Biomimetic Self-Adhesive Structures for Wearable Sensors
Abstract
:1. Introduction
2. Materials and Methods
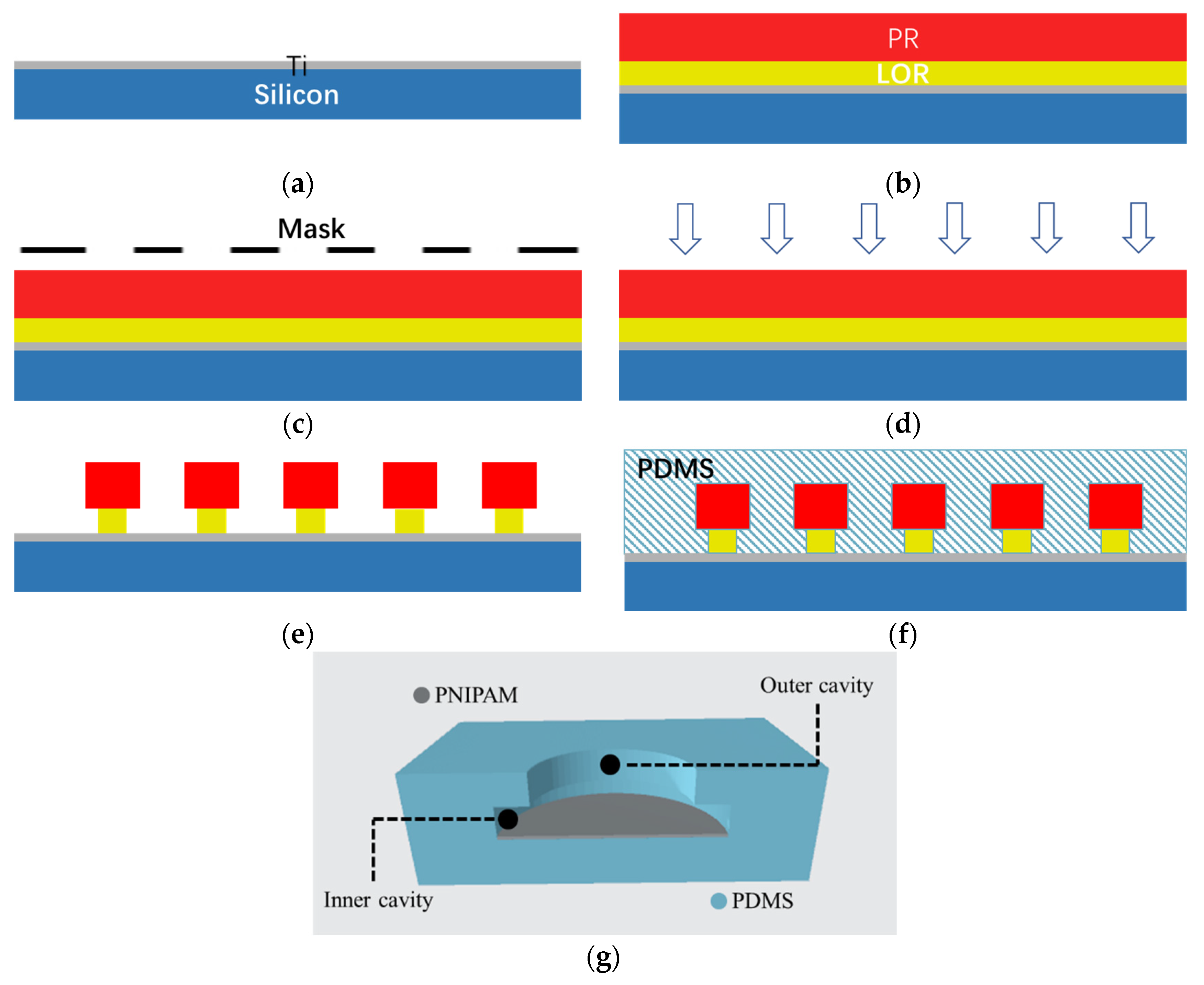
2.1. The Biomimetic Octopus Sucker Structure
2.1.1. Preparation of the Biomimetic Structure
2.1.2. Coating of the Thermosensitive Hydrogel
2.2. The Biomimetic Mussel Mucus Hydrogel
2.3. The Biomimetic Micro–Nano Fusion Structure
3. Results and Discussion
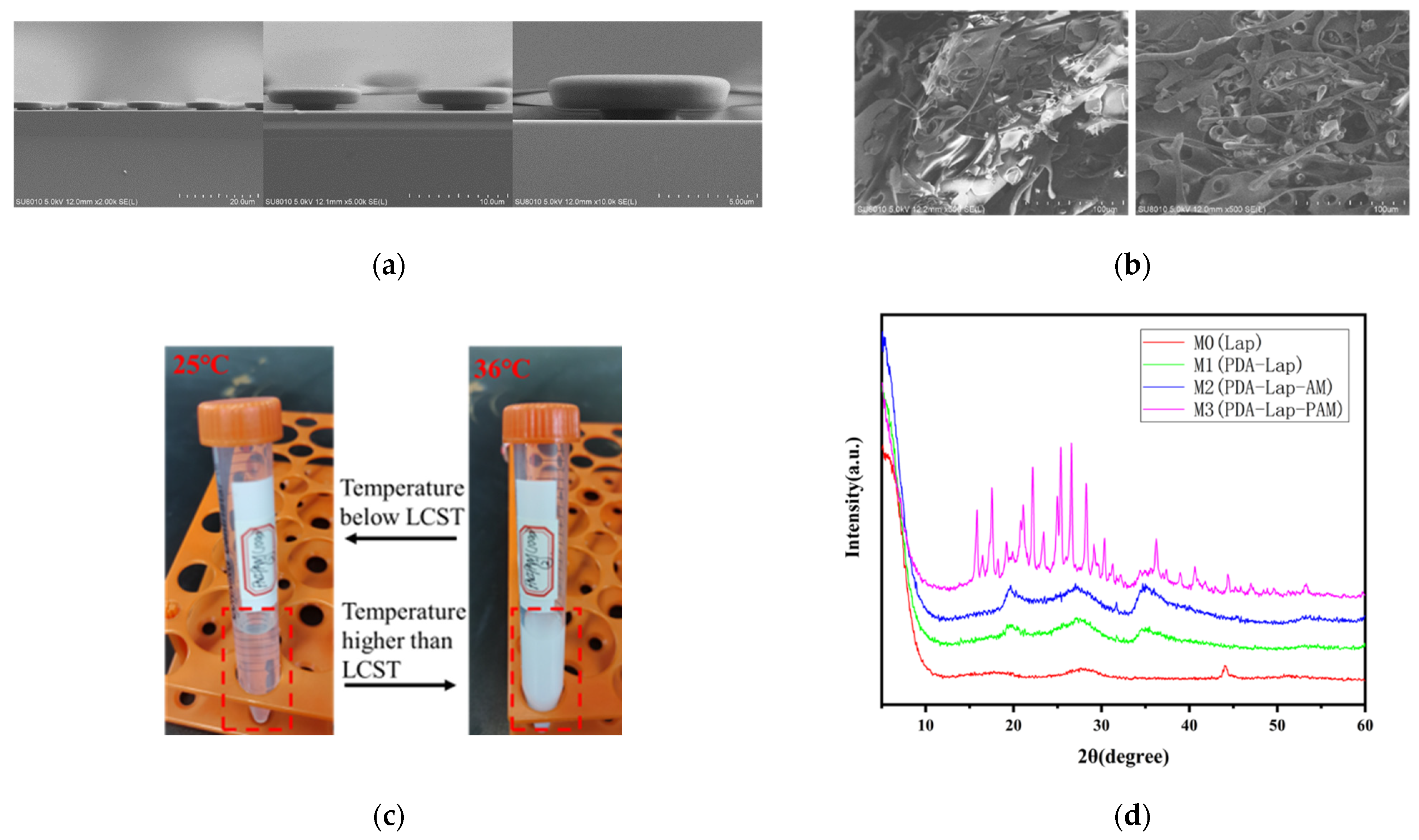
3.1. Preparation Result Characterization
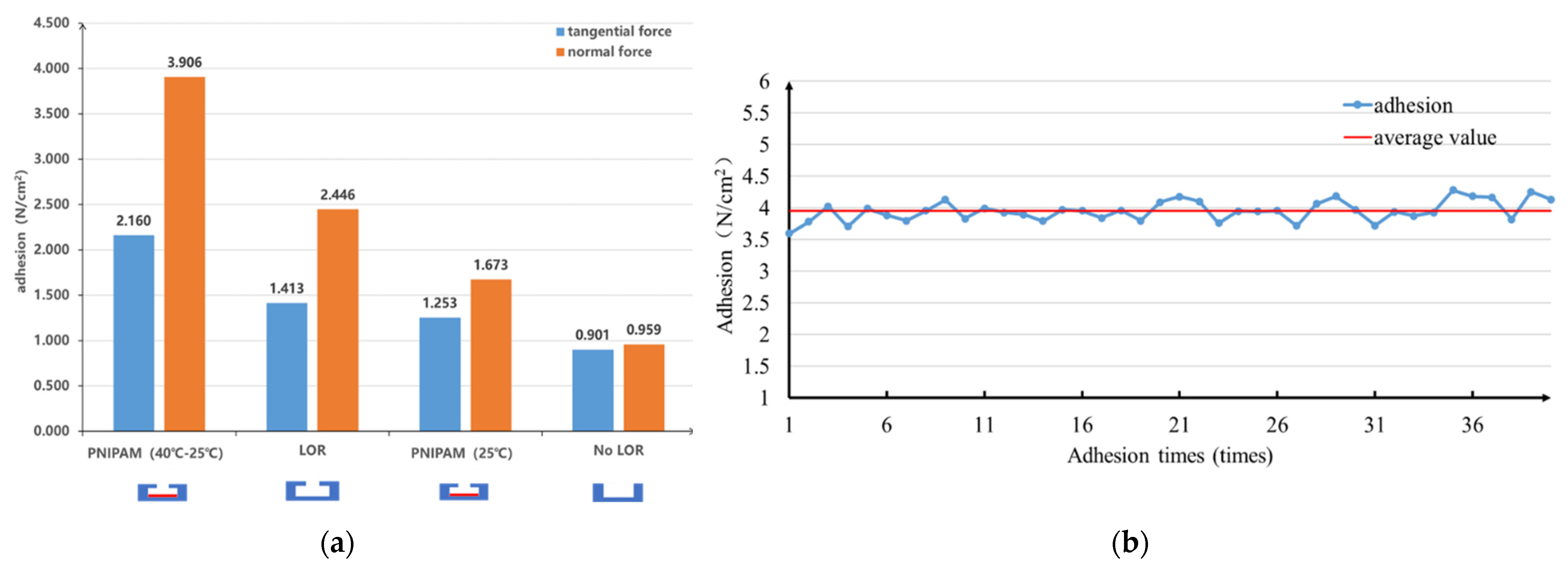
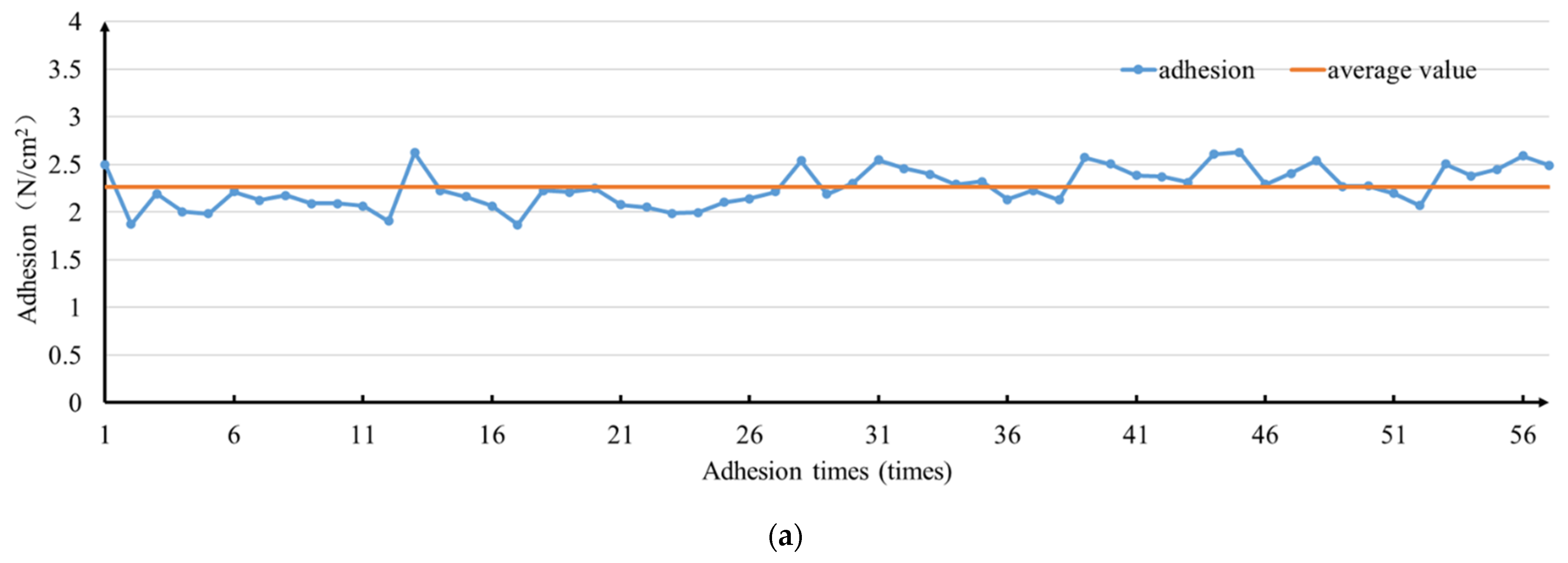
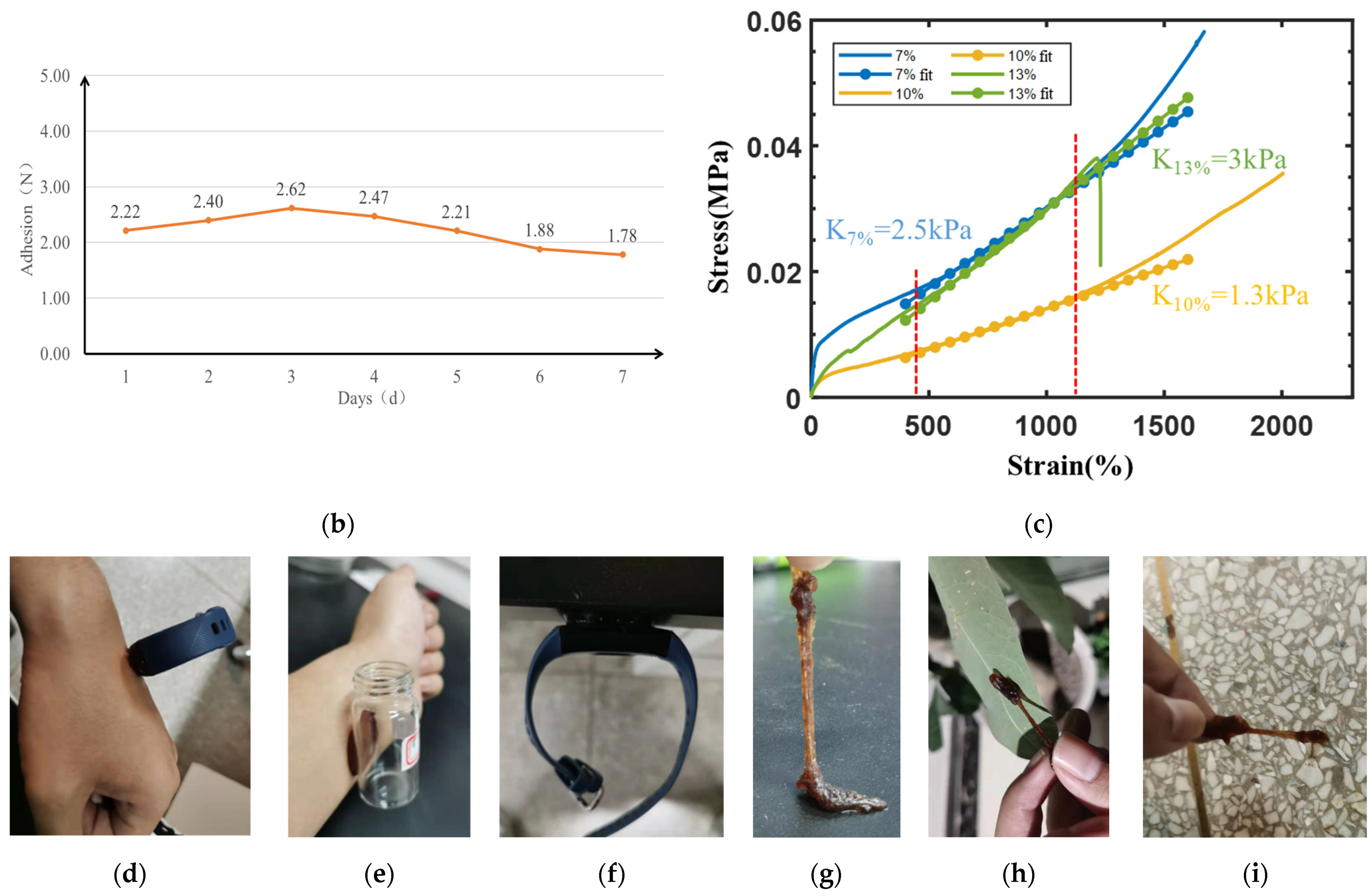
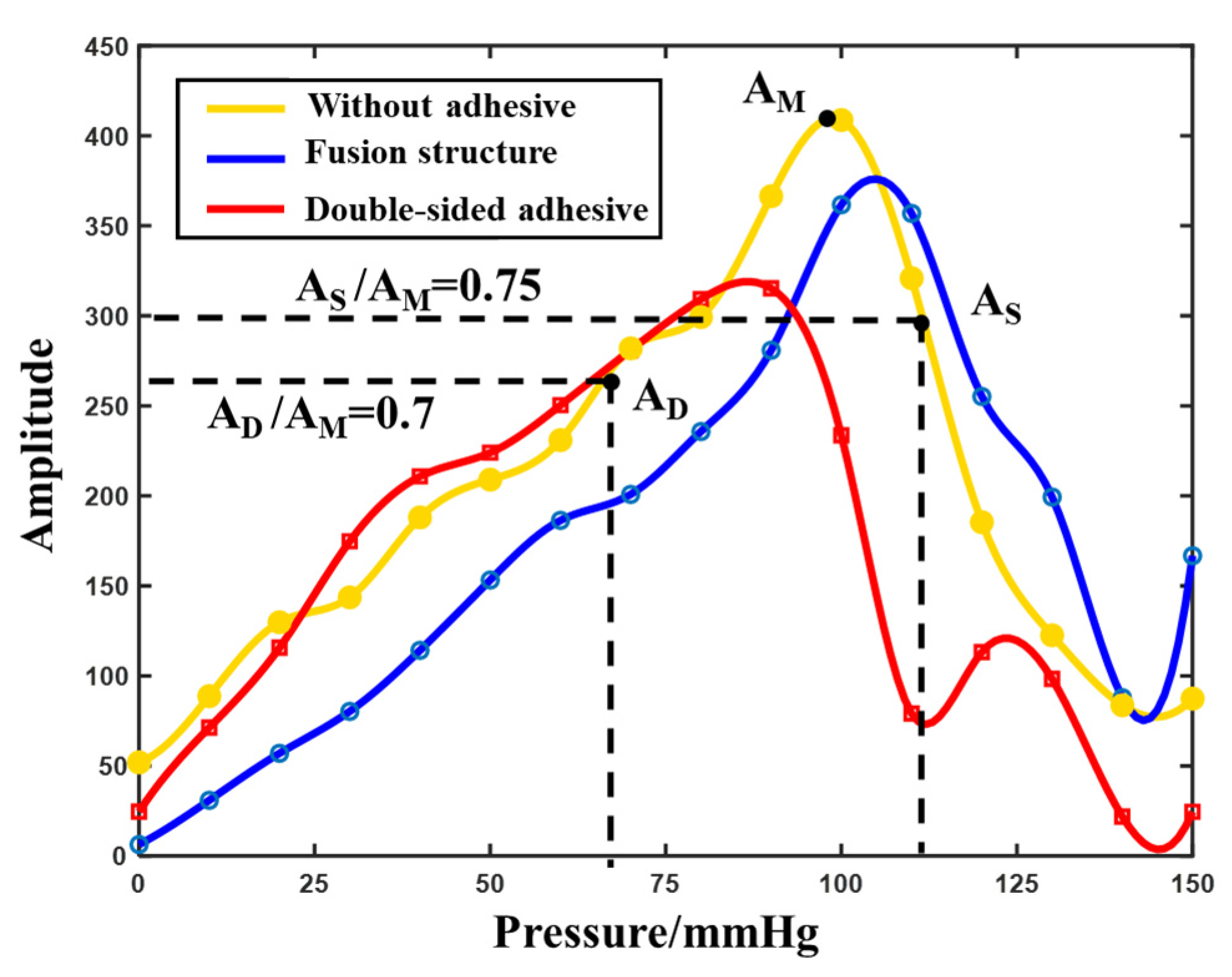
3.2. Adhesion
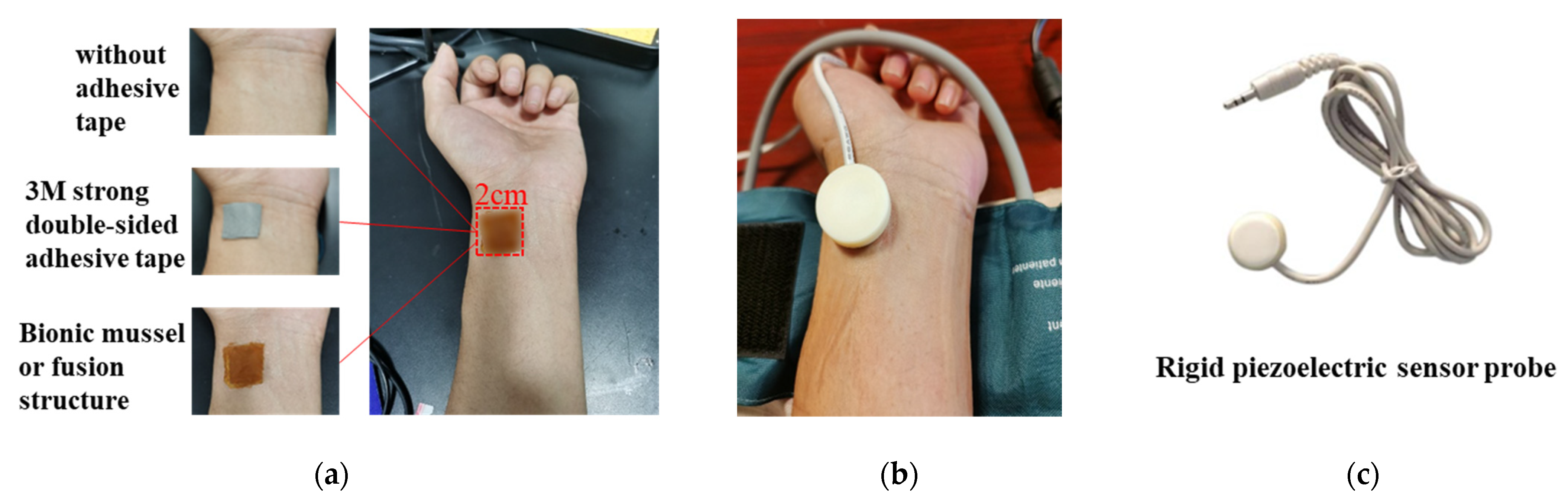
3.3. Pulsewave Signal Acquisition
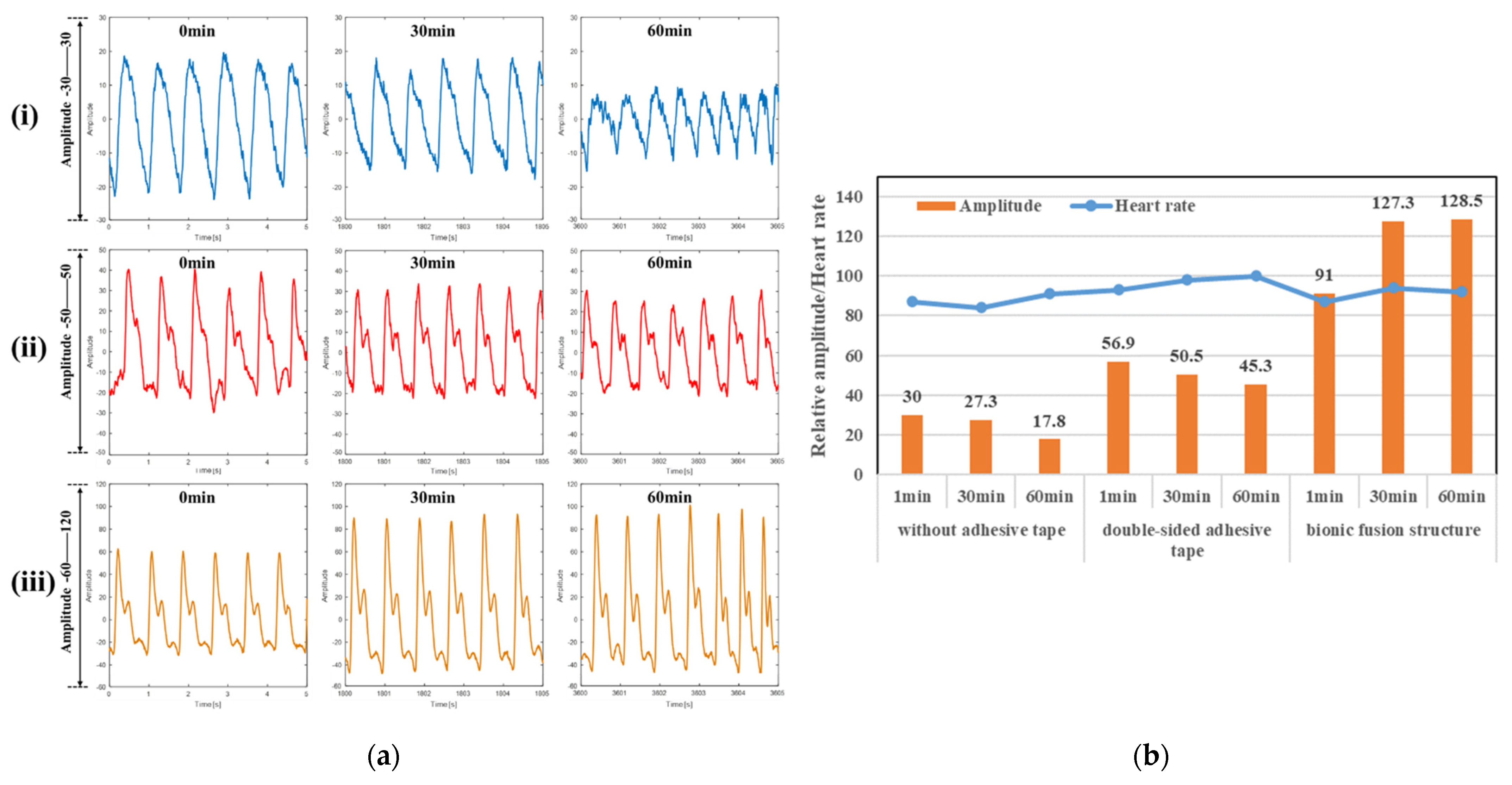
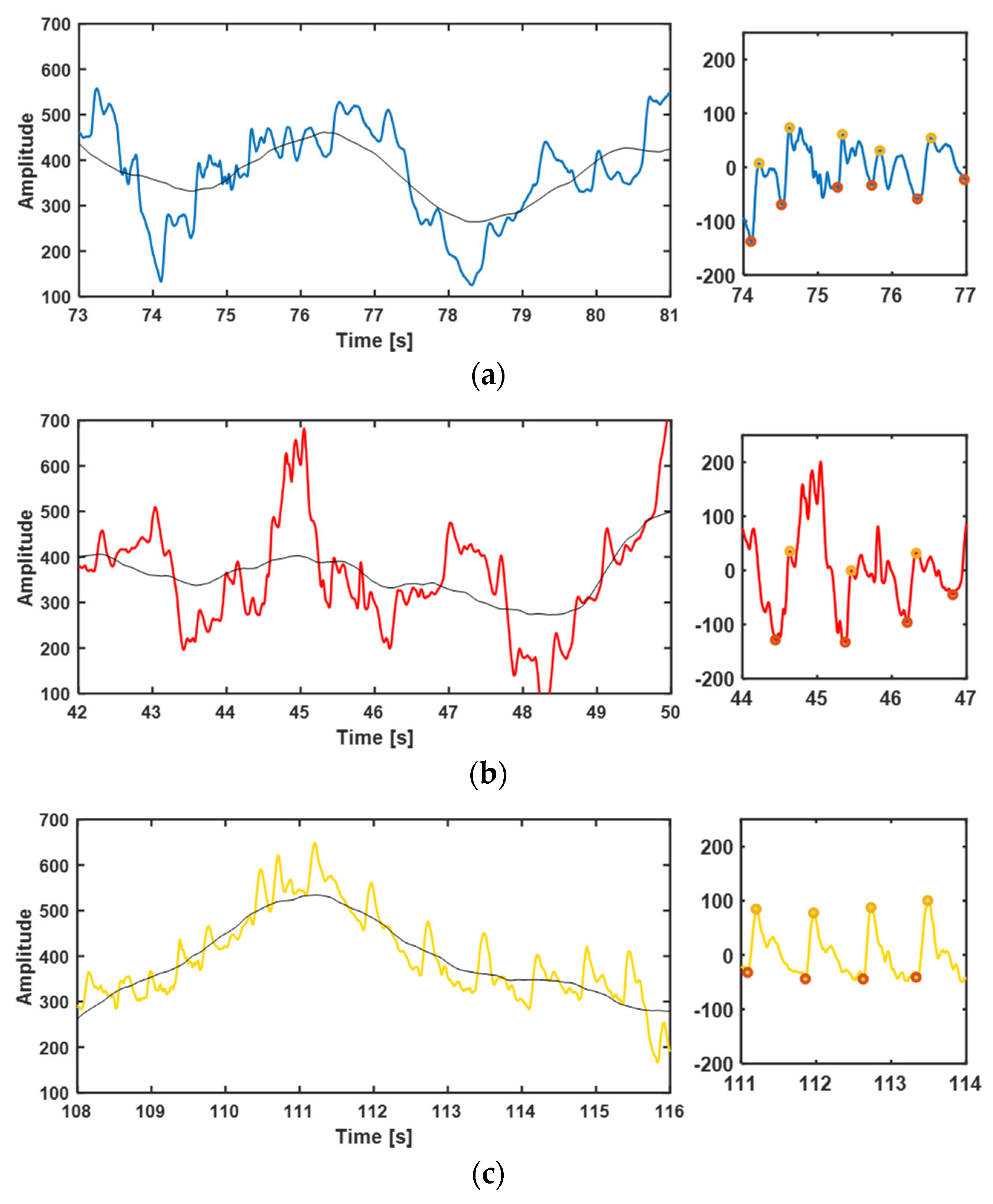
3.3.1. Heart Rate Measurement
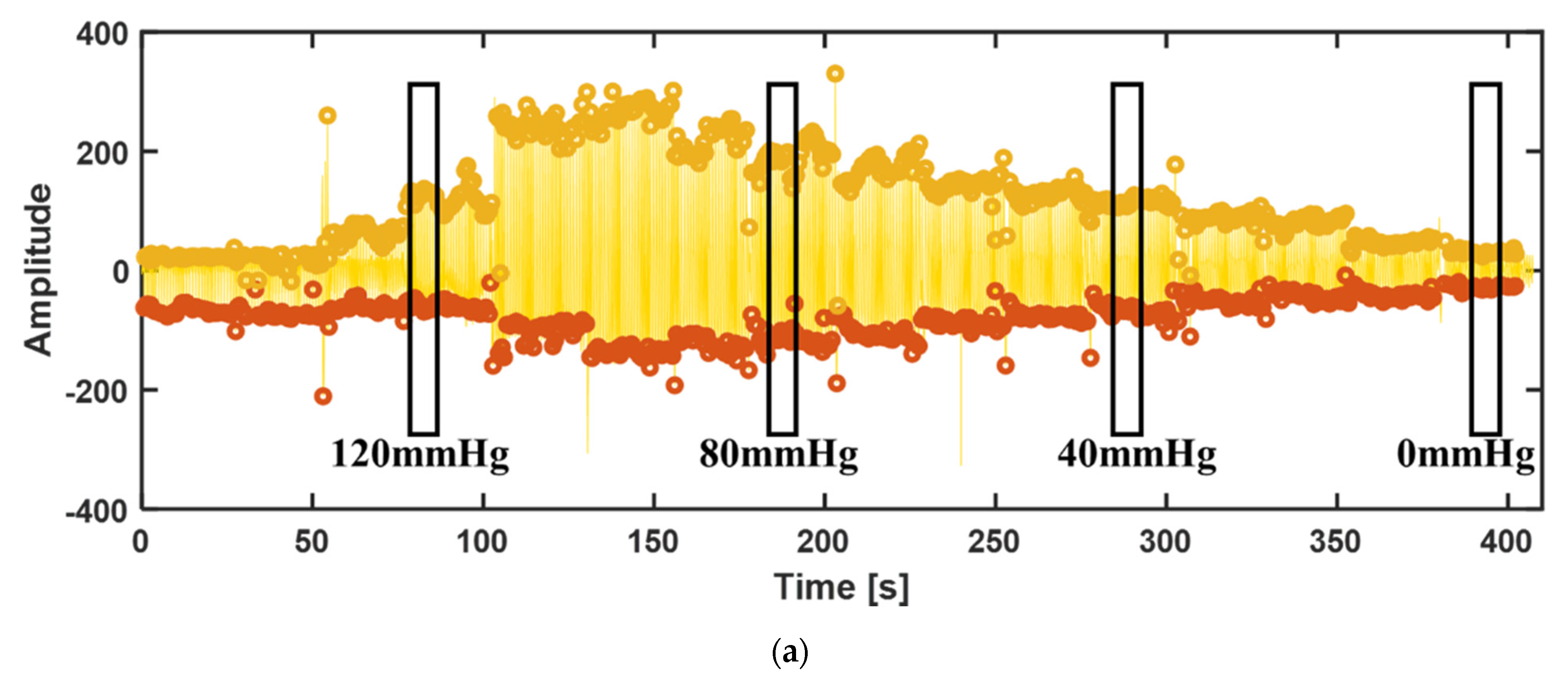
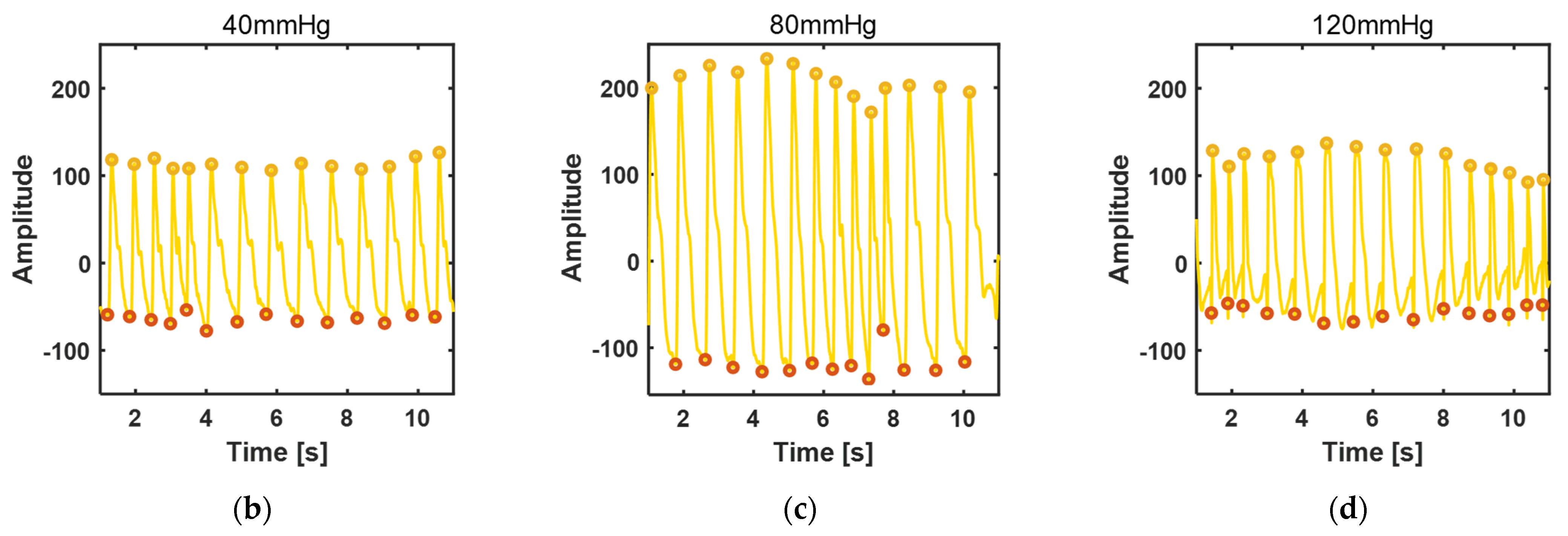
3.3.2. Blood Pressure Measurement
