G-Optrode Bio-Interfaces for Non-Invasive Optical Cell Stimulation: Design and Evaluation
Abstract
1. Introduction
2. Materials and Methods for G-Optrode Electrical Characterization and Cell/Graphene Interfaces Preparation (Sample Preparation)
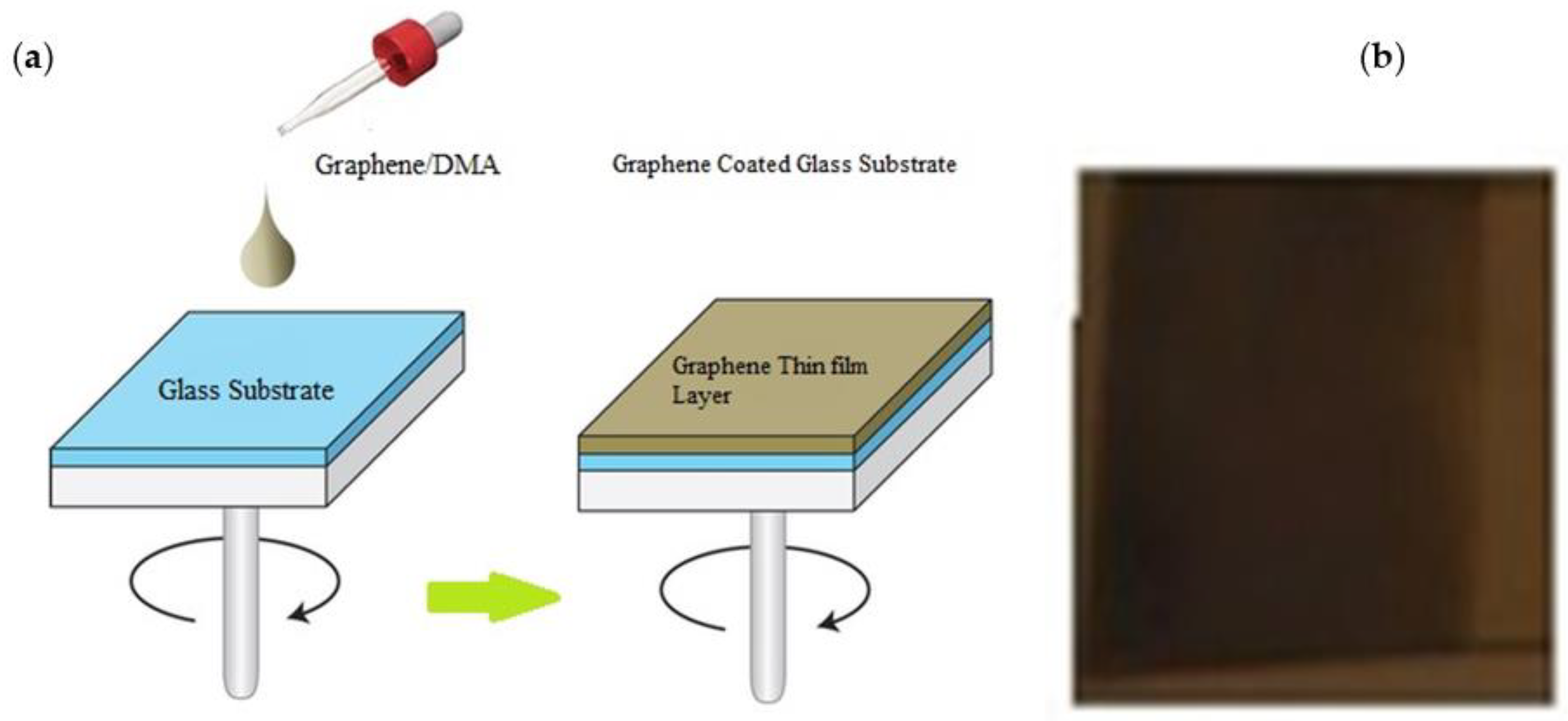
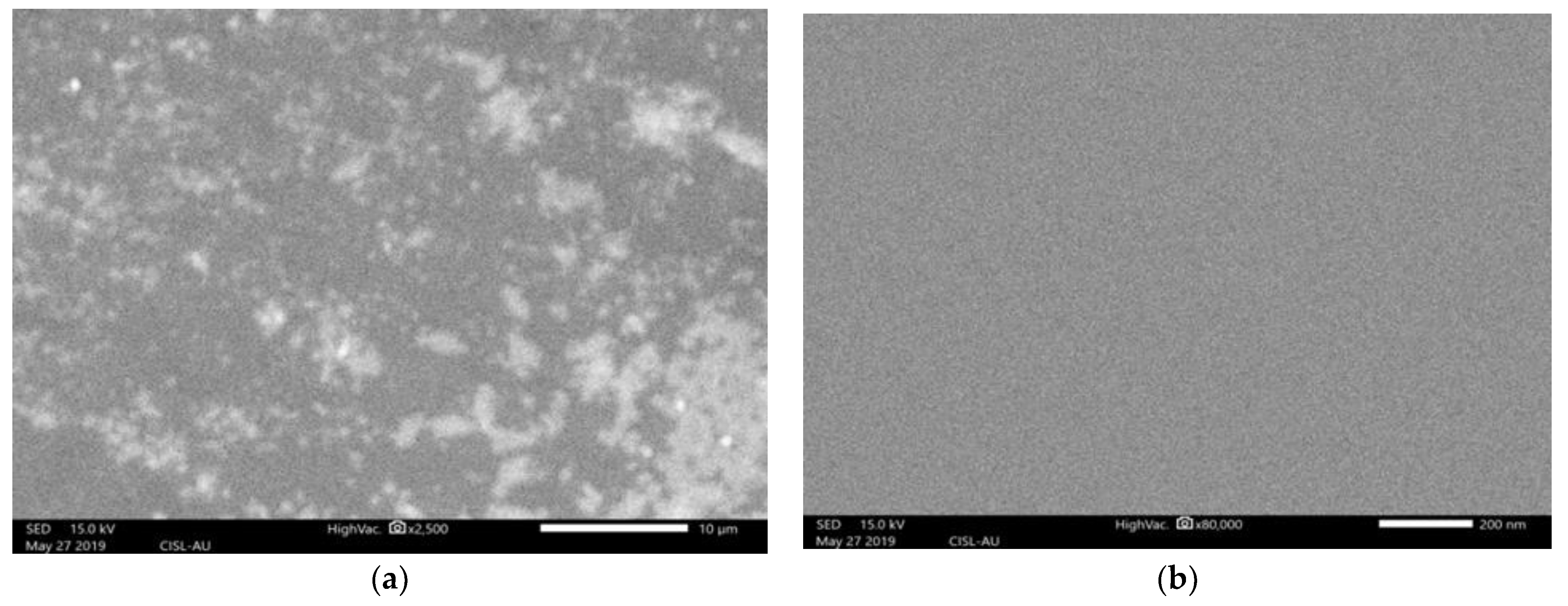
2.1. G-Optrode Thin Film Physical Realization
2.2. This Present Research Work Analyses the Effect of G-Optrode Thin Film
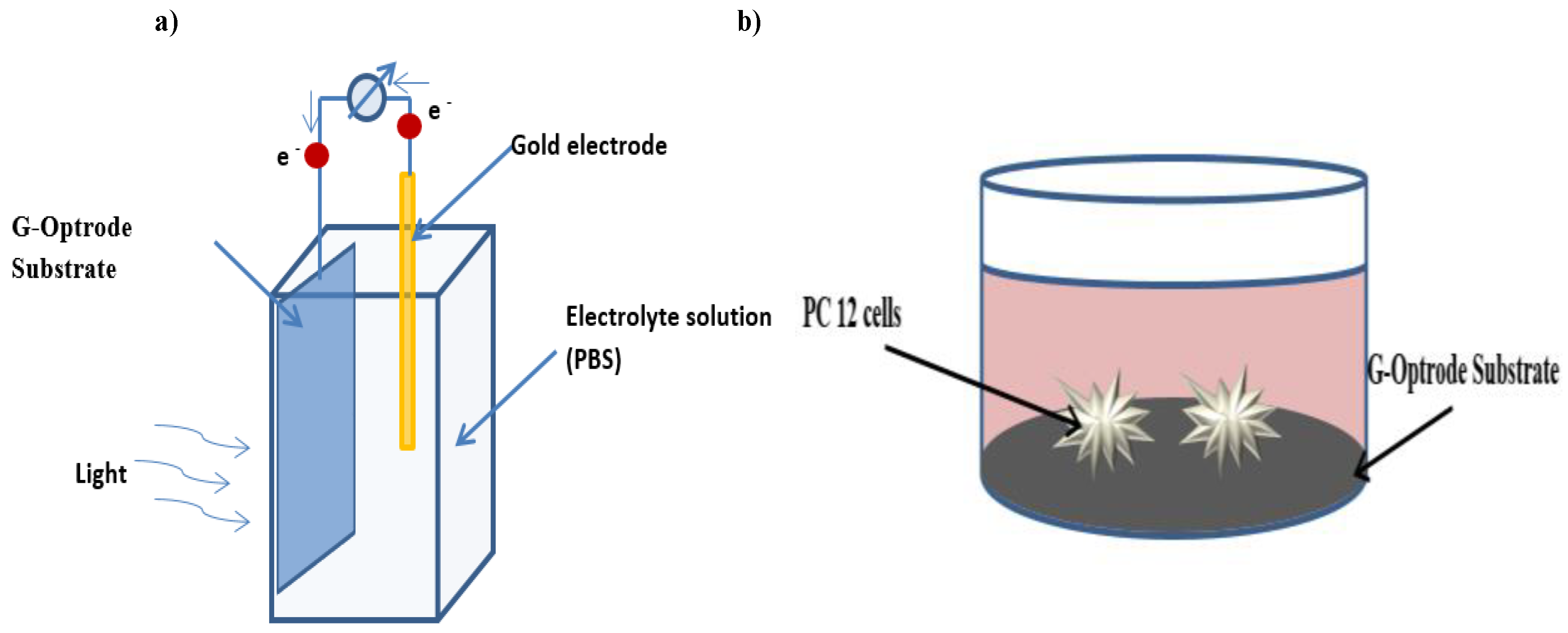
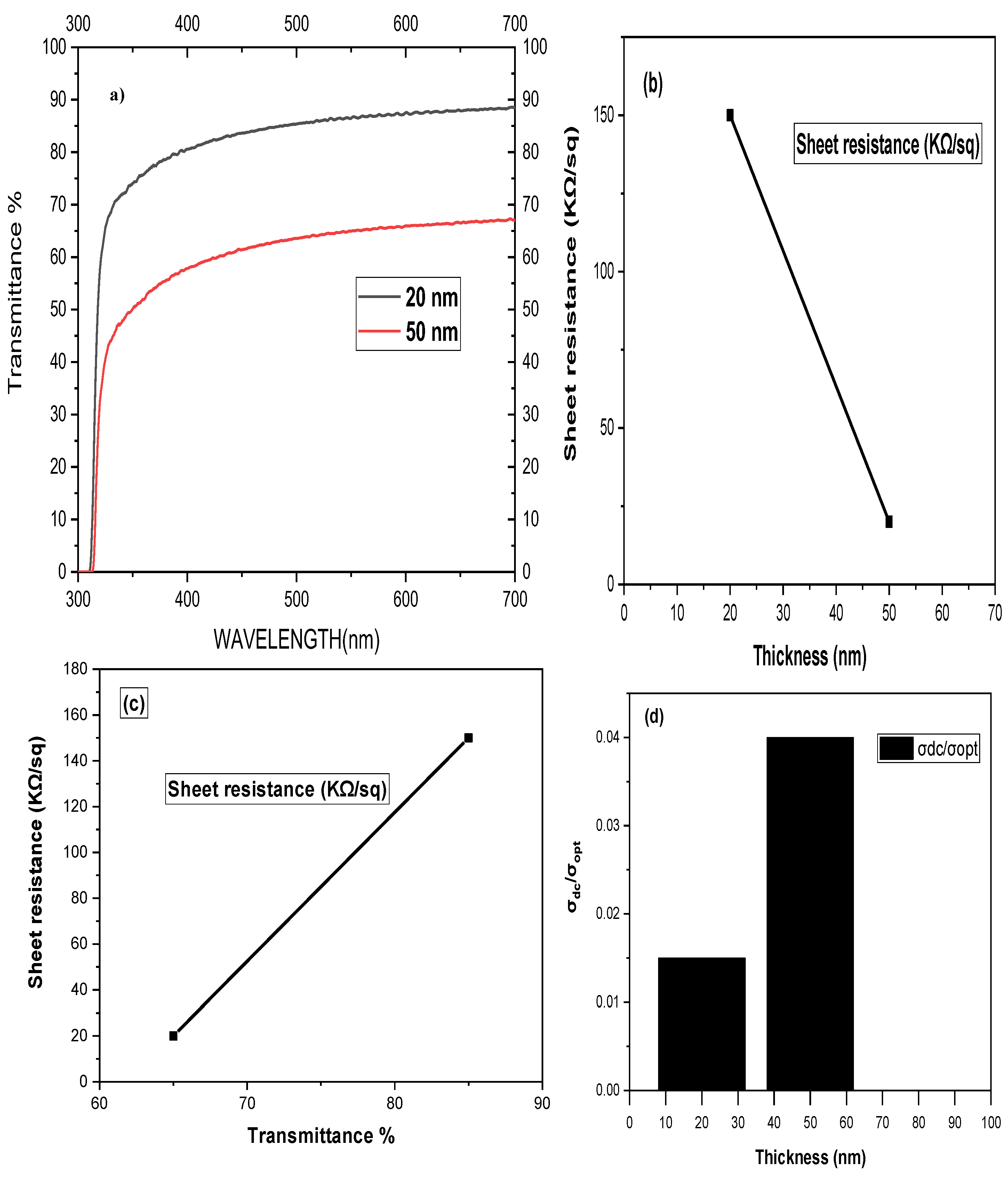
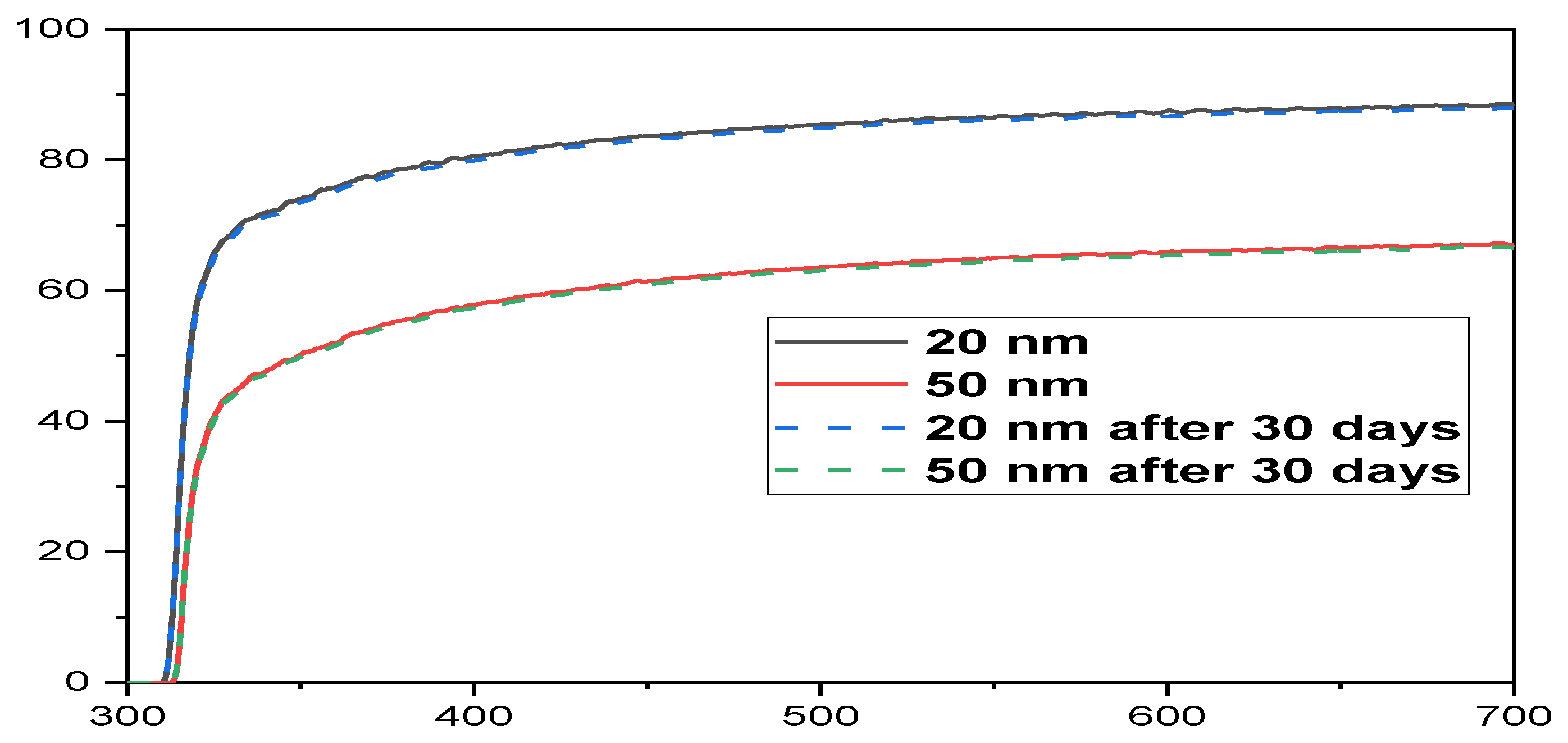
3. Electrical Characterization of G-Optrodes Thin Films
Stability Evaluation of G-Optrode Thin Film’s
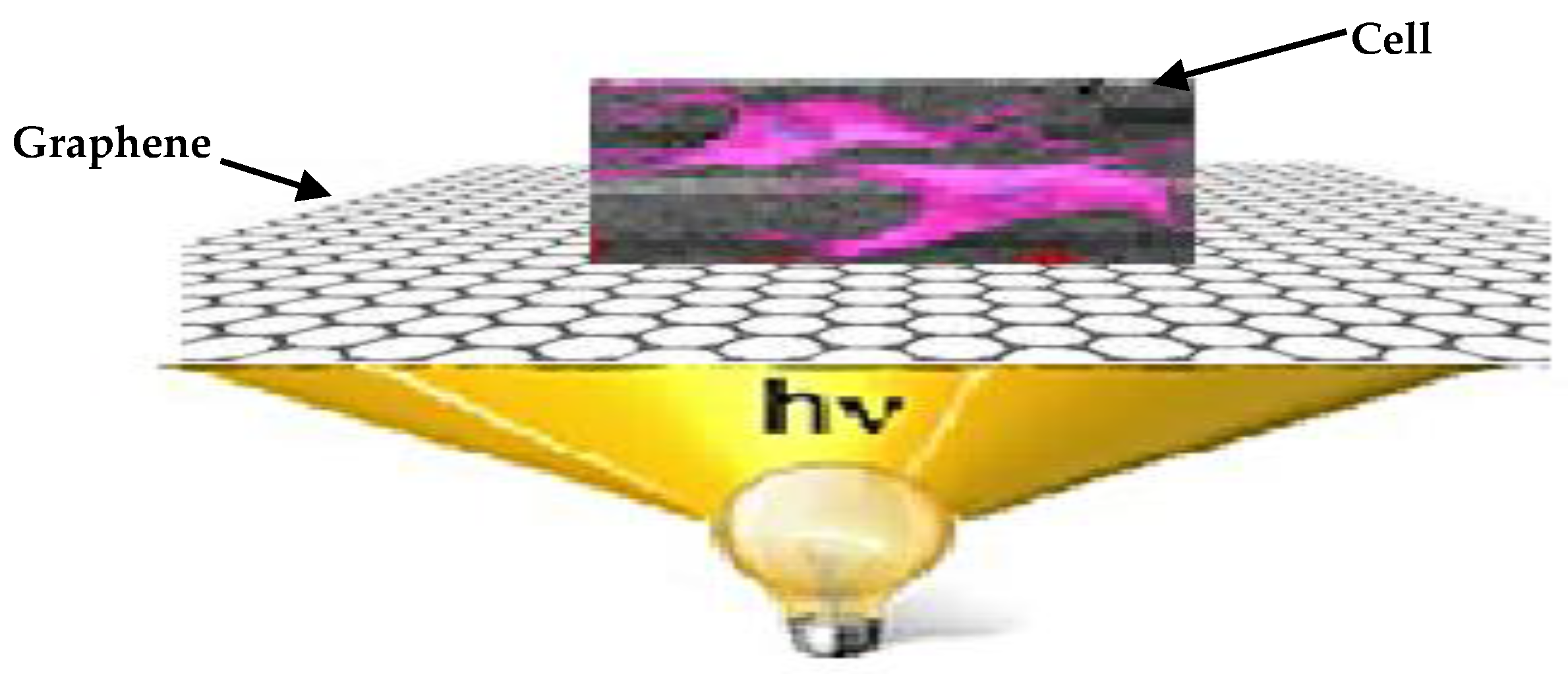
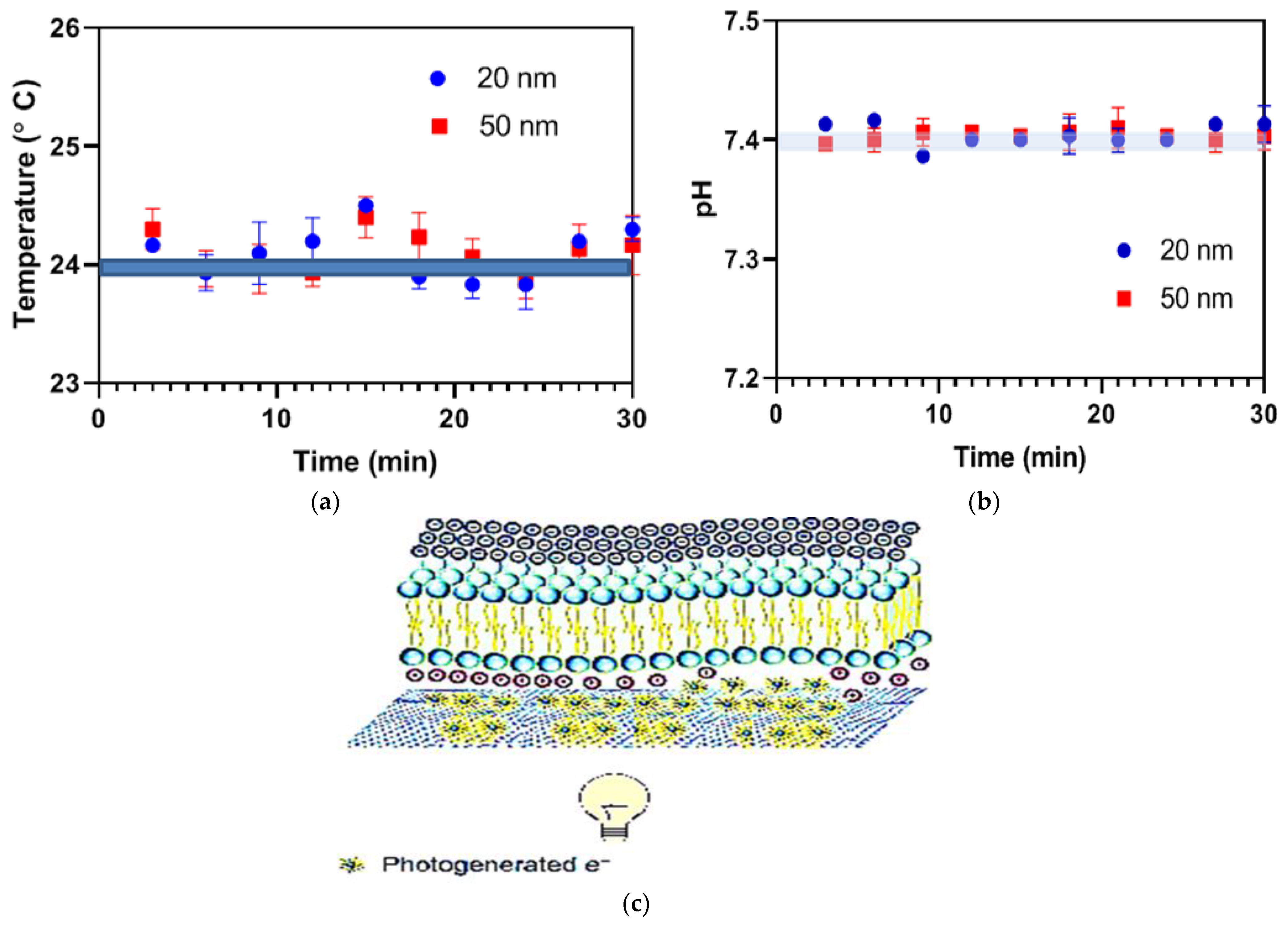
4. Optical Stimulation via G-Optrode Substrate Bio Interfaces: Potential Mechanisms and Analysis
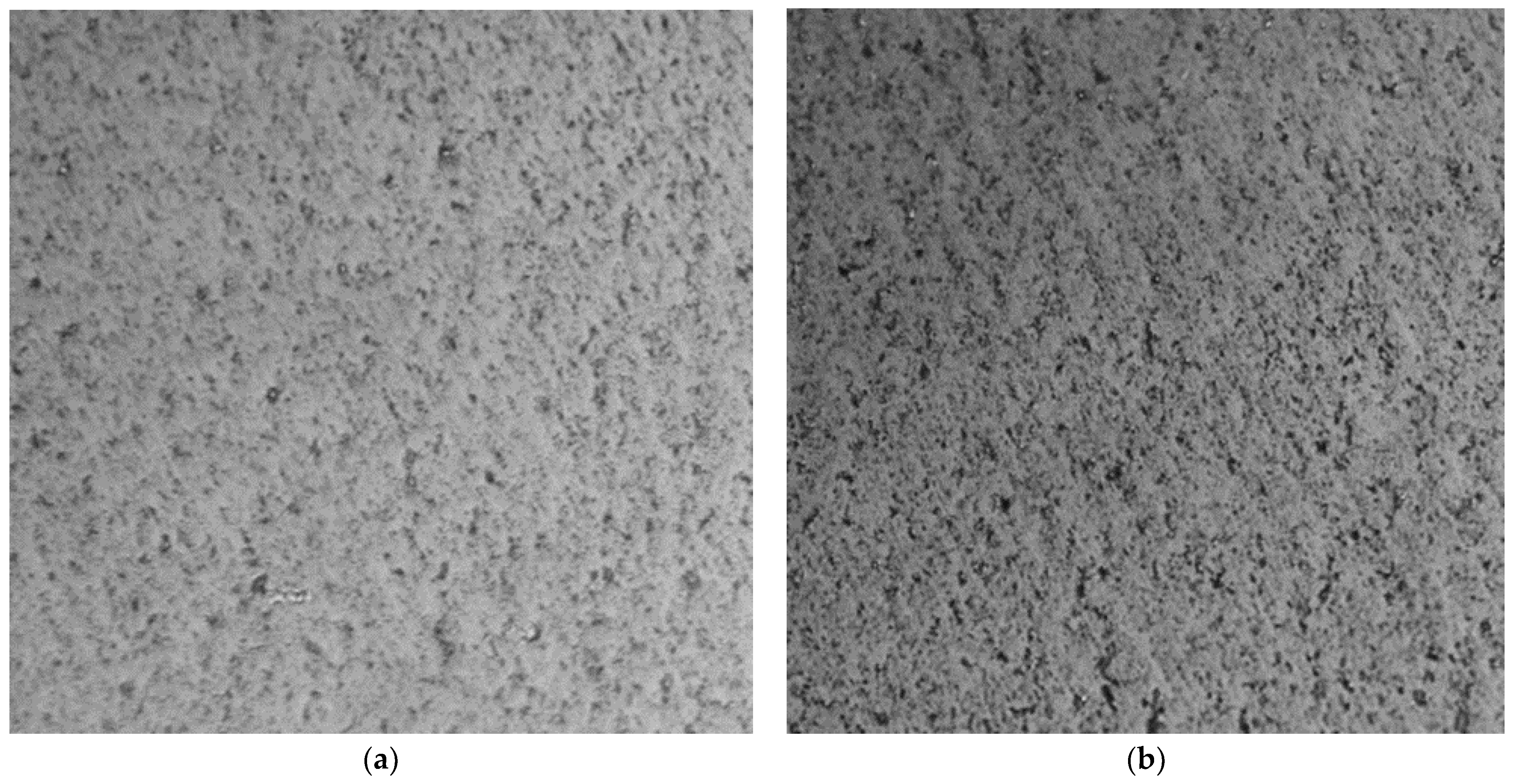
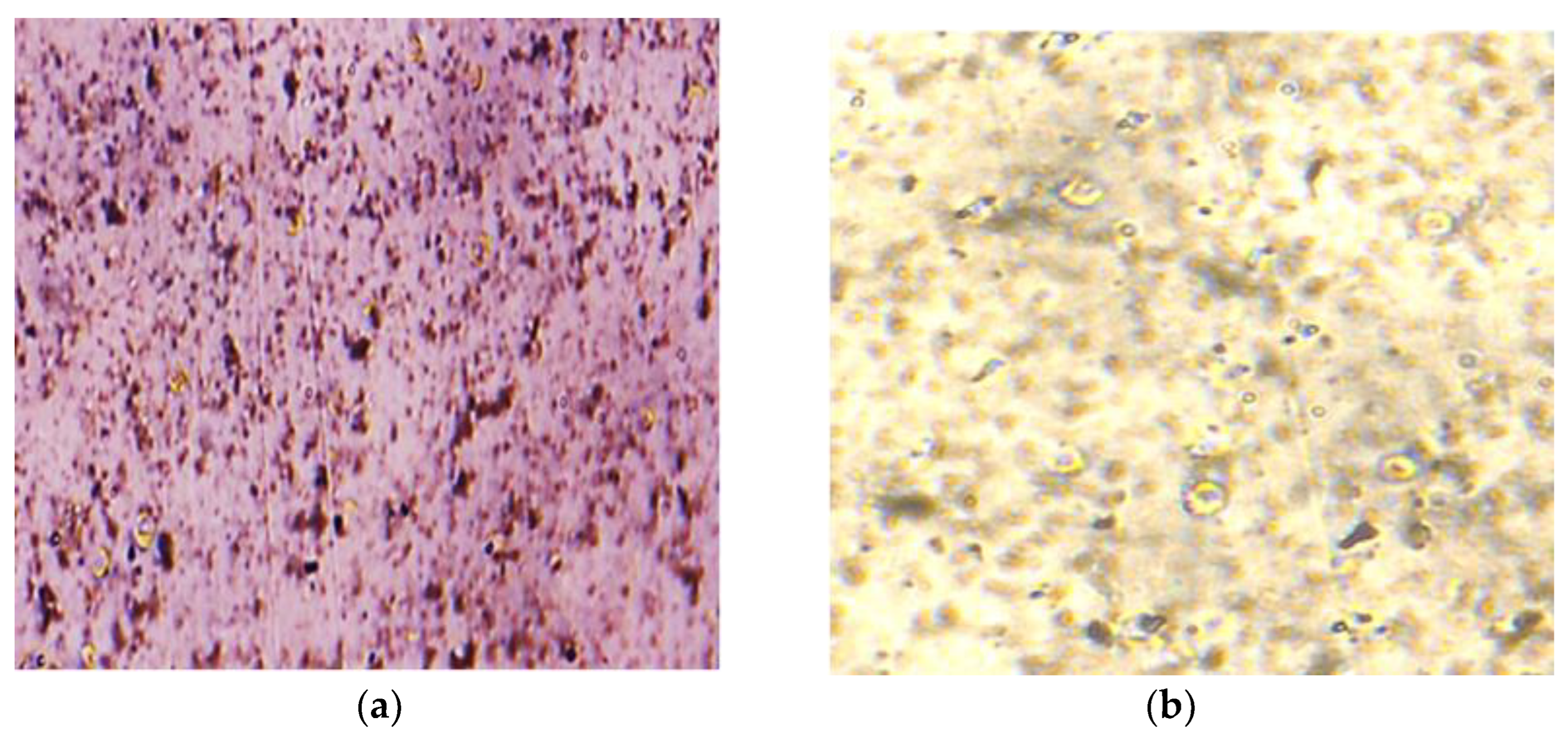
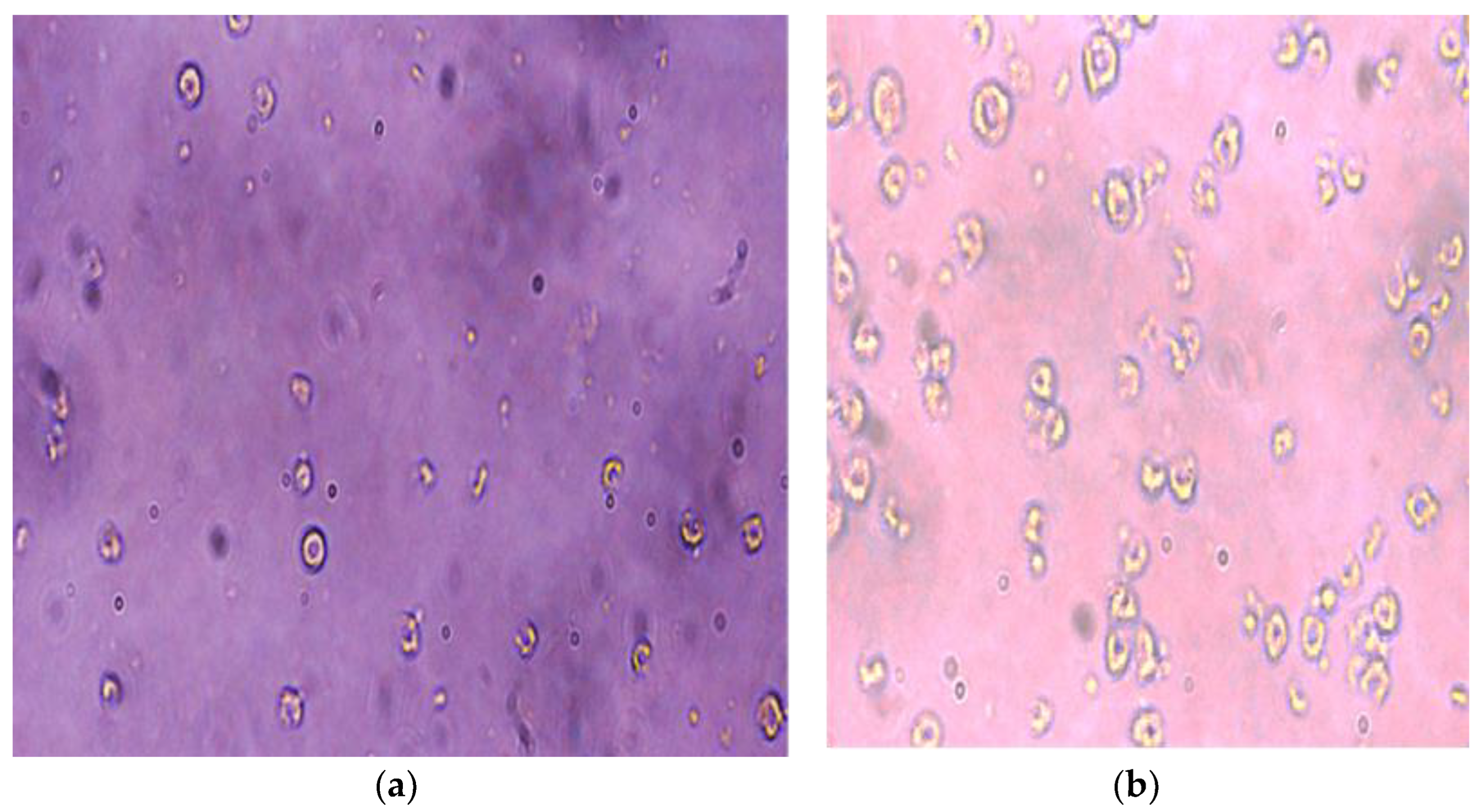
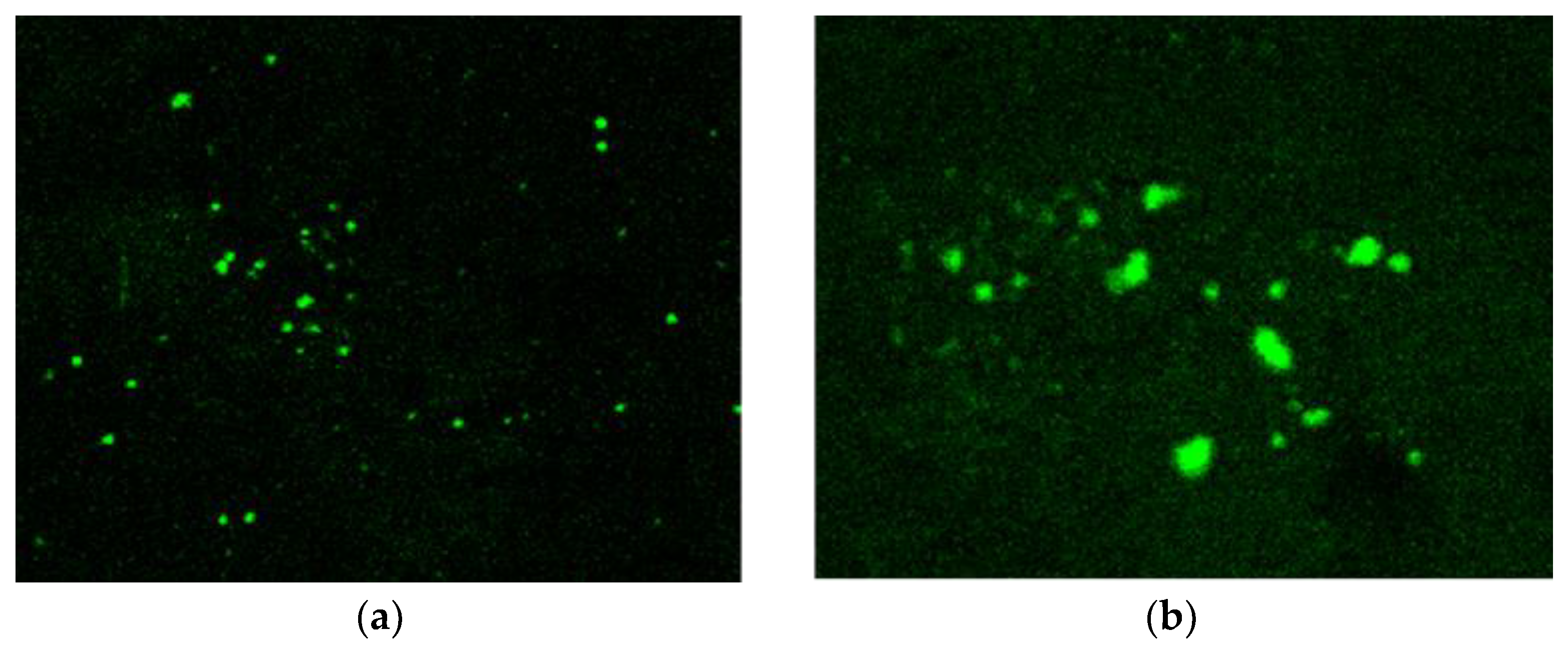
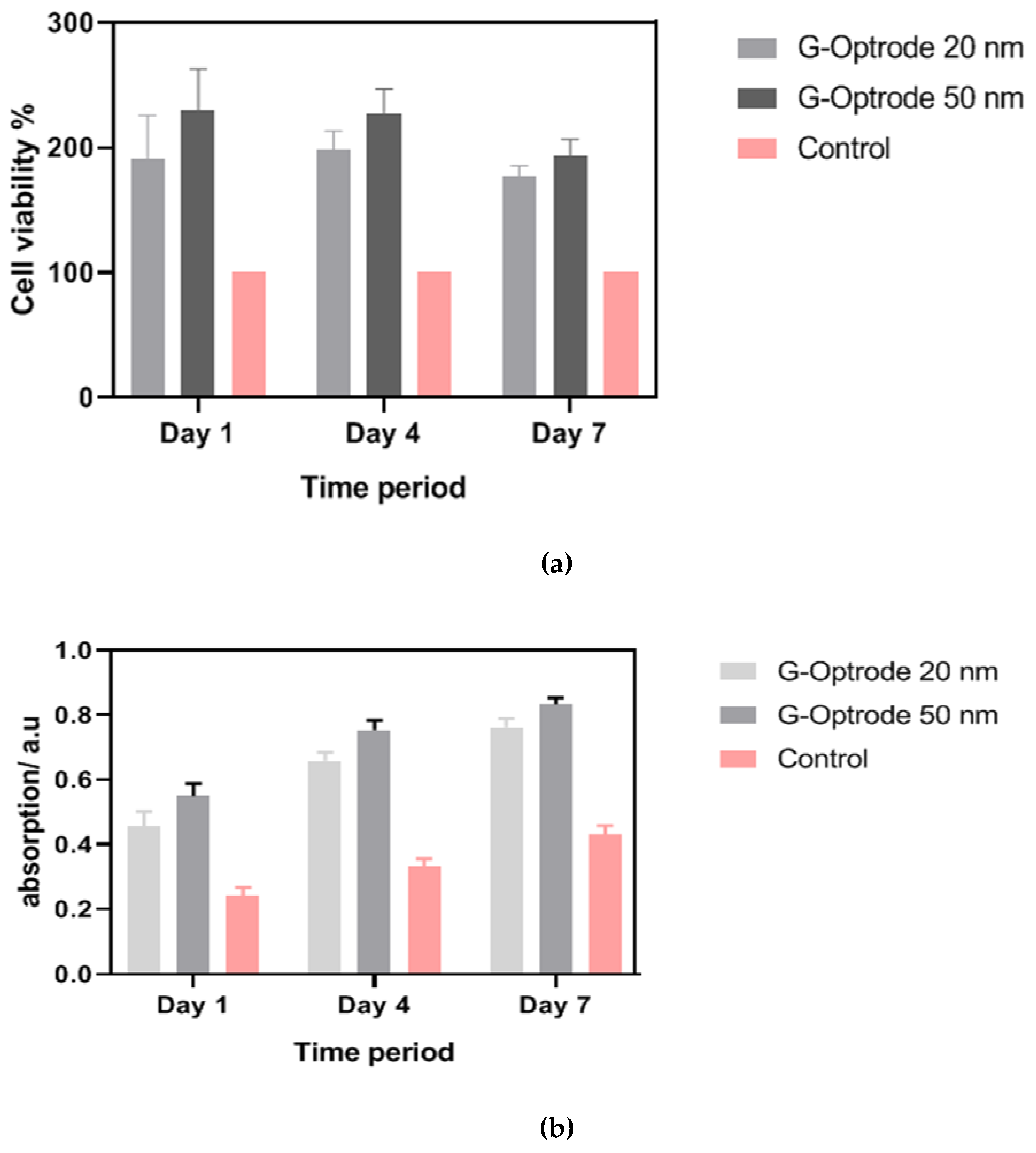
Evaluation of G-Optrode Thin Film Bio-Organic Interface Behaviour
5. Conclusions and Future Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gowthaman, N.; Srivastava, V.M. Mathematical modeling of drain current estimation in CSDG MOSFET based on La2O3 oxide layer with fabrication specifications—A nanomaterial, approach. Nanomaterials 2022, 12, 3374. [Google Scholar] [CrossRef]
