Developments of Conventional and Microfluidic Flow Cytometry Enabling High-Throughput Characterization of Single Cells
Abstract
:1. Introduction
2. Scientific Meaning of Single-Cell Analysis
3. Clinical Demands of Single-Cell Analysis
4. Well-Established Optoelectronic Flow Cytometry (Hematology Analyzer)
4.1. Historical Development
4.2. DxH 900 of Beckman Coulter
4.3. XN-1000 of Sysmex
4.4. ADVIA 2120i of Siemens
4.5. CELL-DYN Ruby of Abbott
5. Microfluidic Optoelectronic Flow Cytometry for Characterizing Individual Blood Cells
5.1. Microfluidic Impedance Flow Cytometry
5.2. Microfluidic Imaging Flow Cytometry
6. Future Directions of Optoelectronic Flow Cytometry
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keating, S.M.; Taylor, D.L.; Plant, A.L.; Litwack, E.D.; Kuhn, P.; Greenspan, E.J.; Hartshorn, C.M.; Sigman, C.C.; Kelloff, G.J.; Chang, D.D.; et al. Opportunities and challenges in implementation of multiparameter single cell analysis platforms for clinical translation. Clin. Transl. Sci. 2018, 11, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mincarelli, L.; Lister, A.; Lipscombe, J.; Macaulay, I.C. Defining cell identity with single-cell omics. Proteomics 2018, 18, e1700312. [Google Scholar] [CrossRef] [PubMed]
- Strzelecka, P.M.; Ranzoni, A.M.; Cvejic, A. Dissecting human disease with single-cell omics: Application in model systems and in the clinic. Dis. Models Mech. 2018, 11, dmm036525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, P.; Gierahn, T.; Roederer, M.; Love, J.C. Single-cell technologies for monitoring immune systems. Nat. Immunol. 2014, 15, 128–135. [Google Scholar] [CrossRef]