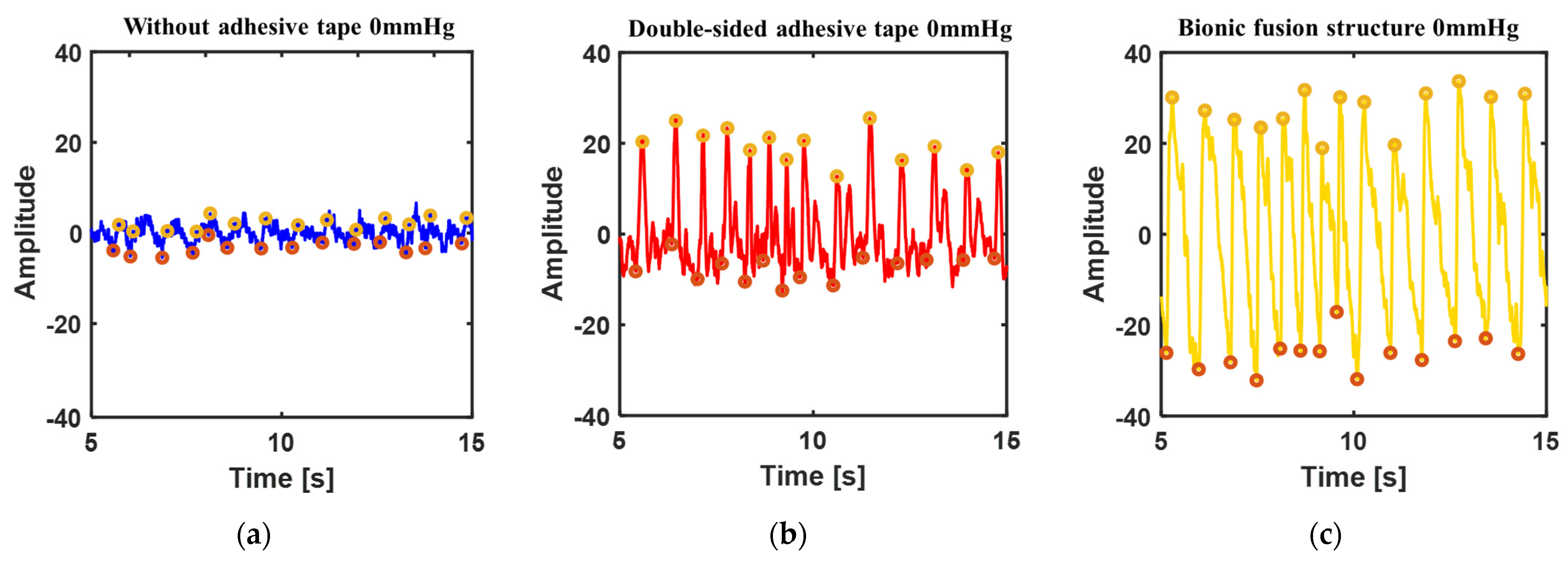
3.3.3. Disturbance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Zhang, L.; Xu, G.; Li, X.; Li, Y. Application and prospect of new bionic materials. Sci. Technol. Rev. 2019, 37, 74–78. [Google Scholar]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.H.; Feng, L.; Feng, X.J.; Zhai, J.; Jiang, L.; Bai, Y.B.; Li, T.J. Super-hydrophobicity of aligned polymer nanopole films. Chem. J. Chin. Univ. Chin. 2004, 25, 1375–1377. [Google Scholar]
- Bormashenko, E.; Stein, T.; Pogreb, R.; Aurbach, D. “Petal effect” on surfaces based on lycopodium: High-stick surfaces demonstrating high apparent contact angles. J. Phys. Chem. C 2009, 113, 5568–5572. [Google Scholar] [CrossRef]
- Tian, S.; Li, L.; Sun, W.; Xia, X.; Han, D.; Li, J.; Gu, C. Robust adhesion of flower-like few-layer graphene nanoclusters. Sci. Rep. 2012, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Dubonos, S.V.; Grigorieva, I.V.; Novoselov, K.S.; Zhukov, A.A.; Shapoval, S.Y. Microfabricated adhesive mimicking gecko foot-hair. Nat. Mater. 