Solid-Phase Synthesis of Red Fluorescent Carbon Dots for the Dual-Mode Detection of Hexavalent Chromium and Cell Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
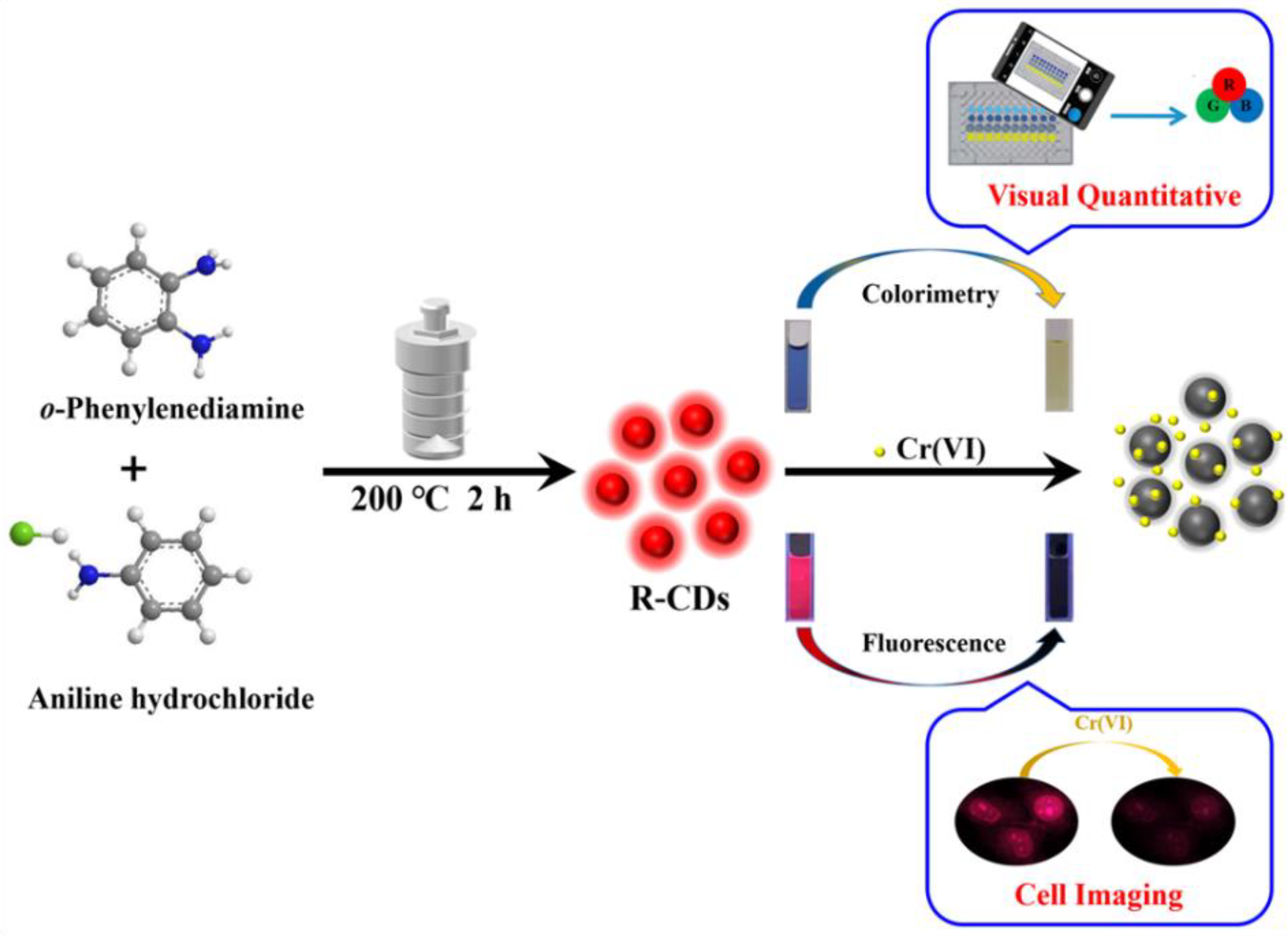
2.3. Preparation of R-CDs
2.4. Determination of Cr(VI)
2.5. Cellular Imaging
3. Results and Discussion
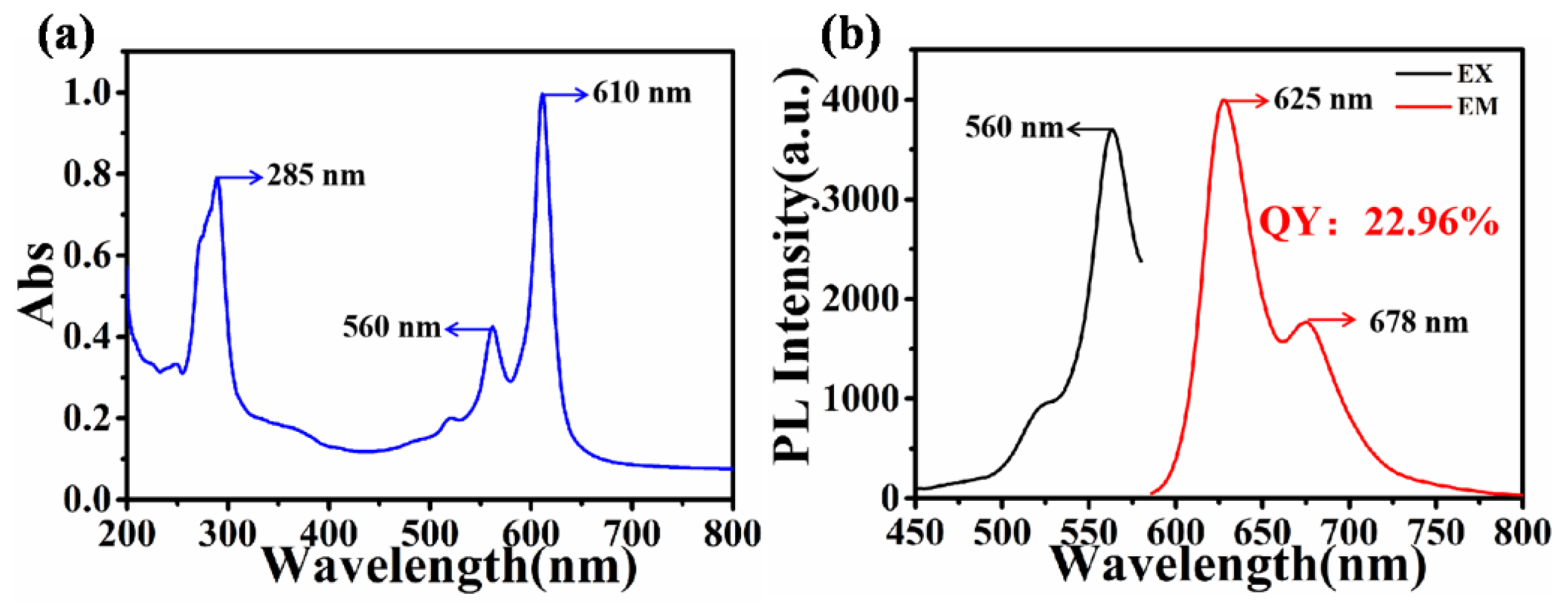
3.1. Characterization of R-CDs
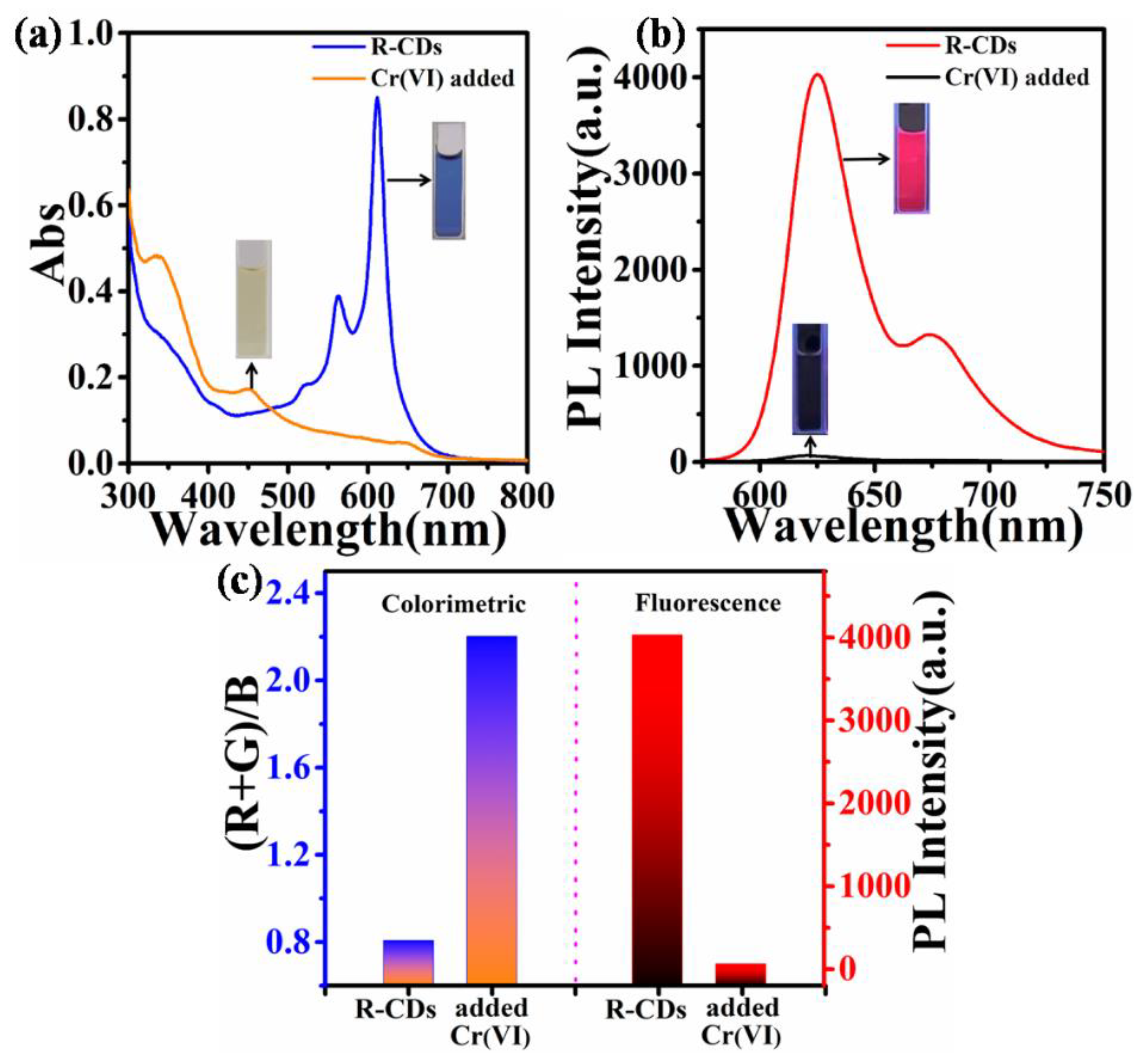
3.2. Dual-Mode Detection of Cr(VI)
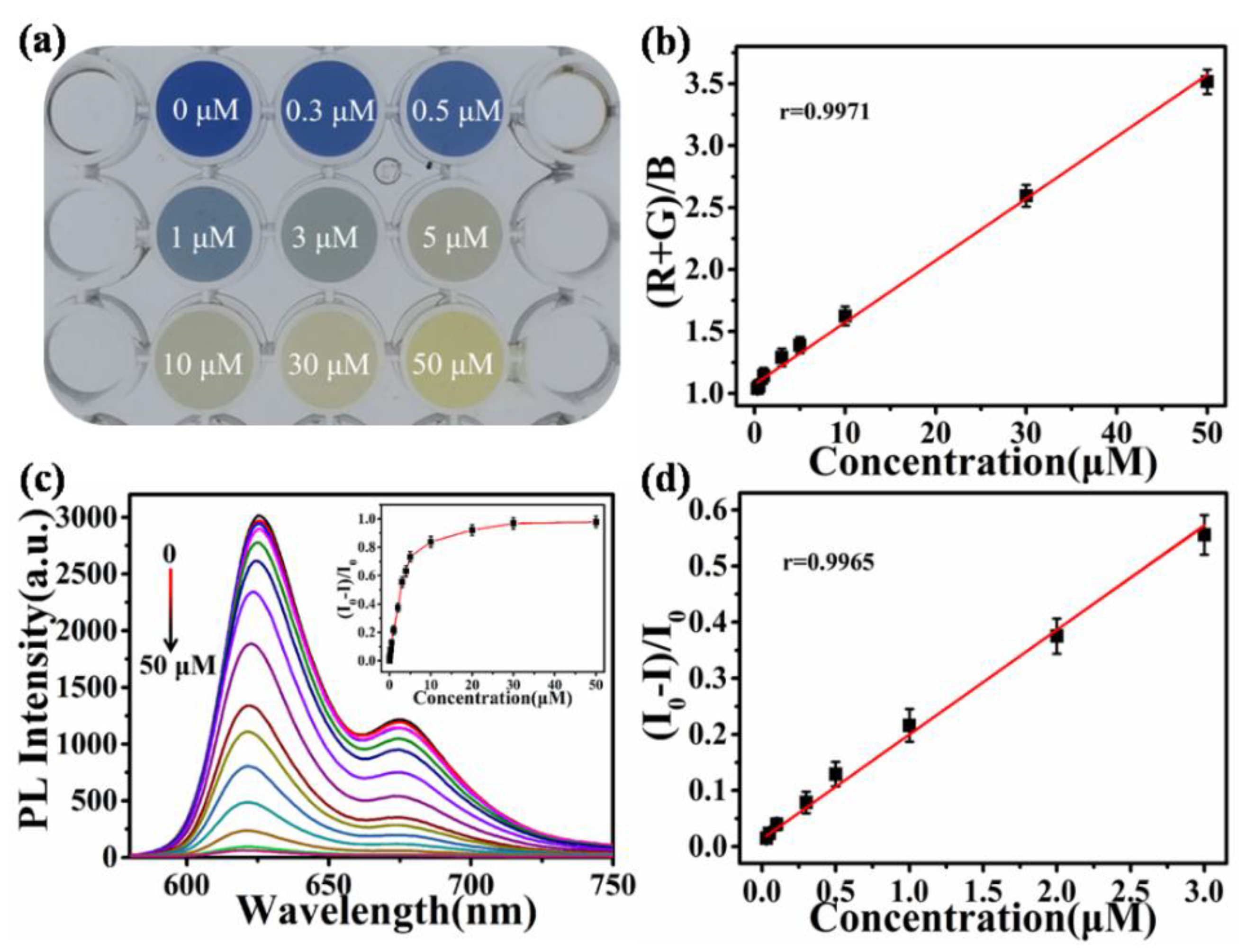
3.3. Analytical Performance
3.4. Selectivity