- Satija, R.; Shalek, A. Heterogeneity in immune responses: From populations to single cells. Trends Immunol. 2014, 35, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Efremova, M.; Vento-Tormo, R.; Park, J.E.; Teichmann, S.A.; James, K.R. Immunology in the era of single-cell technologies. Annu. Rev. Immunol. 2020, 38, 727–757. [Google Scholar] [CrossRef] [Green Version]
- Kuipers, J.; Jahn, K.; Beerenwinkel, N. Advances in understanding tumour evolution through single-cell sequencing. Biochim. Biophys. Acta 2017, 1867, 127–138. [Google Scholar] [CrossRef]
- Lawson, D.A.; Kessenbrock, K.; Davis, R.T.; Pervolarakis, N.; Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 2018, 20, 1349–1360. [Google Scholar] [CrossRef]
- González-Silva, L.; Quevedo, L.; Varela, I. Tumor Functional Heterogeneity Unraveled by scRNA-seq Technologies. Trends Cancer 2020, 6, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Chabot-Richards, D.; George, T. White blood cell counts: Reference methodology. Clin. Lab. Med. 2015, 35, 11–24. [Google Scholar] [CrossRef]
- Green, R.; Wachsmann-Hogiu, S. Development, history, and future of automated cell counters. Clin. Lab. Med. 2015, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Brugnara, C. Automated hematology analyzers: State of the art. Clin. Lab. Med. 2015, 35, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Dong, H.; Berner, A.; Risberg, B. The diagnostic and research applications of flow cytometry in cytopathology. Diagn. Cytopathol. 2012, 40, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Virgo, P.; Gibbs, G. Flow cytometry in clinical pathology. Ann. Clin. Biochem. Int. J. Lab. Med. 2011, 49, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J.; Baumann, A.; Arguello, V. Recent advancements of flow cytometry: New applications in hematology and oncology. Expert Rev. Mol. Diagn. 2013, 14, 67–81. [Google Scholar] [CrossRef]
