Research: The In Vivo/In Vitro Electrochemical Detection of Neurochemicals. Biosensors 2024, 14, 125. https://doi.org/10.3390/bios14030125
Xu X, Zuo Y, Chen S, Hatami A, Gu H. Advancements in Brain Research: The In Vivo/In Vitro Electrochemical Detection of Neurochemicals. Biosensors. 2024; 14(3):125. https://doi.org/10.3390/bios14030125
Chicago/Turabian StyleXu, Xiaoxuan, Yimei Zuo, Shu Chen, Amir Hatami, and Hui Gu. 2024. "Advancements in Brain Research: The In Vivo/In Vitro Electrochemical Detection of Neurochemicals" Biosensors 14, no. 3: 125. https://doi.org/10.3390/bios14030125
APA StyleXu, X., Zuo, Y., Chen, S., Hatami, A., & Gu, H. (2024). Advancements in Brain Research: The In Vivo/In Vitro Electrochemical Detection of Neurochemicals. Biosensors, 14(3), 125. https://doi.org/10.3390/bios14030125