- Huang, P.; Kong, Y.; Li, Z.; Gao, F.; Cui, D. Copper Selenide Nanosnakes: Bovine Serum Albumin-Assisted Room Temperature Controllable Synthesis and Characterization. Nanoscale Res. Lett. 2010, 5, 949–956. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, H.; Yang, H.; Huang, P.; Cui, D.; Peng, Y.; Zhang, J.; Lu, F.; Lian, J.; Shi, D. Unique role of ionic liquid in microwave-assisted synthesis of monodisperse magnetite nanoparticles. Chem. Commun. 2010, 46, 3866–3868. [Google Scholar] [CrossRef] [PubMed]
- Brunton, E.K.; Winther-Jensen, B.; Ewang, C.; Yan, E.B.; Gooie, S.E.; Lowery, A.; Rajan, R. In vivo comparison of the charge densities required to evoke motor responses using novel annular penetrating microelectrodes. Front. Neurosci. 2015, 9, 00271. [Google Scholar] [CrossRef]
- Moorthy, V.M.; Srivastava, V.M. Device modelling and optimization of nanomaterial-based planar heterojunction solar cell (by varying the device dimensions and material parameters). Nanomaterials 2022, 12, 3031. [Google Scholar] [CrossRef]
- Green, R.A.; Matteucci, P.B.; Hassarati, R.T.; Giraud, B.; Dodds, C.W.D.; Chen, S.; Byrnes-Preston, P.J.; Suaning, G.J.; Poole-Warren, L.; Lovell, N. Performance of conducting polymer electrodes for stimulating neuroprosthetics. J. Neural Eng. 2013, 10, 016009. [Google Scholar] [CrossRef]
- Zhang, W.; Lee, S.; McNear, K.L.; Chung, T.-F.; Lee, S.; Lee, K.; Crist, S.A.; Ratliff, T.L.; Zhong, Z.; Chen, Y.P.; et al. Use of graphene as protection film in biological environments. Sci. Rep. 2014, 4, 4097. [Google Scholar] [CrossRef]
- Savchenko, A.; Cherkas, V.; Liu, C.; Braun, G.B.; Kleschevnikov, A.; Miller, Y.I.; Molokanova, E. Graphene biointerfaces for optical stimulation of cells. Sci. Adv. 2018, 4, eaat0351. [Google Scholar] [CrossRef]
- Song, H.; He, R.; Wang, K.; Ruan, J.; Bao, C.; Li, N.; Ji, J.; Cui, D. Anti-HIF-1 a antibody-conjugated pluronic triblock copolymers encapsulated with Paclitaxel for tumor targeting therapy. Biomaterials 2010, 31, 2302–2312. [Google Scholar] [CrossRef]
