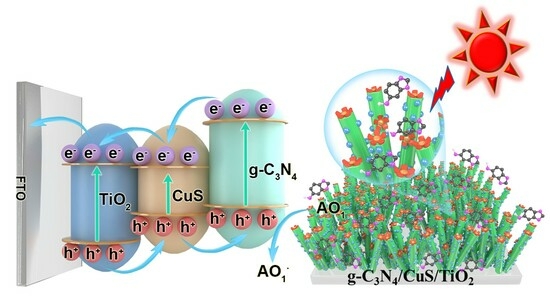
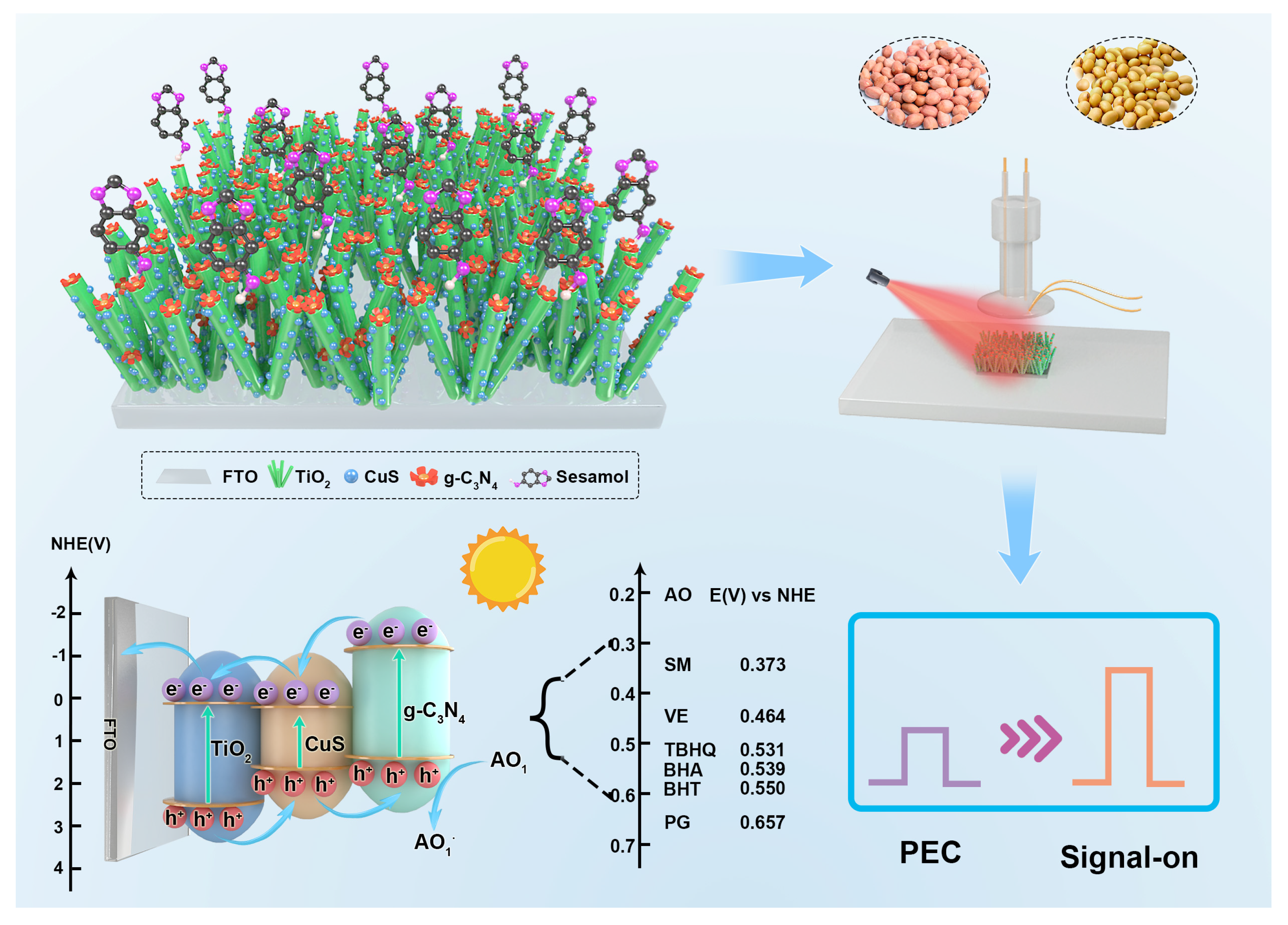
Ternary Heterojunction Graphitic Carbon Nitride/Cupric Sulfide/Titanium Dioxide Photoelectrochemical Sensor for Sesamol Quantification and Antioxidant Synergism
Abstract
:1. Introduction
2. Experimental Procedure
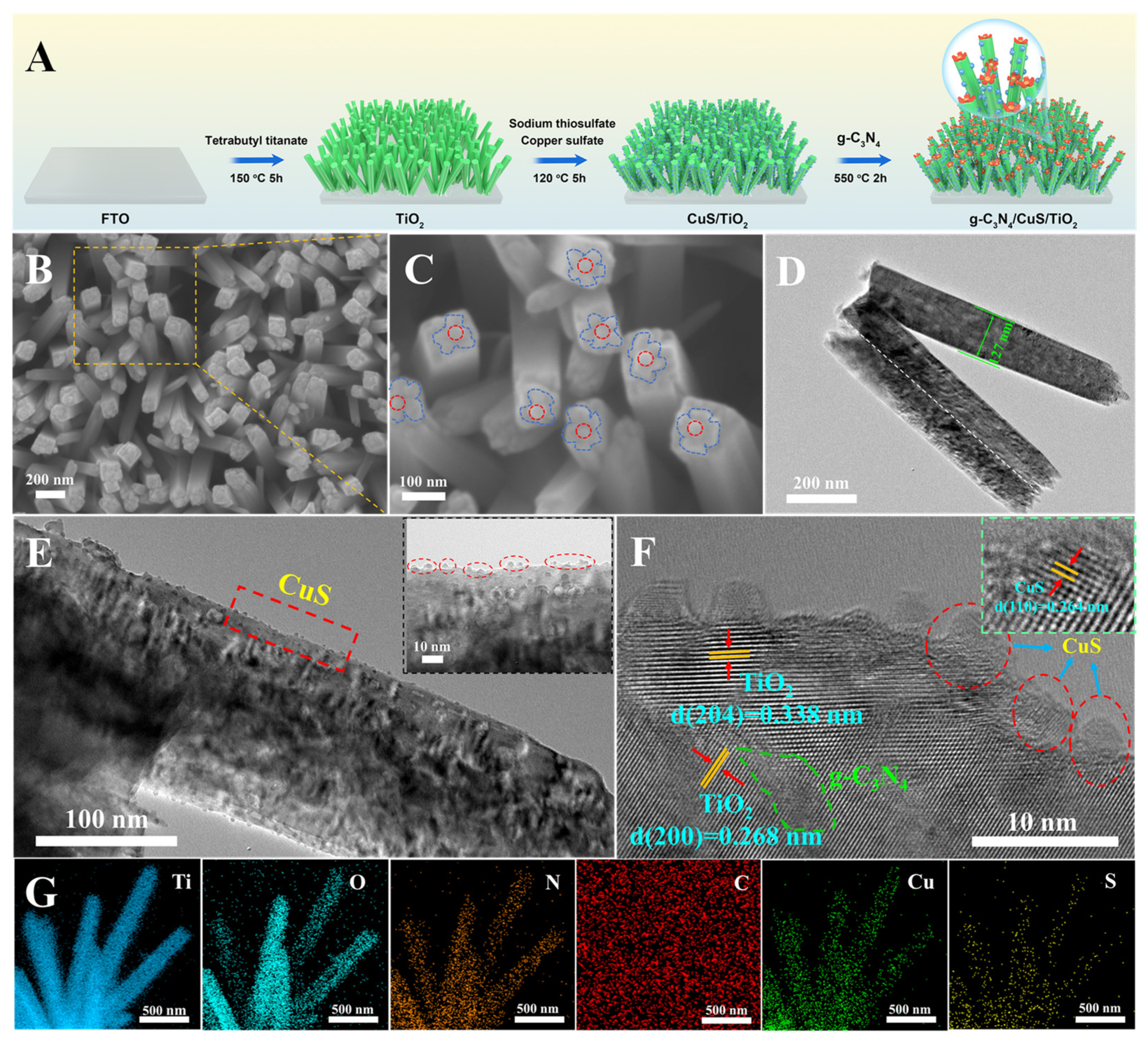
2.1. Synthesis of g-C3N4/CuS/TiO2 Composites
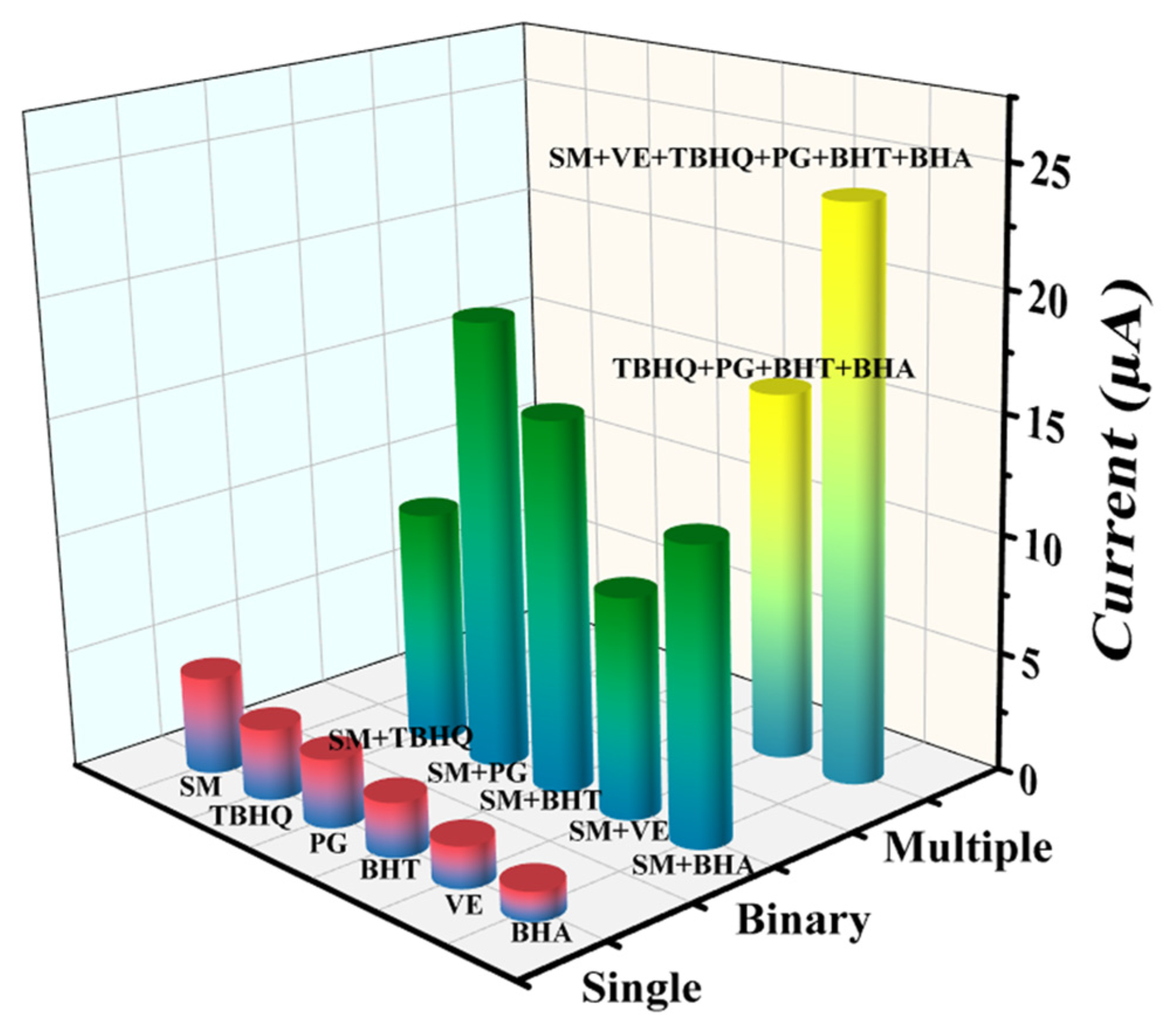
2.2. Antioxidant Capacity and Synergistic Effects
3. Results and Discussion
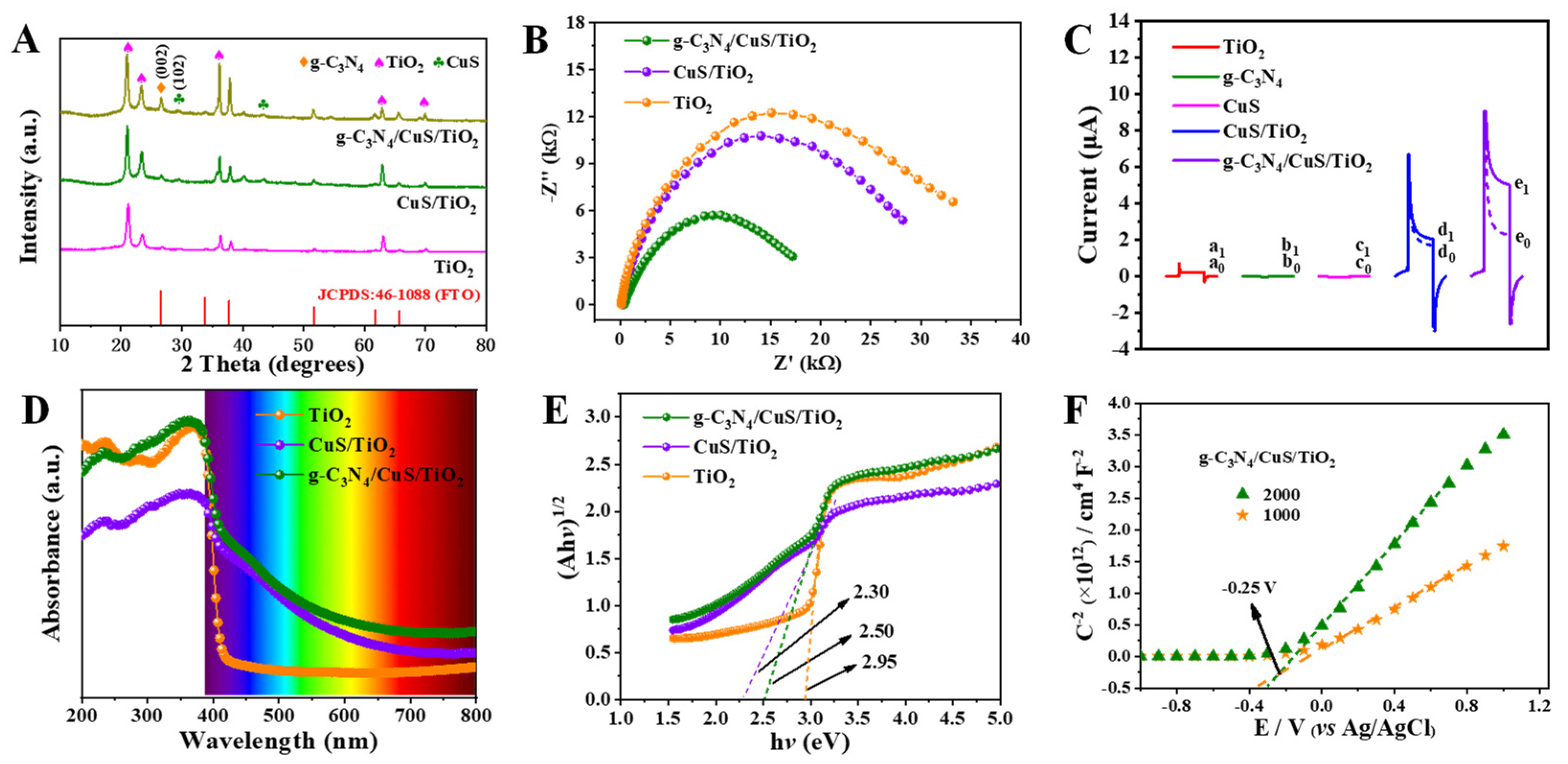
3.1. Characterization of g-C3N4/CuS/TiO2 Composites
3.2. Photoelectrochemical Properties of the g-C3N4/CuS/TiO2 PEC Sensing Platform
3.3. Optimization of Experimental Conditions
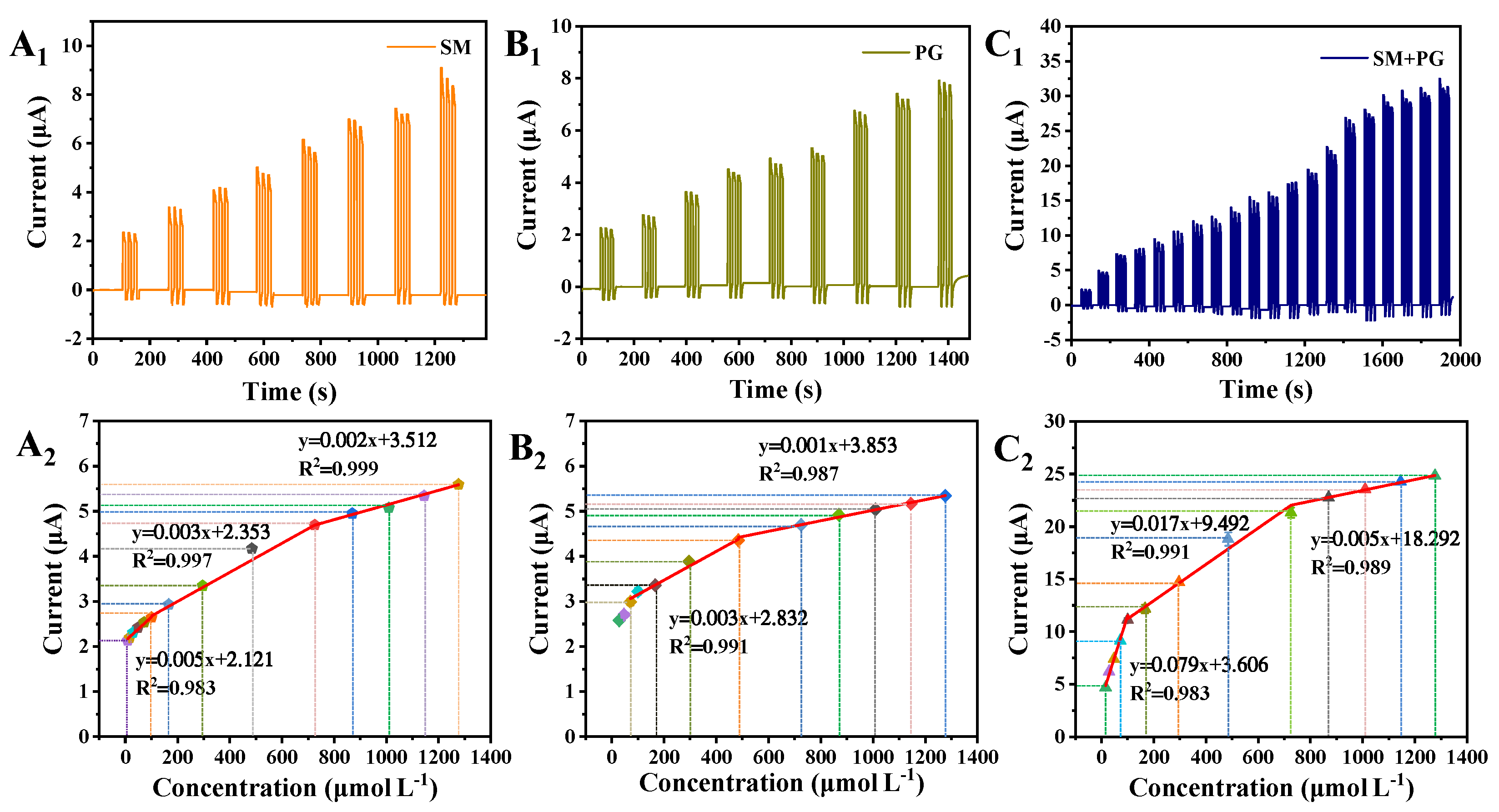
3.4. Antioxidant Assay and Detection Mechanism of the g-C3N4/CuS/TiO2 PEC Sensing Platform
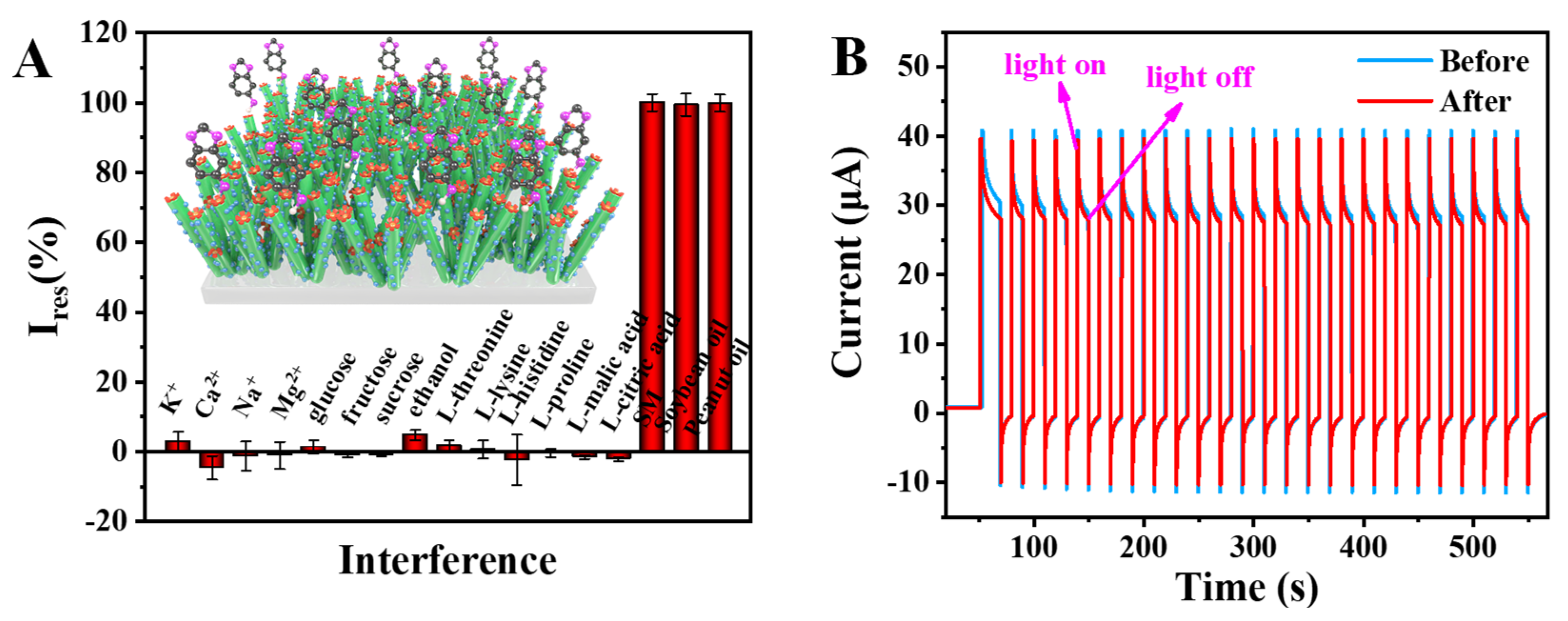
3.5. Detection Selectivity Stability and Reusability ofg-C3N4/CuS/TiO2 PEC Sensing Platform
3.6. SM Detection in Soybean and Peanut Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Motaghed Mazhabi, R.; Ge, L.; Jiang, H.; Wang, X.M. A facile photoelectrochemical sensor for high sensitive ROS and AA detection based on graphitic carbon nitride nanosheets. Biosens. Bioelectron. 2018, 107, 54–61. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.H.; Wang, H.; Liu, K.; Zhang, S.Y.; Chen, B.; Liu, H.; Tong, F.; Peng, F.; Tu, Y.F.; et al. Magnesium-Based micromotors as hydrogen generators for precise rheumatoid arthritis therapy. Nano Lett. 2021, 21, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Han, X.M.; Liu, L.; Gong, H.Y.; Luo, L.P.; Han, Y.R.; Fan, J.W.; Xu, C.F.; Yue, T.L.; Wang, J.L.; Zhang, W.T. Dextran-stabilized Fe-Mn bimetallic oxidase-like nanozyme for total antioxidant capacity assay of fruit and vegetable food. Food Chem. 2022, 371, 131115. [Google Scholar] [CrossRef]
- Abbas, S.; Sharif, M.K.; Sibt-e-Abbas, M.; Fikre Teferra, T.; Sultan, M.T.; Anwar, M.J. Nutritional and therapeutic potential of sesame seeds. J. Food Qual. 2022, 2022, 6163753. [Google Scholar] [CrossRef]
- Fabre, G.; Bayach, I.; Berka, K.; Paloncýová, M.; Starok, M.; Rossi, C.; Duroux, J.-L.; Otyepka, M.; Trouillas, P. Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes. Chem. Commun. 2015, 51, 7713–7716. [Google Scholar] [CrossRef]
- Yuan, T.; Chu, C.; Shi, R.; Cui, T.; Zhang, X.; Zhao, Y.; Shi, X.; Hui, Y.; Pan, J.; Qian, R.; et al. ApoE-Dependent protective effects of sesamol on high-fat diet-induced behavioral disorders: Regulation of the microbiome-gut–brain axis. J. Agri. Food Chem. 2019, 67, 6190–6201. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Mansour, Z.R. Sesamol, a major lignan in sesame seeds (sesamum indicum): Anti-cancer properties and mechanisms of action. Eur. J. Pharmacol. 2019, 855, 75–89. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, T.Z.; Xi, Y.J.; Li, L.; Mo, F.F.; Liu, X.B.; Liu, Z.G.; Gao, J.M.; Yuan, T. Sesamol attenuates amyloid peptide accumulation and cognitive deficits in APP/PS1 mice: The mediating role of the gut–brain axis. J. Agri. Food Chem. 2021, 69, 12717–12729. [Google Scholar] [CrossRef]