- Tan, H.; Su, X.; Chen, D.; Wang, J.; Lu, Y.; Chen, J. Future Directions of Biosensors for Single-Cell Analysis from the Perspective of Instrument Developments. In Biosensors for Single-Cell Analysis; Chen, J., Lu, Y., Eds.; Academic Press: London, UK, 2022; pp. 159–164. [Google Scholar]
- Honrado, C.; Bisegna, P.; Swami, N.S.; Caselli, F. Single-cell microfluidic impedance cytometry: From raw signals to cell phenotypes using data analytics. Lab Chip 2021, 21, 22–54. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, X.; Zhou, Z.; Han, Y.; Xiang, N.; Ni, Z. Microfluidic impedance cytometry for single-cell sensing: Review on electrode configurations. Talanta 2021, 233, 122571. [Google Scholar] [CrossRef]
- Han, Y.; Gu, Y.; Zhang, A.C.; Lo, Y.-H. Review: Imaging technologies for flow cytometry. Lab Chip 2016, 16, 4639–4647. [Google Scholar] [CrossRef]
- Stavrakis, S.; Holzner, G.; Choo, J.; DeMello, A. High-throughput microfluidic imaging flow cytometry. Curr. Opin. Biotechnol. 2018, 55, 36–43. [Google Scholar] [CrossRef]
- Gawad, S.; Schild, L.; Renaud, P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 2001, 1, 76–82. [Google Scholar] [CrossRef]
- Cheung, K.; Gawad, S.; Renaud, P. Impedance spectroscopy flow cytometry: On-chip label-free cell differentiation. Cytom. Part A 2005, 65A, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.; Pettigrew, D.; Reccius, C.H.; Gwyer, J.D.; Berkel, C.v.; Holloway, J.; Davies, D.E.; Morgan, H. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab Chip 2009, 9, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Goda, K.; Ayazi, A.; Gossett, D.R.; Sadasivam, J.; Lonappan, C.K.; Sollier, E.; Fard, A.M.; Hur, S.C.; Adam, J.; Murray, C.; et al. High-throughput single-microparticle imaging flow analyzer. Proc. Natl. Acad. Sci. USA 2012, 109, 11630–11635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Chen, D.; Li, H.; Luo, Y.; Deng, B.; Huang, S.