Magnetic Relaxation Switching Assay Using IFNα-2b-Conjugated Superparamagnetic Nanoparticles for Anti-Interferon Antibody Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Biochemical Characterization of Superparamagnetic Conjugates
2.2. NMR Relaxometry Assessment of the Magnetic Conjugates and MRSw
2.3. Assessment of the Superparamagnetic State of NPs
2.4. Evaluation of Biological Activity of Magnetic Conjugates
2.5. Cells
2.6. Animals
2.7. Assessment of the SPIONs@IFNα-2b Accumulation in Intact Animals
2.8. Statistics
3. Results
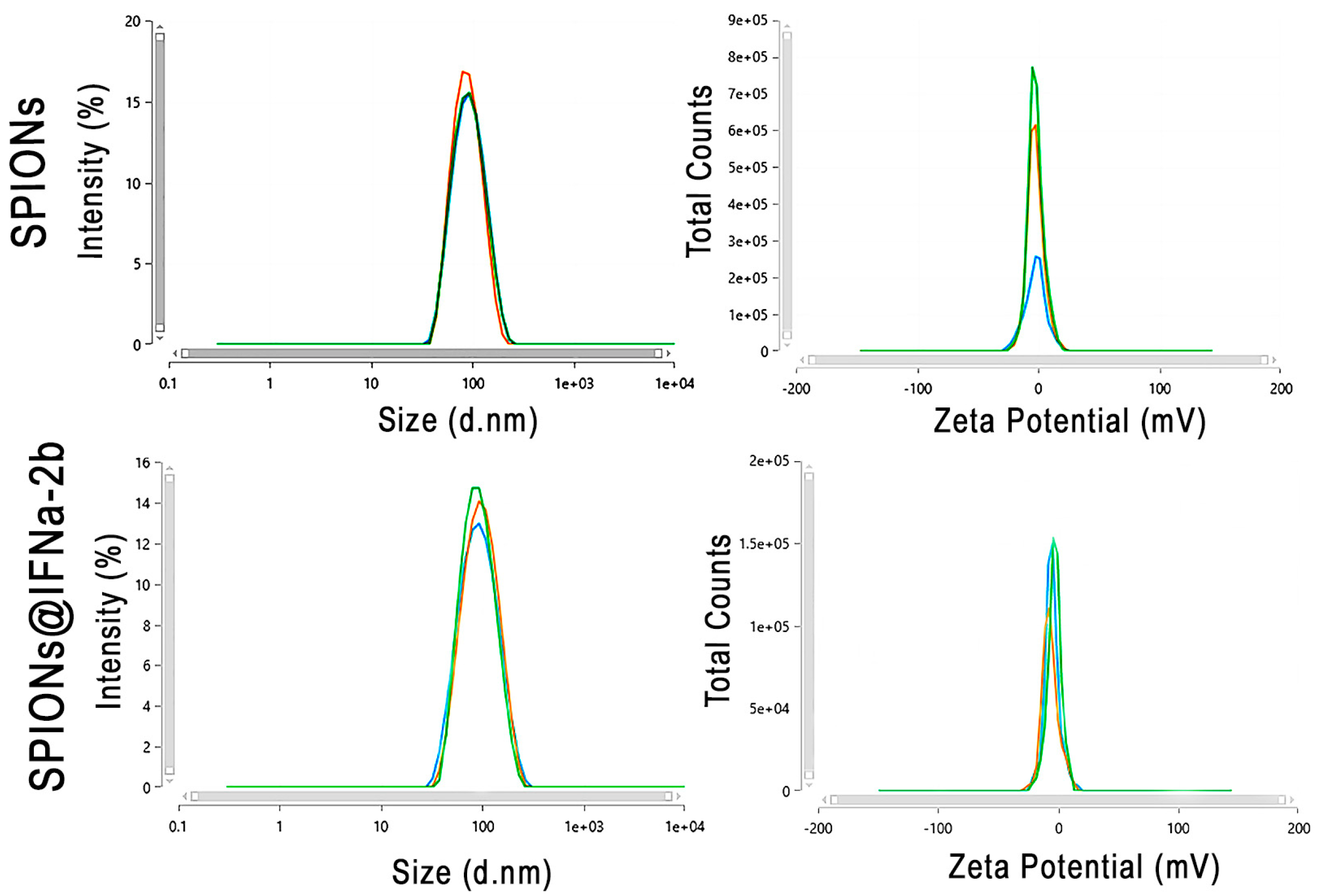
3.1. Physico-Chemical Characterization of Magnetic Conjugates
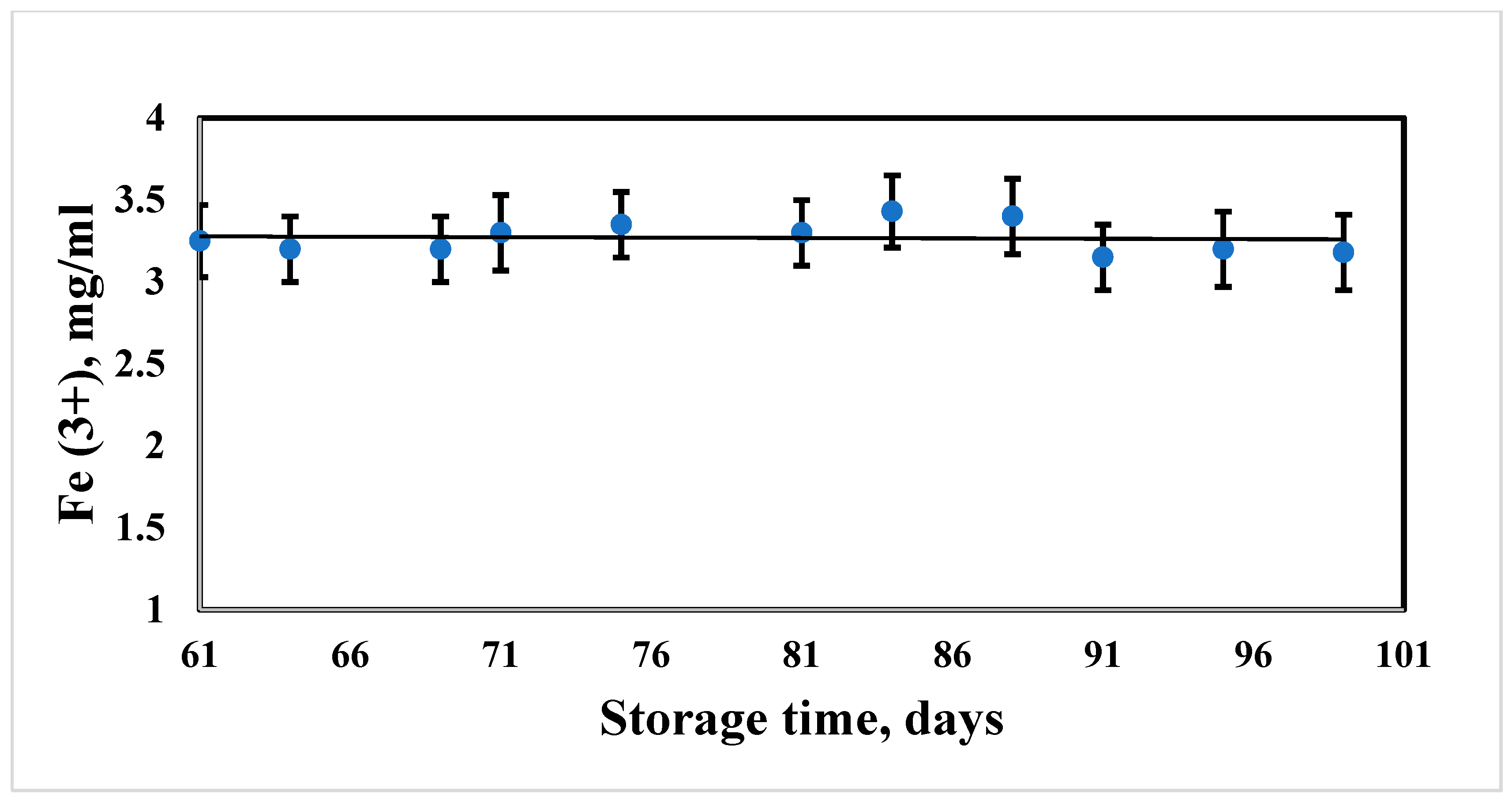
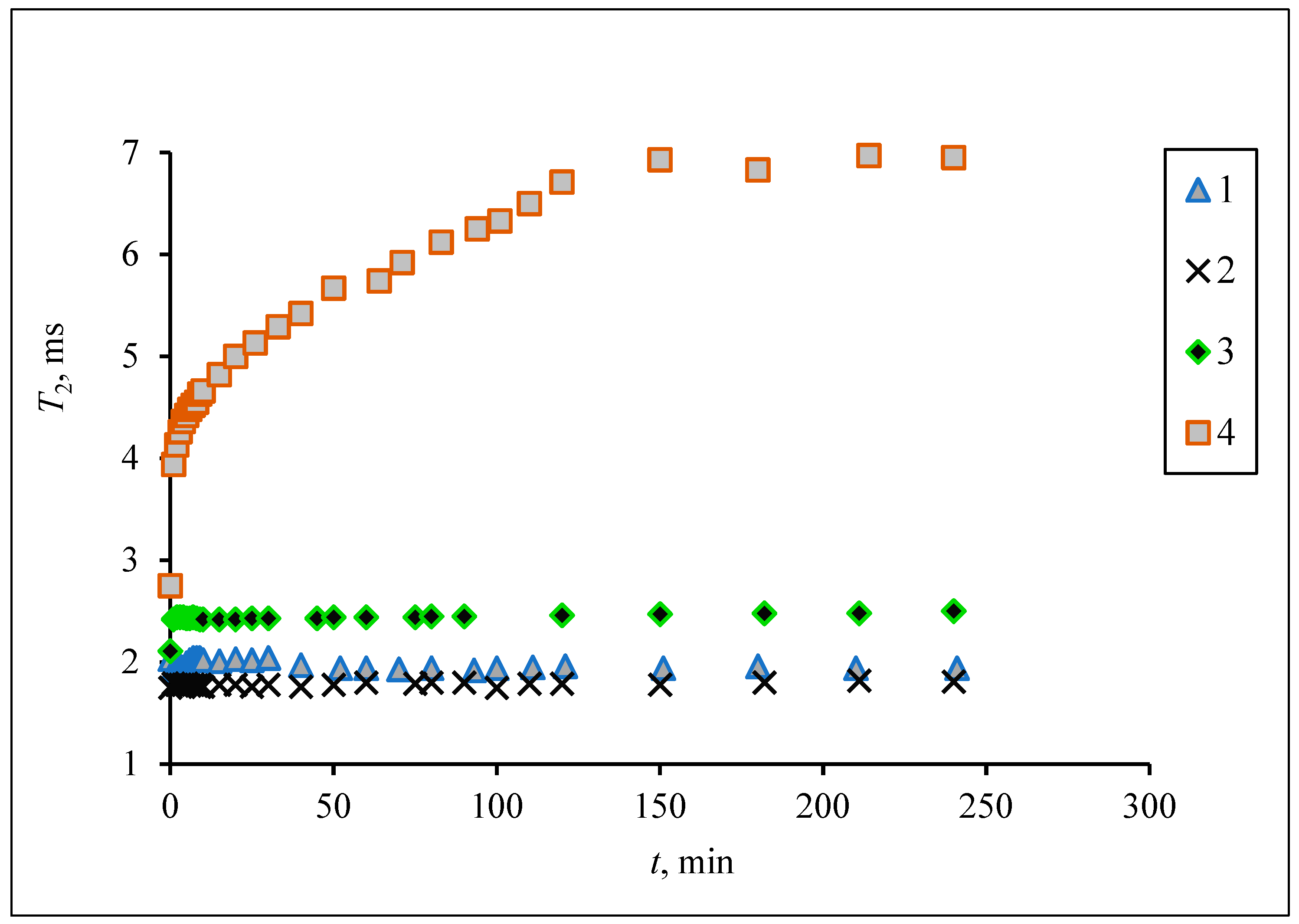
3.2. Measurement of Aggregate Stability of Hybrid Composites of MNPs during Long-Term Storage
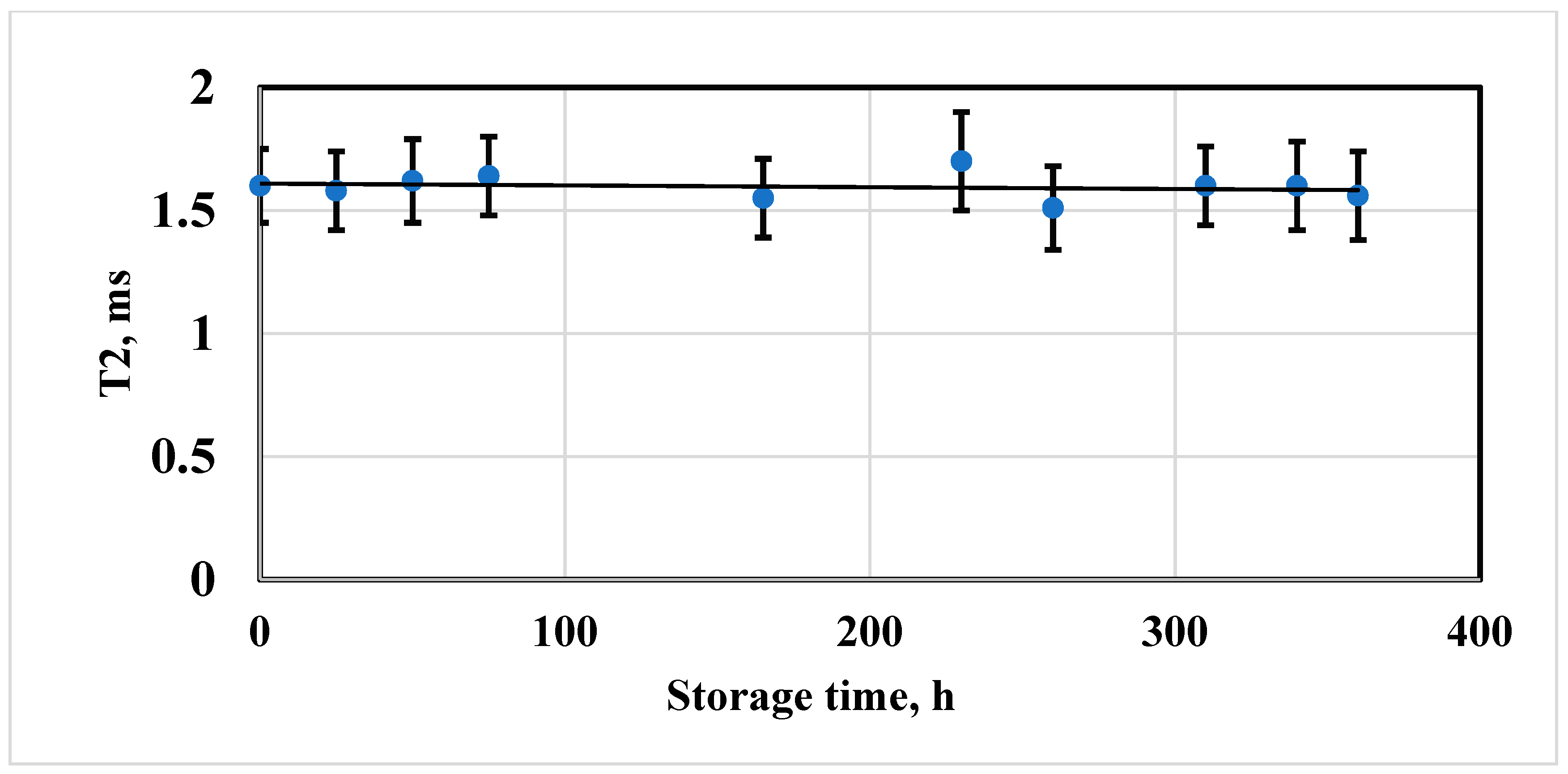
3.3. Evaluation of the Stability of the Magnetic Properties of Iron Oxide Particles Coated with Dextran
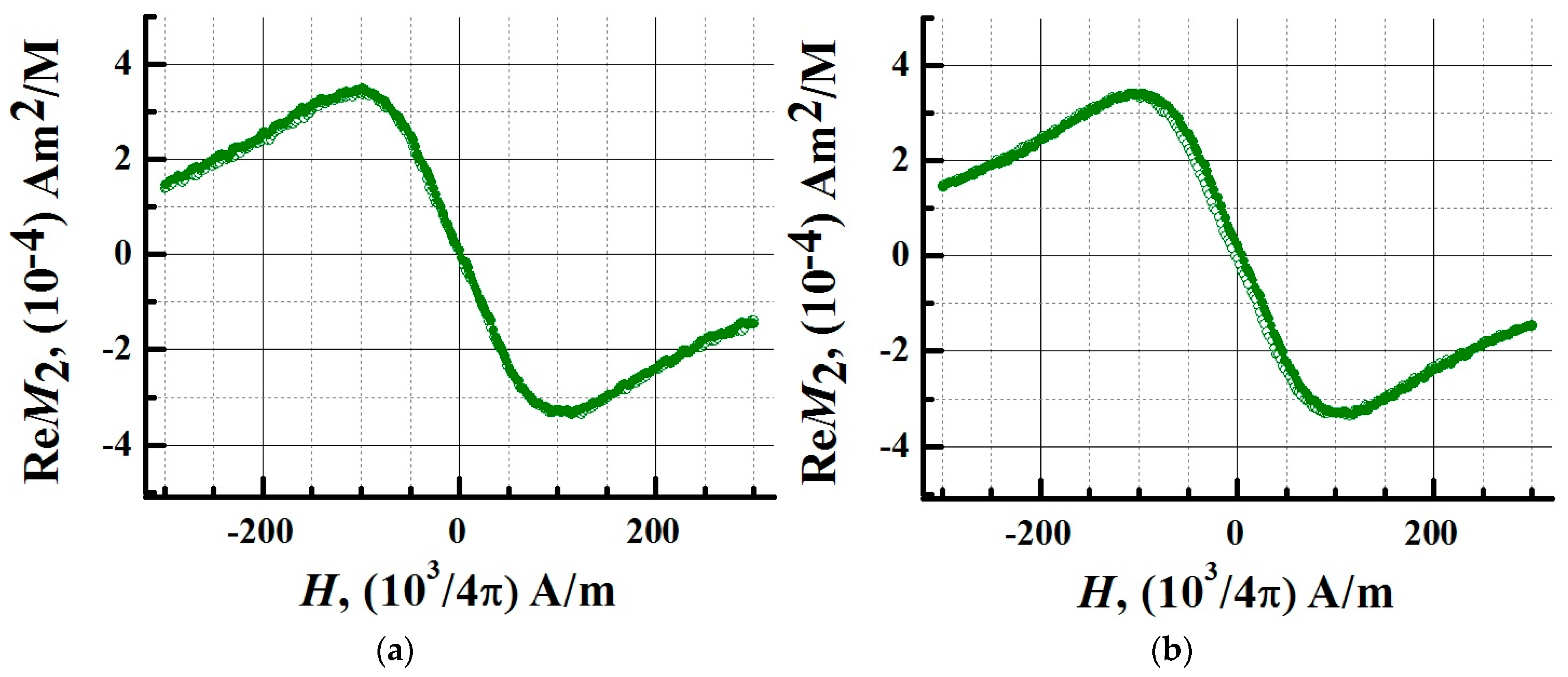
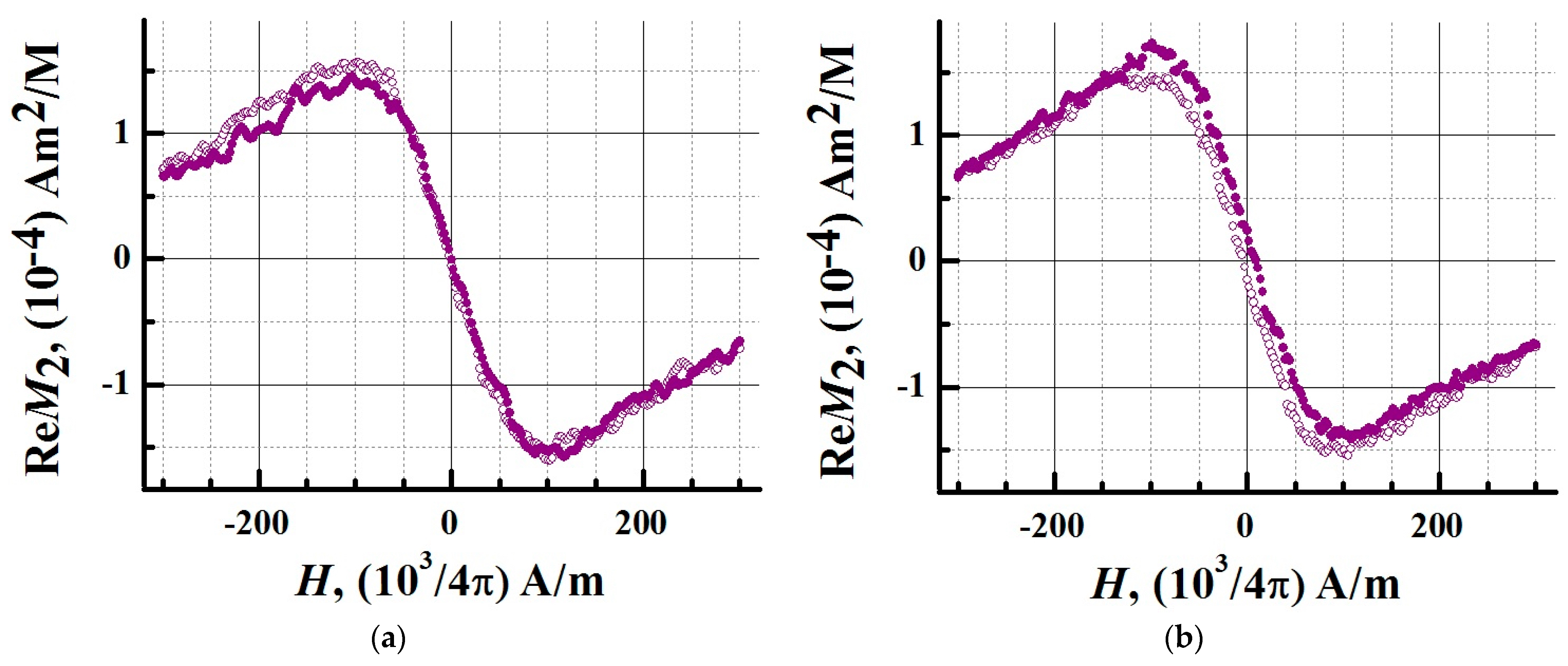
3.4. Superparamagnetic Properties of the SPIONs@IFNα-2b
3.5. Determination of the Biological Activity of SPIONs@IFNα-2b
3.6. NMR Characterization of IFNα-2b Magnetic Conjugates
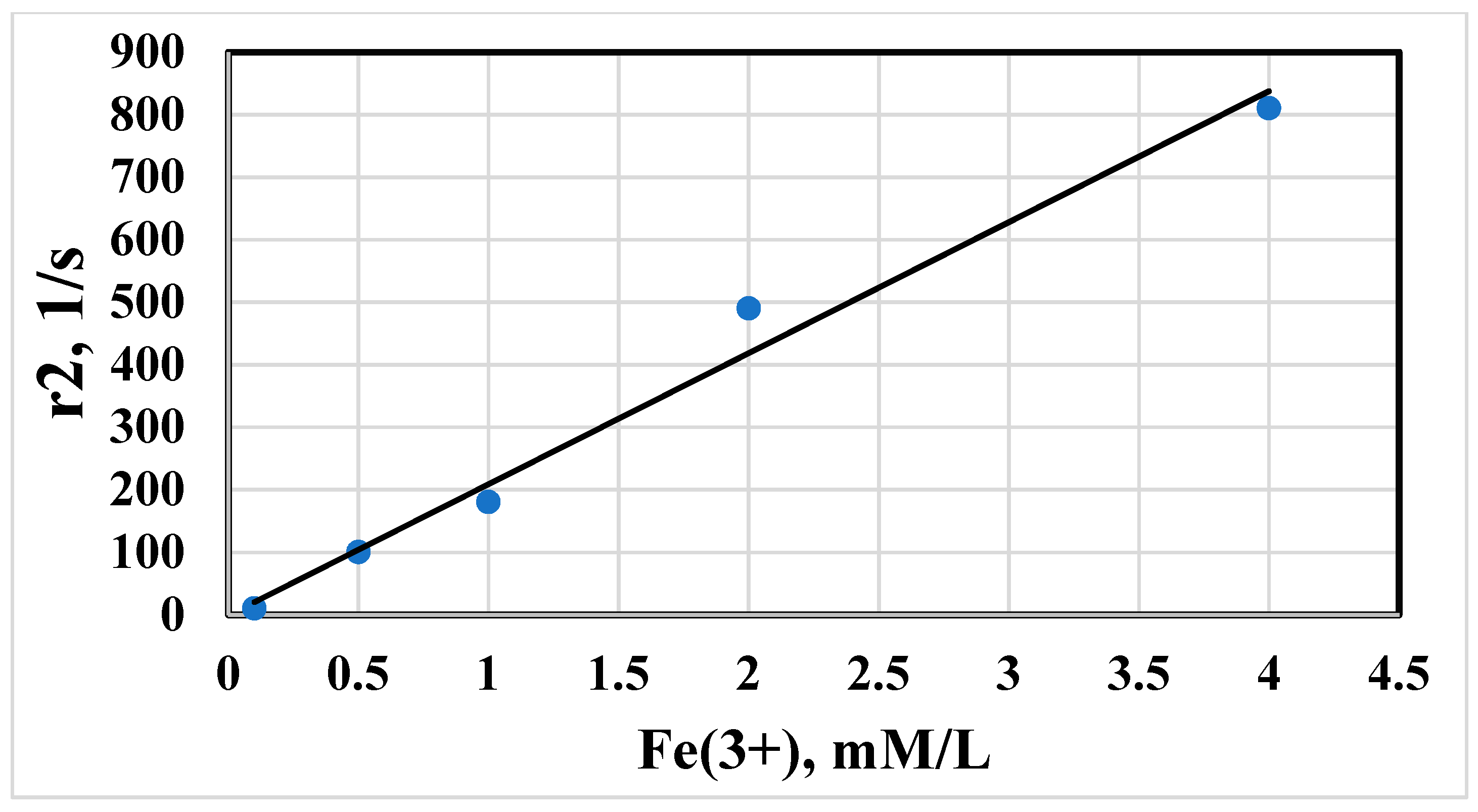
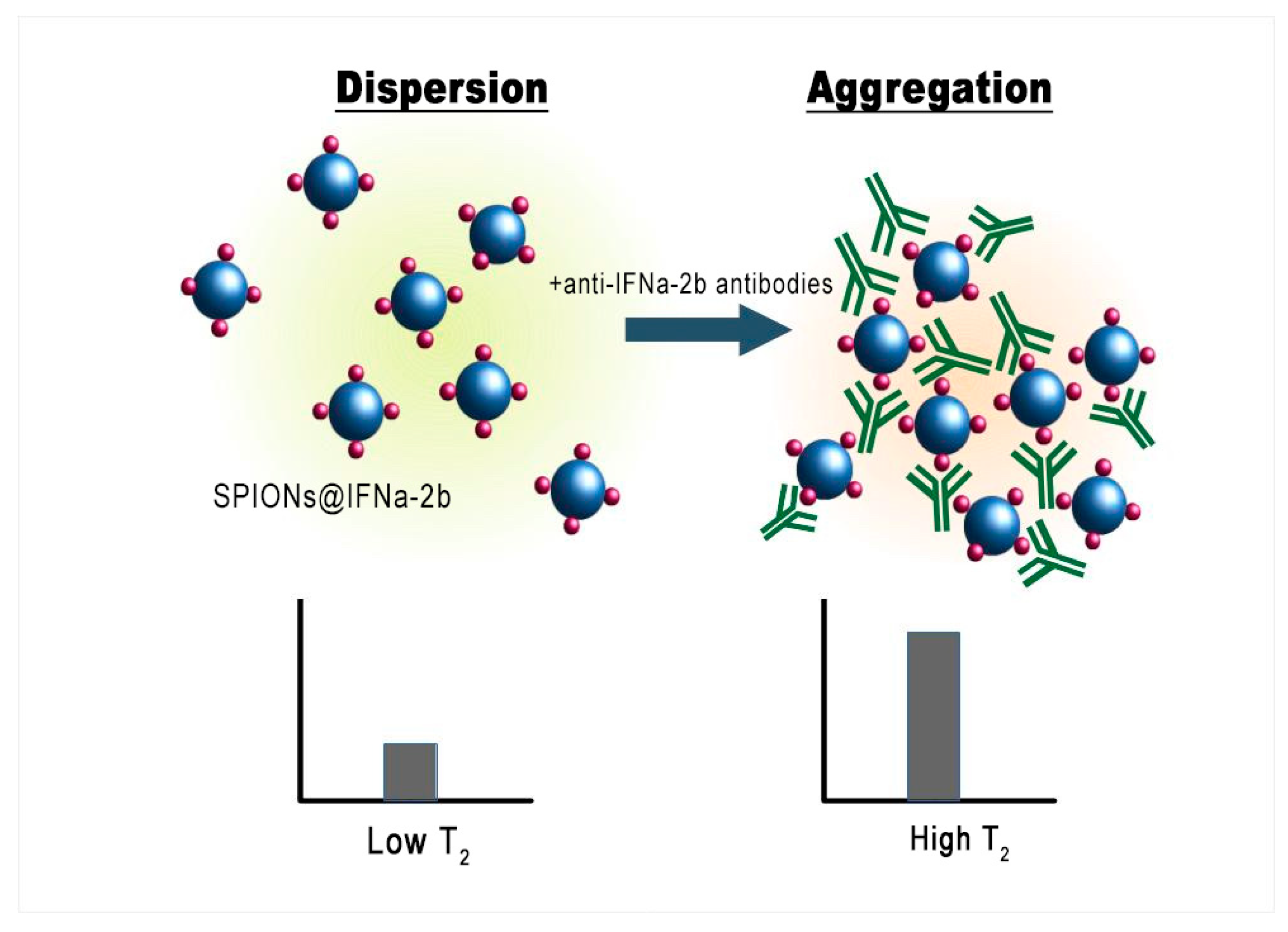
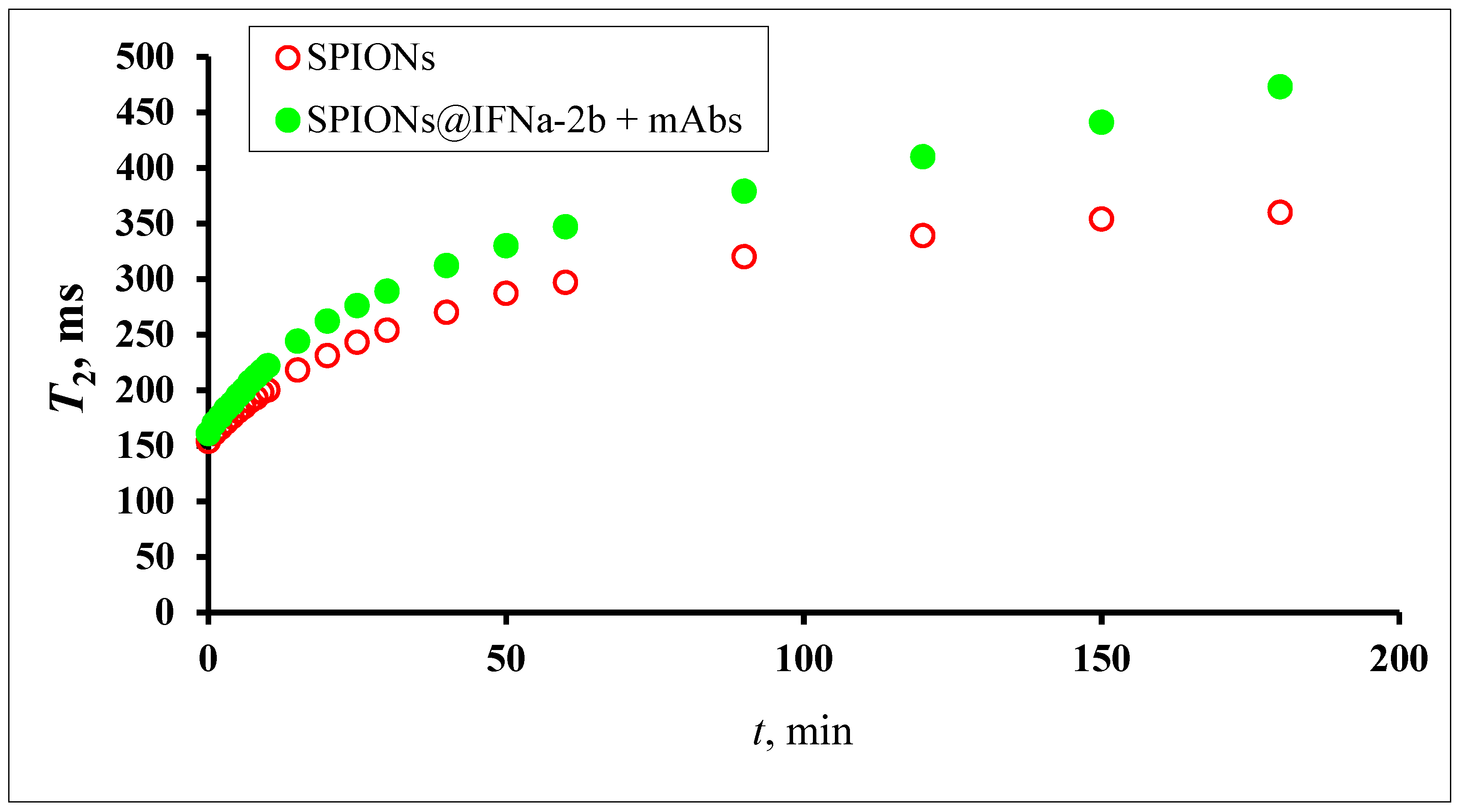
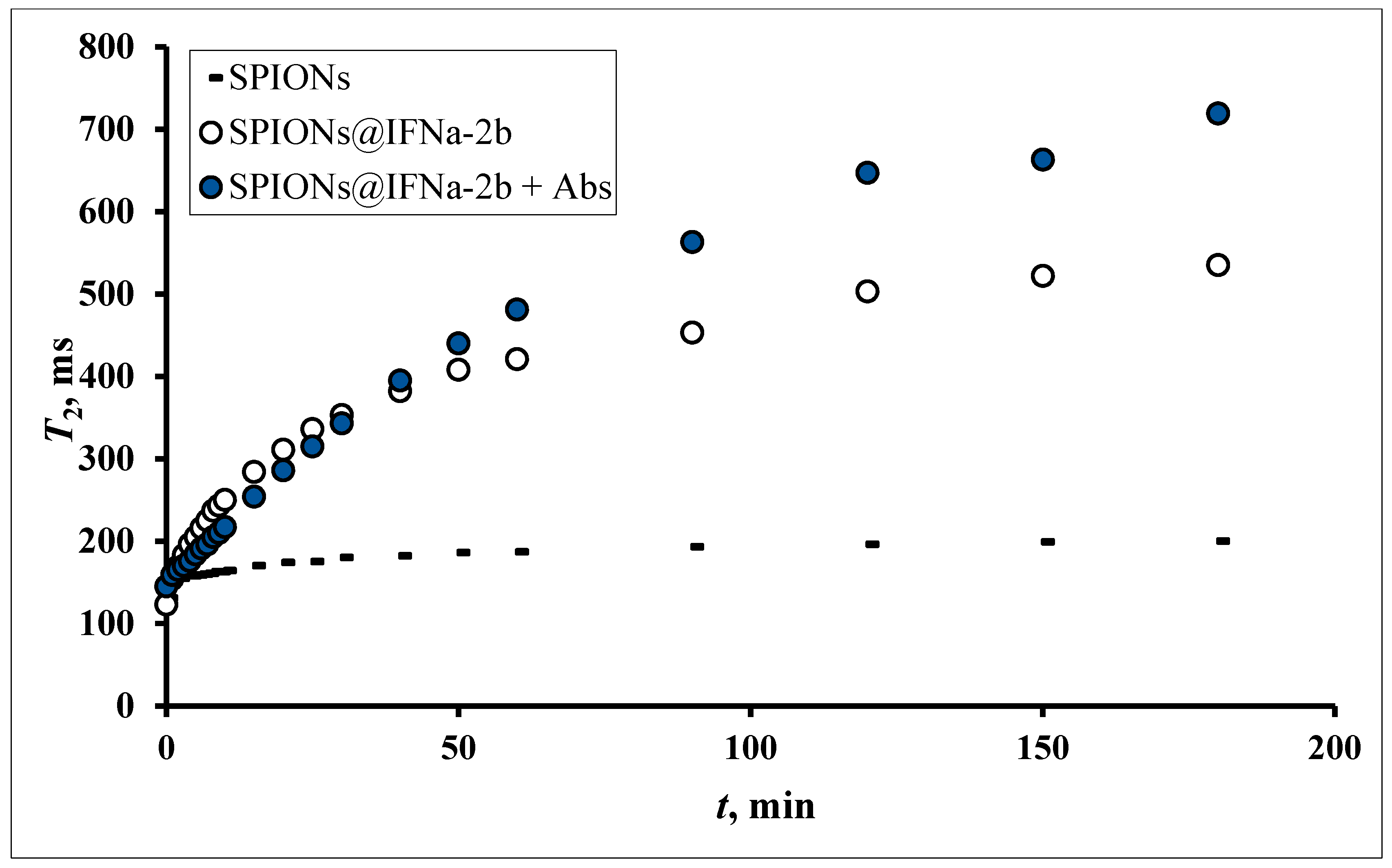
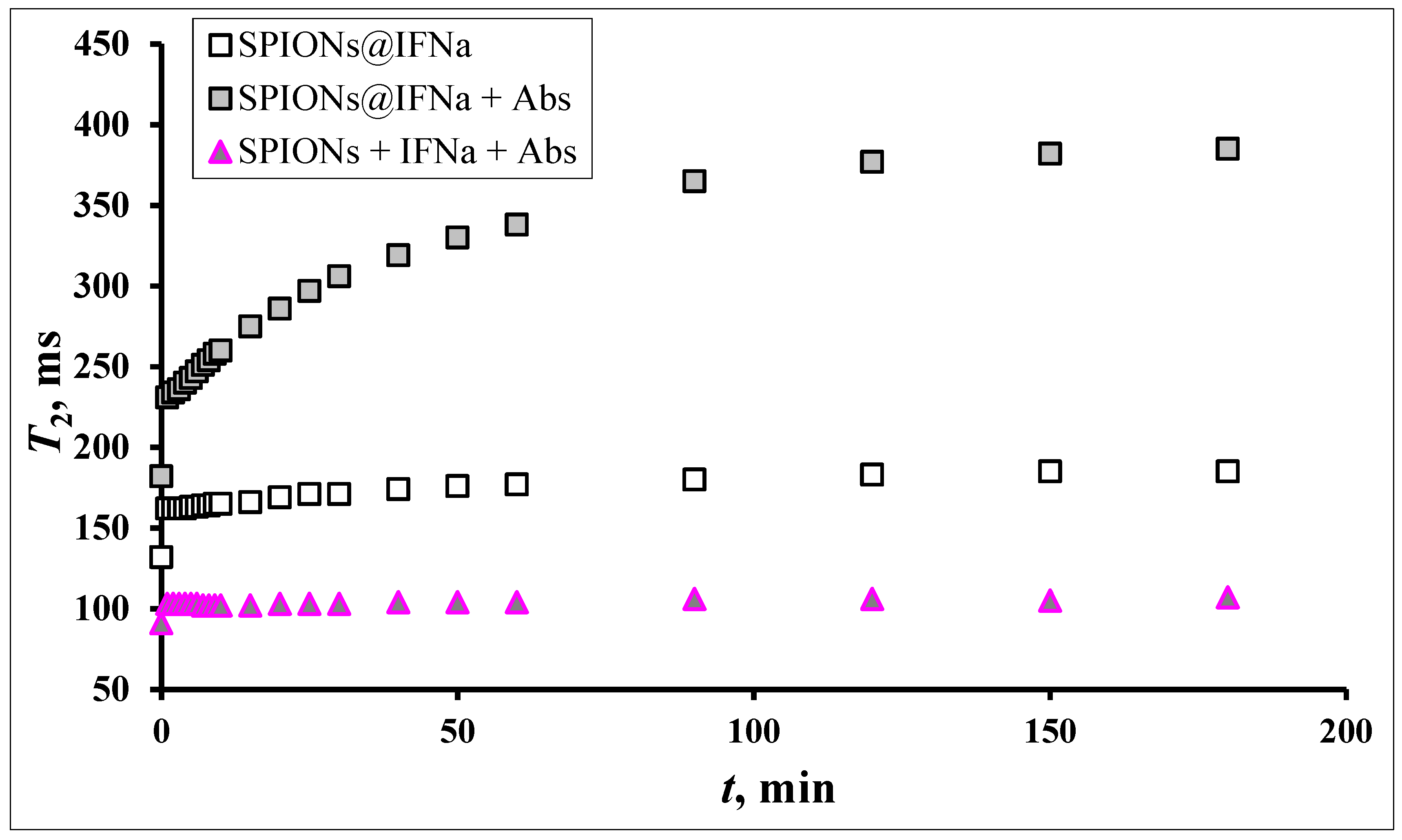
3.7. MRSw for the Detection of Anti-IFNα-2b Antibodies
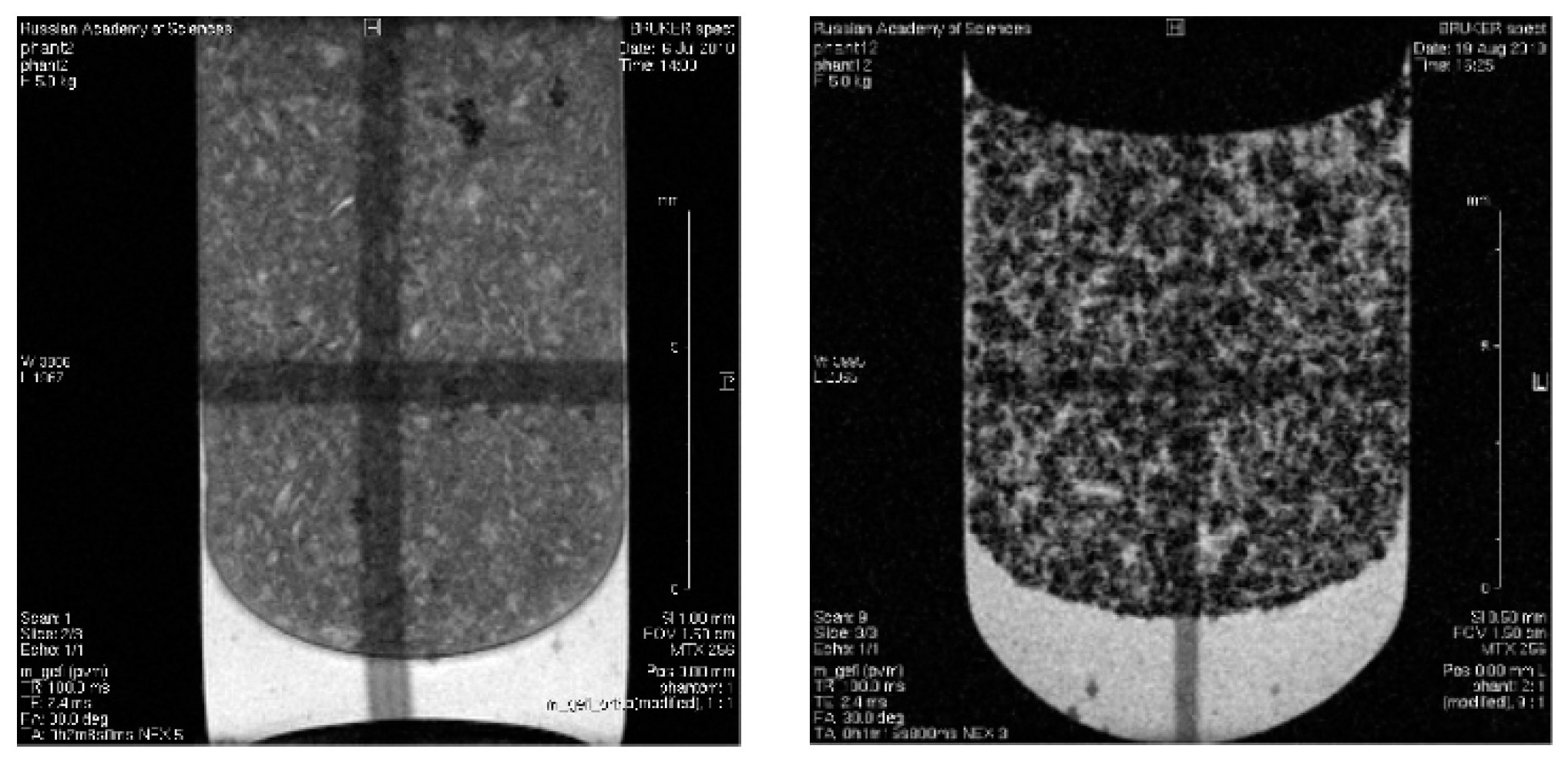
3.8. In Vitro Magnetic Contrast Properties of the SPIONs@IFNα-2b Conjugate
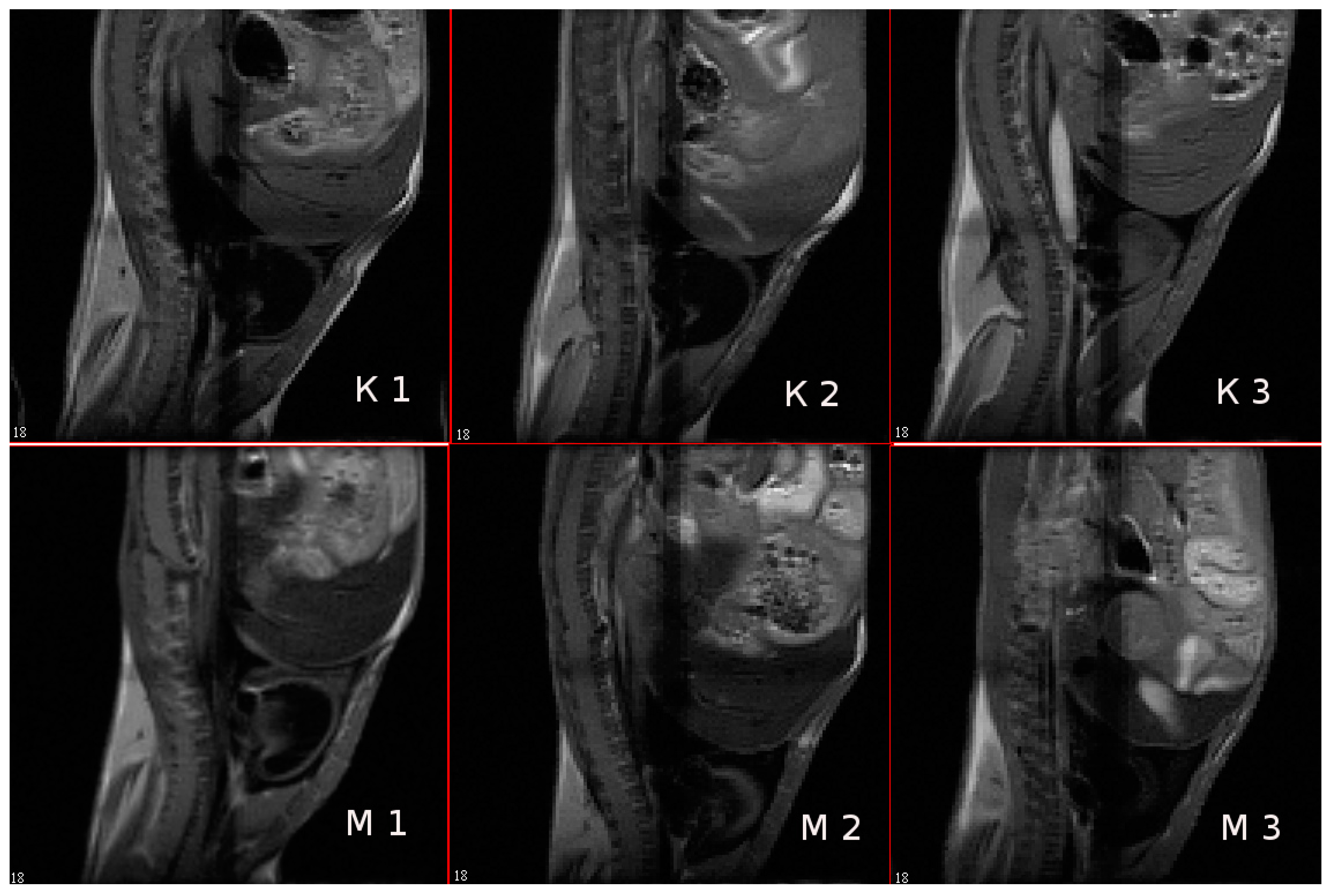
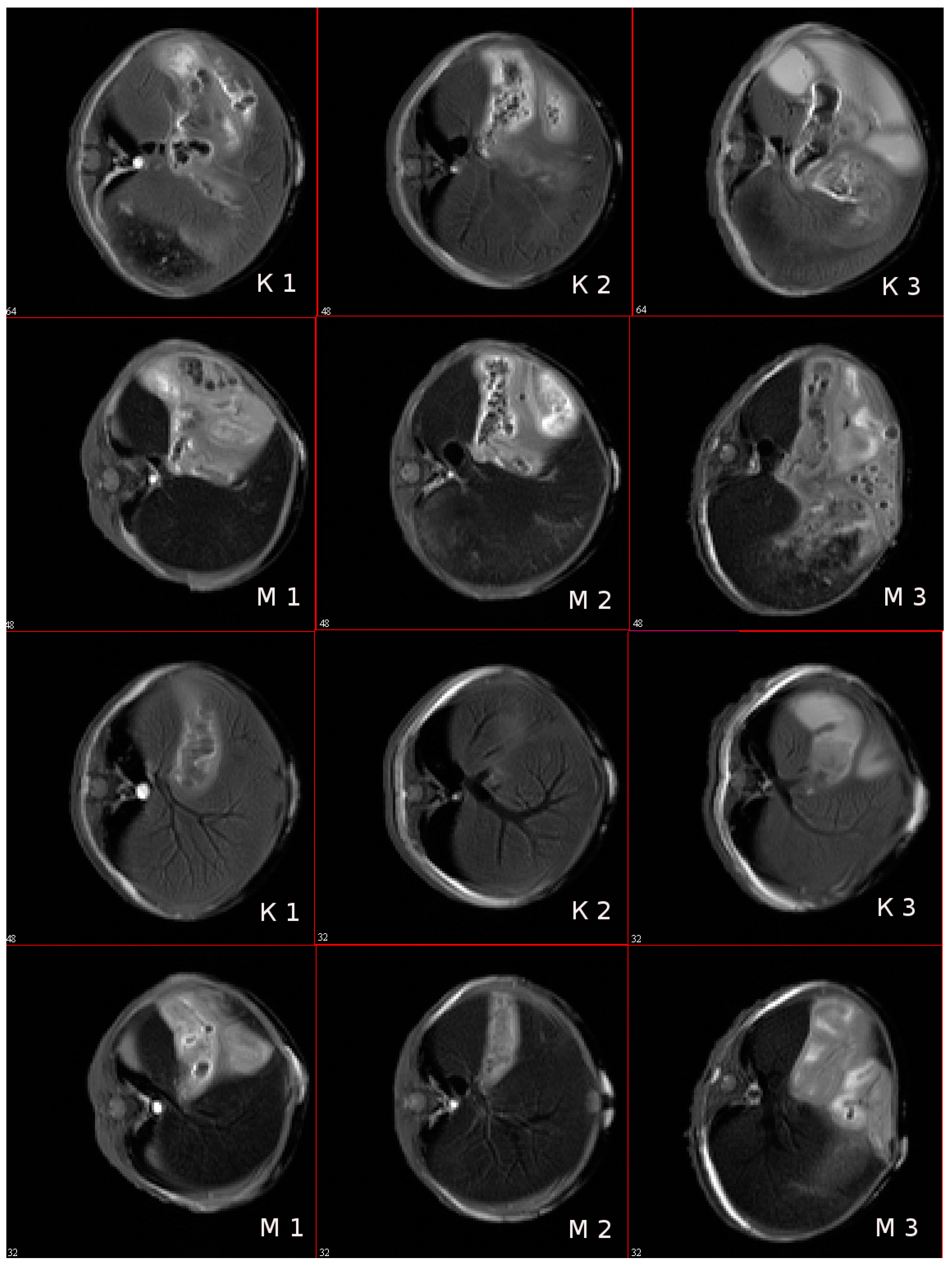
3.9. In Vivo MR-Contrast Enhancing Properties of SPIONs@IFNα-2b Conjugates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parker, B.S.; Rautela, J.; Hertzog, P.J. Antitumour actions of interferons: Implications for cancer therapy. Nat. Rev. Cancer 2016, 16, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, B.; Zhuo, Y.; Jiang, Z.; Li, R.; Lin, C.; Jin, Y.; Gao, Y.; Wang, D. Interferon and interferon-stimulated genes in HBV treatment. Front. Immunol. 2022, 13, 1034968. [Google Scholar] [CrossRef] [PubMed]
- Sebina, I.; Haque, A. Effects of type I interferons in malaria. Immunology 2018, 155, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Eto, T.; Takahashi, H. Enhanced inhibition of hepatitis B virus production by asialoglycoprotein receptor-directed interferon. Nat. Med. 1999, 5, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Suginoshita, Y.; Tabata, Y.; Moriyasu, F.; Ikada, Y.; Chiba, T. Liver targeting of interferon-beta with a liver-affinity polysaccharide based on metal coordination in mice. J. Pharmacol. Exp. Ther. 2001, 298, 805–811. [Google Scholar] [PubMed]
