Ultrasensitive Electrochemical Detection of Salmonella typhimurium in Food Matrices Using Surface-Modified Bacterial Cellulose with Immobilized Phage Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Cell Culture
2.3. Isolation and Purification of S. typhimurium-Specific Phage
2.4. Reduction of Graphene Oxide
2.5. Bacterial Cellulose Production and Purification
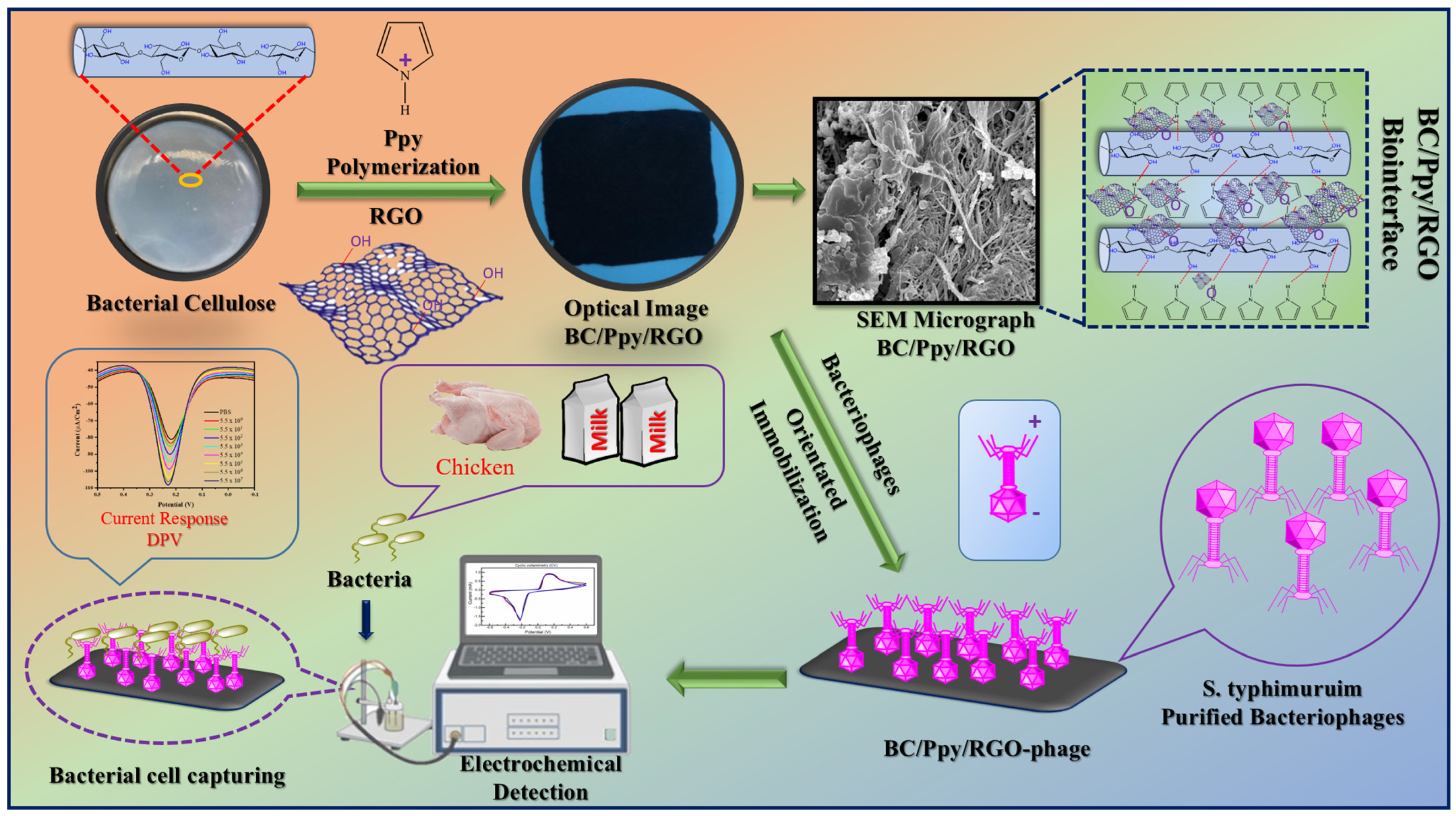
2.6. Development of BC/Ppy/RGO Biointerface
2.7. Immobilization of S. typhimurium-Specific Phages on BC/Ppy/RGO Biointerface
2.8. Characterization of BC/Ppy/RGO Biointerface
2.9. Plaque Assay and Cell Lysis Efficiency of Immobilized Phage
2.10. Fluorescent Characterization of Immobilized Phages
2.11. Electrochemical Characterization
2.12. Specificity, Reproducibility, and Stability of BC/Ppy/RGO Biosensor
2.13. Biosensor Application in Real Sample and Live/Dead Cell Discrimination
3. Results and Discussion
3.1. Principle and Design of the Phage-Based Biosensor
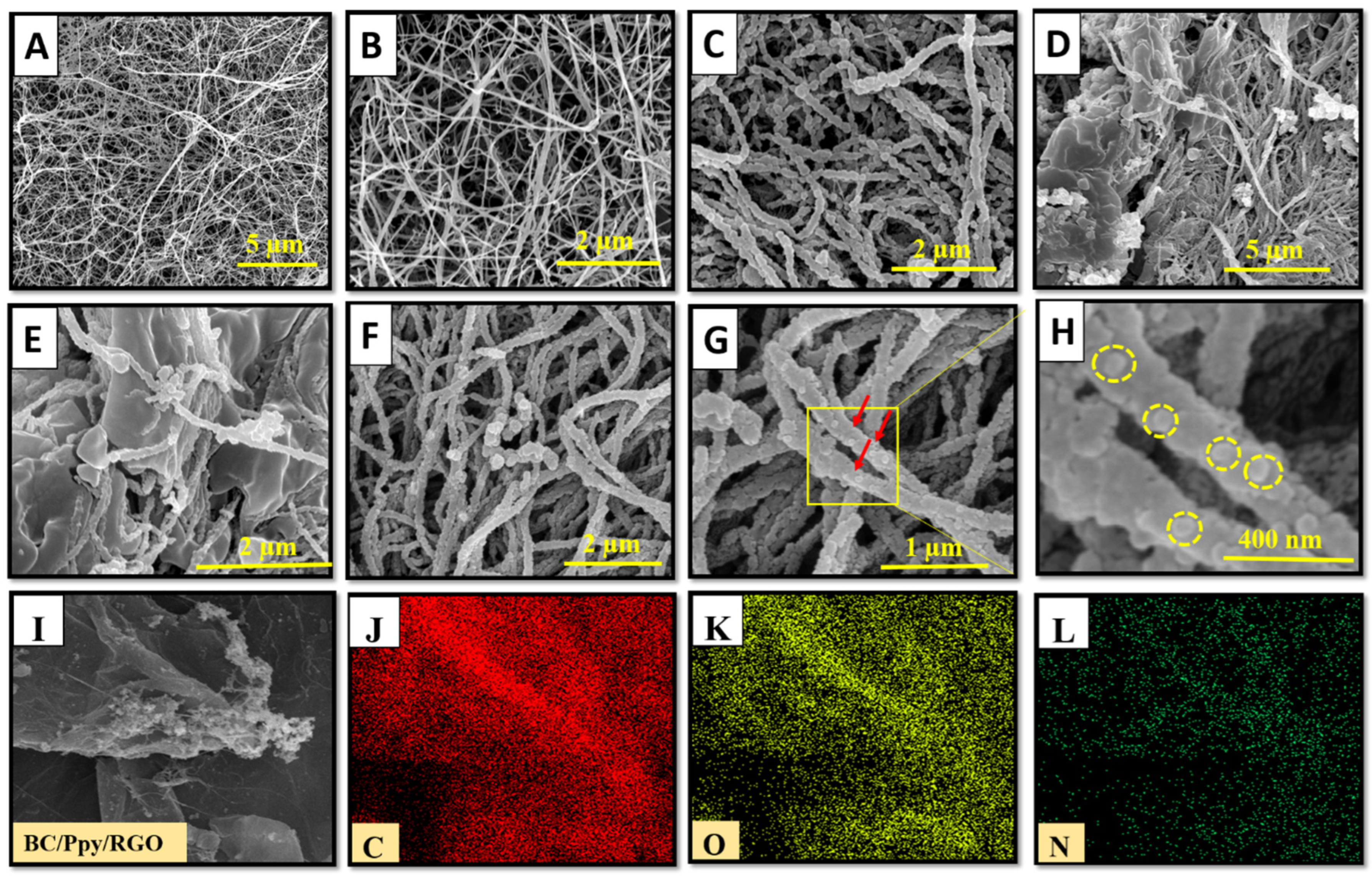
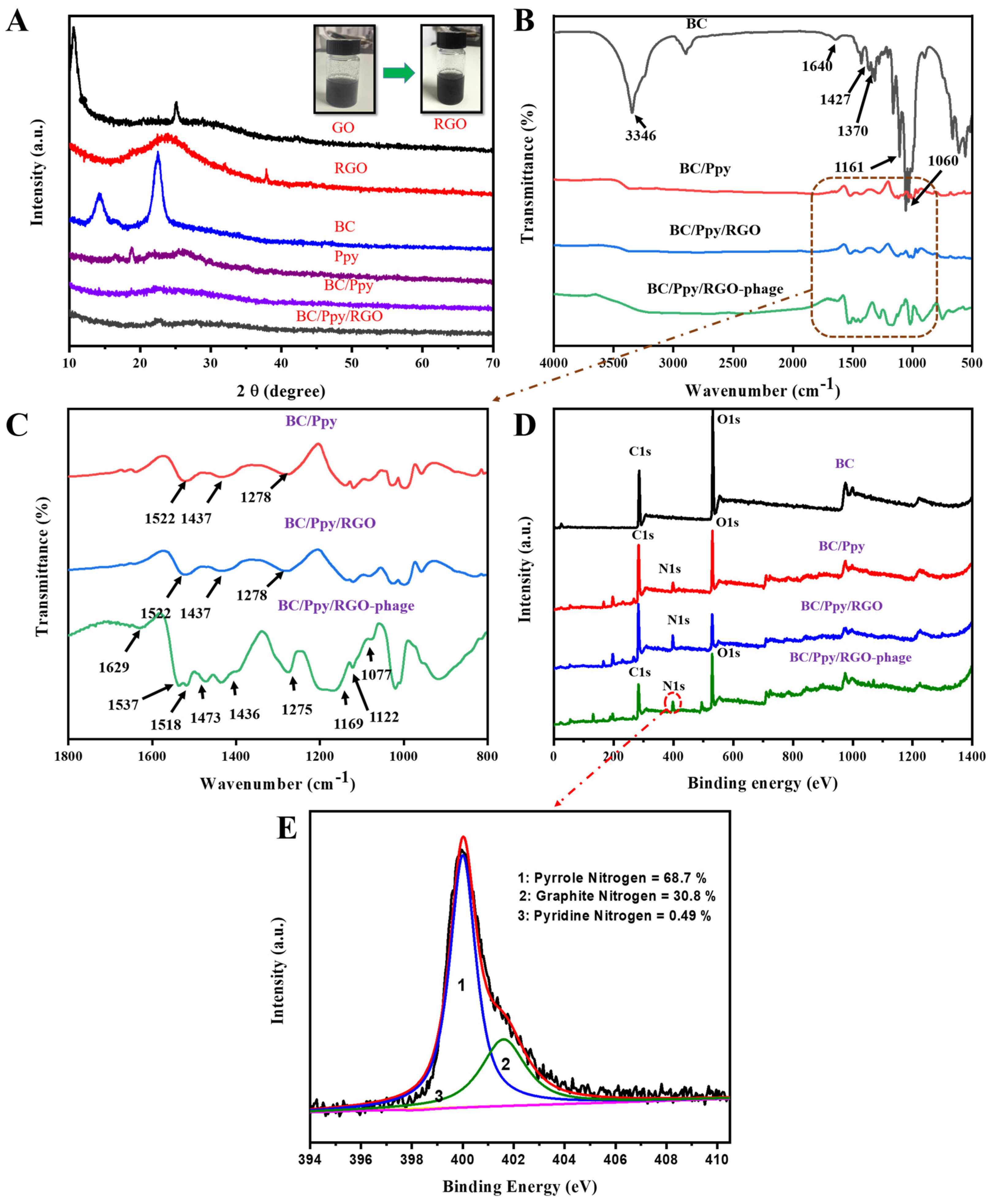
3.2. Morphology and Chemical Structure of the BC/Ppy/RGO–Phage Biointerface
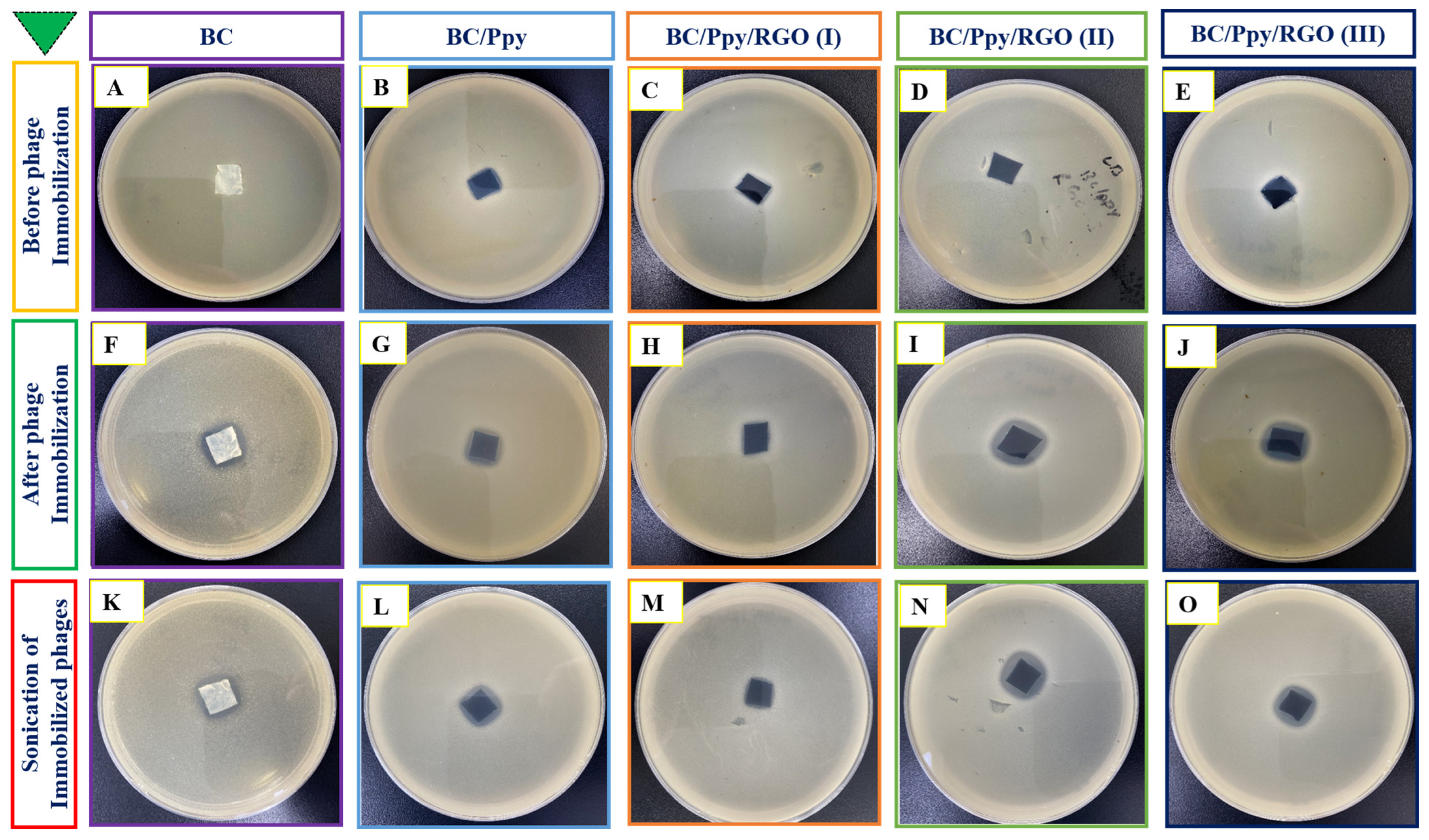
3.3. Anti-Salomenlla Activity of the Phage-Based Biointerface
3.4. Phage Density on BC/Ppy/RGO Biointerface
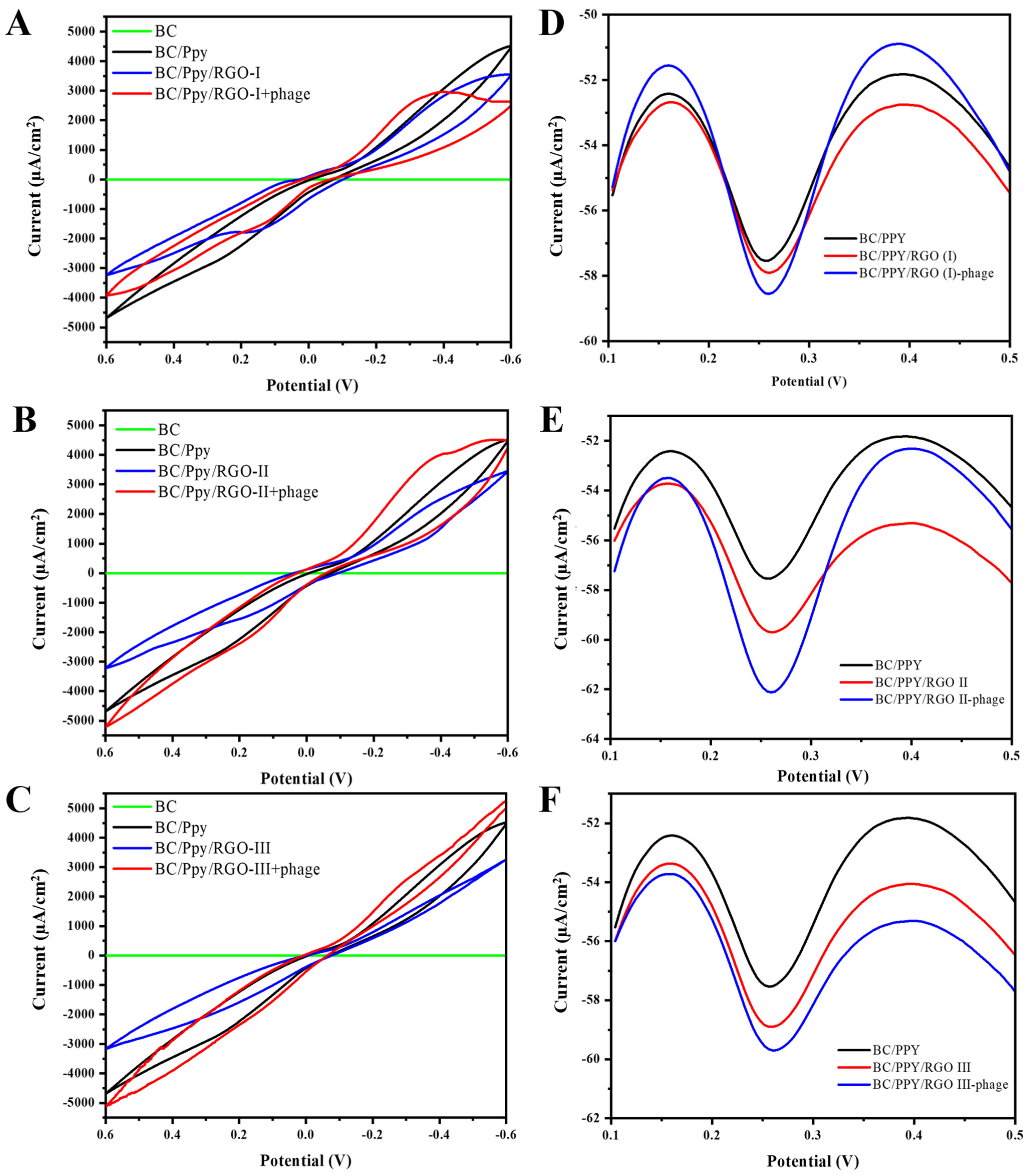
3.5. Electrochemical Characterization and Optimization of BC/Ppy/RGO Electrode
3.6. Development of BC/Ppy/RGO–Phage Biosensor
3.6.1. Optimization of Biosensor with Respect to Time and pH
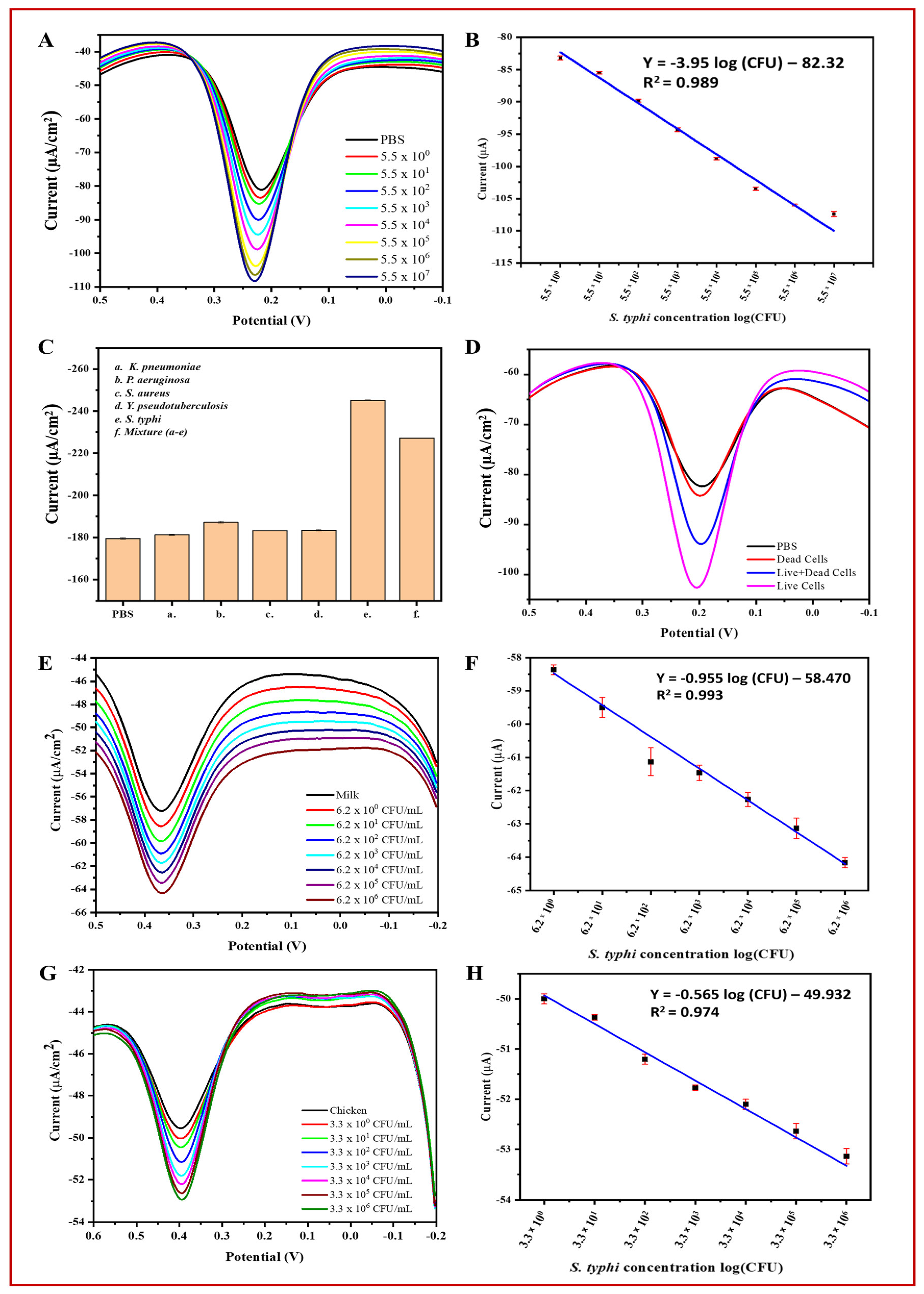
3.6.2. Specificity, Reproducibility, and Stability of the BC/Ppy/RGO–Phage Biosensor
3.6.3. Biosensor Application in Real Samples and Discrimination of Live/Dead Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, H.; Wang, Y.; Zhao, X. Research on the Drug Resistance Mechanism of Foodborne Pathogens. Microb. Pathog. 2022, 162, 105306. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Khaki, P.; Jahanban-Esfahlan, A.; de la Guardia, M.; Mokhtarzadeh, A. State of the Art: Lateral Flow Assays toward the Point-of-Care Foodborne Pathogenic Bacteria Detection in Food Samples. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1868–1912. [Google Scholar] [CrossRef]
- Yu, D.; Li, R.; Rong, K.; Fang, Y.; Liu, L.; Yu, H.; Dong, S. A Novel, Environmentally Friendly Dual-Signal Water Toxicity Biosensor Developed through the Continuous Release of Fe3+. Biosens. Bioelectron. 2023, 220, 114864. [Google Scholar] [CrossRef]
- Habib, I.; Mohamed, M.-Y.I.; Khan, M. Current State of Salmonella, Campylobacter and Listeria in the Food Chain across the Arab Countries: A Descriptive Review. Foods 2021, 10, 2369. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Fakhraei, M.; Jahanban-Esfahlan, A.; Hejazi, M.; Oroojalian, F.; Baradaran, B.; Tohidast, M.; de la Guardia, M.; Mokhtarzadeh, A. Lateral Flow Assays (LFA) for Detection of Pathogenic Bacteria: A Small Point-of-Care Platform for Diagnosis of Human Infectious Diseases. Talanta 2022, 243, 123330. [Google Scholar] [CrossRef]
- Nilghaz, A.; Mousavi, S.M.; Li, M.; Tian, J.; Cao, R.; Wang, X. Paper-Based Microfluidics for Food Safety and Quality Analysis. Trends Food Sci. Technol. 2021, 118, 273–284. [Google Scholar] [CrossRef]
- Bayat, F.; Didar, T.F.; Hosseinidoust, Z. Emerging Investigator Series: Bacteriophages as Nano Engineering Tools for Quality Monitoring and Pathogen Detection in Water and Wastewater. Environ. Sci. Nano 2021, 8, 367–389. [Google Scholar] [CrossRef]