3.5. Detection of Cr(VI) in Real Water Samples
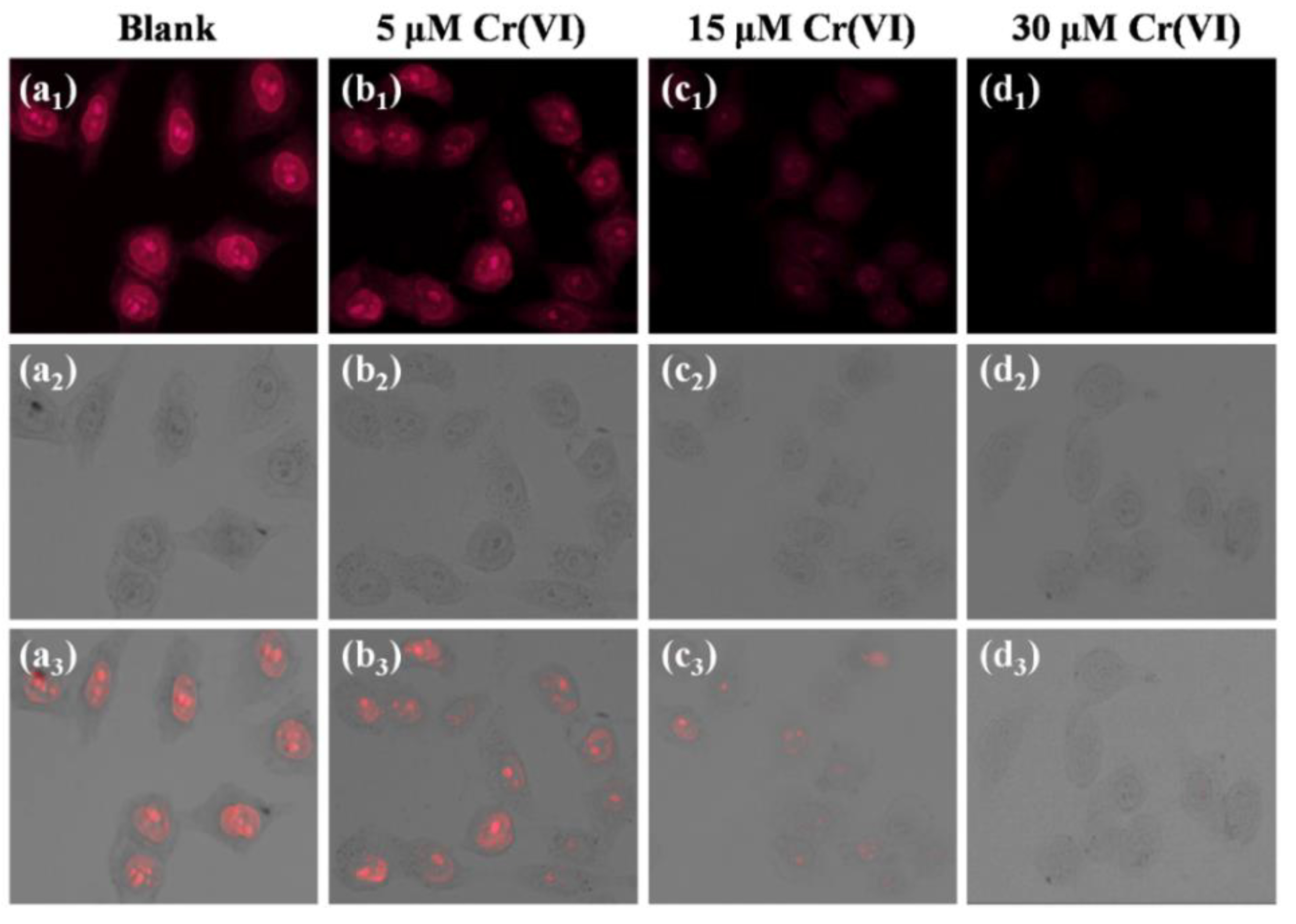
3.6. Intracellular Imaging of Cr(VI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.G.; Zhao, G.H.; Zhang, G.Y.; Zhang, Y.; Wang, H.; Cao, W.; Li, T.D.; Wei, Q. An electrochemical aptasensor based on gold-modified MoS2/rGO nanocomposite and gold-palladium-modified Fe-MOFs for sensitive detection of lead ions. Sens. Actuators B Chem. 2020, 319, 128313. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wang, Y.Y.; Wang, F.Z.; Chi, H.; Zhao, G.H.; Zhang, Y.; Li, T.D.; Wei, Q. Electrochemical