- Yasukawa, T.; Kimura, H.; Tabata, Y.; Kamizuru, H.; Miyamoto, H.; Honda, Y.; Ogura, Y. Targeting of interferon to choroidal neovascularization by use of dextran and metal coordination. Investig. Ophthalmol. Vis. Sci. 2002, 43, 842–848. [Google Scholar] [PubMed]
- Thitinan, S.; McConville, J.T. Interferon alpha delivery systems for the treatment of hepatitis C. Int. J. Pharm. 2009, 369, 121–135. [Google Scholar] [CrossRef]
- Yeh, M.L.; Huang, J.F.; Dai, C.Y.; Yu, M.L.; Chuang, W.L. Pharmacokinetics and pharmacodynamics of pegylated interferon for the treatment of hepatitis B. Expert. Opin. Drug. Metab. Toxicol. 2019, 15, 779–785. [Google Scholar] [CrossRef]
- Foster, G.R. Pegylated interferons for the treatment of chronic hepatitis C: Pharmacological and clinical differences between peginterferon-alpha-2a and peginterferon-alpha-2b. Drugs 2010, 70, 147–165. [Google Scholar] [CrossRef]
- Xiong, F.; Wang, Q.; Wu, G.H.; Liu, W.Z.; Wang, B.; Chen, Y.J. Direct and indirect effects of IFN-α2b in malignancy treatment: Not only an archer but also an arrow. Biomark. Res. 2022, 10, 69. [Google Scholar] [CrossRef]
- Agarwala, S.S. An update on pegylated IFN-α2b for the adjuvant treatment of melanoma. Expert Rev. Anticancer Ther. 2012, 12, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Bekisz, J.; Baron, S.; Balinsky, C.; Morrow, A.; Zoon, K.C. Antiproliferative Properties of Type I and Type II Interferon. Pharmaceuticals 2010, 3, 994–1015. [Google Scholar] [CrossRef] [PubMed]
- Krown, S.E.; Li, P.; Von Roenn, J.H.; Paredes, J.; Huang, J.; Testa, M.A. Efficacy of low-dose interferon with antiretroviral therapy in Kaposi’s sarcoma: A randomized phase II AIDS clinical trials group study. J. Interferon Cytokine Res. 2002, 22, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Ali Khan, W. Recombinant interferon alpha 2b in rheumatoid arthritis: Good antigen for rheumatoid arthritis antibodies. Cent. Eur. J. Immunol. 2018, 43, 58–68. [Google Scholar] [CrossRef]
- Khan, W.A. Recombinant Interferon Alpha-2b is a High-Affinity Antigen for Type 1 Diabetes Autoantibodies. Can. J. Diabetes 2017, 41, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.A.; Qureshi, J.A. Increased binding of circulating systemic lupus erythematosus autoantibodies to recombinant interferon alpha 2b. Apmis 2015, 123, 1016–1024. [Google Scholar] [CrossRef]