- Farooq, U.; Ullah, M.W.; Yang, Q.; Aziz, A.; Xu, J.; Zhou, L.; Wang, S. High-Density Phage Particles Immobilization in Surface-Modified Bacterial Cellulose for Ultra-Sensitive and Selective Electrochemical Detection of Staphylococcus Aureus. Biosens. Bioelectron. 2020, 157, 112163. [Google Scholar] [CrossRef]
- Pebdeni, A.B.; Roshani, A.; Mirsadoughi, E.; Behzadifar, S.; Hosseini, M. Recent Advances in Optical Biosensors for Specific Detection of E. Coli Bacteria in Food and Water. Food Control 2022, 135, 108822. [Google Scholar] [CrossRef]
- Pandey, R.; Lu, Y.; Osman, E.; Saxena, S.; Zhang, Z.; Qian, S.; Pollinzi, A.; Smieja, M.; Li, Y.; Soleymani, L.; et al. DNAzyme-Immobilizing Microgel Magnetic Beads Enable Rapid, Specific, Culture-Free, and Wash-Free Electrochemical Quantification of Bacteria in Untreated Urine. ACS Sens. 2022, 7, 985–994. [Google Scholar] [CrossRef]
- Farooq, U.; Yang, Q.; Ullah, M.W.; Wang, S. Bacterial Biosensing: Recent Advances in Phage-Based Bioassays and Biosensors. Biosens. Bioelectron. 2018, 118, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.M.; Gondil, V.S.; Li, C.; Jiang, M.; Li, J.; Yu, J.; Wei, H.; Yang, H. A Novel Acinetobacter Baumannii Bacteriophage Endolysin LysAB54 With High Antibacterial Activity Against Multiple Gram-Negative Microbes. Front. Cell. Infect. Microbiol. 2021, 11, 637313. [Google Scholar] [CrossRef] [PubMed]
- Filik, K.; Szermer-Olearnik, B.; Niedziółka-Jönson, J.; Roźniecka, E.; Ciekot, J.; Pyra, A.; Matyjaszczyk, I.; Skurnik, M.; Brzozowska, E. ΦYeO3-12 Phage Tail Fiber Gp17 as a Promising High Specific Tool for Recognition of Yersinia Enterocolitica Pathogenic Serotype O:3. AMB Express 2022, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Carmody, C.M.; Goddard, J.M.; Nugen, S.R. Bacteriophage Capsid Modification by Genetic and Chemical Methods. Bioconjug. Chem. 2021, 32, 466–481. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Li, D.; Xu, Y.; Xu, F. Flexible and Sensitivity-Adjustable Pressure Sensors Based on Carbonized Bacterial Nanocellulose/Wood-Derived Cellulose Nanofibril Composite Aerogels. ACS Appl. Mater. Interfaces 2021, 13, 8754–8763. [Google Scholar] [CrossRef]
- Patel, D.; Zhou, Y.; Ramasamy, R.P. A Bacteriophage-Based Electrochemical Biosensor for Detection of Methicillin-Resistant Staphylococcus Aureus. J. Electrochem. Soc. 2021, 168, 57523. [Google Scholar] [CrossRef]
- Zhou, Y.; Marar, A.; Kner, P.; Ramasamy, R.P. Charge-Directed Immobilization of Bacteriophage on Nanostructured Electrode for Whole-Cell Electrochemical Biosensors. Anal. Chem. 2017, 89, 5734–5741. [Google Scholar] [CrossRef]
- Patel, D.R.; Bhartiya, S.K.; Kumar, R.; Shukla, V.K.; Nath, G. Use of Customized Bacteriophages in the Treatment of Chronic Nonhealing Wounds: A Prospective Study. Int. J. Low. Extrem. Wounds 2021, 20, 37–46. [Google Scholar] [CrossRef]
- Raja, I.S.; Vedhanayagam, M.; Preeth, D.R.; Kim, C.; Lee, J.H.; Han, D.W. Development of Two-Dimensional Nanomaterials Based Electrochemical Biosensors on Enhancing the Analysis of Food Toxicants. Int. J. Mol. Sci. 2021, 22, 3277. [Google Scholar] [CrossRef]
- Jo, H.J.; Robby, A.I.; Kim, S.G.; Lee, G.; Lee, B.C.; Park, S.Y. Reusable Biosensor-Based Polymer Dot-Coated Electrode Surface for Wireless Detection of Bacterial Contamination. Sens. Actuators B Chem. 2021, 346, 130503. [Google Scholar] [CrossRef]