- Arrenberg, A.B.; Stainier, D.Y.R.; Baier, H.; Huisken, J. Optogenetic control of cardiac function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef]
- Richardson, R.T.; Ibbotson, M.R.; Thompson, A.C.; Wise, A.K.; Fallon, J.B. Optical stimulation of neural tissue. Healthc. Technol. Lett. 2020, 7, 58–65. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, N.; Srivastava, V.M. Parametric analysis of CSDG MOSFET with La2O3 gate oxide: Based on electrical field estimation. IEEE Access 2021, 9, 159421–159431. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Fan, Z.; Liu, J.; Zhang, H. Graphene-Based Electrodes. Adv. Mater. 2012, 24, 5979–6004. [Google Scholar] [CrossRef]
- Ziąbka, M.; Menaszek, E.; Tarasiuk, J.; Wroński, S. Biocompatible nanocomposite implant with silver nanoparticles for otology—In vivo evaluation. Nanomaterials 2018, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Periasamy, A.P.; Lin, C.Y.; Her, G.M.; Chiu, W.J.; Li, C.L.; Shu, C.L.; Huang, C.C.; Liang, C.T.; Chang, H.T. Photoluminescent graphene quantum dots for in vivo imaging of apoptotic cells. Nanoscale 2015, 7, 2504–2510. [Google Scholar] [CrossRef]
- Moorthy, V.M.; Srivastava, V.M. Device modeling of organic photovoltaic cells with traditional and inverted cells using s-SWCNT:C60 as active layer. Nanomaterials 2022, 12, 2844. [Google Scholar] [CrossRef]
- Iijima, S.; Brabec, C.; Maiti, A.; Bernholc, J. Structural flexibility of carbon nanotubes. J. Chem. Phys. 1996, 104, 2089–2092. [Google Scholar] [CrossRef]