- Han, L.P.; Li, J.H.; Wang, S.J.; Cheng, W.W.; Ma, L.K.; Liu, G.Q.; Han, D.X.; Niu, L. Sesamol can inhibit the formation of glycidyl ester in deep frying palm oil. J. Food Process. Pres. 2022, 46, e16236. [Google Scholar] [CrossRef]
- Ohno, K.-i.; Sato, K.; Kumano, M.; Watanabe, K.; Fujimura, T. Electrochemical detection of sesamol dimer and its application to measurement of radicals. Anal. Sci. 2021, 37, 633–635. [Google Scholar] [CrossRef]
- Huang, J.; Sun, Q.; Song, G.; Qi, S.; Chen, J.; Zhang, P.; Geng, T.; Lin, Q.; Duan, Y. Antioxidant and anti-isomerization effects of sesamol and resveratrol on high oleic acid peanut oil. LWT 2020, 123, 109077. [Google Scholar] [CrossRef]
- Sallam, K.I.; Abd-Elghany, S.M.; Imre, K.; Morar, A.; Herman, V.; Hussein, M.A.; Mahros, M.A. Ensuring safety and improving keeping quality of meatballs by addition of sesame oil and sesamol as natural antimicrobial and antioxidant agents. Food Microbiol. 2021, 99, 103834. [Google Scholar] [CrossRef]
- El-Bindary, M.A.; Shahat, A.; El-Deen, I.M.; El-Afify, M.A.M.; Hassan, N. Synthesis and application of a novel self-smart sensor based on a modified amino-functionalized Zr-metal–organic framework for rapid and selective detection of some toxic metals in wastewater. Appl. Organomet. Chem. 2023, 37, e7029. [Google Scholar] [CrossRef]
- Huang, L.K.; Liang, Z.S.; Zhang, F.; Luo, H.; Liang, R.L.; Han, F.J.; Wu, Z.F.; Han, D.X.; Shen, J.; Niu, L. Upconversion NaYF4:Yb/Er–TiO2–Ti3C2 heterostructure-based near-infrared light-driven photoelectrochemical biosensor for highly sensitive and selective d-serine detection. Anal. Chem. 2022, 94, 16246–16253. [Google Scholar] [CrossRef] [PubMed]