- Rodero, M.P.; Decalf, J.; Bondet, V.; Hunt, D.; Rice, G.I.; Werneke, S.; McGlasson, S.L.; Alyanakian, M.A.; Bader-Meunier, B.; Barnerias, C.; et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J. Exp. Med. 2017, 214, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.K. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res. Ther. 2010, 12 (Suppl. S1), S5. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.G. Autoantibodies targeting type I interferons: Prevalence, mechanisms of induction, and association with viral disease susceptibility. Eur. J. Immunol. 2023, 53, e2250164. [Google Scholar] [CrossRef]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012, 19, 3794–3804. [Google Scholar] [CrossRef]
- Baghban, N.; Khoradmehr, A.; Afshar, A.; Jafari, N.; Zendehboudi, T.; Rasekh, P.; Abolfathi, L.G.; Barmak, A.; Mohebbi, G.; Akmaral, B.; et al. MRI Tracking of Marine Proliferating Cells In Vivo Using Anti-Oct4 Antibody-Conjugated Iron Nanoparticles for Precision in Regenerative Medicine. Biosensors 2023, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.A.; Nikolaev, B.P.; Ryzhov, V.A.; Yakovleva, L.Y.; Marchenko, Y.Y.; Parr, M.A.; Rolich, V.I.; Mikhrina, A.L.; Dobrodumov, A.V.; Pitkin, E.; et al. Ionizing radiation improves glioma-specific targeting of superparamagnetic iron oxide nanoparticles conjugated with cmHsp70.1 monoclonal antibodies (SPION-cmHsp70.1). Nanoscale 2015, 7, 20652–20664. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Masud, M.K.; Islam, M.N.; Gopalan, V.; Lam, A.K.; Tanaka, S.; Nguyen, N.T.; Hossain, M.S.A.; Li, C.; Yamauchi, Y.; et al. Gold-loaded nanoporous iron oxide nanocubes: A novel dispersible capture agent for tumor-associated autoantibody analysis in serum. Nanoscale 2017, 9, 8805–8814. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Z.; Li, G. A Review of Magnetic Nanoparticle-Based Surface-Enhanced Raman Scattering Substrates for Bioanalysis: Morphology, Function and Detection Application. Biosensors 2022, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, H.; Buchanan, J.K.; Kandile, N.G.; Plieger, P.G. Iron Oxide Nanoparticles: Physicochemical Characteristics and Historical Developments to Commercialization for Potential Technological Applications. ACS Biomater. Sci. Eng. 2021, 7, 5432–5450. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.A.; Nikolaev, B.P.; Ryzhov, V.A.; Yakovleva, L.Y.; Dobrodumov, A.V.; Marchenko, Y.Y.; Margulis, B.A.; Pitkin, E.; Mikhrina, A.L.; Guzhova, I.V.; et al. Detection of experimental myocardium infarction in rats by MRI using heat shock protein 70 conjugated superparamagnetic iron oxide nanoparticle. Nanomedicine 2016, 12, 611–621. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Zhou, Z.; Huang, K.; Yang, S.; Han, G. Recent Advances on Magnetic Relaxation Switching Assay-Based Nanosensors. Bioconjug Chem. 2017, 28, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Shao, H.; Liong, M.; Yoon, T.J.; Weissleder, R.; Lee, H. Mechanism of magnetic relaxation switching sensing. ACS Nano 2012, 6, 6821–6828. [Google Scholar] [CrossRef]
- Alcantara, D.; Lopez, S.; García-Martin, M.L.; Pozo, D. Iron oxide nanoparticles as magnetic relaxation switching (MRSw) sensors: Current applications in nanomedicine. Nanomedicine 2016, 12, 1253–1262. [Google Scholar] [CrossRef]
- Koh, I.; Hong, R.; Weissleder, R.; Josephson, L. Nanoparticle-target interactions parallel antibody-protein interactions. Anal. Chem. 2009, 81, 3618–3622. [Google Scholar] [CrossRef]
- de Haan, H.W. Mechanisms of proton spin dephasing in a system of magnetic particles. Magn. Reson. Med. 2011, 66, 1748–1758. [Google Scholar] [CrossRef]
- Hong, R.; Cima, M.J.; Weissleder, R.; Josephson, L. Magnetic microparticle aggregation for viscosity determination by MR. Magn. Reson. Med. 2008, 59, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Yudintceva, N.; Lomert, E.; Mikhailova, N.; Tolkunova, E.; Agadzhanian, N.; Samochernych, K.; Multhoff, G.; Timin, G.; Ryzhov, V.; Deriglazov, V.; et al. Targeting Brain Tumors with Mesenchymal Stem Cells in the Experimental Model of the Orthotopic Glioblastoma in Rats. Biomedicines 2021, 9, 1592. [Google Scholar] [CrossRef]
- Shevtsov, M.; Stangl, S.; Nikolaev, B.; Yakovleva, L.; Marchenko, Y.; Tagaeva, R.; Sievert, W.; Pitkin, E.; Mazur, A.; Tolstoy, P.; et al. Granzyme B Functionalized Nanoparticles Targeting Membrane Hsp70-Positive Tumors for Multimodal Cancer Theranostics. Small 2019, 15, e1900205. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Wang, Y.; Li, Q.; Gao, G.; Dong, N.; Hu, H.; Wang, K.; Wu, J.; Gao, Z.; et al. Establishment of a method to determine the magnetic particles in mouse tissues. Nanoscale Res. Lett. 2012, 7, 665. [Google Scholar] [CrossRef]
- Shamsipour, F.; Zarnani, A.H.; Ghods, R.; Chamankhah, M.; Forouzesh, F.; Vafaei, S.; Bayat, A.A.; Akhondi, M.M.; Ali Oghabian, M.; Jeddi-Tehrani, M. Conjugation of Monoclonal Antibodies to Super Paramagnetic Iron Oxide Nanoparticles for Detection of her2/neu Antigen on Breast Cancer Cell Lines. Avicenna J. Med. Biotechnol. 2009, 1, 27–31. [Google Scholar]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Ryzhov, V.A.; Pleshakov, I.V.; Nechitailov, A.A.; Glebova, N.V.; Pyatyshev, E.N.; Malkova, A.V.; Kiselev, I.A.; Matveev, V.V. Magnetic Study of Nanostructural Composite Material Based on Cobalt Compounds and Porous Silicon. Appl. Magn. Reson. 2014, 45, 339–352. [Google Scholar] [CrossRef]