2003, 2, 461–463. [Google Scholar] [CrossRef]
- Kustandi, T.S.; Samper, V.D.; Yi, D.K.; Ng, W.S.; Neuzil, P.; Sun, W. Self-assembled nanoparticles based fabrication of gecko foot-hair-inspired polymer nanofibers. Adv. Funct. Mater. 2007, 17, 2211–2218. [Google Scholar] [CrossRef]
- Aksak, B.; Sahin, K.; Sitti, M. The optimal shape of elastomer mushroom-like fibers for high and robust adhesion. Beilstein J. Nanotechnol. 2014, 5, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Dai, L.; Stone, M.; Xia, Z.; Wang, Z.L. Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science 2008, 322, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, M.L.; Mccabe, B.M. Diet specialization in Octopus vulgaris at SanSalvador, Bahamas. Mar. Ecol. Prog. Ser. 2014, 516, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Tomokazu, T.; Kikuchi, S.; Suzuki, M.; Aoyagi, S. Vacuum gripper imitated octopus sucker-effect of liquid membrane for absorption. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots & Systems, Hamburg, Germany, 28 September–2 October 2015; pp. 2929–2936. [Google Scholar]
- Chang, W.Y.; Wu, Y.; Chung, Y.C. Facile Fabrication of Ordered Nanostructures from Protruding Nanoballs to Recessional Nanosuckers via Solvent Treatment on Covered Nanosphere Assembled Monolayers. Nano Lett. 2014, 14, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.; Kim, D.W.; Park, Y.; Lee, T.J.; Ho Bhang, S.; Pang, C. A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 2017, 546, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Park, O.K.; Choi, C.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, D.J.; Kim, M.; Hyun, W.; Him, S.J.; et al. Cephalopod-inspired miniaturized suction cups for smart medical skin. Adv. Healthc. Mater. 