Simultaneous Visualization of MiRNA-221 and Caspase-3 in Cancer Cells for Investigating the Feasibility of MiRNA-Targeted Therapy with a Dual-Color Fluorescent Nanosensor
Abstract
:1. Introduction
2. Materials and Methods
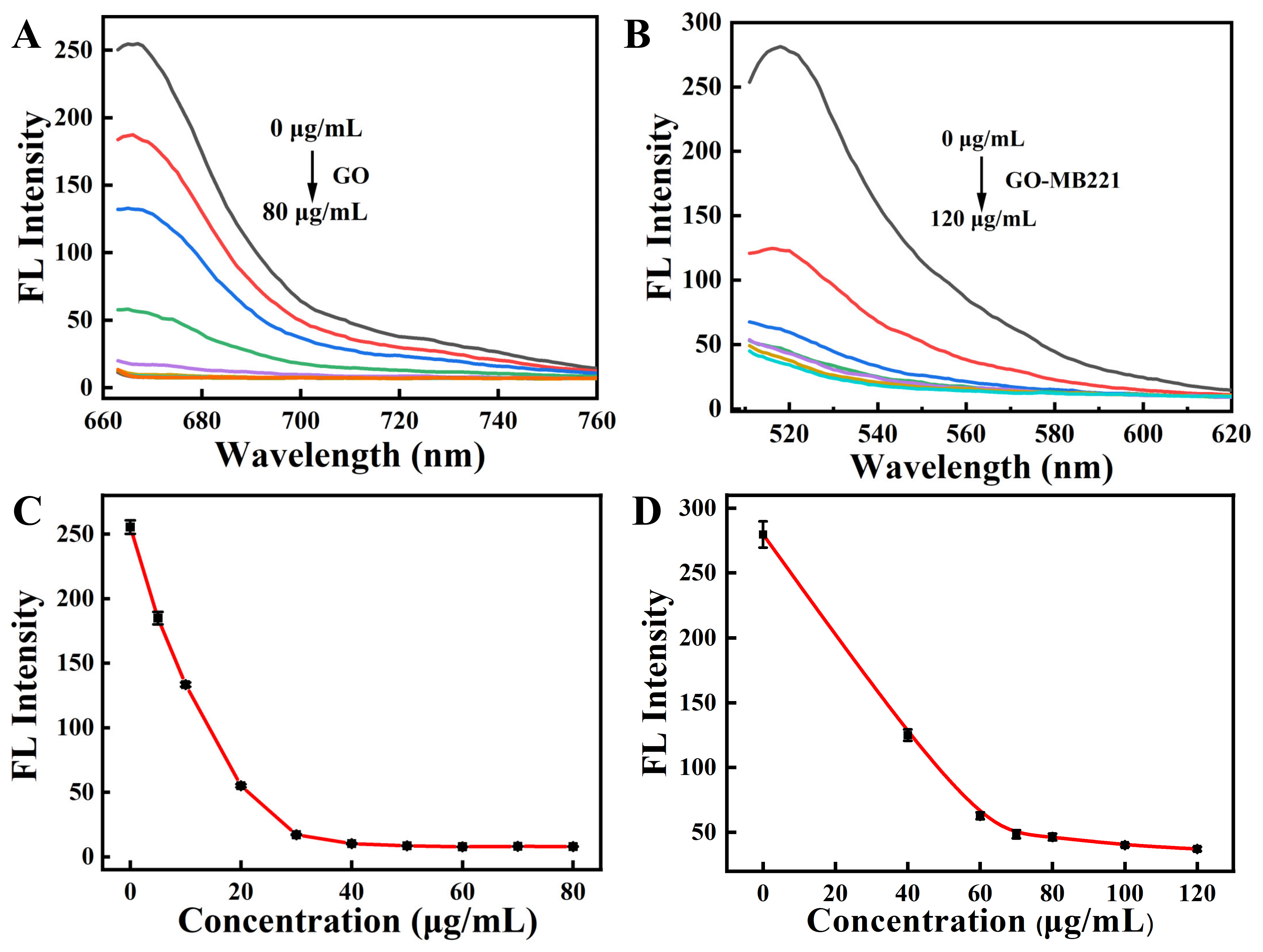
2.1. Fluorescence Quenching
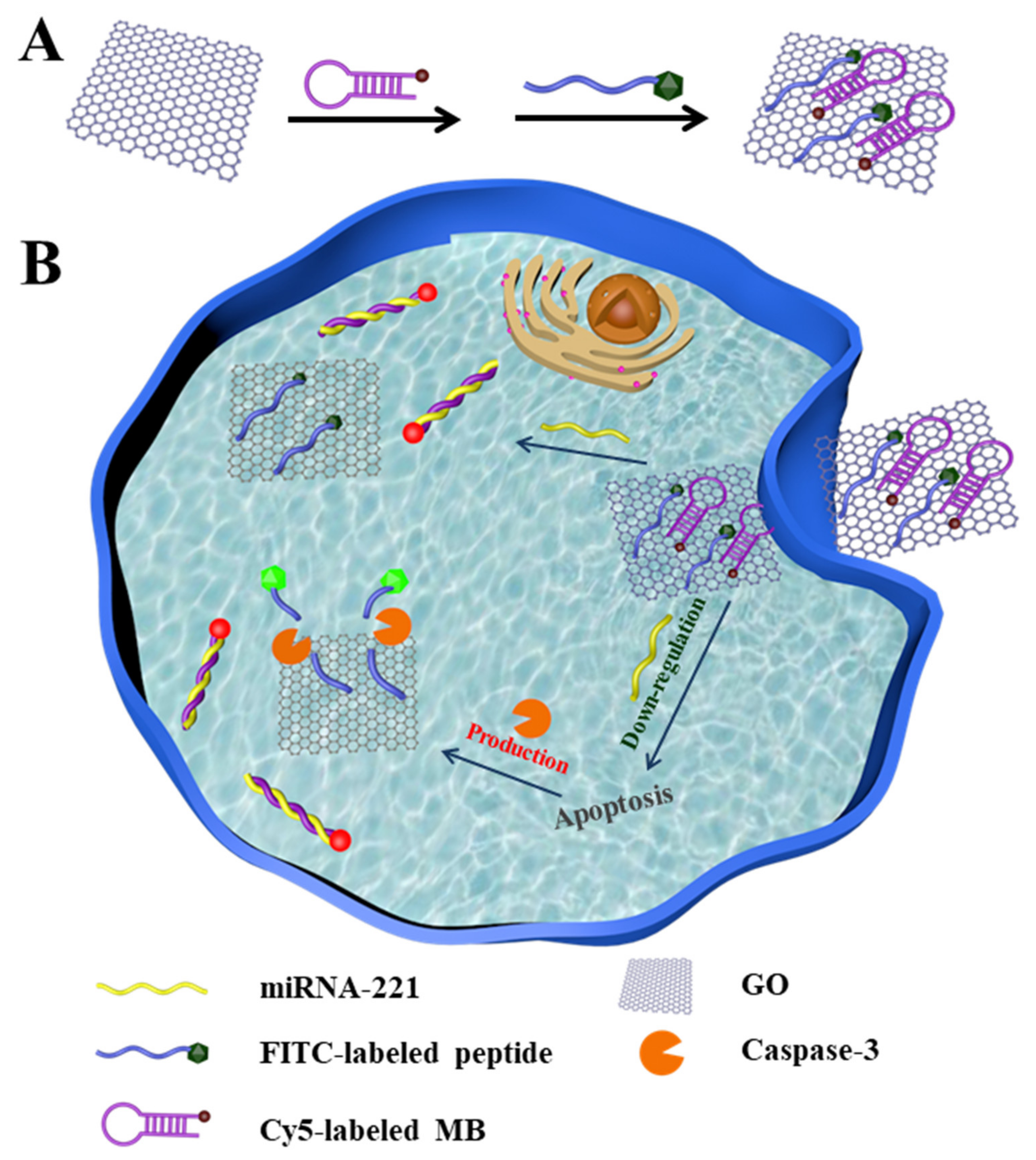
2.2. Preparation of the Fluorescent Nanosensor
2.3. Fluorescence Response of the Nanosensor
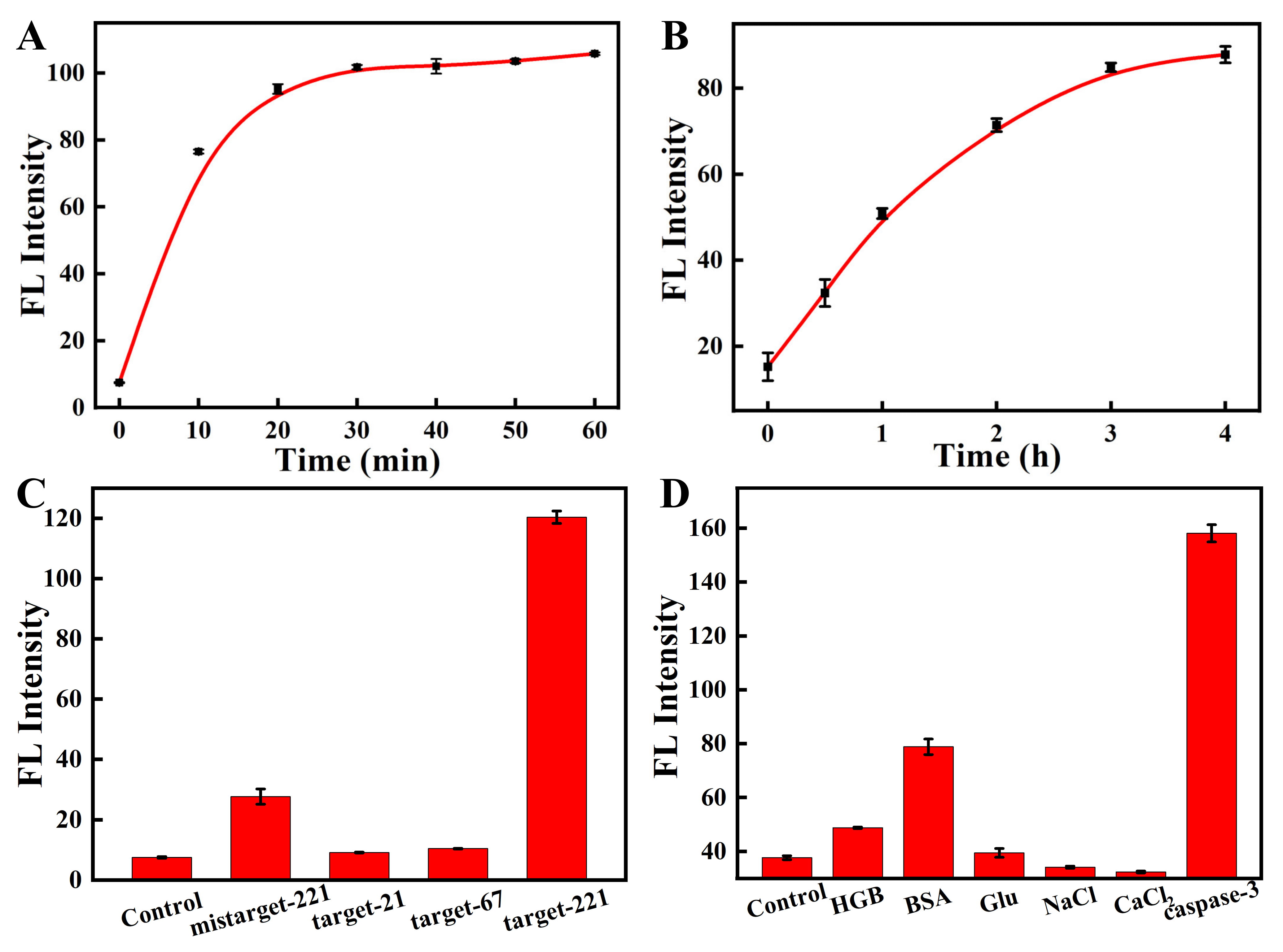
2.4. Kinetic Experiments
2.5. Specificity of the Nanosensor
2.6. Confocal Fluorescence Imaging
3. Results and Discussion
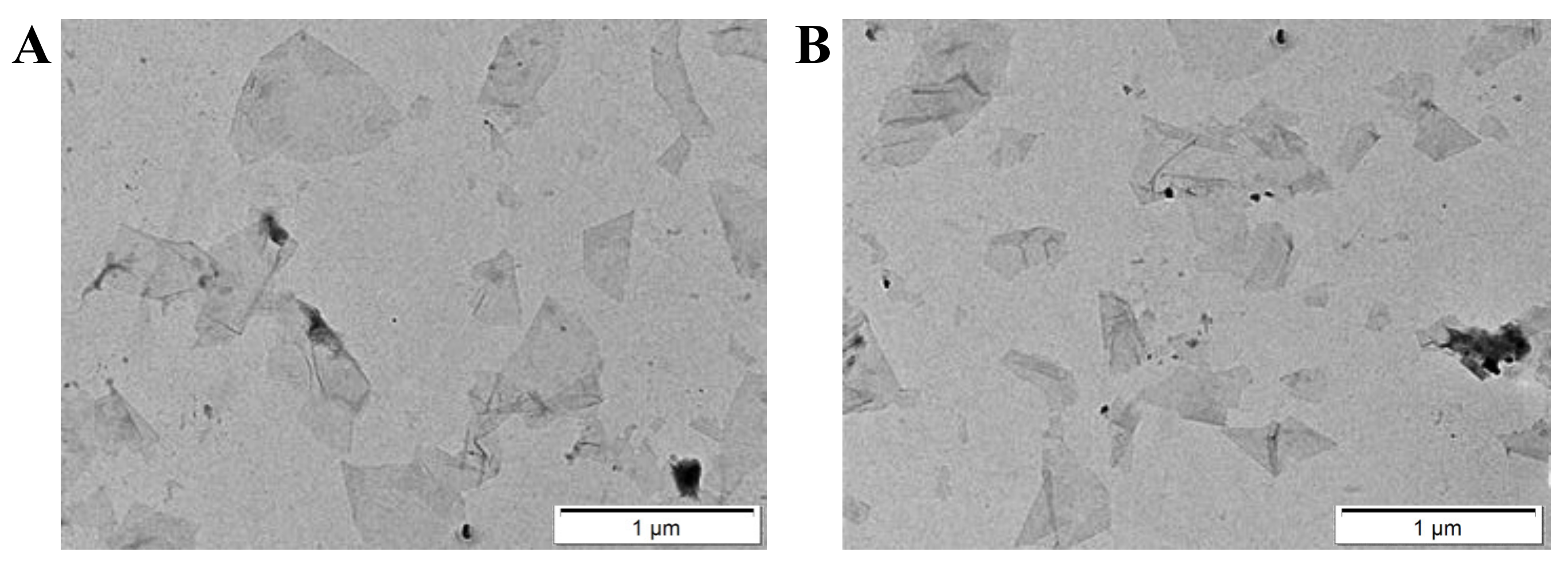
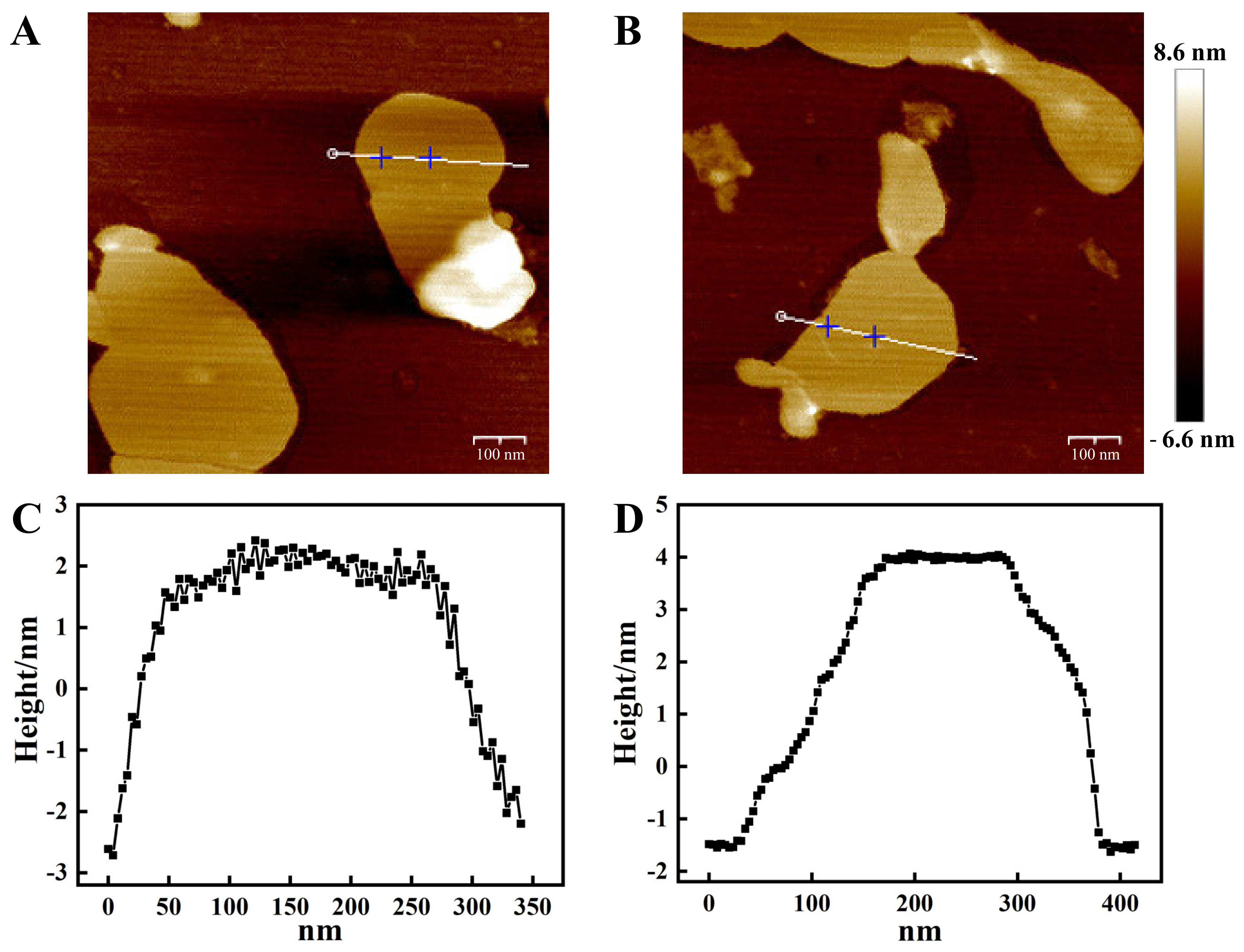
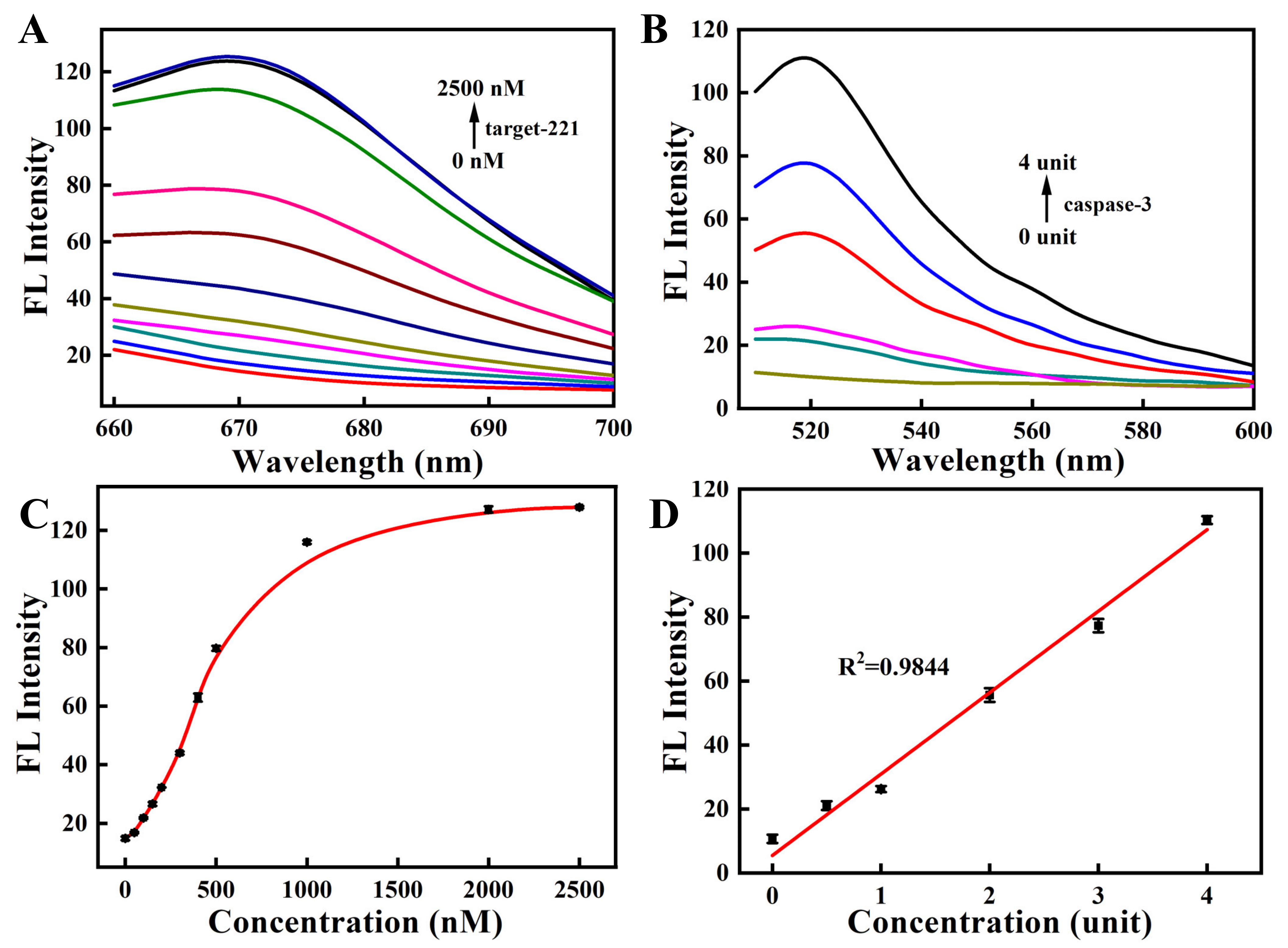
3.1. Preparation and Characterization of the Nanosensor
3.2. In Vitro Studies of the Nanosensor
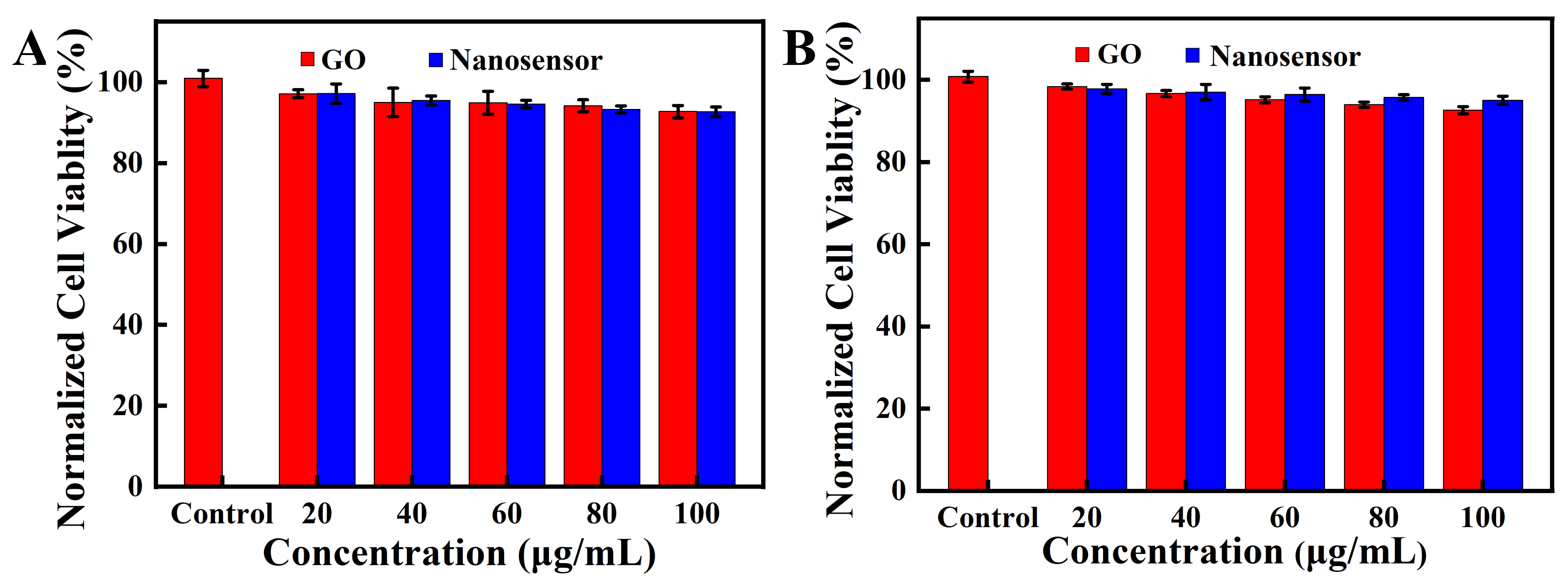
3.3. MTT Experiment
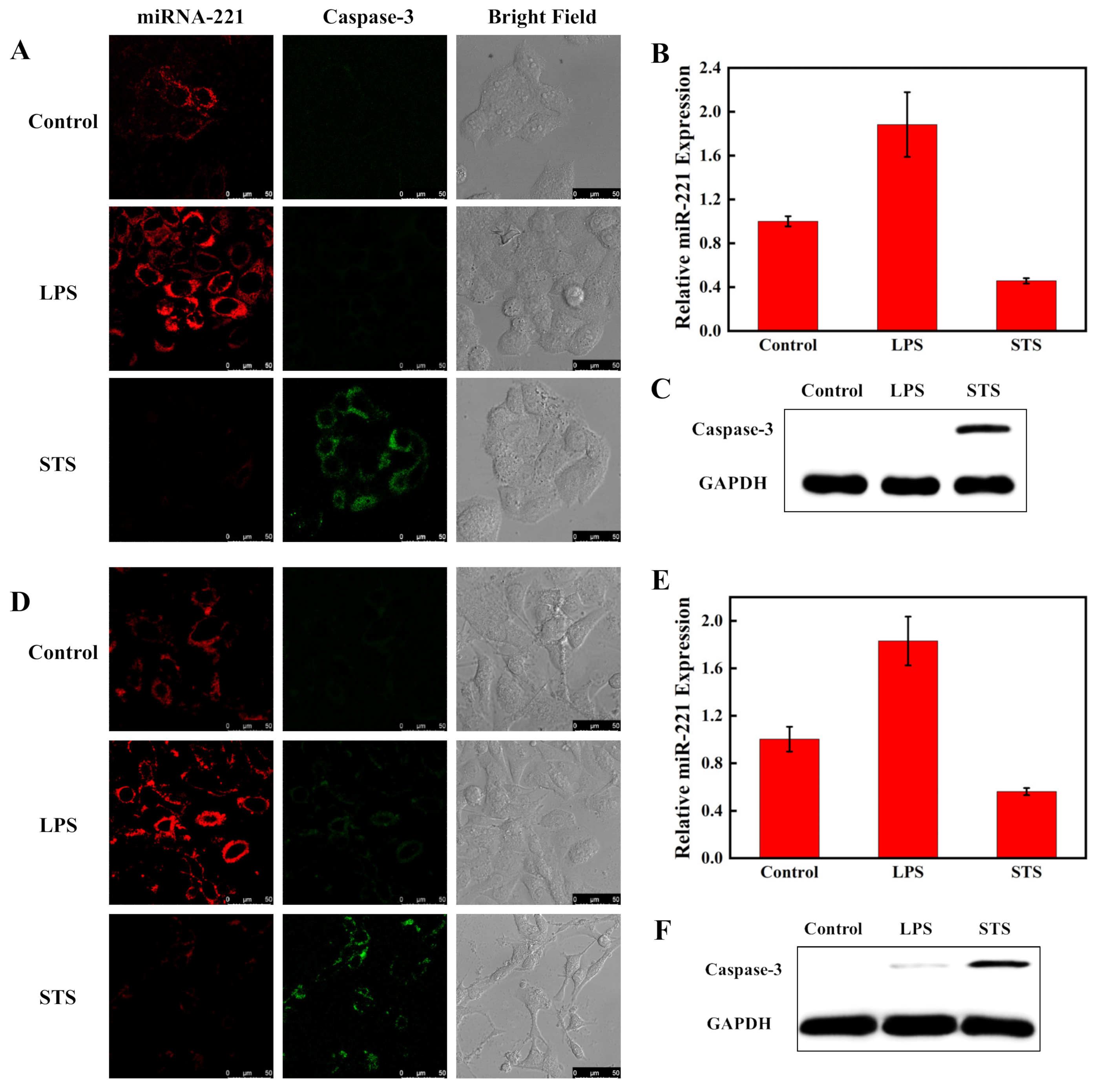
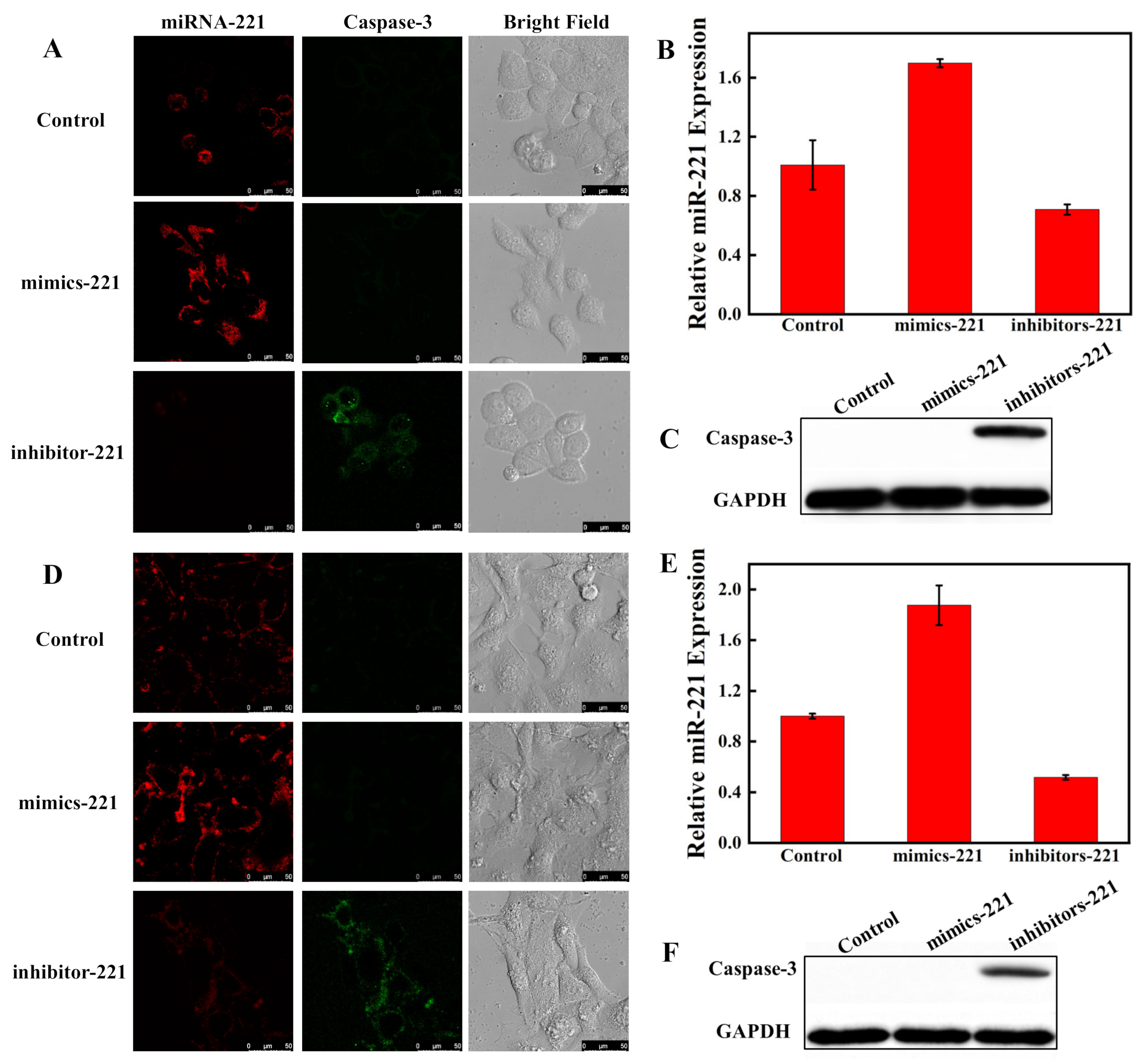
3.4. Imaging of MiRNA-221 and Caspase-3 in Living Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, A.A.; Letai, A.; Fisher, D.E.; Flaherty, K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 2015, 15, 747–756. [Google Scholar] [CrossRef]
- Hodson, R. Precision medicine. Nature 2016, 537, S49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in cancer. Annu. Rev. Pathol-Mech. 2014, 9, 287–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selcuklu, S.D.; Donoghue; Mark, T.A.; Spillane, C. miR-21 as a keyiew regulator of oncogenic