-B.; Chiu, T.-K.; Wu, M.-H.; Long, R.; Hu, H.; et al. A microfluidic system enabling continuous characterization of specific membrane capacitance and cytoplasm conductivity of single cells in suspension. Biosens. Bioelectron. 2013, 43, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Ha, S.; Diez-Silva, M.; Dao, M.; Suresh, S.; Chandrakasan, A.P. Electric impedance microflow cytometry for characterization of cell disease states. Lab Chip 2013, 13, 3903–3909. [Google Scholar] [CrossRef] [Green Version]
- Watkins, N.; Hassan, U.; Damhorst, G.; Ni, H.; Vaid, A.; Rodriguez, W.; Bashir, R. Microfluidic CD4(+) and CD8(+) T lymphocyte counters for point-of-care HIV diagnostics using whole blood. Sci. Transl. Med. 2013, 5, 214ra170. [Google Scholar] [CrossRef]
- Spencer, D.; Elliott, G.; Morgan, H. A sheath-less combined optical and impedance micro-cytometer. Lab Chip 2014, 14, 3064–3073. [Google Scholar] [CrossRef]
- Han, Y.; Lo, Y.-H. Imaging cells in flow cytometer using spatial-temporal transformation. Sci. Rep. 2015, 5, 13267. [Google Scholar] [CrossRef] [Green Version]
- Hassan, U.; Ghonge, T.; Reddy, J.B.; Patel, M.; Rappleye, M.; Taneja, I.; Tanna, A.; Healey, R.; Manusry, N.; Price, Z.; et al. A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat. Commun. 2017, 8, 15949. [Google Scholar] [CrossRef]
- Wang, K.; Chang, C.-C.; Chiu, T.-K.; Zhao, X.; Chen, D.; Chou, W.-P.; Zhao, Y.; Wang, H.-M.; Wang, J.; Wu, M.-H.; et al. Membrane capacitance of thousands of single white blood cells. J. R. Soc. Interface 2017, 14, 20170717. [Google Scholar] [CrossRef]