- Zayats, M.; Kharitonov, A.B.; Pogorelova, S.P.; Lioubashevski, O.; Katz, E.; Willner, I. Probing photoelectrochemical processes in Au−CdS nanoparticle arrays by surface plasmon resonance: application for the detection of acetylcholine esterase inhibitors. J. Am. Chem. Soc. 2003, 125, 16006–16014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Yang, H.K.; Du, C.C.; Liu, S.Y.; Zhang, X.H.; Chen, J.H. Bifunctional magnetic Fe3O4@Cu2O@TiO2 nanosphere-mediated dual-mode assay of PTP1B activity based on photocurrent polarity switching and nanozyme-engineered biocatalytic precipitation strategies. Anal. Chem. 2022, 94, 13342–13349. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Tang, D.P. Recent advances in photoelectrochemical sensing: From engineered photoactive materials to sensing devices and detection modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Dai, W.X.; Zhang, L.; Zhao, W.W.; Yu, X.D.; Xu, J.J.; Chen, H.Y. Hybrid PbS quantum dot/nanoporous NiO film nanostructure: Preparation, characterization, and application for a self-powered cathodic photoelectrochemical biosensor. Anal. Chem. 2017, 89, 8070–8078. [Google Scholar] [CrossRef]
- Xiao, F.X.; Zeng, Z.; Liu, B. Bridging the gap: Electron relay and plasmonic sensitization of metal nanocrystals for metal clusters. J. Am. Chem. Soc. 2015, 137, 10735–10744. [Google Scholar] [CrossRef]
- Zhao, W.W.; Yu, X.D.; Xu, J.J.; Chen, H.Y. Recent advances in the use of quantum dots for photoelectrochemical bioanalysis. Nanoscale 2016, 8, 17407–17414. [Google Scholar] [CrossRef]