- Ryzhov, V.A.; Multhoff, G.; Shevtsov, M.A. Detection of Magnetosome-Like Structures in Eukaryotic Cells Using Nonlinear Longitudinal Response to ac Field. Appl. Magn. Reson. 2019, 50, 943–957. [Google Scholar] [CrossRef]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, J.; Cheng, X.; Wang, X.; Zhang, X.; Chen, Y. Polydopamine-mediated quantity-based magnetic relaxation sensing for the rapid and sensitive detection of chloramphenicol in fish samples. Food Res. Int. 2022, 162, 111919. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, R.; Wu, L.; Wang, X.; Jiang, F.; Fan, Z.; Huang, C.; Chen, Y. Magnetic relaxation switching biosensor via polydopamine nanoparticle mediated click chemistry for detection of chlorpyrifos. Biosens. Bioelectron. 2022, 207, 114127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, P.; Tao, Q.; Krause, H.J.; Yang, S.; Ding, G.; Dong, H.; Xie, X. Magnetic graphene quantum dots facilitate closed-tube one-step detection of SARS-CoV-2 with ultra-low field NMR relaxometry. Sens. Actuators B Chem. 2021, 337, 129786. [Google Scholar] [CrossRef] [PubMed]
- Xianyu, Y.; Dong, Y.; Wang, Z.; Xu, Z.; Huang, R.; Chen, Y. Broad-Range Magnetic Relaxation Switching Bioassays Using Click Chemistry-Mediated Assembly of Polystyrene Beads and Magnetic Nanoparticles. ACS Sens. 2019, 4, 1942–1949. [Google Scholar] [CrossRef]
- Chen, Y.P.; Zou, M.; Qi, C.; Xie, M.X.; Wang, D.N.; Wang, Y.F.; Xue, Q.; Li, J.F.; Chen, Y. Immunosensor based on magnetic relaxation switch and biotin-streptavidin system for the detection of Kanamycin in milk. Biosens. Bioelectron. 2013, 39, 112–117. [Google Scholar] [CrossRef]
- Liang, G.; Chen, H.; Zhang, S.; Wu, W.; Kong, J. Magnetic nanosensors for highly sensitive and selective detection of bacillus Calmette-Guérin. Analyst 2012, 137, 675–679. [Google Scholar] [CrossRef]
- Kim, G.Y.; Josephson, L.; Langer, R.; Cima, M.J. Magnetic relaxation switch detection of human chorionic gonadotrophin. Bioconjug. Chem. 2007, 18, 2024–2028. [Google Scholar] [CrossRef]
- Haun, J.B.; Yoon, T.J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Kadam, U.S.; Cho, Y.; Park, T.Y.; Hong, J.C. Aptamer-based CRISPR-Cas powered diagnostics of diverse biomarkers and small molecule targets. Appl. Biol. Chem. 2023, 66, 13. [Google Scholar] [CrossRef]
- Chen, D.X.; Taboada, E.; Roig, A. Experimental study on T2 relaxation time of protons in water suspensions of iron-oxide nanoparticles: Cases of composite nanospheres. J. Magn. Magn. Mater. 2011, 323, 2487–2492. [Google Scholar] [CrossRef]