- Rane, A.S.; Rutkauskaite, J.; deMello, A.; Stavrakis, S. High-throughput multi-parametric imaging flow cytometry. Chem 2017, 3, 588–602. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Tang, R.; Gu, Y.; Zhang, A.C.; Cai, W.; Castor, V.; Cho, S.H.; Alaynick, W.; Lo, Y.-H. Cameraless high-throughput three-dimensional imaging flow cytometry. Optica 2019, 6, 1297–1304. [Google Scholar] [CrossRef]
- Spencer, D.; Morgan, H. High-speed single-cell dielectric spectroscopy. ACS Sens. 2020, 5, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Holzner, G.; Mateescu, B.; Leeuwen, D.v.; Cereghetti, G.; Dechant, R.; Stavrakis, S.; deMello, A. High-throughput multiparametric imaging flow cytometry: Toward diffraction-limited sub-cellular detection and monitoring of sub-cellular processes. Cell Rep. 2021, 34, 108824. [Google Scholar] [CrossRef]
- Tan, H.; Wang, M.; Zhang, Y.; Huang, X.; Chen, D.; Li, Y.; Wu, M.-H.; Wang, K.; Wang, J.; Chen, J. Inherent bioelectrical parameters of hundreds of thousands of single leukocytes based on impedance flow cytometry. Cytom. Part A 2022, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Tan, H.; Chen, D.; Lei, Y.; Li, Y.; Wang, J.; Chen, J. Inherent single-cell bioelectrical parameters of thousands of neutrophils, eosinophils and basophils derived from impedance flow cytometry. Cytom. Part A 2022. [Google Scholar] [CrossRef]
- Caselli, F.; Reale, R.; Ninno, A.D.; Spencer, D.; Morgan, H.; Bisegna, P. Deciphering impedance cytometry signals with neural networks. Lab Chip 2022, 22, 1714–1722. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, R.; Chen, X.; Waller, L.; Kau, A.; Fung, A.A.; Gutierrez, B.; An, C.; Cho, S.H.; Shi, L.; et al. A high-throughput technique to map cell images to cell positions using a 3D imaging flow cytometer. Proc. Natl. Acad. Sci. USA 2022, 119, e2118068119. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Y.; Chen, D.; Li, Y.; Wang, Y.; Wang, J.; Chen, J. Development of microfluidic flow cytometry capable of characterization of single-cell intrinsic structural and electrical parameters. J. Micromech. Microeng. 2022, 32, 035007. [Google Scholar] [CrossRef]