processes. Biochem. Soc. T. 2009, 37, 918–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayoral, R.J.; Pipkin, M.E.; Pachkov, M.; van Nimwegen, E.; Rao, A.; Monticelli, S. MicroRNA-221–222 Regulate the Cell Cycle in Mast Cells. J. Immunol. 2009, 182, 433. [Google Scholar] [CrossRef]
- Boyerinas, B.; Park, S.-M.; Hau, A.; Murmann, A.E.; Peter, M.E. The role of let-7 in cell differentiation and cancer. Endocr-Relat. Cancer 2010, 17, F19–F36. [Google Scholar] [CrossRef]
- Chen, W.-X.; Hu, Q.; Qiu, M.-T.; Zhong, S.-L.; Xu, J.-J.; Tang, J.-H.; Zhao, J.-H. miR-221/222: Promising biomarkers for breast cancer. Tumor Biol. 2013, 34, 1361–1370. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [Green Version]
- Gibson, U.E.; Heid, C.A.; Williams, P.M. A novel method for real time quantitative RT-PCR. Genome Res. 1996, 6, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, R.; Duraiyan, J.; Kaliyappan, K.; Palanisamy, M. Microarray and its applications. J. Pharm. Bioallied Sci. 2012, 4, S310–S312. [Google Scholar] [CrossRef]
- Streit, S.; Michalski, C.W.; Erkan, M.; Kleeff, J.; Friess, H. Northern blot analysis for detection and quantification of RNA in pancreatic cancer cells and tissues. Nat. Protoc. 2009, 4, 37–43. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.-C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef]