aptasensor based on gold modified thiol graphene as sensing platform and gold-palladium modified zirconium metal-organic frameworks nanozyme as signal enhancer for ultrasensitive detection of mercury ions. J. Colloid Interface Sci. 2022, 606, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Madhwal, D.; Jain, V.K.; Suman, A. POC device for on-the-spot detection of hexavalent chromium in wastewater. J. Environ. Chem. Eng. 2020, 8, 104342. [Google Scholar] [CrossRef]
- Jia, M.Y.; Peng, L.L.; Yang, M.H.; Wei, H.Z.; Zhang, M.; Wang, Y. Carbon dots with dual emission: A versatile sensing platform for rapid assay of Cr(VI). Carbon 2021, 182, 42–50. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.Q. α-Fe2O3/polyaniline nanocomposites as an effective catalyst for improving the electrochemical performance of microbial fuel cell. Chem. Eng. J. 2018, 339, 539–546. [Google Scholar] [CrossRef]
- Alahmad, W.; Tungkijanansim, N.; Kaneta, T.; Varanusupakul, P. A colorimetric paper-based analytical device coupled with hollow fiber membrane liquid phase microextraction (HF-LPME) for highly sensitive detection of hexavalent chromium in water samples. Talanta 2018, 190, 78–84. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, S.J.; Yao, L.L.; Wang, L.; Qu, H.; Wu, Y.; Chen, Y.; Zheng, L. Single-atom nanozyme enabled fast and highly sensitive colorimetric detection of Cr(VI). J. Hazard. Mater. 2021, 408, 124898. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency, 2020. Chromium in Drinking Water. Available online: https://www.epa.gov/sdwa/chromium-drinking-water (accessed on 5 December 2022).