- Colombo, M.; Ronchi, S.; Monti, D.; Corsi, F.; Trabucchi, E.; Prosperi, D. Femtomolar detection of autoantibodies by magnetic relaxation nanosensors. Anal. Biochem. 2009, 392, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Marchenko, Y.Y.; Dobrodumov, A.V.; Mikhrina, A.L.; Martynova, M.G.; Bystrova, O.A.; Yakovenko, I.V.; Ischenko, A.M. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int. J. Nanomed. 2014, 9, 273–287. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Parr, M.A.; Marchenko, Y.Y.; Eliseev, I.; Yudenko, A.; Dobrodumov, A.V.; Zlobina, O.; Zhakhov, A.; et al. 70-kDa heat shock protein coated magnetic nanocarriers as a nanovaccine for induction of anti-tumor immune response in experimental glioma. J. Control. Release 2015, 220, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Kwon, Y.S.; Yoon, T.J.; Lee, J.K. Relaxivity control of magnetic nanoclusters for efficient magnetic relaxation switching assay. Chem. Commun. 2013, 49, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- Raoof, F.; Munawar, A.; Ahmad, M.; Rizvi, S.F.A.; Ali, Z.; Shahid, A.B. Multifunctional Iron Oxide Nanocarriers Synthesis for Drug Delivery, Diagnostic Imaging, and Biodistribution Study. Appl. Biochem. Biotechnol. 2023, 195, 1–16. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Chen, J.; Zhang, X.; Guo, J.; Gu, J.; Mei, C.; Xiao, Y.; Peng, C.; Liu, J.; et al. Arsenic-Loaded Biomimetic Iron Oxide Nanoparticles for Enhanced Ferroptosis-Inducing Therapy of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2023, 15, 6260–6273. [Google Scholar] [CrossRef]
- Grimm, J.; Perez, J.M.; Josephson, L.; Weissleder, R. Novel nanosensors for rapid analysis of telomerase activity. Cancer Res. 2004, 64, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Garrison, J.C.; Poluektova, L.Y.; Bronich, T.K.; Osna, N.A. Liver-targeted antiviral peptide nanocomplexes as potential anti-HCV therapeutics. Biomaterials 2015, 70, 37–47. [Google Scholar] [CrossRef]
- Zhang, J.; Mulvenon, A.; Makarov, E.; Wagoner, J.; Knibbe, J.; Kim, J.O.; Osna, N.; Bronich, T.K.; Poluektova, L.Y. Antiviral peptide nanocomplexes as a potential therapeutic modality for HIV/HCV co-infection. Biomaterials 2013, 34, 3846–3857. [Google Scholar] [CrossRef]
- Khanal, M.; Vausselin, T.; Barras, A.; Bande, O.; Turcheniuk, K.; Benazza, M.; Zaitsev, V.; Teodorescu, C.M.; Boukherroub, R.; Siriwardena, A.; et al. Phenylboronic-acid-modified nanoparticles: Potential antiviral therapeutics. ACS Appl. Mater. Interfaces 2013, 5, 12488–12498. [Google Scholar] [CrossRef]