- Duraiyan, J.; Govindarajan, R.; Kaliyappan, K.; Palanisamy, M. Applications of immunohistochemistry. J. Pharm. Bioallied Sci. 2012, 4, S307–S309. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Chi, H.; Yang, S.; Niu, Q.; Wu, D.; Cao, W.; Li, T.; Ma, H.; Wei, Q. Self-Luminescent Lanthanide Metal–Organic Frameworks as Signal Probes in Electrochemiluminescence Immunoassay. J. Am. Chem. Soc. 2021, 143, 504–512. [Google Scholar] [CrossRef]
- Yang, L.; Yin, X.; An, B.; Li, F. Precise Capture and Direct Quantification of Tumor Exosomes via a Highly Efficient Dual-Aptamer Recognition-Assisted Ratiometric Immobilization-Free Electrochemical Strategy. Anal. Chem. 2021, 93, 1709–1716. [Google Scholar] [CrossRef]
- Pan, W.; Liu, X.; Wan, X.; Li, J.; Li, Y.; Lu, F.; Li, N.; Tang, B. Rapid Preparation of Au–Se–Peptide Nanoprobe Based on a Freezing Method for Bioimaging. Anal. Chem. 2019, 91, 15982–15987. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, F.; Chi, H.; Zhao, G.; Zhang, Y.; Li, T.; Wei, Q. Electrochemical aptasensor based on gold modified thiol graphene as sensing platform and gold-palladium modified zirconium metal-organic frameworks nanozyme as signal enhancer for ultrasensitive detection of mercury ions. J. Colloid Interf. Sci. 2022, 606, 510–517. [Google Scholar] [CrossRef]
- Hong, C.; Baek, A.; Hah, S.S.; Jung, W.; Kim, D.-E. Fluorometric Detection of MicroRNA Using Isothermal Gene Amplification and Graphene Oxide. Anal. Chem. 2016, 88, 2999–3003. [Google Scholar] [CrossRef] [Green Version]
