Progress in the Development of Biosensors Based on Peptide–Copper Coordination Interaction
Abstract
1. Introduction
2. Fluorescent Biosensors
2.1. Peptide Fluorescent Probes for Copper Ions
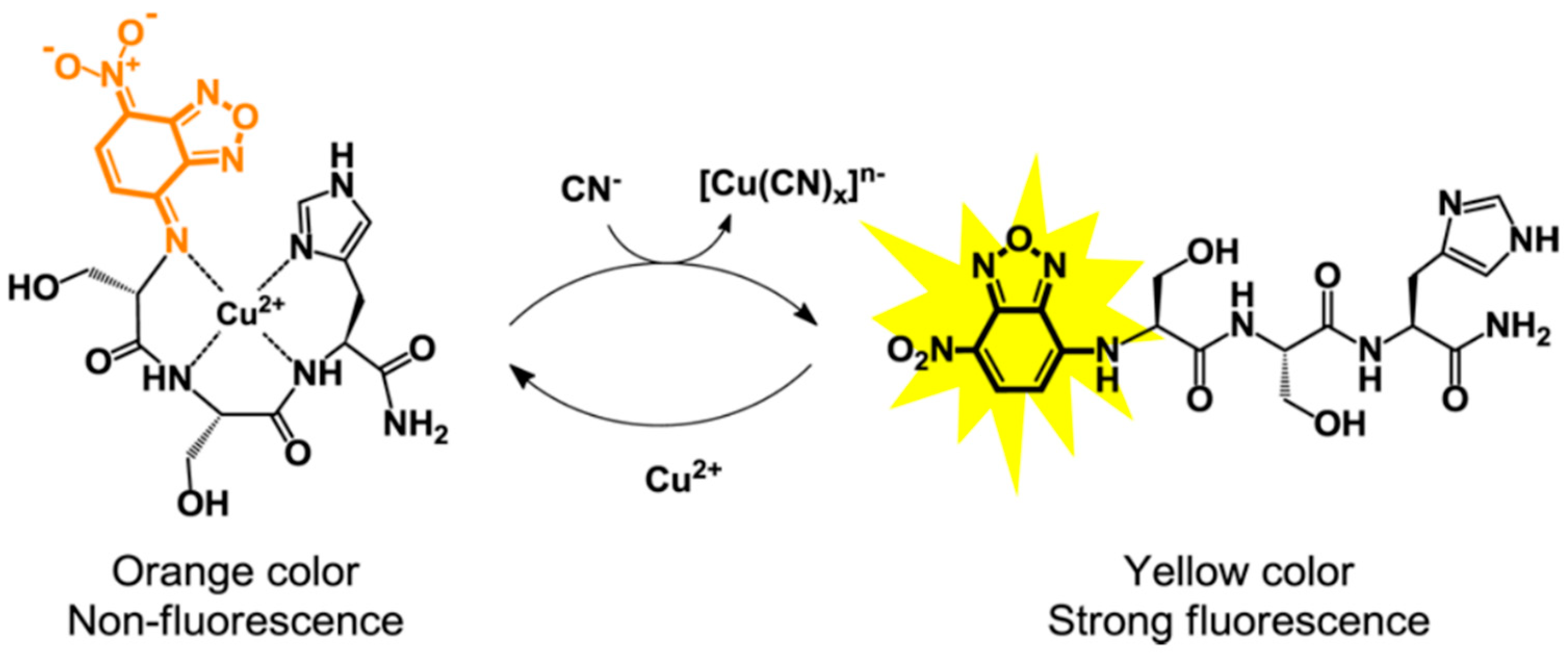
2.2. Peptide-Cu2+ Complexes for Fluorescent Detection of Anions
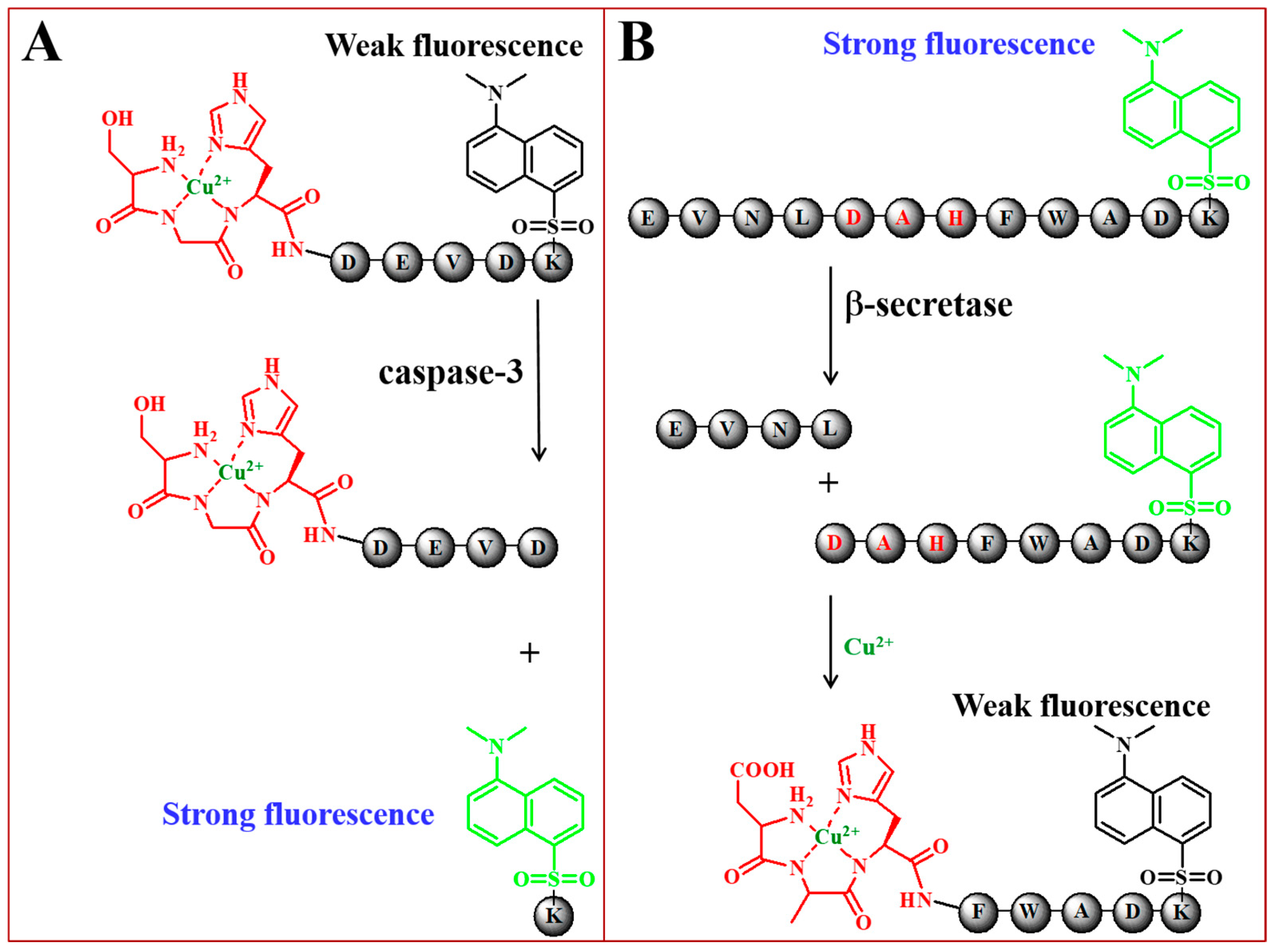
2.3. Other Fluorescent Biosensors Based on Peptide-Cu2+ Complexes
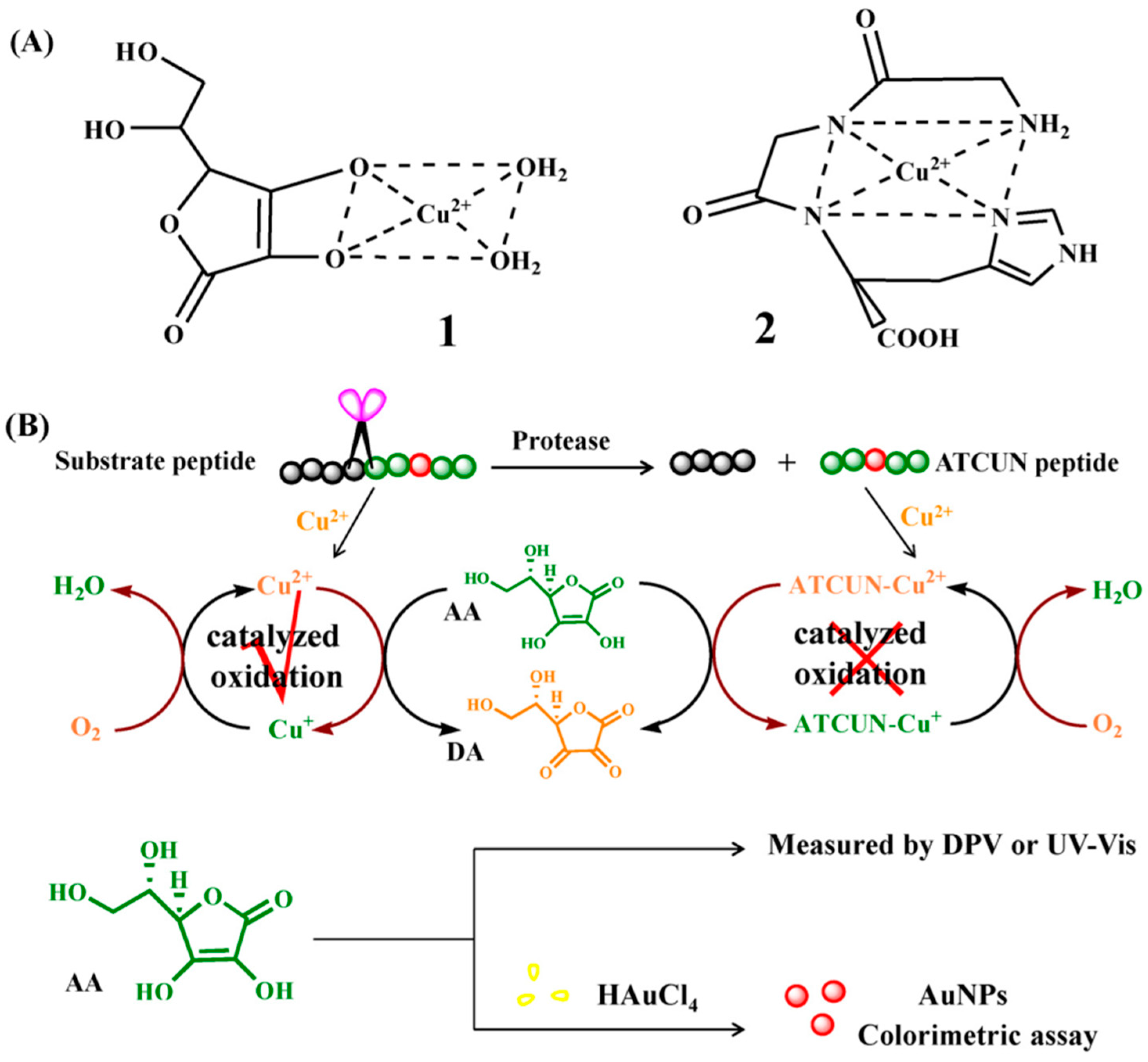
3. Colorimetric Biosensors
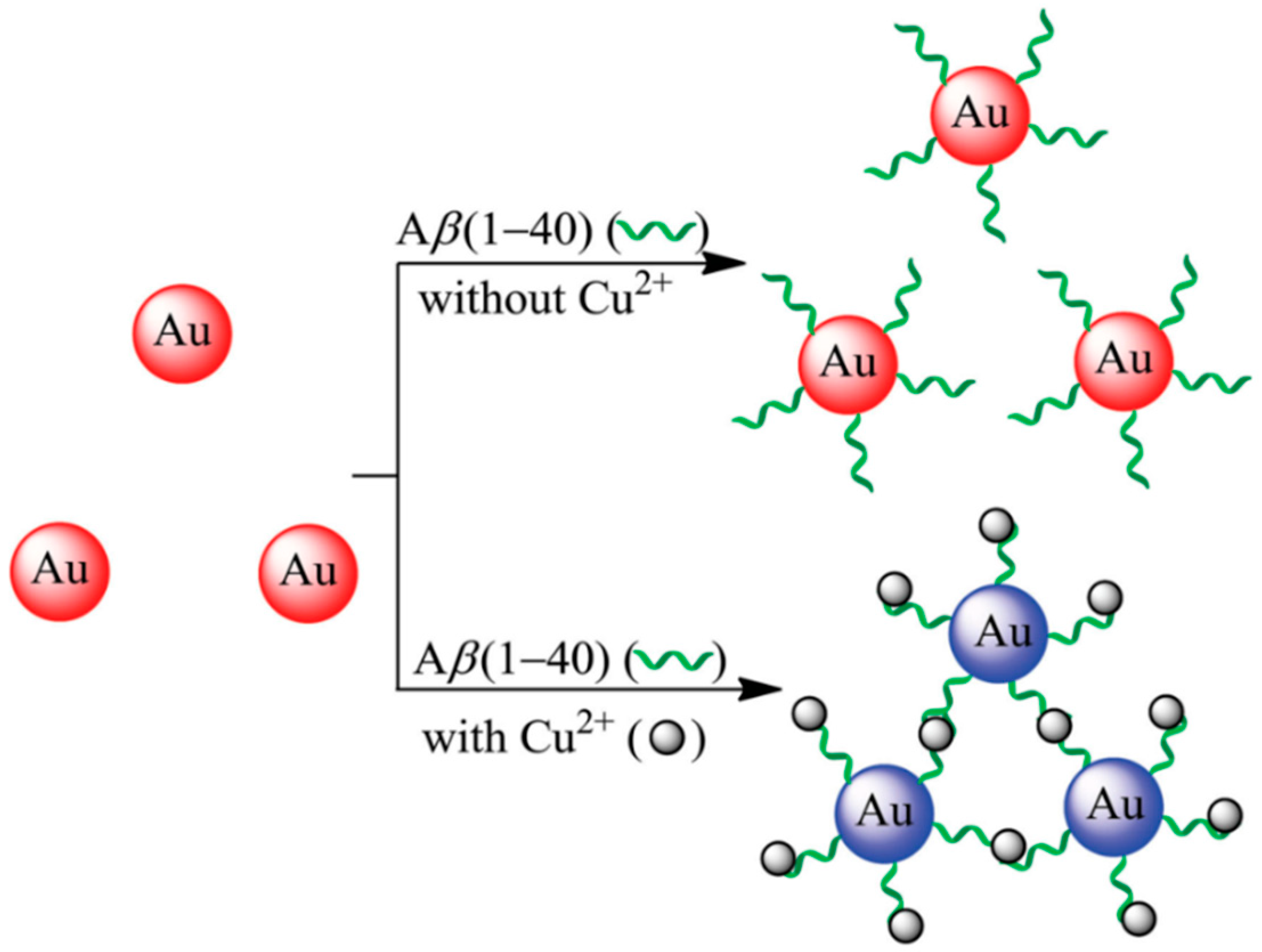
3.1. Aggregation and Growth of Metal Nanoparticles Mediated by Peptide–Cu2+ Complexes
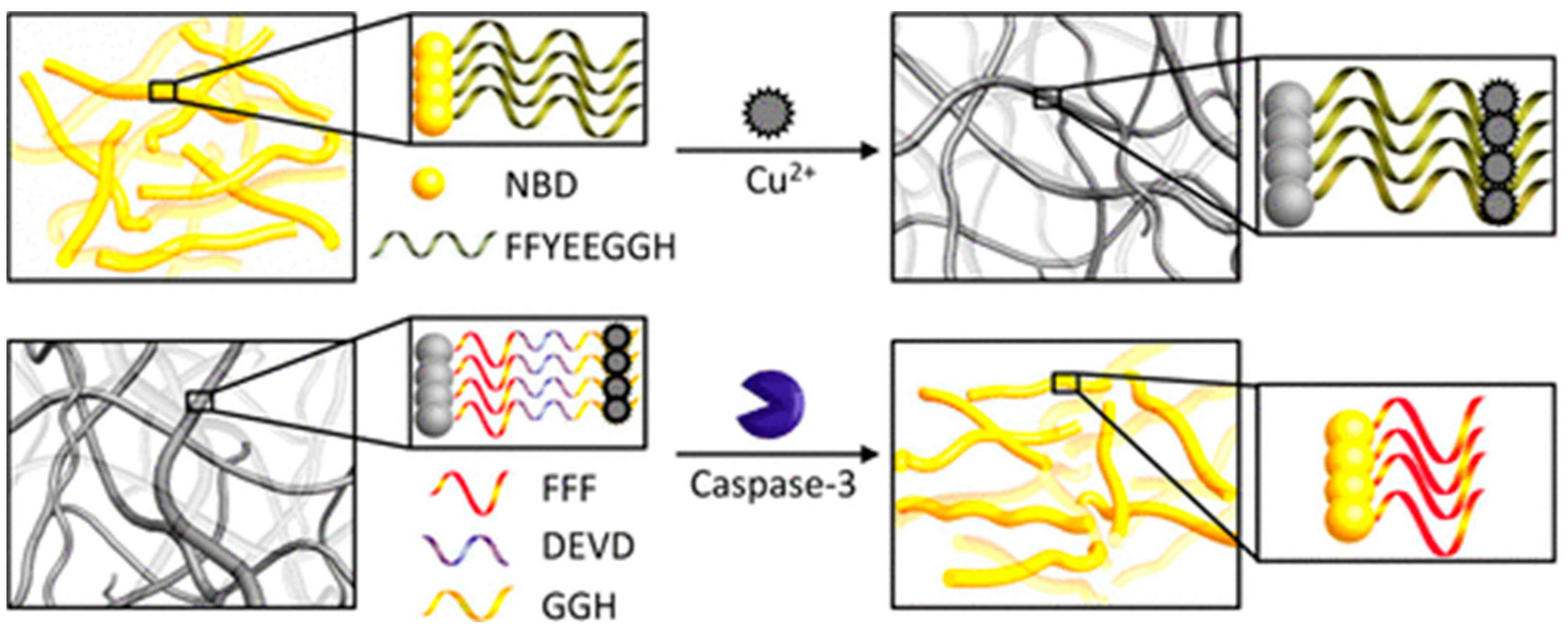