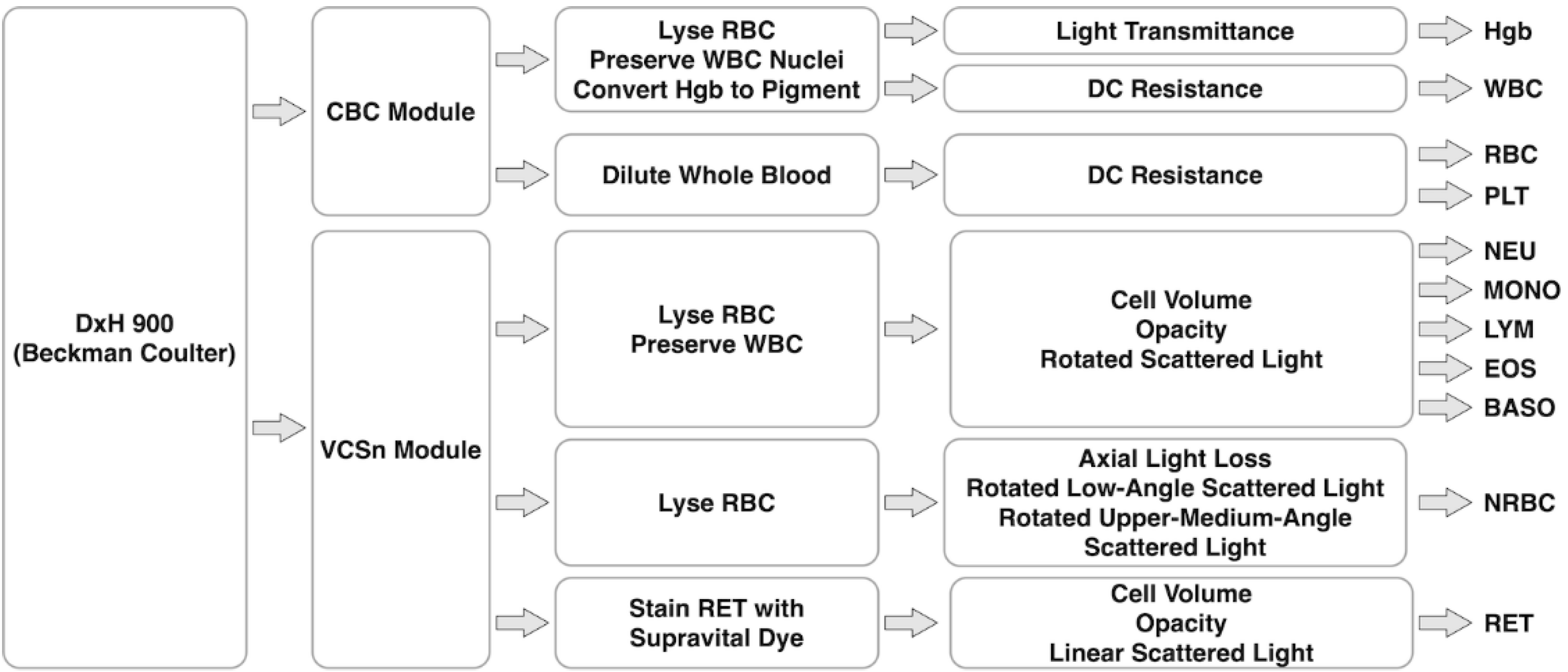

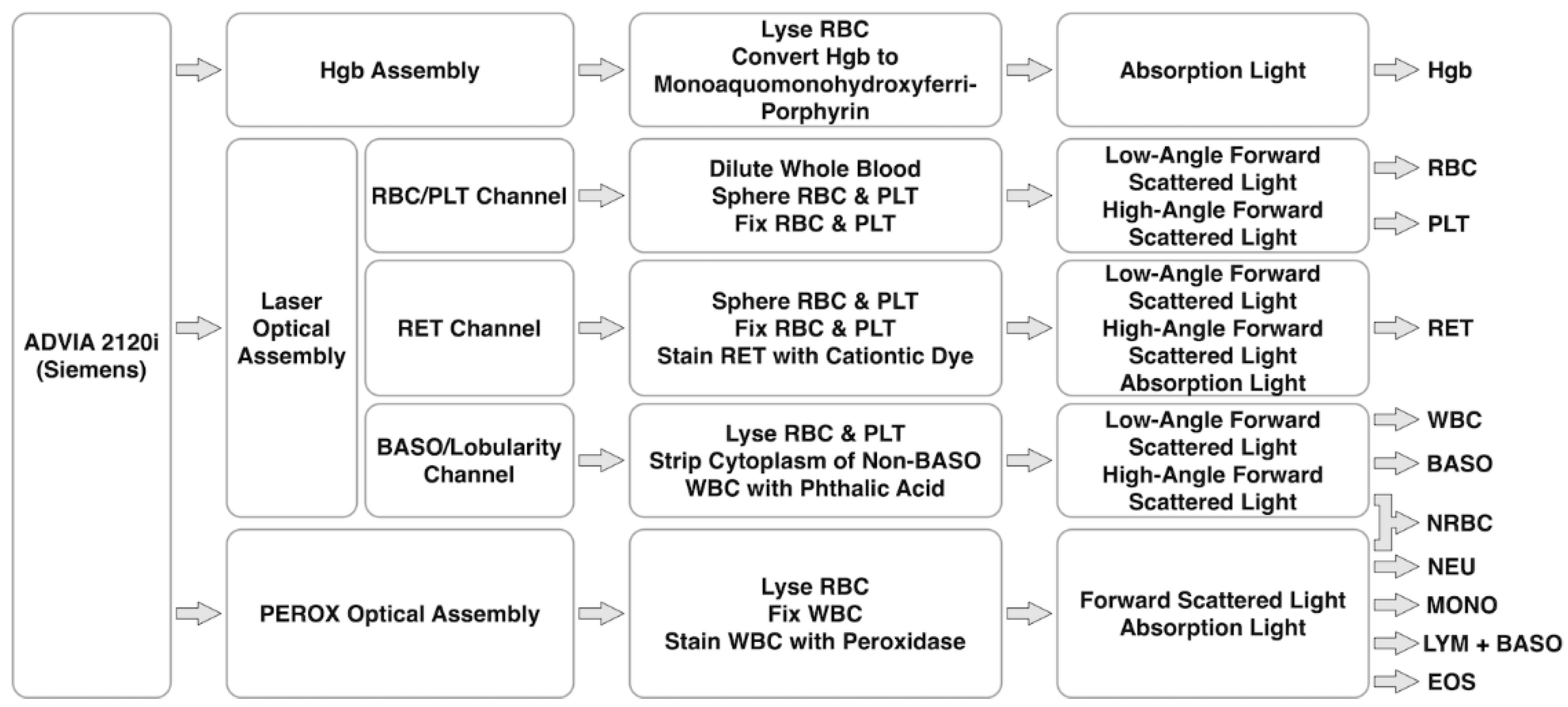

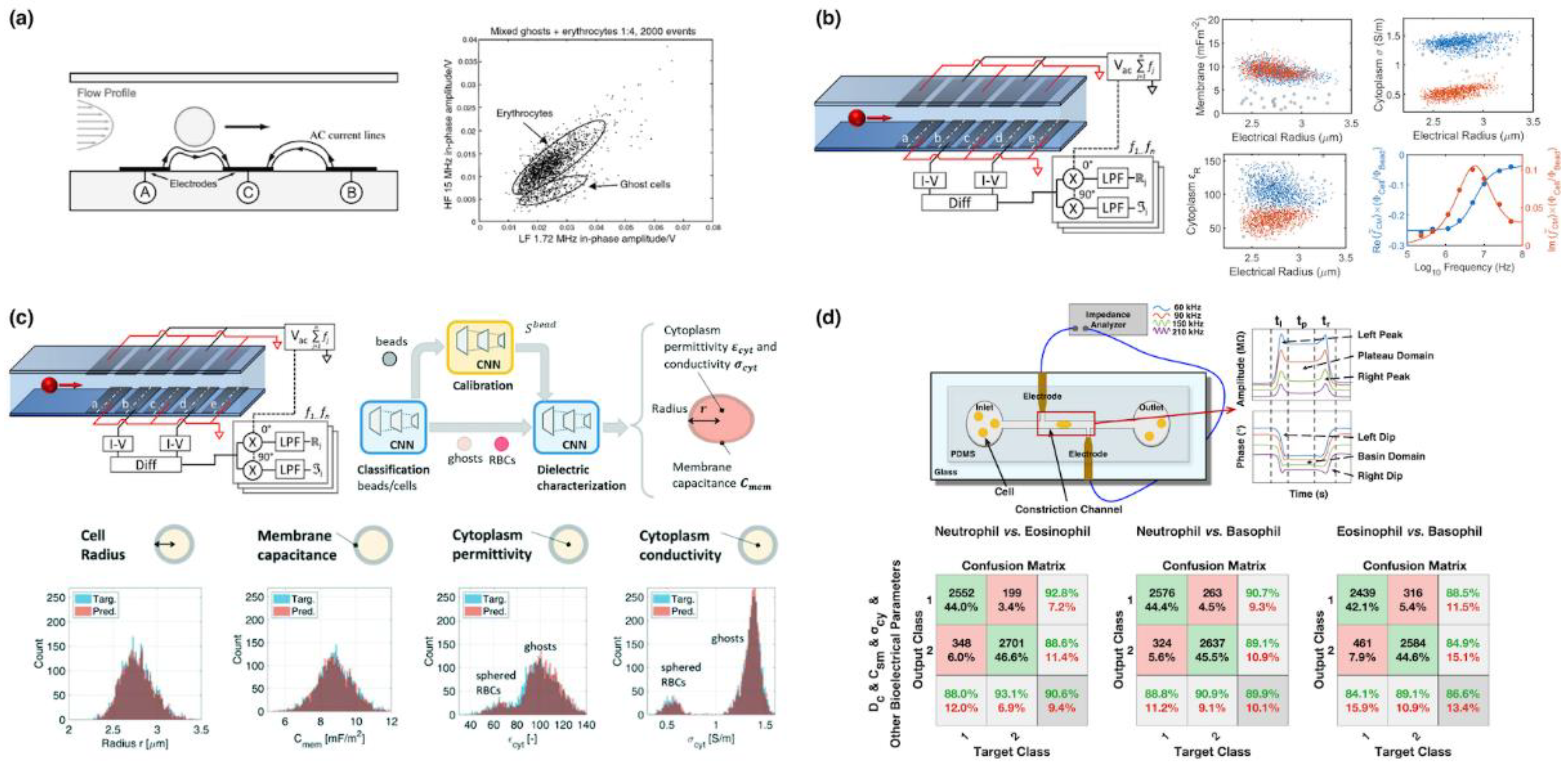
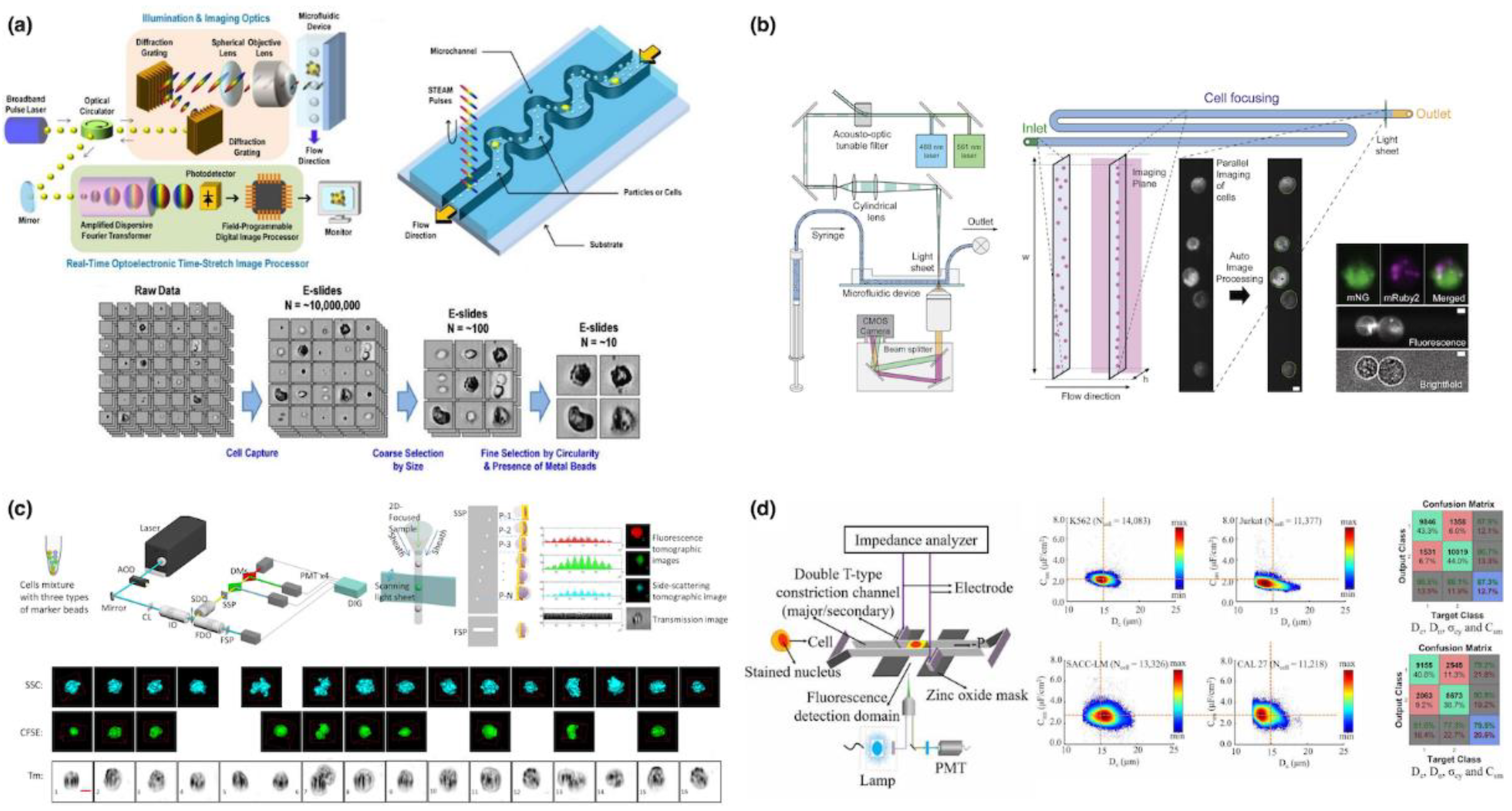
| Year | Instrument | Manufacturer | Methodology | Parameter |
|---|---|---|---|---|
| 1950s | Model A | Coulter Electronics | Direct Current (DC) Resistance | |
| 1970s | Model S Plus | Coulter Electronics | DC Resistance | Three-Part Differential of WBC |
| 1980s | Model STKs | Coulter Electronics | DC/AC (Alternating Current) Impedance & Optical Scattering | Five-Part Differential of WBC |
| 1980s | Sysmex NE-8000 | TOA Medical Electronics | DC/AC Impedance & Cell Treatment | Five-Part Differential of WBC |
| 1980s | CELL-DYN 3000 | Abbott | Multiple-Angle Optical Scattering | Five-Part Differential of WBC |
| 2000s | ADVIA 2120i | Siemens | Multiple-Angle Optical Scattering & Cell Treatment | Five-Part Differential of WBC, NRBC, RET |
| 2010s | DxH 900 | Beckman Coulter | DC/AC Impedance & Multiple-Angle Optical Scattering & Cell Treatment | Five-Part Differential of WBC, NRBC, RET |
| 2010s | XN-1000 | Sysmex | Multiple-Angle Optical Scattering and Fluorescence & Cell Treatment | Five-Part Differential of WBC, NRBC, RET, IG |
| Year | Group | Methodology | Result | Ref |
|---|---|---|---|---|
| 2001 | Renaud@EPFL | Coplanar Microelectrode + Impedance | RBC vs. Ghost Based on AC Impedance | [21] |
| 2005 | Renaud@EPFL | Parallel Microelectrode + Impedance | RBC vs. Fixed RBC vs. Ghost Based on AC Impedance | [22] |