2016, 5, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, X.; He, H.; Ding, Y.; Lu, X. Mussel-inspired hydrogels for self-adhesive bioelectronics. Adv. Funct. Mater. 2020, 30, 1909954. [Google Scholar] [CrossRef]
- Bell, E.C.; Gosline, J.M. Mechanical design of mussel byssus: Material yield enhances attachment strength. J. Exp. Biol. 1996, 199, 1005–1017. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ryu, S.B.; Park, K.D. Preparation and characterization of dual-crosslinked gelatin hydrogel via Dopa-Fe3+ complexation and fenton reaction. J. Ind. Eng. Chem. 2018, 58, 105–112. [Google Scholar] [CrossRef]
- Lee, B.P.; Huang, K.; Nunalee, F.N.; Shull, K.R.; Messersmith, P.B. Synthesis of 3, 4-dihydroxyphenylalanine (DOPA) containing monomers and their co-polymerization with PEG-diacrylate to form hydrogels. Journal of Biomaterials Science. Polym. Ed. 2004, 15, 449–464. [Google Scholar]
- Ai, Y.; Nie, J.; Wu, G.; Yang, D. The Dopa-functionalized bioadhesive with properties of photocrosslinked and thermoresponsive. J. Appl. Polym. Sci. 2014, 131, 135526633. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef]