- Tian, T.; He, Y.; Ge, Y.L.; Song, G.W. One-pot synthesis of boron and nitrogen co-doped carbon dots as the fluorescence probe for dopamine based on the redox reaction between Cr(VI) and dopamine. Sensor Actuators B-Chem. 2017, 240, 1265–1271. [Google Scholar] [CrossRef]
- Liang, P.; Shi, T.Q.; Lu, H.B.; Jiang, Z.C.; Hu, B. Speciation of Cr(III) and Cr(VI) by nanometer titanium dioxide micro-column and inductively coupled plasma atomic emission spectrometry. Spectrochim. Acta B 2003, 58, 1709–1714. [Google Scholar] [CrossRef]
- Puthusseri, R.M.; Nair, H.P.; Johny, T.K.; Bhat, S.G. Insights into the response of mangrove sediment microbiomes to heavy metal pollution: Ecological risk assessment and metagenomics perspectives. J. Environ. Manag. 2021, 298, 113492. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhao, G.H.; Chi, H.; Yang, S.H.; Niu, Q.F.; Wu, D.; Cao, W.; Li, T.D.; Ma, H.M.; Wei, Q. Self-luminescent Lanthanide metal-organic frameworks as signal probes in electrochemiluminescence immunoassay. J. Am. Chem. Soc. 2021, 143, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thahur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Q.; Tan, Z.; Chen, X.; Chen, X.M.; Cheng, L.; Wu, H.M.; Li, P.W.; Zhang, Z.W. Carnation-like morphology of BiVO4-7 enables sensitive photoelectrochemical determination of Cr(VI) in the food and environment. Biosensors 2022, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, X.; Liu, J.; Wang, K.; Zhao, R.S.; Yang, S.H. Ingenious aspartic acid-functionalized gold nanoparticles by one-pot protocol for the sensitive detection of chromium (III) ions. Microchem. J. 2020, 159, 105359. [Google Scholar] [CrossRef]
- Othong, J.; Boonmak, J.; Kielar, F.; Youngme, S. Dual function based on switchable colorimetric luminescence for water and temperature sensing in two-dimensional metal-organic framework nanosheets. ACS Appl. Mater. Inter. 2020, 12, 41776–41784. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Han, X.Y.; Zhang, L.W.; Wang, X.Y.; Chen, L.X. Fluorescent probe for mercury ion imaging analysis: Strategies and applications. Chem. Eng. J. 2021, 406, 127166. [Google Scholar] [CrossRef]
- Xia, C.L.; Zhu, S.J.; Feng, T.L.; Yang, M.X.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Su, D.D.; Han, X.S.; Yan, X.; Jin, R.; Li, H.X.; Kong, D.S.; Gao, H.; Liu, F.M.; Sun, P.; Lu, G.Y. Smartphone-assisted robust sensing platform for on-site quantitation of 2,4-dichlorophenoxyacetic acid using red emissive carbon dots. Anal. Chem. 2020, 92, 12716–12724. [Google Scholar] [CrossRef]
- Tang, J.Z.; Chen, S.Q.; Jia, Y.L.; Xie, H.M.; Quan, X.; Ding, Q. Carbon dots as an additive for improving performance in water-based lubricants for amorphous carbon (a-C) coatings. Carbon 2020, 156, 272–281. [Google Scholar] [CrossRef]
