Proof-of-Concept: Smartphone- and Cloud-Based Artificial Intelligence Quantitative Analysis System (SCAISY) for SARS-CoV-2-Specific IgG Antibody Lateral Flow Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. LFA Kit and Human Blood Sampling
2.2. Display of Results
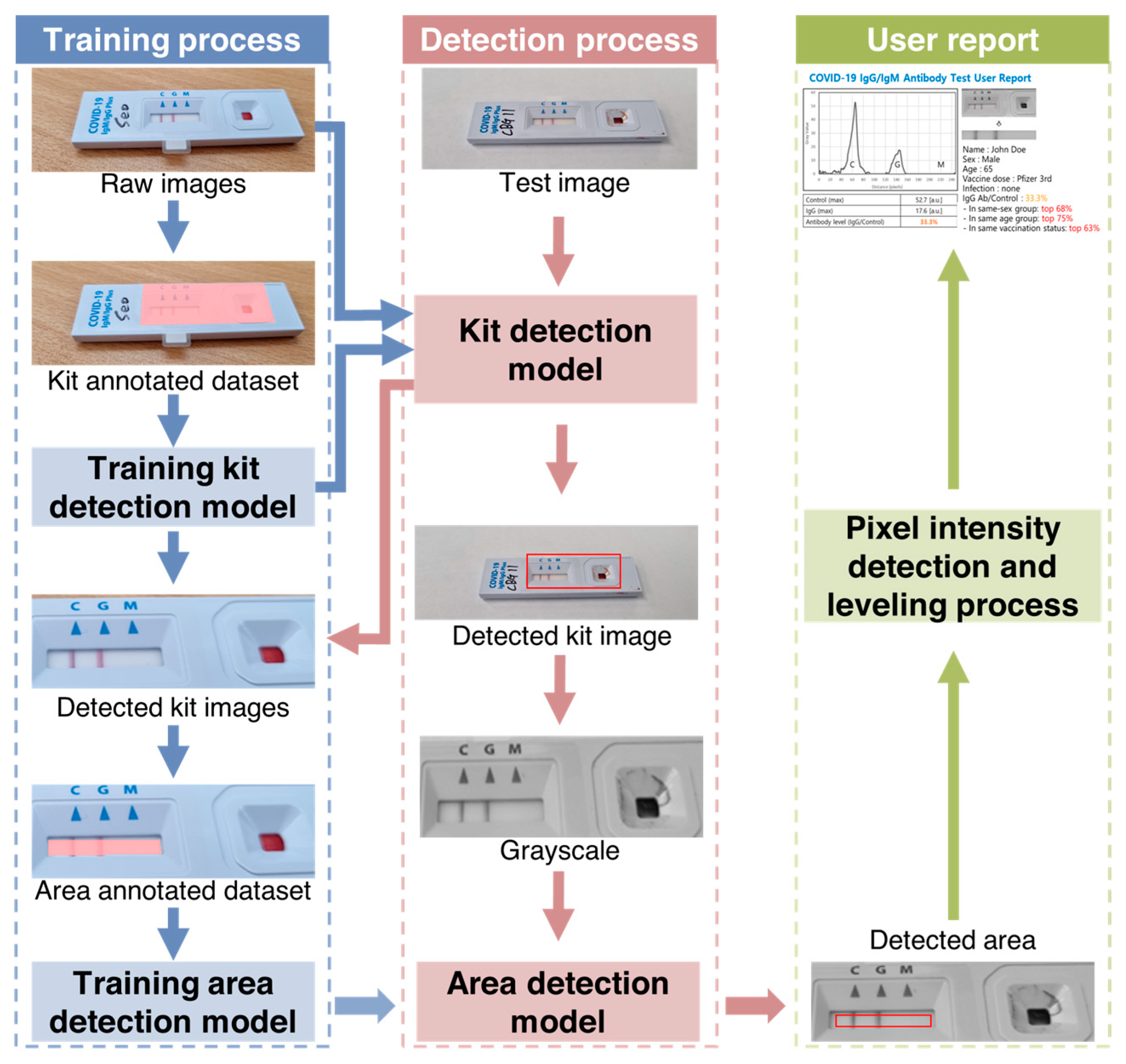
2.3. Data Acquisition Using a Smartphone Camera and Image Analysis
2.4. Feature Extraction
2.5. Test to Control Line Signal Intensity (T/C) Quantification Using AI
2.6. Comparative Analysis with ELISA
3. Results
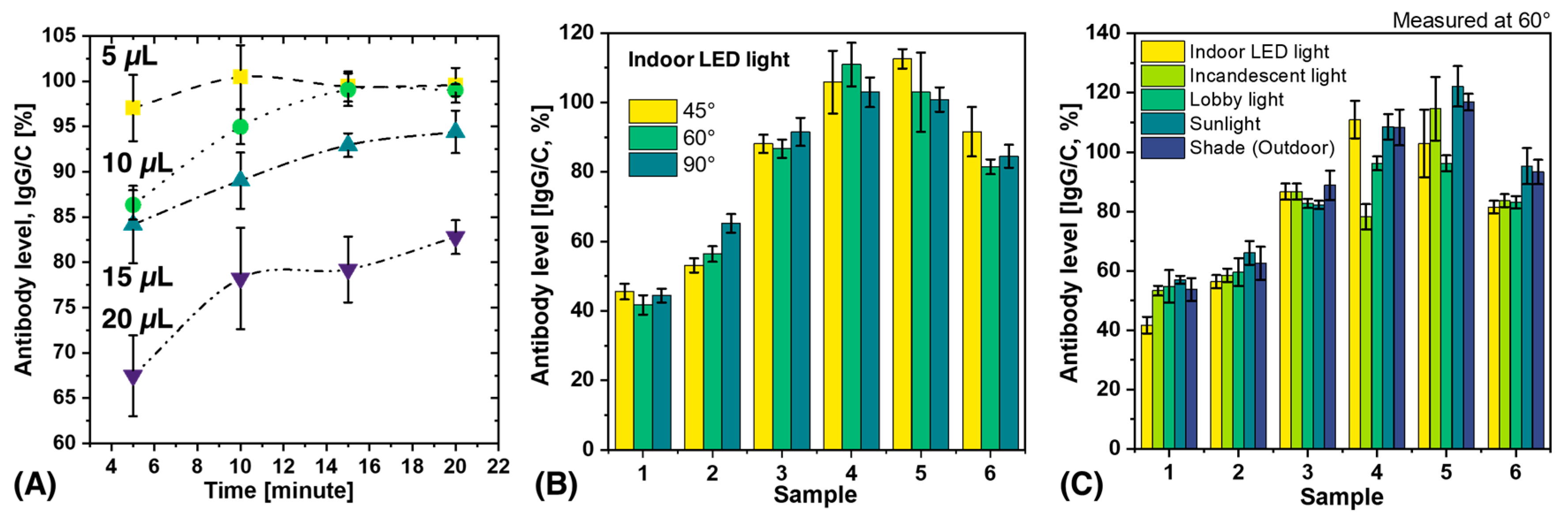
3.1. Effect of Blood Volume and Measurement Time on Antibody Level
3.2. Analysis of Variability Caused by Different Lighting Conditions and Shooting Angles
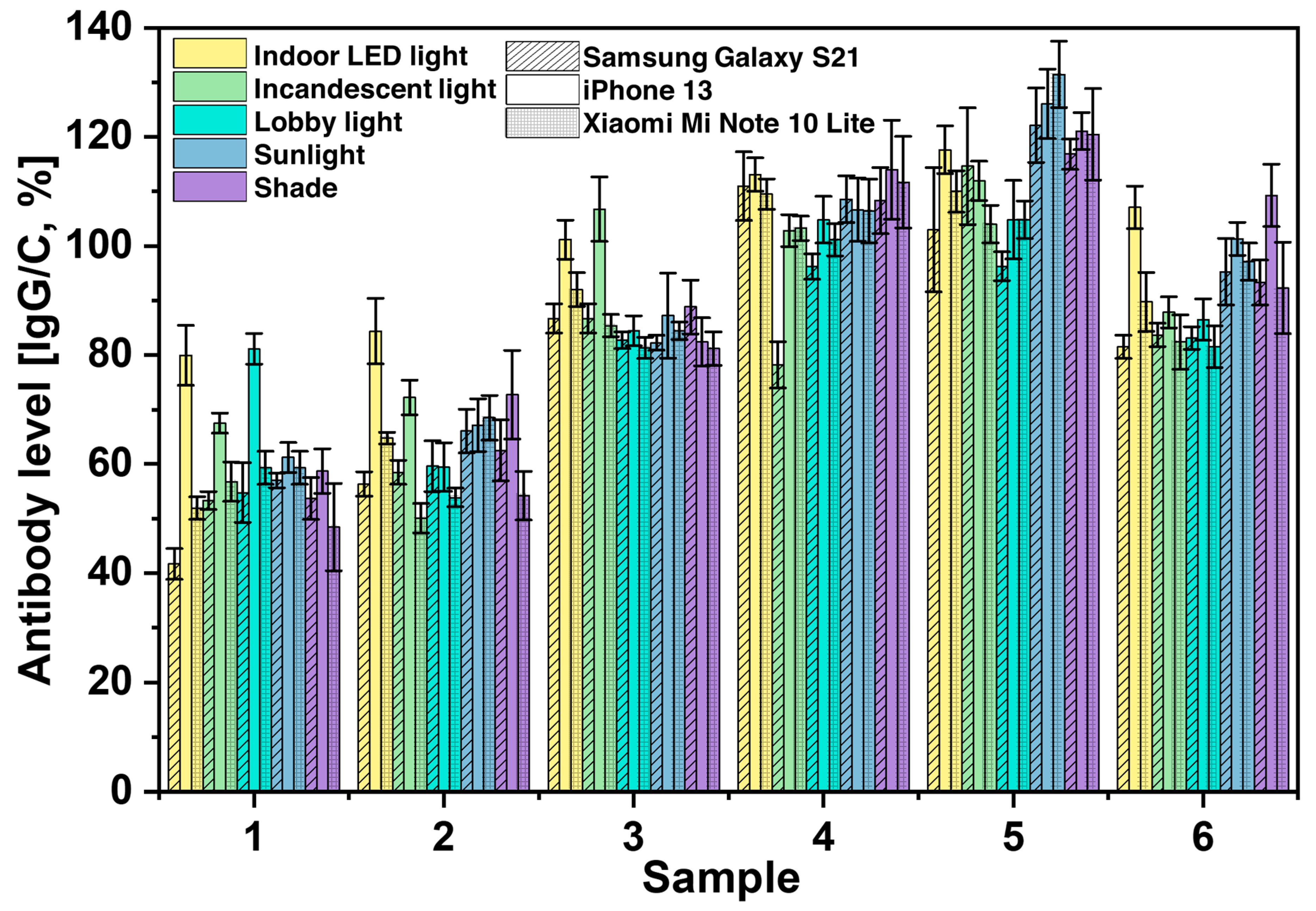
3.3. Analysis of Variability Caused by Smartphone Cameras
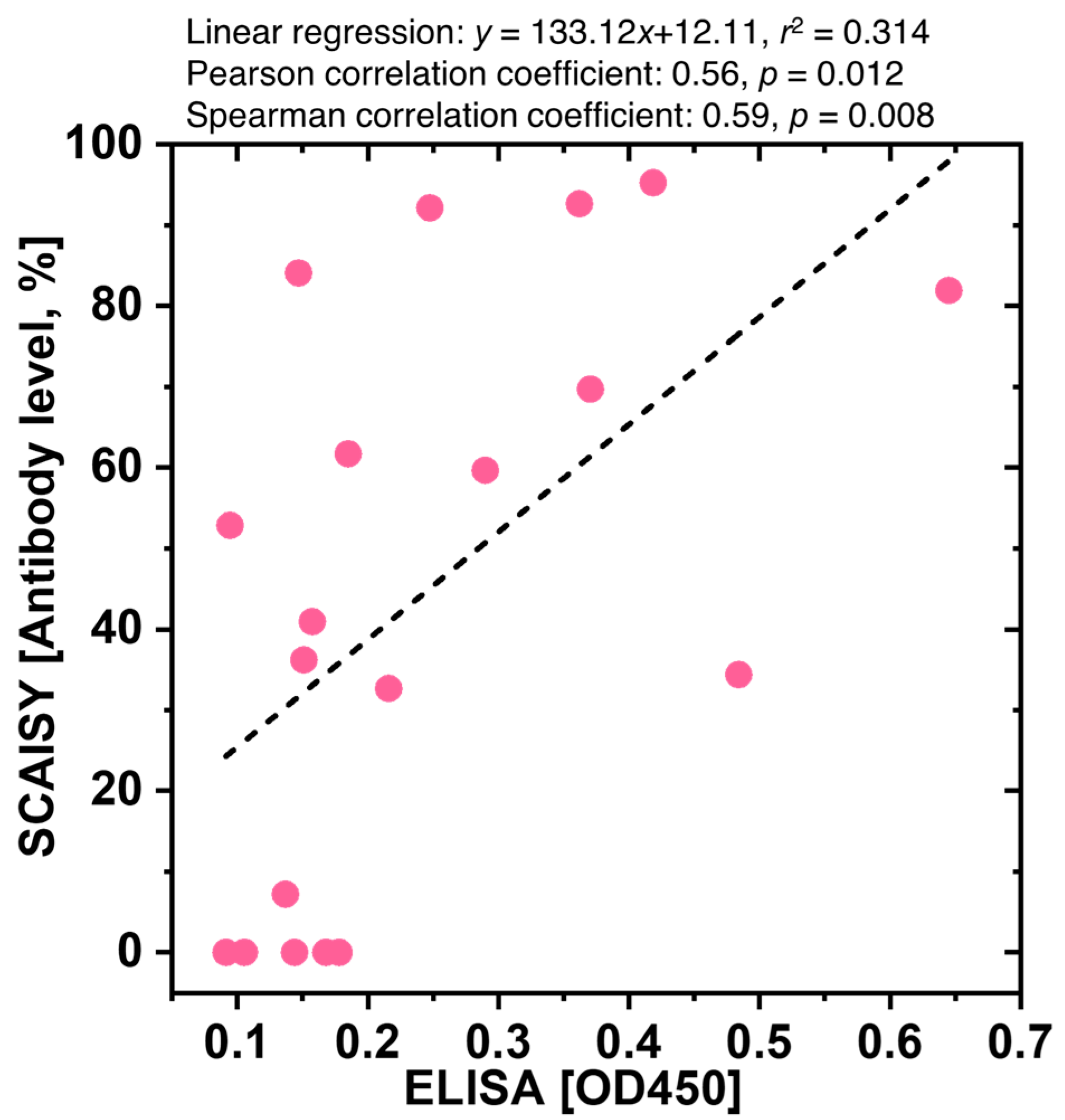
3.4. Comparative Analysis of SCAISY and ELISA for Quantification of SARS-CoV-2 Antibody Levels
3.5. Capabilities of SCAISY
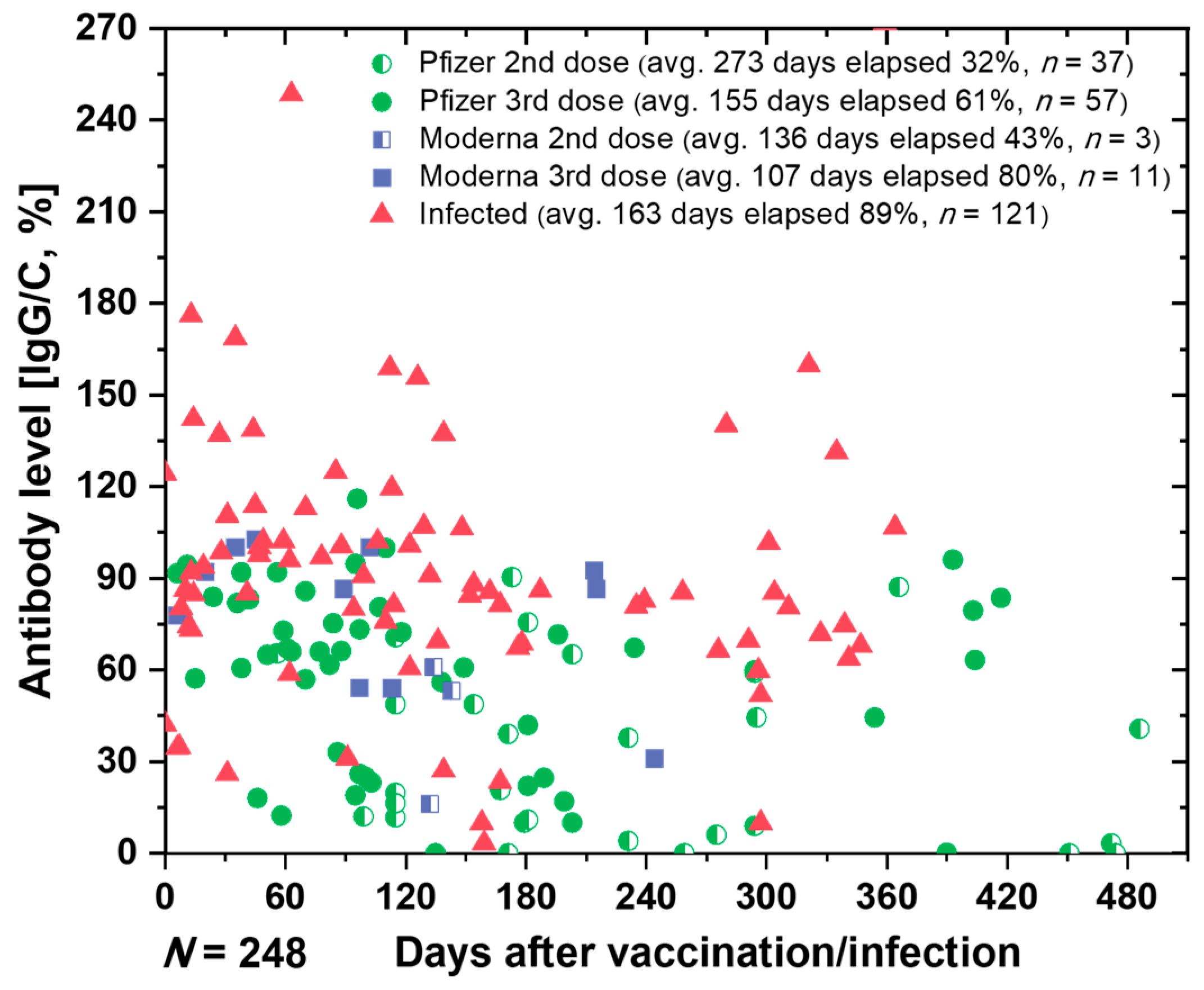
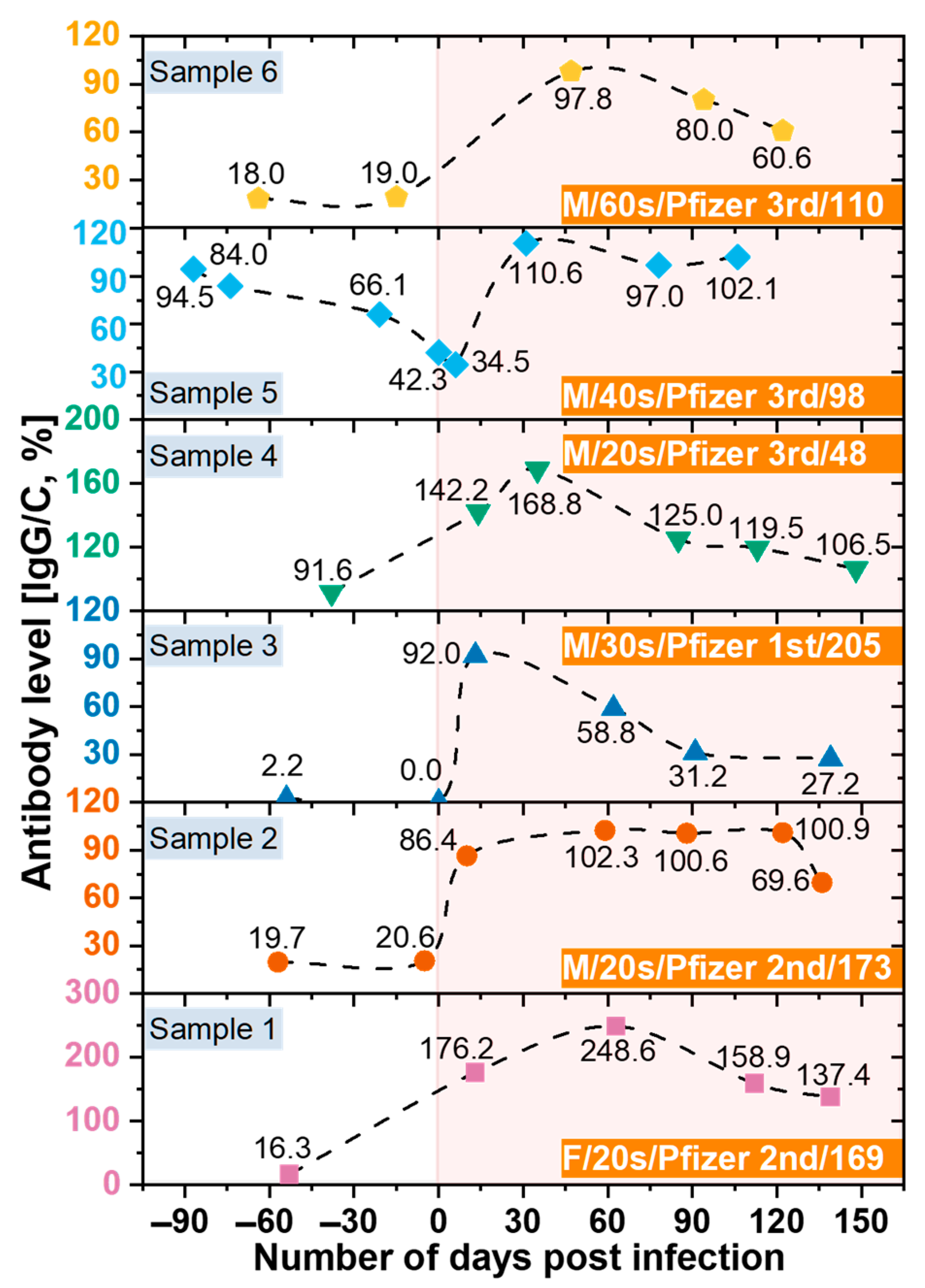
3.6. Monitoring the Antibodies against SARS-CoV-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 April 2023).
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Yau, H.S.; Yu, M.Y.; Tsang, H.F.; Chan, L.W.C.; Cho, W.C.S.; Yu, A.C.S.; Yim, A.K.Y.; Li, M.J.W.; Wong, Y.K.E.; et al. The diagnostic methods in the COVID-19 pandemic, today and in the future. Expert Rev. Mol. Diagn. 2020, 20, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef]