- Kung, L.A.; Groves, J.T.; Ulman, N.; Boxer, S.G. Printing via Photolithography on Micropartitioned Fluid Lipid Membranes. Adv. Mater. 2010, 12, 731–734. [Google Scholar] [CrossRef]
- Chen, Y.T.; Lin, I.K.; Lo, T.N.; Su, C.I.; Liu, C.J.; Je, J.H.; Margaritondo, G.; Hwu, Y. Study of LOR5B resist for the Fabrication of Hard X-ray Zone Plates by E-beam Lithography and ICP. AIP Conf. Proc. 2007, 879, 1516. [Google Scholar]
- Chen, G.; Ying, Y.U.; Luo, Z.Z.; Wu, Q. Study the performance of AZ5214E image-reversal photoresist and its uses in lift-off technics. J. Funct. Mater. 2005, 3, 431–433. [Google Scholar]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; Matsko, N.B.; Butte, A.; Morbidelli, M. Synthesis of temperature responsive polymer brushes from polystyrene latex particles functionalized with ATRP initiator. Eur. Polym. J. 2007, 43, 4868–4881. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, T.I.; Guo, X.I.; Zhou, X.I.; Nie, J.I.; Xu, J.U.; Fan, Z.H.; Du, B.I. Synthesis and properties of organic-inorganic hybrid P(NIPAM-co-AM-co-TMSPMA) microgels. Chin. J. Polym. Sci. 2011, 29, 439–449. [Google Scholar] [CrossRef]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Möhwald, H. Adhesion and mechanical properties of PNIPAM microgel films and their potential use as switchable cell culture substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Schaef, H.T.; Ilton, E.S.; Qafoku, O.; Martin, P.F.; Felmy, A.R.; Rosso, K.M. In situ XRD Study of Ca2+ Saturated Montmorillonite (STX-1) Exposed to Anhydrous and Wet Supercritical Carbon Dioxide. Int. J. Greenh. Gas Control 2012, 6, 220–229. [Google Scholar] [CrossRef]
- Kryzhanovsky, B.V.; Litinskii, L.B. Generalized bragg-williams equation for systems with an arbitrary long-range interaction. Dokl. Math. 2015, 90, 784–787. [Google Scholar] [CrossRef]
- Chandrasekhar, A.; Natarajan, K.; Yavarimanesh, M.; Mukkamala, R. An iPhone application for blood pressure monitoring via the oscillometric finger pressing method. Sci. Rep. 2018, 8, 1–6. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Han, L.; Dong, Y.; Wang, X. Biomimetic Self-Adhesive Structures for Wearable Sensors. Biosensors 2022, 12, 431. https://doi.org/10.3390/bios12060431
Chen F, Han L, Dong Y, Wang X. Biomimetic Self-Adhesive Structures for Wearable Sensors. Biosensors. 2022; 12(6):431. https://doi.org/10.3390/bios12060431
Chicago/Turabian StyleChen, Feihu, Liuyang Han, Ying Dong, and Xiaohao Wang. 2022. "Biomimetic Self-Adhesive Structures for Wearable Sensors" Biosensors 12, no. 6: 431. https://doi.org/10.3390/bios12060431
APA StyleChen, F., Han, L., Dong, Y., & Wang, X. (2022). Biomimetic Self-Adhesive Structures for Wearable Sensors. Biosensors, 12(6), 431. https://doi.org/10.3390/bios12060431