- Phan, L.M.T.; Hoang, T.X.; Cho, S.B. Fluorescent carbon dots for sensitive and rapid monitoring of intracellular ferrous ion. Biosensors 2022, 12, 41. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Zhai, Y.L.; Li, Z.H.; Zhu, P.Y.; Mao, S.; Zhu, C.Z.; Du, D.; Belfiore, L.A.; Tang, J.G.; Lin, Y.H. Red carbon dots: Optical property regulations and applications. Mater. Today 2019, 30, 52–79. [Google Scholar] [CrossRef]
- Wang, R.N.; Huang, Z.H.; Ding, L.F.; Yang, F.Y.; Peng, D. Carbon dot powders with cross-linking-based long-wavelength emission for multicolor imaging of latent fingerprints. ACS Appl. Nano Mater. 2022, 5, 2214–2221. [Google Scholar] [CrossRef]
- Soni, N.; Singh, S.; Sharma, S.; Batra, G.; Kaushik, K.; Rao, C.; Verma, N.C.; Mandal, B.; Yadav, A.; Nandi, C.K. Absorption and emission of light in red emissive carbon nanodots. Chem. Sci. 2021, 12, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.L.; Ma, Y.S.; Yuan, G.J.; Chen, X.; Mei, J.; Zhang, L.; Ren, L.L. Solvent-controlled and solvent-dependent strategies for the synthesis of multicolor carbon dots for pH sensing and cell imaging. J. Mater. Chem. C 2019, 7, 9709. [Google Scholar] [CrossRef]
- Sun, Z.H.; Yan, F.Y.; Xu, J.; Zhang, H.; Chen, L. Solvent-controlled synthesis strategy of multicolor emission carbon dots and its applications in sensing and light-emitting devices. Nano Res. 2022, 15, 414–422. [Google Scholar] [CrossRef]
- Zhao, C.X.; Li, X.; Cheng, C.S.; Yang, Y.L. Green and microwave-assisted synthesis of carbon dots and application for visual detection of cobalt(II) ions and pH sensing. Microchem. J. 2019, 147, 183–190. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Z.X.; Li, C.M.; Xiao, X.C.; Li, J.; Liu, X.M.; Cheng, H. Yellow-emitting hydrophobic carbon dots via solid-phase synthesis and their applications. ACS Omega 2020, 5, 22587–22595. [Google Scholar] [CrossRef]
- Crista, D.M.A.; Mragui, A.E.; Algarra, M.; Silva, J.C.G.; Luque, R.; Silva, L.P.D. Turning spent coffee grounds into sustainable precursors for the fabrication of carbon dots. Nanomaterials 2020, 10, 1209. [Google Scholar] [CrossRef]
- Ji, C.Y.; Han, Q.R.; Zhou, Y.Q.; Wu, J.J.; Shi, W.Q.; Gao, L.P.; Leblanc, R.M.; Peng, Z.L. Phenylenediamine-derived near infrared carbon dots: The kilogram-scale preparation, formation process, photoluminescence tuning mechanism and application as red phosphors. Carbon 2022, 192, 198–208. [Google Scholar] [CrossRef]
- Li, X.C.; Xing, X.J.; Zhao, S.J.; Zhu, S.H.; Wang, B.H.; Lan, M.H.; Song, X.Z. Carbon dot-based fluorescent and colorimetric sensor for sensitive and selective visual detection of benzoyl peroxide. Chin. Chem. Lett. 2022, 33, 1632–1636. [Google Scholar] [CrossRef]