- Afzalinia, A.; Mirzaee, M. Ultrasensitive Fluorescent miRNA Biosensor Based on a “Sandwich” Oligonucleotide Hybridization and Fluorescence Resonance Energy Transfer Process Using an Ln(III)-MOF and Ag Nanoparticles for Early Cancer Diagnosis: Application of Central Composite Design. ACS Appl. Mater. Inter. 2020, 12, 16076–16087. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Qiu, Q.; Wen, Q.; Zhang, Q.; Yang, W.; Yuwen, L.; Weng, L.; Wang, L. Efficient biofunctionalization of MoS2 nanosheets with peptides as intracellular fluorescent biosensor for sensitive detection of caspase-3 activity. J. Colloid Interf. Sci. 2019, 543, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qiu, L.; Lv, P.; Han, A.; Fang, G.; Liu, J.; Wang, S. AuNP-peptide probe for caspase-3 detection in living cells by SERS. Analyst 2019, 144, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Guo, Y.; Li, J.; Peng, Z.; Bai, S.; Yang, S.; Wang, D.; Chen, H.; Xie, G. A nanoprobe for fluorescent monitoring of microRNA and targeted delivery of drugs. RSC Adv. 2021, 11, 8871–8878. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Y.-L.; Deng, W.-J.; Pan, Q.-S.; Yi, J.-T.; Chen, T.-T.; Chu, X. A graphene oxide nanosensor enables the co-delivery of aptamer and peptide probes for fluorescence imaging of a cascade reaction in apoptotic signaling. Analyst 2018, 143, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Li, N.; Pan, W.; Yang, L.; Yu, Z.; Tang, B. Simultaneous detection of multiple targets involved in the PI3K/AKT pathway for investigating cellular migration and invasion with a multicolor fluorescent nanoprobe. Chem. Commun. 2017, 53, 356–359. [Google Scholar] [CrossRef]