| 2009 | Morgan@Southampton | Parallel Microelectrode + Impedance | Three-Part Differential of WBC Based on AC Impedance | [23] |
| 2012 | Goda@UCLA | Inertial Focusing + PMT | WBC vs. MCF-7, 100,000 cells/s, Differentiation | [24] |
| 2013 | Chen@CAS and Sun@Toronto | Constriction Microchannel + Impedance | RBC vs. Neonatal RBC Based on Cell Diameter, Specific Membrane Capacitance and Cytoplasmic Conductivity | [25] |
| 2013 | Dao@MIT | Coplanar Microelectrode + Impedance | RBC vs. P. falciparum Infected RBC Based on AC Impedance | [26] |
| 2013 | Bashir@UIUC | Coplanar Microelectrode + Impedance | CD4+ and CD8+ LYM Based on AC Impedance | [27] |
| 2014 | Morgan@Southampton | Parallel Microelectrode + Optical Waveguide | Three-Part Differential of WBC Based on AC Impedance, Optical Scattering and Fluorescence | [28] |
| 2015 | Lo@UCSD | Microfabricated Window + PMT | A549, 1000 cells/s, Imaging | [29] |
| 2017 | Bashir@UIUC | Coplanar Microelectrode + Impedance | CD64+ NEU and MONO Based on AC Impedance | [30] |
| 2017 | Chen@CAS | Constriction Microchannel + Impedance | GRA vs. LYM Based on Membrane Capacitance and Specific Membrane Capacitance | [31] |
| 2017 | deMello@ETH | Inertial Focusing + sCMOS | HL-60, HeLa, Live, Early and Late Apoptotic Jurkat, 50,000 cells/s, Imaging | [32] |
| 2019 | Lo@UCSD | 3D Microfabricated Window + PMT | HEK-293, CMK3, 500 cells/s, Imaging | [33] |
| 2020 | Morgan@Southampton | Parallel Microelectrode + Maxwell’s Mixture Theory | RBC vs. Ghost Based on Cell Diameter, Specific Membrane Capacitance, Cytoplasmic Conductivity and Cytoplasm Permittivity | [34] |
| 2021 | deMello@ETH | Viscoelastic Focusing + sCMOS | Yeasts, 293T, B-Lymphoid, Jurkat, 60,000 cells/s, Imaging | [35] |
| 2022 | Chen@CAS | Constriction Microchannel + Impedance | Three-Part Differential of WBC Based on Cell Diameter, Specific Membrane Capacitance and Cytoplasmic Conductivity | [36] |