- Cassaniti, I.; Novazzi, F.; Giardina, F.; Salinaro, F.; Sachs, M.; Perlini, S.; Bruno, R.; Mojoli, F.; Baldanti, F.; Members of the San Matteo Pavia COVID-19 Task Force. Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020, 92, 1724–1727. [Google Scholar] [CrossRef]
- Kierkegaard, P.; McLister, A.; Buckle, P. Rapid point-of-care testing for COVID-19: Quality of supportive information for lateral flow serology assays. BMJ Open 2021, 11, e047163. [Google Scholar] [CrossRef]
- Rasmi, Y.; Li, X.; Khan, J.; Ozer, T.; Choi, J.R. Emerging point-of-care biosensors for rapid diagnosis of COVID-19: Current progress, challenges, and future prospects. Anal. Bioanal. Chem. 2021, 413, 4137–4159. [Google Scholar] [CrossRef]
- Uwamino, Y.; Wakui, M.; Aoki, W.; Kurafuji, T.; Yanagita, E.; Morita, M.; Nagata, M.; Inose, R.; Noguchi, M.; Yokota, H.; et al. Evaluation of the usability of various rapid antibody tests in the diagnostic application for COVID-19. Ann. Clin. Biochem. 2021, 58, 174–180. [Google Scholar] [CrossRef]
- Xu, J.; Kerr, L.; Jiang, Y.; Suo, W.; Zhang, L.; Lao, T.; Chen, Y.; Zhang, Y. Rapid antigen diagnostics as frontline testing in the COVID-19 pandemic. Small Sci. 2022, 2, 2200009. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Tsagkaris, A.S.; Dillon, M.J.; Hajslova, J.; Elliott, C.T. Smartphone-Based Optical Assays in the Food Safety Field. Trends Analyt. Chem. 2020, 129, 115934. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Cao, C.; You, M.; Han, S.; Liu, Z.; Xiao, Y.; He, W.; Liu, C.; Peng, P.; Xue, Z.; et al. Artificial Intelligence-Assisted Colorimetric Lateral Flow Immunoassay for Sensitive and Quantitative Detection of COVID-19 Neutralizing Antibody. Biosens. Bioelectron. 2022, 213, 114449. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Vaccine Equity. Available online: https://www.who.int/campaigns/vaccine-equity (accessed on 12 April 2023).