- Vadanan, S.V.; Basu, A.; Lim, S. Bacterial Cellulose Production, Functionalization, and Development of Hybrid Materials Using Synthetic Biology. Polym. J. 2022, 54, 481–492. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial Cellulose: A Smart Biomaterial with Diverse Applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Umar Aslam Khan, M.; Haider, S.; Haider, A.; Izwan Abd Razak, S.; Rafiq Abdul Kadir, M.; Shah, S.A.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of Porous, Antibacterial and Biocompatible GO/n-HAp/Bacterial Cellulose/β-Glucan Biocomposite Scaffold for Bone Tissue Engineering. Arab. J. Chem. 2021, 14, 102924. [Google Scholar] [CrossRef]
- Drozd, R.; Szymańska, M.; Przygrodzka, K.; Hoppe, J.; Leniec, G.; Kowalska, U. The Simple Method of Preparation of Highly Carboxylated Bacterial Cellulose with Ni- and Mg-Ferrite-Based Versatile Magnetic Carrier for Enzyme Immobilization. Int. J. Mol. Sci. 2021, 22, 8563. [Google Scholar] [CrossRef] [PubMed]
- Jasim, A.; Ullah, M.W.; Shi, Z.; Lin, X.; Yang, G. Fabrication of Bacterial Cellulose/Polyaniline/Single-Walled Carbon Nanotubes Membrane for Potential Application as Biosensor. Carbohydr. Polym. 2017, 163, 62–69. [Google Scholar] [CrossRef]
- Kiangkitiwan, N.; Srikulkit, K. Preparation and Properties of Bacterial Cellulose/Graphene Oxide Composite Films Using Dyeing Method. Polym. Eng. Sci. 2021, 61, 1854–1863. [Google Scholar] [CrossRef]
- Song, Q.; Zhan, Z.; Chen, B.; Zhou, Z.; Lu, C. Biotemplate Synthesis of Polypyrrole@bacterial Cellulose/MXene Nanocomposites with Synergistically Enhanced Electrochemical Performance. Cellulose 2020, 27, 7475–7488. [Google Scholar] [CrossRef]
- Booth, M.A.; Harbison, S.; Travas-Sejdic, J. Development of an Electrochemical Polypyrrole-Based DNA Sensor and Subsequent Studies on the Effects of Probe and Target Length on Performance. Biosens. Bioelectron. 2011, 28, 362–367. [Google Scholar] [CrossRef]
- Li, Y.; Yu, C. One-Step Electrosynthesis of Graphene Oxide-Doped Polypyrrole Nanocomposite as a Nanointerface for Electrochemical Impedance Detection of Cell Adhesion and Proliferation Using Two Approaches. J. Nanomater. 2016, 2016, 8932908. [Google Scholar] [CrossRef]
- Moon, J.-M.; Hui Kim, Y.; Cho, Y. A Nanowire-Based Label-Free Immunosensor: Direct Incorporation of a PSA Antibody in Electropolymerized Polypyrrole. Biosens. Bioelectron. 2014, 57, 157–161. [Google Scholar] [CrossRef]
- Fahlgren, A.; Bratengeier, C.; Gelmi, A.; Semeins, C.M.; Klein-Nulend, J.; Jager, E.W.H.; Bakker, A.D. Biocompatibility of Polypyrrole with Human Primary Osteoblasts and the Effect of Dopants. PLoS ONE 2015, 10, e0134023. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, K.; Shen, S.; Yin, J. Preparation and Thermoelectric Properties of Multi-Walled Carbon Nanotubes/Polypyrrole Composites. Synth. Met. 2014, 195, 132–136. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.-Y. Polypyrrole Nanostructures and Their Thermoelectric Performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Baghdadi, N.; Zoromba, M.S.; Abdel-Aziz, M.H.; Al-Hossainy, A.F.; Bassyouni, M.; Salah, N. One-Dimensional Nanocomposites Based on Polypyrrole-Carbon Nanotubes and Their Thermoelectric Performance. Polymers 2021, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-Polymer-Based Supercapacitor Devices and Electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Yang, C.; Shen, J.; Wang, C.; Fei, H.; Bao, H.; Wang, G. All-Solid-State Asymmetric Supercapacitor Based on Reduced Graphene Oxide/Carbon Nanotube and Carbon Fiber Paper/Polypyrrole Electrodes. J. Mater. Chem. A 2014, 2, 1458–1464. [Google Scholar] [CrossRef]