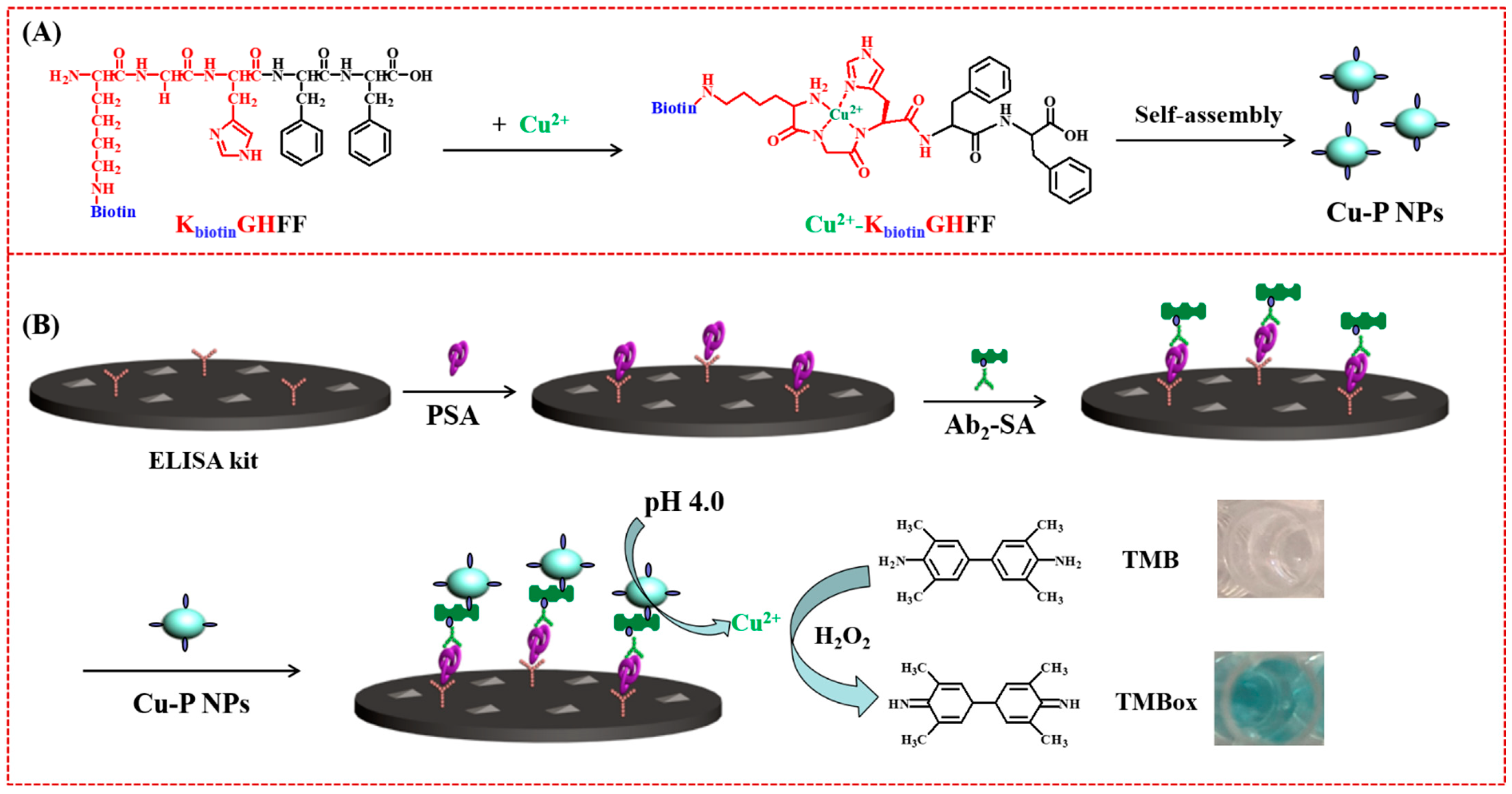
3.2. Peptide-Cu2+ Self-Assembles as the Signal Tags
4. Electrochemical Biosensors
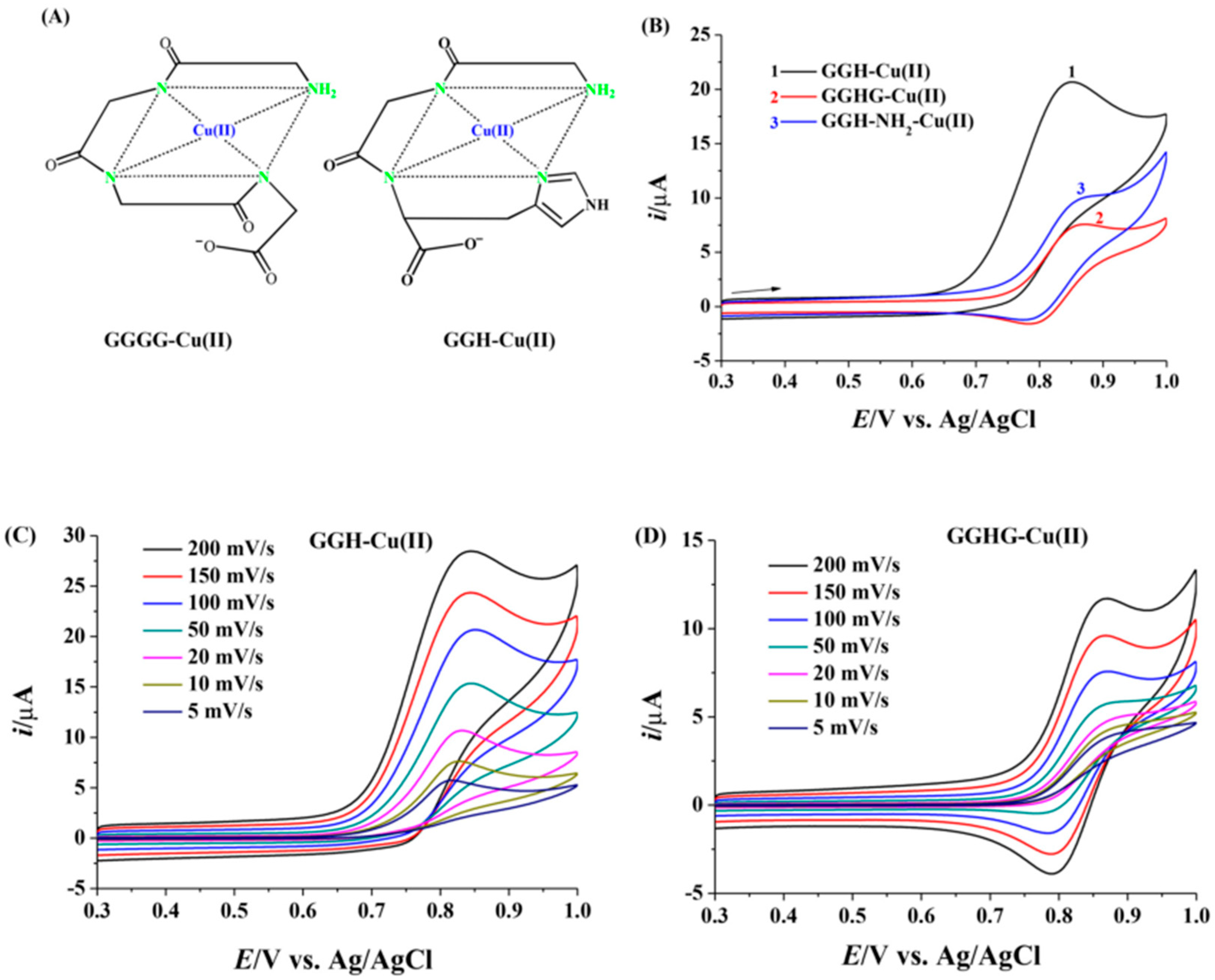
4.1. Peptide-Modified Electrodes for Copper Detection
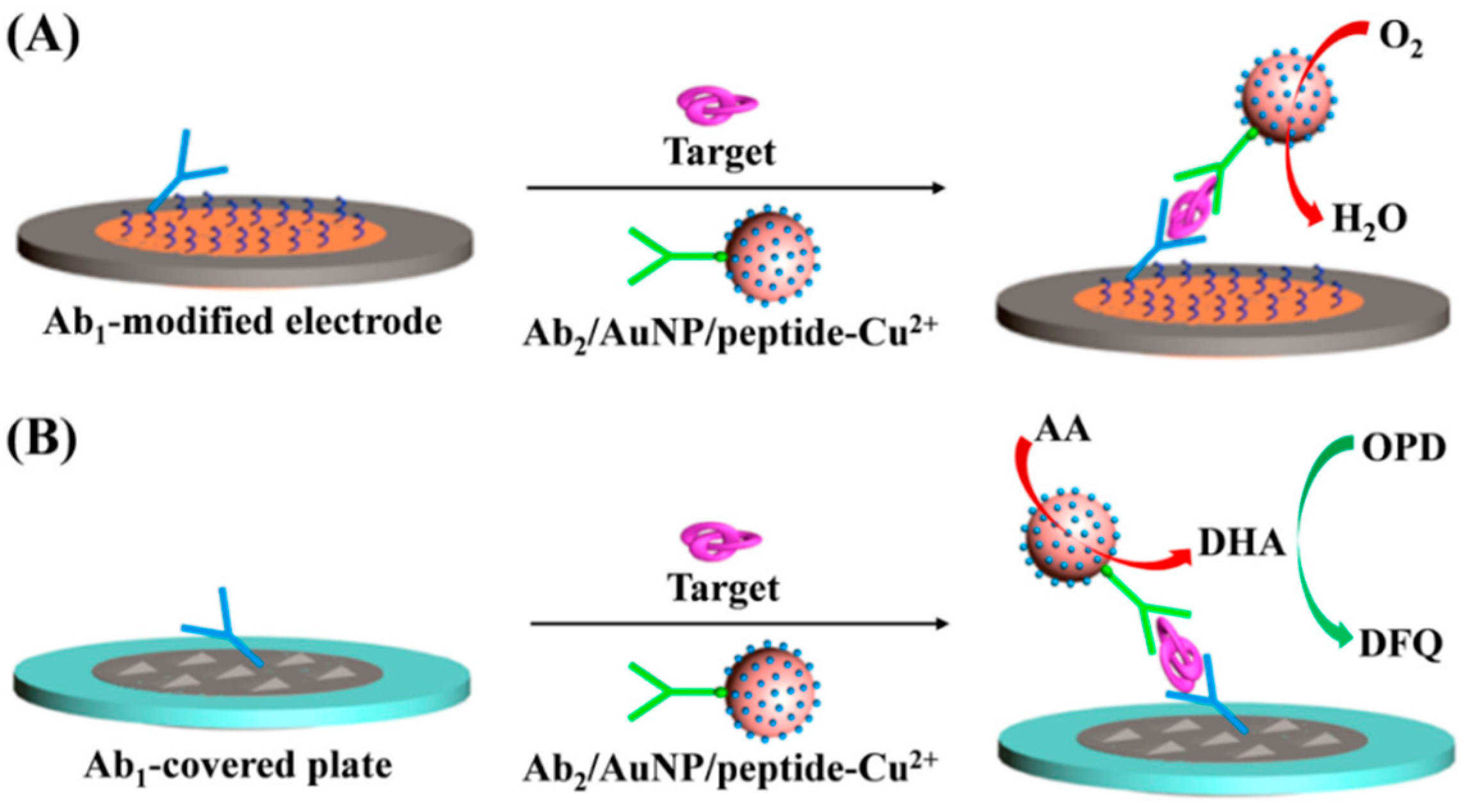
4.2. Electrochemical Biosensors with Peptide-Cu2+ Complexes as the Signal Reporters
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708. [Google Scholar] [CrossRef]
- Kozlowski, H.; Potocki, S.; Remelli, M.; Rowinska-Zyrek, M.; Valensin, D. Specific metal ion binding sites in unstructured regions of proteins. Coordin. Chem. Rev. 2013, 257, 2625. [Google Scholar] [CrossRef]