- Krishnan, A.; Dujardin, E.; Ebbesen, T.W.; Yianilos, P.N.; Treacy, M.M.J. Young’s modulus of single-walled nanotubes. Phys. Rev. B 1998, 58, 13–19. [Google Scholar] [CrossRef]
- Dargar, S.K.; Srivastava, V.M. Analysis of short channel effects in multiple-gate (n,0)-carbon nanotube FETs. J. Eng. Sci. Technol. 2019, 14, 3282–3293. [Google Scholar]
- Keren, L.B.; Hanein, Y. Carbon nanotube-based multi electrode arrays for neuronal interfacing: Progress and prospects. Front. Neural Circuits 2013, 6, 122. [Google Scholar]
- Tsukahara, T.; Matsuda, Y.; Haniu, H. The Role of Autophagy as a Mechanism of Toxicity Induced by Multi-Walled Carbon Nanotubes in Human Lung Cells. Int. J. Mol. Sci. 2015, 16, 40–48. [Google Scholar] [CrossRef]
- Long, J.; Xiao, Y.; Liu, L.; Cao, Y. The adverse vascular effects of multi-walled carbon nanotubes (MWCNTs) to human vein endothelial cells (HUVECs) in vitro: Role of length of MWCNTs. J. Nanobiotechnol. 2017, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Healthc. 2017, 59, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Poderyte, M.; Valiunien, A.; Ramanavicius, A. Scanning electrochemical microscope as a tool for the electroporation of living yeast cells. Biosens. Bioelectron. 2022, 205, 114096. [Google Scholar] [CrossRef]
- Chan, K.W.; Liao, C.Z.; Wong, H.M.; Wai, K.; Yeung, K.; Tjong, S.C. Preparation of polyetheretherketone composites with nanohydroxyapatite rods and carbon nanofibers having high strength, good biocompatibility and excellent thermal stability. RSC Adv. 2016, 6, 19417–19429. [Google Scholar] [CrossRef]
- Dasari, T.P.; Danielle, S.; Asok, M.; Paul, K.D. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef]