- Chen, C.; Cai, W.M.; Long, M.C.; Zhou, B.X.; Wu, Y.H.; Wu, D.Y.; Feng, Y.J. Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction. ACS Nano 2010, 4, 6425–6432. [Google Scholar] [CrossRef]
- Qi, H.; Hou, Y.; Wang, W.J.; Deng, W.; Tang, L.; Zhang, C.Y. Single-crystalline nanoflakes assembled CuS microspheres with improved sodium ion storage. J. Alloys Compd. 2023, 942, 168884. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, Y.Y.; Zhang, W.S.; Zhang, X.J.; Han, D.H.; Niu, L.; Ivaska, A. Nanoengineering construction of Cu2O nanowire arrays encapsulated with g-C3N4 as 3D spatial reticulation all-solid-state direct Z-scheme photocatalysts for photocatalytic reduction of carbon dioxide. ACS Catal. 2020, 10, 6367–6376. [Google Scholar] [CrossRef]
- Dekkouche, S.; Morales-Torres, S.; Ribeiro, A.R.; Faria, J.L.; Fontàs, C.; Kebiche-Senhadji, O.; Silva, A.M.T. In situ growth and crystallization of TiO2 on polymeric membranes for the photocatalytic degradation of diclofenac and 17α-ethinylestradiol. Chem. Eng. J. 2022, 427, 131476. [Google Scholar] [CrossRef]
- Lei, G.C.; Zhao, W.T.; Shen, L.J.; Liang, S.J.; Au, C.; Jiang, L.L. Isolated iron sites embedded in graphitic carbon nitride (g-C3N4) for efficient oxidative desulfurization. Appl. Catal. B-Environ. 2020, 267, 118663. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Guo, S.E.; Tian, G.H.; Jiang, B.J.; Ren, Z.Y.; Tian, C.G.; Li, W.; Fu, H.G. Synergetic enhancement of surface reactions and charge separation over holey C3N4/TiO2 2D heterojunctions. Sci. Bull. 2021, 66, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; He, Y.N.; Zhang, H.; Cao, K.Z.; Wang, S.D.; Jiang, Y.; Jing, Q.S.; Jiao, L.F. Lowering the voltage-hysteresis of CuS anode for Li-ion batteries via constructing heterostructure. Chem. Eng. J. 2021, 425, 130548. [Google Scholar] [CrossRef]