- COVID Vaccines: Widening Inequality and Millions Vulnerable. Available online: https://news.un.org/en/story/2021/09/1100192 (accessed on 12 April 2023).
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—22 December 2021. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---22-december-2021 (accessed on 12 April 2023).
- Alhamid, G.; Tombuloglu, H.; Rabaan, A.A.; Al-Suhaimi, E. SARS-CoV-2 Detection Methods: A Comprehensive Review. Saudi J. Biol. Sci. 2022, 29, 103465. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Alipour, M.; Chodari, L.; Maleki Dizaj, S.; Ardalan, M.; Samiei, M.; Sharifi, S.; Zununi Vahed, S.; Huseynova, I.; Khalilov, R.; et al. A Comprehensive Review of Detection Methods for SARS-CoV-2. Microorganisms 2021, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Safiabadi Tali, S.H.; LeBlanc, J.J.; Sadiq, Z.; Oyewunmi, O.D.; Camargo, C.; Nikpour, B.; Armanfard, N.; Sagan, S.M.; Jahanshahi-Anbuhi, S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin. Microbiol. Rev. 2021, 34, e00228-20. [Google Scholar] [CrossRef]
- Pu, R.; Liu, S.; Ren, X.; Shi, D.; Ba, Y.; Huo, Y.; Zhang, W.; Ma, L.; Liu, Y.; Yang, Y.; et al. The Screening Value of RT-LAMP and RT-PCR in the Diagnosis of COVID-19: Systematic Review and Meta-Analysis. J. Virol. Methods 2022, 300, 114392. [Google Scholar] [CrossRef]