- Chen, D.; Tang, L.; Li, J. Graphene-Based Materials in Electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef]
- Feng, H.; Cheng, R.; Zhao, X.; Duan, X.; Li, J. A Low-Temperature Method to Produce Highly Reduced Graphene Oxide. Nat. Commun. 2013, 4, 1539. [Google Scholar] [CrossRef]
- Sui, C.; Tan, R.; Liu, Z.; Li, X.; Xu, W. Smart Chemical Oxidative Polymerization Strategy To Construct Au@PPy Core–Shell Nanoparticles for Cancer Diagnosis and Imaging-Guided Photothermal Therapy. Bioconjug. Chem. 2023, 34, 257–268. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Xu, X.; Hua, L.; Lu, Z. In Situ Loading of Polypyrrole onto Aramid Nanofiber and Carbon Nanotube Aerogel Fibers as Physiology and Motion Sensors. ACS Nano 2022, 16, 8161–8171. [Google Scholar] [CrossRef]
- Bai, T.; Guo, Y.; Wang, D.; Liu, H.; Song, G.; Wang, Y.; Guo, Z.; Liu, C.; Shen, C. A Resilient and Lightweight Bacterial Cellulose-Derived C/RGO Aerogel-Based Electromagnetic Wave Absorber Integrated with Multiple Functions. J. Mater. Chem. A 2021, 9, 5566–5577. [Google Scholar] [CrossRef]
- Liu, J.; Tian, C.; Xiong, J.; Wang, L. Polypyrrole Blending Modification for PVDF Conductive Membrane Preparing and Fouling Mitigation. J. Colloid Interface Sci. 2017, 494, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Skotheim, T.A.; Elsenbaumer, R.L.; Reynolds, J.R. Handbook of Conducting Polymers; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-Based Conducting Polymers and Interactions with Biological Tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, B.; Bozkurt, S.; Cellat, K.; Arıkan, K.; Yılmaz, M.; Şavk, A.; Çalımlı, M.H.; Nas, M.S.; Atalar, M.N.; Alma, M.H.; et al. Palladium Supported on Polypyrrole/Reduced Graphene Oxidenanoparticles for Simultaneous Biosensing Application of Ascorbic Acid, Dopamine, Anduric Acid. Sci. Rep. 2020, 10, 2946. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, R.; Sun, Q.; Li, C. Isolation, Characterization, and Application of Salmonella Paratyphi Phage KM16 against Salmonella Paratyphi Biofilm. Biofouling 2021, 37, 276–288. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Yoshimura, M. Progress of Reduction of Graphene Oxide by Ascorbic Acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Alhajaim, W.; Fatima, A.; Yasir, S.; Kamal, T.; Abbas, Y.; Khan, S.; Khan, A.H.; Manan, S.; Ullah, M.W.; et al. Development of Low-Cost Bacterial Cellulose-Pomegranate Peel Extract-Based Antibacterial Composite for Potential Biomedical Applications. Int. J. Biol. Macromol. 2023, 231, 123269. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Niu, H.; Wang, F.; Liu, L.; Huang, Y. Freestanding Conductive Film Based on Polypyrrole/Bacterial Cellulose/Graphene Paper for Flexible Supercapacitor: Large Areal Mass Exhibits Excellent Areal Capacitance. Electrochim. Acta 2016, 222, 429–437. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Liu, L.; Zhang, Y.; Shi, Q.; Zhao, Q.; Cheng, Y.; Zhou, C.; Yang, S.; Song, X. Spongy P-Toluenesulfonic Acid-Doped Polypyrrole with Extraordinary Rate Performance as Durable Anodes of Sodium-Ion Batteries at Different Temperatures. Langmuir 2020, 36, 15075–15081. [Google Scholar] [CrossRef]