- Liu, C.; Wong, H.M.; Wai, K.; Yeung, K.; Tjong, S.C. Novel electrospun polylactic acid nanocomposite fiber mats with hybrid graphene oxide and nanohydroxyapatite reinforcements having enhanced biocompatibility. Polymer 2016, 8, 287. [Google Scholar] [CrossRef]
- Cui, X.; Wan, B.; Yang, Y.; Ren, X.; Guo, L. Length effects on the dynamic process of cellular uptake and exocytosis of single-walled carbon nanotubes in murine macrophage cells. Sci. Rep. 2017, 7, 1518. [Google Scholar] [CrossRef]
- Pillay, D.; Srivastava, V.M. Realization with fabrication of dual-gate MOSFET based source follower. Springer Silicon 2022. [Google Scholar] [CrossRef]
- Nakano, H.; Tetsuka, H.; Spencer, M.J.S. Chemical modification of group IV graphene analogues. Sci. Technol. Adv. Mater. 2018, 19, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Some, S.; Kim, J.; Lee, K.; Kulkarni, A.; Yoon, Y.; Lee, S.; Kim, T.; Lee, H. Highly Air-Stable Phosphorus-Doped n-Type Graphene Field-Effect Transistors. Adv. Mater. 2012, 24, 5481–5486. [Google Scholar] [CrossRef]
- Lu, Y.F.; Lo, S.T.; Lin, J.C.; Zhang, W.; Lu, J.Y.; Liu, F.H.; Tseng, C.M.; Lee, Y.H.; Liang, C.T.; Li, L.J. Nitrogen-doped graphene sheets grown by chemical vapor deposition: Synthesis and influence of nitrogen impurities on carrier transport. ACS Nano 2013, 7, 6522–6532. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Singh, S.N.; Srivastava, V.M. Comparative analysis of ZnO quantum dots synthesized on PVA and PVP capping matrix. Nanosyst. Nanomater. Nanotechnol. 2021, 19, 337–345. [Google Scholar]
- Lammel, T.; Boisseaux, P.; Cruz, M.F.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef]