- Nawattanapaiboon, K.; Pasomsub, E.; Prombun, P.; Wongbunmak, A.; Jenjitwanich, A.; Mahasupachai, P.; Vetcho, P.; Chayrach, C.; Manatjaroenlap, N.; Samphaongern, C.; et al. Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) as a Visual Diagnostic Platform for the Detection of the Emerging Coronavirus SARS-CoV-2. Analyst 2021, 146, 471–477. [Google Scholar] [CrossRef]
- Inaba, M.; Higashimoto, Y.; Toyama, Y.; Horiguchi, T.; Hibino, M.; Iwata, M.; Imaizumi, K.; Doi, Y. Diagnostic Accuracy of LAMP versus PCR over the Course of SARS-CoV-2 Infection. Int. J. Infect. Dis. 2021, 107, 195–200. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, Point-of-Care Antigen and Molecular-Based Tests for Diagnosis of SARS-CoV-2 Infection. Cochrane Database Syst. Rev. 2020, 8, CD013705. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Ferrante di Ruffano, L.; et al. Antibody Tests for Identification of Current and Past Infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, A.V.; De Summa, S.; Loconsole, D.; Procacci, V.; Sallustio, A.; Centrone, F.; Silvestris, N.; Cafagna, V.; De Palma, G.; Tufaro, A.; et al. Rapid Serological Assays and SARS-CoV-2 Real-Time Polymerase Chain Reaction Assays for the Detection of SARS-CoV-2: Comparative Study. J. Med. Internet Res. 2020, 22, e19152. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Ding, L.; Huang, X.; Xiong, Y. Point-of-care COVID-19 diagnostics powered by lateral flow assay. Trends Analyt. Chem. 2021, 145, 116452. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Drain, P.K. Rapid Diagnostic Testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272. [Google Scholar] [CrossRef]
- Labmedica Expo. Available online: https://mobile.labmedica.com/expo/product/5344/fluorescent-reader-model-hrdr-300 (accessed on 12 August 2022).