- Vonasek, E.; Lu, P.; Hsieh, Y.-L.; Nitin, N. Bacteriophages Immobilized on Electrospun Cellulose Microfibers by Non-Specific Adsorption, Protein–Ligand Binding, and Electrostatic Interactions. Cellulose 2017, 24, 4581–4589. [Google Scholar] [CrossRef]
- Choi, I.; Yoo, D.S.; Chang, Y.; Kim, S.Y.; Han, J. Polycaprolactone Film Functionalized with Bacteriophage T4 Promotes Antibacterial Activity of Food Packaging toward Escherichia coli. Food Chem. 2021, 346, 128883. [Google Scholar] [CrossRef]
- Guo, X.; Bai, N.; Tian, Y.; Gai, L. Free-Standing Reduced Graphene Oxide/Polypyrrole Films with Enhanced Electrochemical Performance for Flexible Supercapacitors. J. Power Sources 2018, 408, 51–57. [Google Scholar] [CrossRef]
- Dekanovsky, L.; Khezri, B.; Rottnerova, Z.; Novotny, F.; Plutnar, J.; Pumera, M. Chemically Programmable Microrobots Weaving a Web from Hormones. Nat. Mach. Intell. 2020, 2, 711–718. [Google Scholar] [CrossRef]
- Samanta, D.; Meiser, J.L.; Zare, R.N. Polypyrrole Nanoparticles for Tunable, PH-Sensitive and Sustained Drug Release. Nanoscale 2015, 7, 9497–9504. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization Model for Hydrogen Peroxide Initiated Synthesis of Polypyrrole Nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, R.; Li, E.; Li, X.; Liu, Y.; Yuan, G. Graphene/Carbon Nanotube/Bacterial Cellulose Assisted Supporting for Polypyrrole towards Flexible Supercapacitor Applications. J. Alloys Compd. 2019, 777, 524–530. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized Bacterial Cellulose Derivatives and Nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- Xiao, S.-P.; Huang, H.-X. In Situ Vitamin C Reduction of Graphene Oxide for Preparing Flexible TPU Nanocomposites with High Dielectric Permittivity and Low Dielectric Loss. Polym. Test. 2018, 66, 334–341. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, C.; Lu, G.; Ni, Y.; Lu, C.; Xu, Z. Synthesis of PPy/RGO-Based Hierarchical Material with Super-Paramagnetic Behavior and Understanding Its Robust Photo Current Driven by Visible Light. Synth. Met. 2018, 241, 17–25. [Google Scholar] [CrossRef]
- Pattanayak, P.; Papiya, F.; Kumar, V.; Pramanik, N.; Kundu, P.P. Deposition of Ni–NiO Nanoparticles on the Reduced Graphene Oxide Filled Polypyrrole: Evaluation as Cathode Catalyst in Microbial Fuel Cells. Sustain. Energy Fuels 2019, 3, 1808–1826. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, T.; Park, J.K. Chapter 30—Bacterial Cellulose: Trends in Synthesis, Characterization, and Applications. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2021; pp. 923–974. ISBN 978-0-12-820104-6. [Google Scholar]
- Xu, Z.; He, M.; Zhou, Y.; Nie, S.; Wang, Y.; Huo, Y.; Kang, Y.; Wang, R.; Xu, R.; Peng, H.; et al. Spider Web-like Carbonized Bacterial Cellulose/MoSe2 Nanocomposite with Enhanced Microwave Attenuation Performance and Tunable Absorption Bands. Nano Res. 2021, 14, 738–746. [Google Scholar] [CrossRef]
- Peng, H.; Liang, C. Electrochemical Determination of Hydrazine Based on Polydopamine-Reduced Graphene Oxide Nanocomposite. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 29–33. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Preparation and Characterization of Cellulose Nanocrystals from Bacterial Cellulose Produced in Sugar Beet Molasses and Cheese Whey Media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Structural and Physico-Mechanical Characterization of Bio-Cellulose Produced by a Cell-Free System. Carbohydr. Polym. 2016, 136, 908–916. [Google Scholar] [CrossRef]