- Gharibkandi, N.A.; Żuk, M.; Muftuler, F.Z.B.; Wawrowicz, K.; Żelechowska-Matysiak, K.; Bilewicz, A. (198)Au-Coated Superparamagnetic Iron Oxide Nanoparticles for Dual Magnetic Hyperthermia and Radionuclide Therapy of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 5282. [Google Scholar] [CrossRef]
- Jia, W.; Han, Y.; Mao, X.; Xu, W.; Zhang, Y. Nanotechnology strategies for hepatocellular carcinoma diagnosis and treatment. RSC Adv. 2022, 12, 31068–31082. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jia, X.; Yin, L.; Yang, Z.; Hui, H.; Li, J.; Huang, W.; Tian, J.; Zhang, S. Advances in magnetic particle imaging and perspectives on liver imaging. iLIVER 2022, 1, 237–244. [Google Scholar] [CrossRef]
- Matsushita, T.; Kusakabe, Y.; Fujii, H.; Murase, K.; Yamazaki, Y.; Murase, K. Inflammatory imaging with ultrasmall superparamagnetic iron oxide. Magn. Reson. Imaging 2011, 29, 173–178. [Google Scholar] [CrossRef]
- Macarini, L.; Milillo, P.; Cascavilla, A.; Scalzo, G.; Stoppino, L.; Vinci, R.; Moretti, G.; Ettorre, G. MR characterisation of dysplastic nodules and hepatocarcinoma in the cirrhotic liver with hepatospecific superparamagnetic contrast agents: Pathological correlation in explanted livers. Radiol. Med. 2009, 114, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Chen, Z.G.; Wang, J.C.; Zhang, Z.M. Superparamagnetic iron oxide-enhanced magnetic resonance imaging for focal hepatic lesions: Systematic review and meta-analysis. World J. Gastroenterol. 2015, 21, 4334–4344. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, J.M.; Kim, S.H.; Chang, S.; Kim, S.J.; Han, J.K.; Choi, B.I. Differentiation of well-differentiated hepatocellular carcinomas from other hepatocellular nodules in cirrhotic liver: Value of SPIO-enhanced MR imaging at 3.0 Tesla. J. Magn. Reson. Imaging 2009, 29, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Ito, K.; Shimizu, A.; Fujita, T.; Onoda, H.; Yamatogi, S.; Washida, Y.; Matsunaga, N. Hepatocellular lesions with increased iron uptake on superparamagnetic iron oxide-enhanced magnetic resonance imaging in cirrhosis or chronic hepatitis: Comparison of four magnetic resonance sequences for lesion conspicuity. Magn. Reson. Imaging 2009, 27, 801–806. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, M.J.; Kim, J.H.; Choi, J.Y.; Chung, Y.E.; Park, M.S.; Kim, K.W. Comparison of multi-echo and single-echo gradient-recalled echo sequences for SPIO-enhanced liver MRI at 3 T. Clin. Radiol. 2010, 65, 916–923. [Google Scholar] [CrossRef] [PubMed]