- Xiao, L.M.; Liu, T.F.; Zhang, M.; Li, Q.Y.; Yang, J.J. Interfacial construction of Zero-dimensional/one-dimensional g-C3N4 nanoparticles/TiO2 nanotube arrays with Z-scheme heterostructure for improved photoelectrochemical water splitting. ACS Sustain. Chem. Eng. 2019, 7, 2483–2491. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Qian, Y.; Xiao, Y.; Du, S.J.; Qiu, X.Q. Synergistic antioxidant performance of lignin and quercetin mixtures. ACS Sustain. Chem. Eng. 2017, 5, 8424–8428. [Google Scholar] [CrossRef]
- Iglesias, J.; Pazos, M.; Andersen, M.L.; Skibsted, L.H.; Medina, I. Caffeic acid as antioxidant in fish muscle: Mechanism of synergism with endogenous ascorbic acid and α-Tocopherol. J. Agri. Food Chem. 2009, 57, 675–681. [Google Scholar] [CrossRef]
- Han, F.J.; Song, Z.Q.; Xu, J.N.; Dai, M.J.; Luo, S.L.; Han, D.X.; Niu, L.; Wang, Z.X. Oxidized titanium carbide MXene-enabled photoelectrochemical sensor for quantifying synergistic interaction of ascorbic acid based antioxidants system. Biosens. Bioelectron. 2021, 177, 112978. [Google Scholar] [CrossRef] [PubMed]
- Zanfini, A.; Corbini, G.; La Rosa, C.; Dreassi, E. Antioxidant activity of tomato lipophilic extracts and interactions between carotenoids and α-tocopherol in synthetic mixtures. LWT-Food Sci. Technol. 2010, 43, 67–72. [Google Scholar] [CrossRef]