- Hu, R.; Shao, D.; Wang, X. Graphene Oxide/Polypyrrole Composites for Highly Selective Enrichment of U(vi) from Aqueous Solutions. Polym. Chem. 2014, 5, 6207–6215. [Google Scholar] [CrossRef]
- Jaroniec, M.; Kaneko, K. Physicochemical Foundations for Characterization of Adsorbents by Using High-Resolution Comparative Plots. Langmuir 1997, 13, 6589–6596. [Google Scholar] [CrossRef]
- Xiang, C.; Jiang, D.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Sun, L.; Zheng, L. Ammonia Sensor Based on Polypyrrole–Graphene Nanocomposite Decorated with Titania Nanoparticles. Ceram. Int. 2015, 41, 6432–6438. [Google Scholar] [CrossRef]
- Tiwari, D.C.; Atri, P.; Sharma, R. Sensitive Detection of Ammonia by Reduced Graphene Oxide/Polypyrrole Nanocomposites. Synth. Met. 2015, 203, 228–234. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Sutaria, S.; Konarov, A.; Chen, P. Binding Mechanism and Electrochemical Properties of M13 Phage-Sulfur Composite. PLoS ONE 2013, 8, e82332. [Google Scholar] [CrossRef]
- Archer, M.J.; Liu, J.L. Bacteriophage T4 Nanoparticles as Materials in Sensor Applications: Variables That Influence Their Organization and Assembly on Surfaces. Sensors 2009, 9, 6298–6311. [Google Scholar] [CrossRef]
- Anany, H.; Chen, W.; Pelton, R.; Griffiths, M.W. Biocontrol of Listeria Monocytogenes and Escherichia coli O157:H7 in Meat by Using Phages Immobilized on Modified Cellulose Membranes. Appl. Environ. Microbiol. 2011, 77, 6379–6387. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Olsson, A.L.; Tufenkji, N. Going Viral: Designing Bioactive Surfaces with Bacteriophage. Colloids Surf. B Biointerfaces 2014, 124, 2–16. [Google Scholar] [CrossRef]
- Pittner, F.; Miron, T.; Pittner, G.; Wilchek, M. Enzyme Immobilization on Pyridine Containing Polymers BT—Enzyme Engineering: Volume 5; Weetall, H.H., Royer, G.P., Eds.; Springer US: Boston, MA, USA, 1980; pp. 447–449. ISBN 978-1-4684-3749-2. [Google Scholar]
- Singh, A.; Glass, N.; Tolba, M.; Brovko, L.; Griffiths, M.; Evoy, S. Immobilization of Bacteriophages on Gold Surfaces for the Specific Capture of Pathogens. Biosens. Bioelectron. 2009, 24, 3645–3651. [Google Scholar] [CrossRef]
- Wang, C.; Sauvageau, D.; Elias, A. Immobilization of Active Bacteriophages on Polyhydroxyalkanoate Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 1128–1138. [Google Scholar] [CrossRef]
- Bone, S.; Alum, A.; Markovski, J.; Hristovski, K.; Bar-Zeev, E.; Kaufman, Y.; Abbaszadegan, M.; Perreault, F. Physisorption and Chemisorption of T4 Bacteriophages on Amino Functionalized Silica Particles. J. Colloid Interface Sci. 2018, 532, 68–76. [Google Scholar] [CrossRef]
- Tawil, N.; Sacher, E.; Mandeville, R.; Meunier, M. Strategies for the Immobilization of Bacteriophages on Gold Surfaces Monitored by Surface Plasmon Resonance and Surface Morphology. J. Phys. Chem. C 2013, 117, 6686–6691. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, L.; Bai, Z.; Liang, G.; Liu, L.; Fang, D.; Xu, W. Conductive Polypyrrole–Bacterial Cellulose Nanocomposite Membranes as Flexible Supercapacitor Electrode. Org. Electron. 2013, 14, 3331–3338. [Google Scholar] [CrossRef]