- Bengtson, S.; Kling, K.; Madsen, A.M.; Noergaard, A.W.; Jacobsen, N.R.; Clausen, P.A.; Alonso, B.; Pesquera, A.; Zurutuza, A.; Ramos, R.; et al. No cytotoxicity or genotoxicity of graphene and grapheneoxide in murine lung epithelial FE1 cells in vitro. Environ. Mol. Mutagen. 2016, 57, 469–482. [Google Scholar] [CrossRef]
- Liao, K.; Lin, Y.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Liao, C.Z.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. The development, fabrication, and material characterization of polypropylene composites reinforced with carbon nanofiber and hydroxyapatite nanorod hybrid fillers. Int. J. Nanomed. 2014, 11, 1299–1310. [Google Scholar]
- He, L.; Tjong, S.C. Nanostructured transparent conductive films: Fabrication, characterization and applications. Mater. Sci. Eng. R: Rep. 2016, 109, 1–101. [Google Scholar] [CrossRef]
- Moorthy, M.V.; Parthasarathy, V.; Rathnasami, J.D. Bio-interface behaviour of Graphene and semiconducting SWCNT:C60 blend based nano photodiode for subretinal implant. Biosurface Biotribol. 2020, 6, 53–58. [Google Scholar]
- Swapnaa, P.; Raoa, Y.S. Fabrication and characterization of semiconducting single walled carbon nano tube based bulk hetero junction organic solar cell using spin coating technique. J. Chin. Adv. Mater. Soc. 2015, 3, 161–169. [Google Scholar] [CrossRef]
- Koerbitzer, B.; Krauss, P.; Nick, C.; Yadav, S.; Schneider, J.J.; Thielemann, C. Graphene electrodes for stimulation of neuronal cells. 2d Mater. 2016, 3, 024004. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Zhaoa, Y. Integrated graphene/nanoparticle hybrids for biological and electronic applications. Nanoscale 2014, 6, 6245–6266. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, V.; Arora, S.; Singh, S. Fabrication of ultrathin, free-standing, transparent and conductive graphene/multiwalled carbon nanotube film with superior optoelectronic properties. Thin Solid Film. 2017, 21, 193–199. [Google Scholar] [CrossRef]