- Palheta, I.C.; Borges, R.S. Sesamol is a related antioxidant to the vitamin E. Chem. Data Collect. 2017, 11–12, 77–83. [Google Scholar] [CrossRef]
- Sahu, S.C. Food additives: A special issue of the journal Food and Chemical Toxicology. Food Chem. Toxicol. 2017, 107, 529. [Google Scholar] [CrossRef]
- El-Bindary, M.A.; El-Desouky, M.G.; El-Bindary, A.A. Metal–organic frameworks encapsulated with an anticancer compound as drug delivery system: Synthesis, characterization, antioxidant, anticancer, antibacterial, and molecular docking investigation. Appl. Organomet. Chem. 2022, 36, e6660. [Google Scholar] [CrossRef]
- Nawade, B.; Mishra, G.P.; Radhakrishnan, T.; Dodia, S.M.; Ahmad, S.; Kumar, A.; Kumar, A.; Kundu, R. High oleic peanut breeding: Achievements, perspectives, and prospects. Trends Food Sci. Tech. 2018, 78, 107–119. [Google Scholar] [CrossRef]
- Morawska, K.; Festinger, N.; Chwatko, G.; Głowacki, R.; Ciesielski, W.; Smarzewska, S. Rapid electroanalytical procedure for sesamol determination in real samples. Food Chem. 2020, 309, 125789. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.D.; Qin, Y.Q.; Yu, J.J. A simple and green ultrasonic-assisted liquid–liquid microextraction technique based on deep eutectic solvents for the HPLC analysis of sesamol in sesame oils. Anal. Method. 2017, 9, 4184. [Google Scholar] [CrossRef]
- Liu, H.L.; Wu, D.; Liu, Y.L.; Zhang, H.J.; Ma, T.Z.; Aidaerhan, A.; Wang, J.; Sun, B.G. Application of an optosensing chip based on molecularly imprinted polymer coated quantum dots for the highly selective and sensitive determination of sesamol in sesame oils. J. Agric. Food Chem. 2015, 63, 2545–2549. [Google Scholar] [CrossRef]