- Li, Q.; Tang, R.; Zhou, H.; Hu, X.; Zhang, S. A High-Performance and Flexible Electrode Film Based on Bacterial Cellulose/Polypyrrole/Nitrogen-Doped Graphene for Supercapacitors. Carbohydr. Polym. 2023, 311, 120754. [Google Scholar] [CrossRef]
- Shi, R.; Zou, W.; Zhao, Z.; Wang, G.; Guo, M.; Ai, S.; Zhou, Q.; Zhao, F.; Yang, Z. Development of a Sensitive Phage-Mimotope and Horseradish Peroxidase Based Electrochemical Immunosensor for Detection of O,O-Dimethyl Organophosphorus Pesticides. Biosens. Bioelectron. 2022, 218, 114748. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, L.; Marcoux, P.R.; Roupioz, Y. Strategies for Surface Immobilization of Whole Bacteriophages: A Review. ACS Biomater. Sci. Eng. 2021, 7, 1987–2014. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Mohanta, G.C.; Deep, A. Bacteriophage Immobilized Graphene Electrodes for Impedimetric Sensing of Bacteria (Staphylococcus Arlettae). Anal. Biochem. 2016, 505, 18. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive Microorganisms in Bioelectrochemical Systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Bian, T.; Gardin, A.; Gemen, J.; Houben, L.; Perego, C.; Lee, B.; Elad, N.; Chu, Z.; Pavan, G.M.; Klajn, R. Electrostatic Co-Assembly of Nanoparticles with Oppositely Charged Small Molecules into Static and Dynamic Superstructures. Nat. Chem. 2021, 13, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Molnár, G.; Fendrych, M.; Friml, J. Plasma Membrane: Negative Attraction. Nat. Plants 2016, 2, 16102. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Yang, Q.; Ullah, M.W.; Wang, S. Principle and Development of Phage-Based Biosensors. In Biosensors for Environmental Monitoring; IntechOpen: London, UK, 2019. [Google Scholar]
- Yang, X.; Wisuthiphaet, N.; Young, G.M.; Nitin, N. Rapid Detection of Escherichia coli Using Bacteriophage-Induced Lysis and Image Analysis. PLoS ONE 2020, 15, e0233853. [Google Scholar] [CrossRef]
- Hussain, W.; Yang, X.; Ullah, M.; Wang, H.; Aziz, A.; Xu, F.; Asif, M.; Ullah, M.W.; Wang, S. Genetic Engineering of Bacteriophages: Key Concepts, Strategies, and Applications. Biotechnol. Adv. 2023, 64, 108116. [Google Scholar] [CrossRef]
- Hussain, W.; Ullah, M.W.; Farooq, U.; Aziz, A.; Wang, S. Bacteriophage-Based Advanced Bacterial Detection: Concept, Mechanisms, and Applications. Biosens. Bioelectron. 2021, 177, 112973. [Google Scholar] [CrossRef]
- Abdelhamied, N.; Abdelrahman, F.; El-Shibiny, A.; Hassan, R.Y.A. Bacteriophage-Based Nano-Biosensors for the Fast Impedimetric Determination of Pathogens in Food Samples. Sci. Rep. 2023, 13, 3498. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Huang, J.; Wu, Y.; Mao, X.; Tan, Y.; Liu, H.; Ma, D.; Li, X.; Wang, X. Development of a Phage-Based Electrochemical Biosensor for Detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry 2023, 150, 108345. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Shahrokhian, S.; Nurmohammadi, F. Nanoporous Gold as a Suitable Substrate for Preparation of a New Sensitive Electrochemical Aptasensor for Detection of Salmonella Typhimurium. Sens. Actuators B Chem. 2018, 255, 1536–1544. [Google Scholar] [CrossRef]
- Niyomdecha, S.; Limbut, W.; Numnuam, A.; Kanatharana, P.; Charlermroj, R.; Karoonuthaisiri, N.; Thavarungkul, P. Phage-Based Capacitive Biosensor for Salmonella Detection. Talanta 2018, 188, 658–664. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Y.; Huang, C.; Wang, J.; Li, H.; Wang, X. An Electrochemical Biosensor Based on Phage-Encoded Protein RBP 41 for Rapid and Sensitive Detection of Salmonella. Talanta 2024, 270, 125561. [Google Scholar] [CrossRef]