- Microplate Readers: Multi-Mode and Absorbance Readers. Available online: https://www.biotek.com/products/detection/ (accessed on 4 August 2022).
- Microplate Readers. Available online: https://lifesciences.tecan.com/microplate-readers (accessed on 12 April 2023).
- Varioskan LUX Multimode Microplate Reader—KR. Available online: https://www.thermofisher.com/kr/ko/home/life-science/lab-equipment/microplate-instruments/plate-readers/models/varioskan.html (accessed on 4 August 2022).
- xMAP® Technology. Available online: https://www.luminexcorp.com/xmap-technology/ (accessed on 22 August 2022).
- Fernandez-Pittol, M.; Hurtado, J.C.; Ali, M.; Simarro, Á.; Proaño, F.; Sierra, M.; Vila, J. Assessment of QuantuMDx Q-POC Assay for Rapid Detection of SARS-CoV-2 Using Middle Turbinate Swabs. Microbiol. Spectr. 2023, 11, e04256-22. [Google Scholar] [CrossRef]
- Cooper, D.; Callahan, B.; Callahan, P.; Burnett, L. Mobile Image Ratiometry: A New Method for Instantaneous Analysis of Rapid Test Strips. Nat. Preced. 2012. [Google Scholar] [CrossRef]
- Eltzov, E.; Guttel, S.; Low Yuen Kei, A.; Sinawang, P.D.; Ionescu, R.E.; Marks, R.S. Lateral flow immunoassays—From paper strip to smartphone technology. Electroanalysis 2015, 27, 2116–2130. [Google Scholar] [CrossRef]
- Foysal, K.H.; Seo, S.E.; Kim, M.J.; Kwon, O.S.; Chong, J.W. Analyte Quantity Detection from Lateral Flow Assay Using a Smartphone. Sensors 2019, 19, 4812. [Google Scholar] [CrossRef]