- Bao, W.; Wan, J.; Han, X.; Cai, X.; Zhu, H.; Kim, D.; Ma, D.; Xu, Y.; Munday, J.N.; Drew, H.D.; et al. Approaching the limits of transparency and conductivity in graphitic materials through lithium intercalation. Nat. Commun. 2014, 5, 4224. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, D.; Antognazza, M.R.; Maschio, M.D.; Lanzarini, E.; Benfenati, F.; Lanzani, G. A hybrid bioorganic interface for neuronal photoactivation. Nat. Commun. 2011, 2, 166. [Google Scholar] [CrossRef]
- George, P.A.; Strait, J.; Dawlaty, J.; Shivaraman, S.; Chandrashekhar, M.; Rana, F.; Spencer, M.G. Spectroscopy of the Carrier Relaxation Epitaxial Graphene. Nano Lett. 2008, 8, 4248–4251. [Google Scholar] [CrossRef]
- Dawlaty, J.M.; Shivaraman, S.; Chandrashekhar, M.; Spencer, M.G.; Rana, F. Measurement of ultrafast carrier dynamics in epitaxial Graphene. Appl. Phys. Lett. 2008, 92, 042116. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Duke, A.R.; Gu, S.; Chiel, H.J.; Fujioka, H.; Watanabe, M.; Jansen, E.D.; Rollins, A.M. Optical pacing of the embryonic heart. Nat. Photonics 2010, 4, 623–626. [Google Scholar] [CrossRef]
- Gabor, N.M.; Song, J.C.; Ma, Q.; Nair, N.L.; Taychatanapat, T.; Watanabe, K.; Taniguchi, T.; Levitov, L.S.; Jarillo Herrero, P. Hot Carrier–assisted intrinsic photoresponse in graphene. Science 2011, 334, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Tielrooij, K.J.; Song, J.C.W.; Jensen, S.A.; Centeno, A.; Pesquera, A.; Elorza, A.Z.; Bonn, M.; Levitov, L.S.; Koppens, F.H.L. Photoexcitation cascade and multiple hot-carrier generation in graphene. Nat. Phys. 2013, 9, 248–252. [Google Scholar] [CrossRef]