| SPIONs@IFNα-2b | Characteristics | ||||
| Initial mass ratio [Fe]:[Protein] | Fe3+, µg/mL | Protein, µg/mL | Protein/Fe3+ | Protein, µg/mL (IFA) | |
| 1:0.7 | 2370 | 120 | 0.05 | 0.2 | |
| Sample | Direction of Movement (Cathode “−”; Anode “+”) | Electrophoretic Mobility, cm2V−1s−1 |
|---|---|---|
| Ferrocol | + | 4.0 × 10−4 |
| fluid MAG–DX | + | 3.5 × 10−5 |
| SPIONS | − | 8.2 × 10−6 |
| SPIONs@IFNα-2b | + | 6.8 × 10−6 |
| Sample | Average Hydrodynamic Radius, nm | Particle Charge, C | Charge per Particle Surface Unit, C/m2 |
|---|---|---|---|
| SPIONs | 53 | +9.2 × 10−19 | +2.6 × 10−5 |
| SPIONs@IFNα-2b | 62 | −8.9 × 10−19 | −1.9 × 10−5 |
| fluid MAG–DX | 25 | −1.8 × 10−18 | −2.3 × 10−4 |
| Ferrocol | 76 | −6.5 × 10−17 | −8.9 × 10−4 |
| t, s | r2*, 1/s | t, s | r2, 1/s | t, s | r1, 1/s |
|---|---|---|---|---|---|
| 0 | 1727 | 0 | 1250 | 0 | 5.00 |
| 90 | 1344 | 735 | 1205 | 420 | 5.00 |
| 1128 | 1225 | 1350 | 1190 | 1691 | 4.59 |
| 3519 | 1149 | 4020 | 1111 | 3735 | 4.48 |
| 7380 | 1112 | 7620 | 1042 | 7950 | 4.55 |
| 11,550 | 1074 | 12,110 | 952 | 11,805 | 4.35 |
| 67,080 | 728 | 68,040 | 370 | 67,740 | 2.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Yudintceva, N.; Ryzhov, V.; Marchenko, Y.; Ischenko, A.; Zhakhov, A.; Dobrodumov, A.; Combs, S.E.; et al. Magnetic Relaxation Switching Assay Using IFNα-2b-Conjugated Superparamagnetic Nanoparticles for Anti-Interferon Antibody Detection. Biosensors 2023, 13, 624. https://doi.org/10.3390/bios13060624
Nikolaev B, Yakovleva L, Fedorov V, Yudintceva N, Ryzhov V, Marchenko Y, Ischenko A, Zhakhov A, Dobrodumov A, Combs SE, et al. Magnetic Relaxation Switching Assay Using IFNα-2b-Conjugated Superparamagnetic Nanoparticles for Anti-Interferon Antibody Detection. Biosensors. 2023; 13(6):624. https://doi.org/10.3390/bios13060624
Chicago/Turabian StyleNikolaev, Boris, Ludmila Yakovleva, Viacheslav Fedorov, Natalia Yudintceva, Vyacheslav Ryzhov, Yaroslav Marchenko, Alexander Ischenko, Alexander Zhakhov, Anatoliy Dobrodumov, Stephanie E. Combs, and et al. 2023. "Magnetic Relaxation Switching Assay Using IFNα-2b-Conjugated Superparamagnetic Nanoparticles for Anti-Interferon Antibody Detection" Biosensors 13, no. 6: 624. https://doi.org/10.3390/bios13060624
APA StyleNikolaev, B., Yakovleva, L., Fedorov, V., Yudintceva, N., Ryzhov, V., Marchenko, Y., Ischenko, A., Zhakhov, A., Dobrodumov, A., Combs, S. E., Gao, H., & Shevtsov, M. (2023). Magnetic Relaxation Switching Assay Using IFNα-2b-Conjugated Superparamagnetic Nanoparticles for Anti-Interferon Antibody Detection. Biosensors, 13(6), 624. https://doi.org/10.3390/bios13060624