- Lee, S.; O’Dell, D.; Hohenstein, J.; Colt, S.; Mehta, S.; Erickson, D. NutriPhone: A mobile platform for low-cost point-of-care quantification of vitamin B12 concentrations. Sci. Rep. 2016, 6, 28237. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of Cobalt Ferrite Nanoparticles in Biomedical Nanotechnology. Nanomedicine 2018, 13, 1221–1238. [Google Scholar] [CrossRef]
- Mohamed, S. UCHEK: An Affordable Smartphone Based Point of Care Diagnostic System for a Low Resource Medical Setup. In IET Conference Proceedings, Proceedings of the Appropriate Healthcare Technologies for Low Resource Settings (AHT 2014), London, UK, 17–18 September 2014; IET Digital Library: London, UK, 2014; p. 23. [Google Scholar] [CrossRef]
- Lee, S.; Oncescu, V.; Mancuso, M.; Mehta, S.; Erickson, D. A smartphone platform for the quantification of vitamin D levels. Lab Chip 2014, 14, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; O’Dell, D.; Finkelstein, J.L.; Lee, S.; Erickson, D.; Mehta, S. ironPhone: Mobile device-coupled point-of-care diagnostics for assessment of iron status by quantification of serum ferritin. Biosens. Bioelectron. 2018, 99, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Wang, J.; Liu, Z.; Bai, L.; Ma, L. Effect of sample volume on the sensitivity of lateral flow assays through computational modeling. Anal. Biochem. 2021, 619, 114130. [Google Scholar] [CrossRef] [PubMed]
- Nuntawong, P.; Putalun, W.; Tanaka, H.; Morimoto, S.; Sakamoto, S. Lateral flow immunoassay for small-molecules detection in phytoproducts: A review. J. Nat. Med. 2022, 76, 521–545. [Google Scholar] [CrossRef]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Analyt. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Han, E.; Kumar, S.; Jeon, E.; Nam, M.