- Luan, M.; Chang, J.; Pan, W.; Chen, Y.; Li, N.; Tang, B. Simultaneous Fluorescence Visualization of Epithelial–Mesenchymal Transition and Apoptosis Processes in Tumor Cells for Evaluating the Impact of Epithelial–Mesenchymal Transition on Drug Efficacy. Anal. Chem. 2018, 90, 10951–10957. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Hou, T.; Huang, B.; Yang, L.; Li, F. Aptamer recognition-trigged label-free homogeneous electrochemical strategy for an ultrasensitive cancer-derived exosome assay. Chem. Commun. 2019, 55, 13705–13708. [Google Scholar] [CrossRef]
- Ma, H.; Xue, N.; Li, Z.; Xing, K.; Miao, X. Ultrasensitive detection of miRNA-155 using multi-walled carbon nanotube-gold nanocomposites as a novel fluorescence quenching platform. Sens. Actuat. B-Chem. 2018, 266, 221–227. [Google Scholar] [CrossRef]
- Liu, X.; Song, X.; Luan, D.; Hu, B.; Xu, K.; Tang, B. Real-Time in Situ Visualizing of the Sequential Activation of Caspase Cascade Using a Multicolor Gold–Selenium Bonding Fluorescent Nanoprobe. Anal. Chem. 2019, 91, 5994–6002. [Google Scholar] [CrossRef]
- Ma, S.; Qi, Y.-X.; Jiang, X.-Q.; Chen, J.-Q.; Zhou, Q.-Y.; Shi, G.; Zhang, M. Selective and Sensitive Monitoring of Cerebral Antioxidants Based on the Dye-Labeled DNA/Polydopamine Conjugates. Anal. Chem. 2016, 88, 11647–11653. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Zhang, H.; Chen, H.; Abualrejal, M.M.A.; Song, D.; Wang, Z. Six-in-one peptide functionalized upconversion@polydopamine nanoparticle-based ratiometric fluorescence sensing platform for real-time evaluating anticancer efficacy through monitoring caspase-3 activity. Sensors Actuat. B-Chem. 2021, 333, 129554. [Google Scholar] [CrossRef]
- Oudeng, G.; Au, M.; Shi, J.; Wen, C.; Yang, M. One-Step in Situ Detection of miRNA-21 Expression in Single Cancer Cells Based on Biofunctionalized MoS2 Nanosheets. ACS Appl. Mater. Inter. 2018, 10, 350–360. [Google Scholar] [CrossRef]
- Cetinel, S.; Shen, W.-Z.; Aminpour, M.; Bhomkar, P.; Wang, F.; Borujeny, E.R.; Sharma, K.; Nayebi, N.; Montemagno, C. Biomining of MoS2 with peptide-based smart biomaterials. Sci. Rep. 2018, 8, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Zhang, L.; Deng, Y.; Li, S.; He, N. Graphene oxide for rapid microRNA detection. Nanoscale 2012, 4, 5840–5842. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, B.-C.; Wang, X.-F.; Ye, B.-C. Interaction of peptides with graphene oxide and its application for real-time monitoring of protease activity. Chem. Commun. 2011, 47, 2399–2401. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety Considerations for Graphene: Lessons Learnt from Carbon Nanotubes. Accounts Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.-y.; Liu, Y. Investigation on fluorescence quenching of dyes by graphite oxide and graphene. Appl. Surf. Sci. 2011, 257, 5513–5518. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Zhang, G.; Zhang, Y.; Wang, H.; Cao, W.; Li, T.; Wei, Q. An electrochemical aptasensor based on gold-modified MoS2/rGO nanocomposite and gold-palladium-modified Fe-MOFs for sensitive detection of lead ions. Sens. Actuat. B-Chem. 2020, 319, 128313. [Google Scholar] [CrossRef]