- Wang, J.L.; Zheng, J.X.; Yang, Y.Z.; Liu, X.G.; Qiu, J.S.; Tian, Y. Tunable full-color solid-state fluorescent carbon dots for light emitting diodes. Carbon 2022, 190, 22–31. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, D.H.; Huang, H.W.; Liu, L.F.; Zhou, Y.; Xia, X.D.; Deng, K.Q.; Liu, X.Y. Preparation of gold-carbon dots and ratiometric fluorescence cellular imaging. ACS Appl. Mater. Inter. 2016, 8, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Zhang, S.R.; Cui, J.Z.; Gao, W.S.; Rong, X.; Lu, Y.X.; Gao, C.Z. Preparation of nitrogen-doped carbon quantum dots from chelating agent and used as fluorescent probes for accurate detection of ClO− and Cr(VI). Anal. Chim. Acta 2022, 1195, 339478. [Google Scholar] [CrossRef]
- Gao, Y.F.; Jiao, Y.; Lu, W.J.; Liu, Y.; Han, H.; Gong, X.J.; Xian, M.; Shuang, S.M.; Dong, C. Carbon dots with red emission as a fluorescent and colorimeteric dual-readout probe for the detection of chromium(VI) and cysteine and its logic gate operation. J. Mater. Chem. B 2018, 6, 6099–6107. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.X.; Liu, Q.R.; Zhao, N.; Liu, J.M.; Fang, G.Z.; Wang, S. Ratiometric determination of Cr(VI) based on a dual-emission fluorescent nanoprobe using carbon quantum dots and a smartphone app. Microchim. Acta 2021, 188, 89. [Google Scholar] [CrossRef] [PubMed]


| Sample | Spiked (μM) | Image | Founded (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Tap water | 1.00 |  | 0.97 | 97.4 | 2.1 |
| 10.0 |  | 9.73 | 97.3 | 1.5 | |
| 30.0 |  | 30.54 | 101.8 | 2.3 | |
| Rainwater | 1.00 |  | 1.03 | 103.4 | 3.7 |
| 10.00 |  | 9.67 | 96.7 | 2.8 | |
| 30.00 |  | 29.79 | 99.3 | 1.2 | |
| Pearl Spring | 1.00 |  | 1.07 | 107.2 | 2.5 |
| 10.00 |  | 9.65 | 96.5 | 3.7 | |
| 30.00 |  | 29.54 | 98.5 | 1.3 | |
| Daming Lake | 1.00 |  | 1.05 | 105.8 | 1.6 |
| 10.00 |  | 9.87 | 98.7 | 2.8 | |
| 30.00 |  | 29.21 | 97.4 | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wang, X.; Wei, H.; Zhao, L.; Yao, B.; Zhang, C.; Zhou, J.; Liu, J.; Yang, S. Solid-Phase Synthesis of Red Fluorescent Carbon Dots for the Dual-Mode Detection of Hexavalent Chromium and Cell Imaging. Biosensors 2022, 12, 432. https://doi.org/10.3390/bios12060432
Hu J, Wang X, Wei H, Zhao L, Yao B, Zhang C, Zhou J, Liu J, Yang S. Solid-Phase Synthesis of Red Fluorescent Carbon Dots for the Dual-Mode Detection of Hexavalent Chromium and Cell Imaging. Biosensors. 2022; 12(6):432. https://doi.org/10.3390/bios12060432
Chicago/Turabian StyleHu, Jinshuang, Xin Wang, Hua Wei, Lei Zhao, Boxuan Yao, Caiyun Zhang, Jiarui Zhou, Jian Liu, and Shenghong Yang. 2022. "Solid-Phase Synthesis of Red Fluorescent Carbon Dots for the Dual-Mode Detection of Hexavalent Chromium and Cell Imaging" Biosensors 12, no. 6: 432. https://doi.org/10.3390/bios12060432
APA StyleHu, J., Wang, X., Wei, H., Zhao, L., Yao, B., Zhang, C., Zhou, J., Liu, J., & Yang, S. (2022). Solid-Phase Synthesis of Red Fluorescent Carbon Dots for the Dual-Mode Detection of Hexavalent Chromium and Cell Imaging. Biosensors, 12(6), 432. https://doi.org/10.3390/bios12060432