- Sóvágó, I.; Várnagy, K.; Lihi, N.; Grenács, Á. Coordinating properties of peptides containing histidyl residues. Coordin. Chem. Rev. 2016, 327, 43. [Google Scholar] [CrossRef]
- Weng, Y.C.; Fan, F.-R.F.; Bard, A.J. Combinatorial biomimetics. Optimization of a composition of copper(II) poly-L-histidine complex as an electrocatalyst for O2 reduction by scanning electrochemical microscopy. J. Am. Cheml. Soc. 2005, 127, 17576. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Ho, W.F.; Yang, K.-L. Copper–tripeptides (cuzymes) with peroxidase-mimetic activity. RSC Adv. 2020, 10, 17408. [Google Scholar] [CrossRef]
- Gonzalez, P.; Bossak, K.; Stefaniak, E.; Hureau, C.; Raibaut, L.; Bal, W.; Faller, P. N-Terminal Cu-binding motifs (Xxx-Zzz-His, Xxx-His) and their derivatives: Chemistry, biology and medicinal applications. Chem. Eur. J. 2018, 24, 8029. [Google Scholar] [CrossRef]
- Portelinha, J.; Duay, S.S.; Yu, S.I.; Heilemann, K.; Libardo, M.D.J.; Juliano, S.A.; Klassen, J.L.; Angeles-Boza, A.M. Antimicrobial peptides and copper(II) ions: Novel therapeutic opportunities. Chem. Rev. 2021, 121, 2648. [Google Scholar] [CrossRef]
- Harford, C.; Sarkar, B. Amino terminal Cu(II)- and Ni(II)-binding (ATCUN) motif of proteins and peptides: Metal binding, DNA cleavage, and other properties. Acc. Chem. Res. 1997, 30, 123–130. [Google Scholar] [CrossRef]