| 2022 | Chen@CAS | Constriction Microchannel + Impedance | Five-Part Differential of WBC Based on AC Impedance | [37] |
| 2022 | Morgan@Southampton | Parallel Microelectrode + Convolutional Neural Network | RBC vs. Ghost Based on Cell Diameter, Membrane Capacitance, Cytoplasm Conductivity, Cytoplasm Permittivity | [38] |
| 2022 | Lo@UCSD | 3D Microfabricated Window + PMT | HEK-293, HeLa, MCF-7, MCF-10A, 1000 cells/s, Differentiation | [39] |
| 2022 | Chen@CAS | Constriction Microchannel + Microfabricated Window + Impedance + PMT | K562 vs. Jurkat, SACC-LM vs. CAL-27, Differentiation | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Liang, H.; Chen, X.; Chen, D.; Wang, J.; Zhang, Y.; Chen, J. Developments of Conventional and Microfluidic Flow Cytometry Enabling High-Throughput Characterization of Single Cells. Biosensors 2022, 12, 443. https://doi.org/10.3390/bios12070443
Wang M, Liang H, Chen X, Chen D, Wang J, Zhang Y, Chen J. Developments of Conventional and Microfluidic Flow Cytometry Enabling High-Throughput Characterization of Single Cells. Biosensors. 2022; 12(7):443. https://doi.org/10.3390/bios12070443
Chicago/Turabian StyleWang, Minruihong, Hongyan Liang, Xiao Chen, Deyong Chen, Junbo Wang, Yuan Zhang, and Jian Chen. 2022. "Developments of Conventional and Microfluidic Flow Cytometry Enabling High-Throughput Characterization of Single Cells" Biosensors 12, no. 7: 443. https://doi.org/10.3390/bios12070443
APA StyleWang, M., Liang, H., Chen, X., Chen, D., Wang, J., Zhang, Y., & Chen, J. (2022). Developments of Conventional and Microfluidic Flow Cytometry Enabling High-Throughput Characterization of Single Cells. Biosensors, 12(7), 443. https://doi.org/10.3390/bios12070443