- Pineau, P.; Volinia, S.; McJunkin, K.; Marchio, A.; Battiston, C.; Terris, B.; Mazzaferro, V.; Lowe, S.W.; Croce, C.M.; Dejean, A. miR-221 overexpression contributes to liver tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihle, M.A.; Trautmann, M.; Kuenstlinger, H.; Huss, S.; Heydt, C.; Fassunke, J.; Wardelmann, E.; Bauer, S.; Schildhaus, H.-U.; Buettner, R.; et al. miRNA-221 and miRNA-222 induce apoptosis via the KIT/AKT signalling pathway in gastrointestinal stromal tumours. Mol. Oncol. 2015, 9, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, X.-F.; Fu, D.-S.; Huang, J.-G.; Yang, S.-E. Clinical potential of miRNA-221 as a novel prognostic biomarker for hepatocellular carcinoma. Cancer Biomark. 2017, 18, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Finch, A.; Evan, G. Apoptosis in development. Nature 2000, 407, 796–801. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer Therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Bertrand, R.; Solary, E.; O’Connor, P.; Kohn, K.W.; Pommier, Y. Induction of a Common Pathway of Apoptosis by Staurosporine. Exp. Cell Res. 1994, 211, 314–321. [Google Scholar] [CrossRef]
- Qi, W.; Li, H.; Cai, X.-H.; Gu, J.-Q.; Meng, J.; Xie, H.-Q.; Zhang, J.-L.; Chen, J.; Jin, X.-G.; Tang, Q.; et al. Lipoxin A4 activates alveolar epithelial sodium channel gamma via the microRNA-21/PTEN/AKT pathway in lipopolysaccharide-induced inflammatory lung injury. Lab. Investig. 2015, 95, 1258–1268. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Chen, Z.; Ge, C.; Hu, W.; Wang, N.; Yang, L.; Luan, M.; Xu, J. Simultaneous Visualization of MiRNA-221 and Caspase-3 in Cancer Cells for Investigating the Feasibility of MiRNA-Targeted Therapy with a Dual-Color Fluorescent Nanosensor. Biosensors 2022, 12, 444. https://doi.org/10.3390/bios12070444
Ren M, Chen Z, Ge C, Hu W, Wang N, Yang L, Luan M, Xu J. Simultaneous Visualization of MiRNA-221 and Caspase-3 in Cancer Cells for Investigating the Feasibility of MiRNA-Targeted Therapy with a Dual-Color Fluorescent Nanosensor. Biosensors. 2022; 12(7):444. https://doi.org/10.3390/bios12070444
Chicago/Turabian StyleRen, Mingyao, Zhe Chen, Chuandong Ge, Wei Hu, Nianxing Wang, Limin Yang, Mingming Luan, and Jing Xu. 2022. "Simultaneous Visualization of MiRNA-221 and Caspase-3 in Cancer Cells for Investigating the Feasibility of MiRNA-Targeted Therapy with a Dual-Color Fluorescent Nanosensor" Biosensors 12, no. 7: 444. https://doi.org/10.3390/bios12070444
APA StyleRen, M., Chen, Z., Ge, C., Hu, W., Wang, N., Yang, L., Luan, M., & Xu, J. (2022). Simultaneous Visualization of MiRNA-221 and Caspase-3 in Cancer Cells for Investigating the Feasibility of MiRNA-Targeted Therapy with a Dual-Color Fluorescent Nanosensor. Biosensors, 12(7), 444. https://doi.org/10.3390/bios12070444