- Feng, K.; Li, T.; Ye, C.; Gao, X.; Yang, T.; Liang, X.; Yue, X.; Ding, S.; Dong, Q.; Yang, M.; et al. A Label-Free Electrochemical Immunosensor for Rapid Detection of Salmonella in Milk by Using CoFe-MOFs-Graphene Modified Electrode. Food Control 2021, 130, 108357. [Google Scholar] [CrossRef]
- Hyeon, S.H.; Lim, W.K.; Shin, H.J. Novel Surface Plasmon Resonance Biosensor That Uses Full-Length Det7 Phage Tail Protein for Rapid and Selective Detection of Salmonella Enterica Serovar Typhimurium. Biotechnol. Appl. Biochem. 2021, 68, 5–12. [Google Scholar] [CrossRef]
- He, Y.; Jia, F.; Sun, Y.; Fang, W.; Li, Y.; Chen, J.; Fu, Y. An Electrochemical Sensing Method Based on CRISPR/Cas12a System and Hairpin DNA Probe for Rapid and Sensitive Detection of Salmonella Typhimurium. Sens. Actuators B Chem. 2022, 369, 132301. [Google Scholar] [CrossRef]

| Substrate | Detection Method | Recognition Element | Linear Range (CFU·mL−1) | Detection Time (min) | Limit of Detection (CFU·mL−1) | Sample Type | Live/Dead Cell Discrimination | Ref. |
|---|---|---|---|---|---|---|---|---|
| NPG/Au/GCE | EIS | Aptamer | 6.5 × 102–6.5 × 108 | 40 | 1 | Egg | No | [95] |
| Pty-Au NPs | Capacitive | Phage | 2.0 × 102–1.0 × 107 | 40 | 200 | Chicken | No | [96] |
| GNP/GO | Electrochemical | Phage RBP 41 IS A | 3–3 × 106 | 30 | 2 | PBS, Milk | No | [97] |
| CoFe-MOFs-graphene | Electrochemical | Antibody | 2.4 × 102–2.4 × 108 | 90 | 1.2 × 102 | Milk | No | [98] |
| Det7/Au | SPR | Det7 phage tail protein | 0.5 × 104–5.0 × 107 | 20 | - | Apple juice | No | [99] |
| E-CRISPR | PCR | DNA | 6.7 × 101–6.7 × 105 | 360 | 55 | Poultry | No | [100] |
| BC/Ppy/RGO | Electrochemical | Phage | 5.5 × 100–5.5 × 107 | 30 | 1 | PBS | Yes | Current study |
| 6.2 × 100–6.2 × 106 | 5 | Milk | ||||||
| 3.3 × 100–3.3 × 106 | 3 | Chicken |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, W.; Wang, H.; Yang, X.; Ullah, M.W.; Hussain, J.; Ullah, N.; Ul-Islam, M.; Awad, M.F.; Wang, S. Ultrasensitive Electrochemical Detection of Salmonella typhimurium in Food Matrices Using Surface-Modified Bacterial Cellulose with Immobilized Phage Particles. Biosensors 2024, 14, 500. https://doi.org/10.3390/bios14100500
Hussain W, Wang H, Yang X, Ullah MW, Hussain J, Ullah N, Ul-Islam M, Awad MF, Wang S. Ultrasensitive Electrochemical Detection of Salmonella typhimurium in Food Matrices Using Surface-Modified Bacterial Cellulose with Immobilized Phage Particles. Biosensors. 2024; 14(10):500. https://doi.org/10.3390/bios14100500
Chicago/Turabian StyleHussain, Wajid, Huan Wang, Xiaohan Yang, Muhammad Wajid Ullah, Jawad Hussain, Najeeb Ullah, Mazhar Ul-Islam, Mohamed F. Awad, and Shenqi Wang. 2024. "Ultrasensitive Electrochemical Detection of Salmonella typhimurium in Food Matrices Using Surface-Modified Bacterial Cellulose with Immobilized Phage Particles" Biosensors 14, no. 10: 500. https://doi.org/10.3390/bios14100500
APA StyleHussain, W., Wang, H., Yang, X., Ullah, M. W., Hussain, J., Ullah, N., Ul-Islam, M., Awad, M. F., & Wang, S. (2024). Ultrasensitive Electrochemical Detection of Salmonella typhimurium in Food Matrices Using Surface-Modified Bacterial Cellulose with Immobilized Phage Particles. Biosensors, 14(10), 500. https://doi.org/10.3390/bios14100500