-H.; Jun, H.S.; Seo, S. Field-Portable Leukocyte Classification Device Based on Lens-Free Shadow Imaging Technique. Biosensors 2022, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Oh, S.; Seo, D.; Kumar, S.; Lee, A.; Lee, S.; Kim, Y.-R.; Lee, M.; Seo, S. Field-Portable Seawater Toxicity Monitoring Platform Using Lens-Free Shadow Imaging Technology. Water Res. 2023, 230, 119585. [Google Scholar] [CrossRef]
- Patalon, T.; Saciuk, Y.; Peretz, A.; Perez, G.; Lurie, Y.; Maor, Y.; Gazit, S. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat. Commun. 2022, 13, 3203. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, Y.; Xiao, M.; Chen, L.; Zhao, Y.; Zhang, H.; Long, P.; Zhou, Y.; Xu, X.; Lei, Y.; et al. Dynamics of the SARS-CoV-2 Antibody Response up to 10 Months after Infection. Cell. Mol. Immunol. 2021, 18, 1832–1834. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, M.; Peng, Y.; Liang, Y.; Wei, J.; Xing, L.; Guo, L.; Li, X.; Li, J.; Wang, J.; et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat. Microbiol. 2022, 7, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.D.; DeSantis, S.M.; Yaseen, A.; Brito, F.A.; Valerio-Shewmaker, M.A.; Messiah, S.E.; Leon-Novelo, L.G.; Kohl, H.W.; Pinzon-Gomez, C.L.; Hao, T.; et al. Antibody duration after infection from SARS-CoV-2 in the Texas Coronavirus Antibody Response Survey. J. Infect. Dis. 2022, 227, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Antibody Persistence through 6 Months after the Second Dose of MRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2022, 386, 500. [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Young, M.K.; Kornmeier, C.; Carpenter, R.M.; Natale, N.R.; Sasson, J.M.; Solga, M.D.; Mathers, A.J.; Poulter, M.D.; Qiang, X.; Petri, W.A. IgG antibodies against SARS-CoV-2 correlate with days from symptom onset, viral load and IL-10. medRxiv 2020. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Ko, T.; Chae, Y.; Jang, Y.; Lee, I.; Lee, A.; Shin, S.; Nam, M.-H.; Kim, B.S.; Jun, H.S.; et al. Proof-of-Concept: Smartphone- and Cloud-Based Artificial Intelligence Quantitative Analysis System (SCAISY) for SARS-CoV-2-Specific IgG Antibody Lateral Flow Assays. Biosensors 2023, 13, 623. https://doi.org/10.3390/bios13060623
Kumar S, Ko T, Chae Y, Jang Y, Lee I, Lee A, Shin S, Nam M-H, Kim BS, Jun HS, et al. Proof-of-Concept: Smartphone- and Cloud-Based Artificial Intelligence Quantitative Analysis System (SCAISY) for SARS-CoV-2-Specific IgG Antibody Lateral Flow Assays. Biosensors. 2023; 13(6):623. https://doi.org/10.3390/bios13060623
Chicago/Turabian StyleKumar, Samir, Taewoo Ko, Yeonghun Chae, Yuyeon Jang, Inha Lee, Ahyeon Lee, Sanghoon Shin, Myung-Hyun Nam, Byung Soo Kim, Hyun Sik Jun, and et al. 2023. "Proof-of-Concept: Smartphone- and Cloud-Based Artificial Intelligence Quantitative Analysis System (SCAISY) for SARS-CoV-2-Specific IgG Antibody Lateral Flow Assays" Biosensors 13, no. 6: 623. https://doi.org/10.3390/bios13060623
APA StyleKumar, S., Ko, T., Chae, Y., Jang, Y., Lee, I., Lee, A., Shin, S., Nam, M.-H., Kim, B. S., Jun, H. S., & Seo, S. (2023). Proof-of-Concept: Smartphone- and Cloud-Based Artificial Intelligence Quantitative Analysis System (SCAISY) for SARS-CoV-2-Specific IgG Antibody Lateral Flow Assays. Biosensors, 13(6), 623. https://doi.org/10.3390/bios13060623