| Sample | SM Added (μM) | SM Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Soybean oil | 4 | 3.93 ± 0.01 | 0.31 | 98.28 |
| 20 | 20.40 ± 0.20 | 0.98 | 102.00 | |
| Peanut oil | 4 | 3.97 ± 0.09 | 2.30 | 99.16 |
| 20 | 20.40 ± 0.53 | 2.59 | 102.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Yang, J.; Liang, Z.; Liang, R.; Luo, H.; Sun, Z.; Han, D.; Niu, L. Ternary Heterojunction Graphitic Carbon Nitride/Cupric Sulfide/Titanium Dioxide Photoelectrochemical Sensor for Sesamol Quantification and Antioxidant Synergism. Biosensors 2023, 13, 859. https://doi.org/10.3390/bios13090859
Huang L, Yang J, Liang Z, Liang R, Luo H, Sun Z, Han D, Niu L. Ternary Heterojunction Graphitic Carbon Nitride/Cupric Sulfide/Titanium Dioxide Photoelectrochemical Sensor for Sesamol Quantification and Antioxidant Synergism. Biosensors. 2023; 13(9):859. https://doi.org/10.3390/bios13090859
Chicago/Turabian StyleHuang, Likun, Jingshi Yang, Zhishan Liang, Ruilian Liang, Hui Luo, Zhonghui Sun, Dongxue Han, and Li Niu. 2023. "Ternary Heterojunction Graphitic Carbon Nitride/Cupric Sulfide/Titanium Dioxide Photoelectrochemical Sensor for Sesamol Quantification and Antioxidant Synergism" Biosensors 13, no. 9: 859. https://doi.org/10.3390/bios13090859
APA StyleHuang, L., Yang, J., Liang, Z., Liang, R., Luo, H., Sun, Z., Han, D., & Niu, L. (2023). Ternary Heterojunction Graphitic Carbon Nitride/Cupric Sulfide/Titanium Dioxide Photoelectrochemical Sensor for Sesamol Quantification and Antioxidant Synergism. Biosensors, 13(9), 859. https://doi.org/10.3390/bios13090859