- Shapiro, M.G.; Homma, K.; Villarreal, S.; Richter, C.; Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012, 3, 310–376. [Google Scholar] [CrossRef]
- Freitag, M.; Low, T.; Xia, F.; Avouris, P. Photoconductivity of biased graphene. Nat. Photonics 2012, 7, 53–59. [Google Scholar] [CrossRef]
- Ghezzi, D.; Pedrocchi, A.; Menegon, A.; Mantero, S.; Valtorta, F.; Ferrigno, G. PhotoMEA: An opto-electronic biosensor for monitoring in vitro neuronal network activity. BioSystems 2007, 87, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moorthy, V.M.; Varatharajan, P.; Rathnasami, J.D.; Srivastava, V.M. G-Optrode Bio-Interfaces for Non-Invasive Optical Cell Stimulation: Design and Evaluation. Biosensors 2022, 12, 808. https://doi.org/10.3390/bios12100808
Moorthy VM, Varatharajan P, Rathnasami JD, Srivastava VM. G-Optrode Bio-Interfaces for Non-Invasive Optical Cell Stimulation: Design and Evaluation. Biosensors. 2022; 12(10):808. https://doi.org/10.3390/bios12100808
Chicago/Turabian StyleMoorthy, Vijai M., Parthasarathy Varatharajan, Joseph D. Rathnasami, and Viranjay M. Srivastava. 2022. "G-Optrode Bio-Interfaces for Non-Invasive Optical Cell Stimulation: Design and Evaluation" Biosensors 12, no. 10: 808. https://doi.org/10.3390/bios12100808
APA StyleMoorthy, V. M., Varatharajan, P., Rathnasami, J. D., & Srivastava, V. M. (2022). G-Optrode Bio-Interfaces for Non-Invasive Optical Cell Stimulation: Design and Evaluation. Biosensors, 12(10), 808. https://doi.org/10.3390/bios12100808