- Frączyk, T. Cu(II)-Binding N-terminal sequences of human proteins. Chem. Biodivers. 2021, 18, e210004. [Google Scholar] [CrossRef]
- Deng, D.; Hao, Y.; Xue, J.; Liu, X.; Xu, X.; Liu, L. A colorimetric enzyme-linked immunosorbent assay with CuO nanoparticles as signal labels based on the growth of gold nanoparticles in situ. Nanomaterials 2019, 9, 4. [Google Scholar] [CrossRef]
- Brunner, J.; Kraemer, R. Copper(II)-quenched oligonucleotide probes for fluorescent DNA sensing. J. Am. Chem. Soc. 2004, 126, 13626. [Google Scholar] [CrossRef]
- Moro, A.J.; Santos, M.; Outis, M.; Mateus, P.; Pereira, P.M. Selective coordination of Cu2+ and subsequent anion detection based on a naphthalimide-triazine-(DPA)2 chemosensor. Biosensors 2020, 10, 129. [Google Scholar] [CrossRef]
- Monson, C.F.; Cong, X.; Robison, A.D.; Pace, H.P.; Liu, C.; Poyton, M.F.; Cremer, P.S. Phosphatidylserine reversibly binds Cu2+ with extremely high affinity. J. Am. Chem. Soc. 2012, 134, 7773–7779. [Google Scholar] [CrossRef]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011, 133, 18003. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Zhang, L.; Zhao, J.; Jiang, Z.; Wang, W. Peptide-derived biosensors and their applications in tumor immunology-related detection. Anal. Chem. 2022, 94, 431–441. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, J.; Qin, W. Recent advances in peptide-based electrochemical biosensor. Prog. Chem. 2021, 33, 1756. [Google Scholar]
- Saw, P.E.; Xu, X.; Kim, S.; Jon, S. Biomedical applications of a novel class of high-affinity peptides. Acc. Chem. Res. 2021, 54, 3576–3592. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Moro, G.; Polo, F.; Palchetti, I. The role of peptides in the sesign of electrochemical biosensors for clinical diagnostics. Biosensors 2021, 11, 246. [Google Scholar] [CrossRef]
- Vanova, V.; Mitrevska, K.; Milosavljevic, V.; Hynek, D.; Richtera, L.; Adam, V. Peptide-based electrochemical biosensors utilized for protein detection. Biosens. Bioelectron. 2021, 180, 113087. [Google Scholar] [CrossRef]
- Alies, B.; Renaglia, E.; Rozga, M.; Bal, W.; Faller, P.; Hureau, C. Cu(II) affinity for the Alzheimer’s peptide: Tyrosine fluorescence studies revisited. Anal. Chem. 2013, 85, 1501. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, F.; Zhou, T.; Cao, J.; Xie, Y.; Zhang, M.; Wang, Y.; Cao, D.S.; Lin, Q.; Zhang, L. Label-free, water-soluble fluorescent peptide probe for a sensitive and selective determination of copper ions. Anal. Sci. 2017, 33, 191. [Google Scholar] [CrossRef][Green Version]
- Makowska, J.; Zamojc, K.; Wyrzykowski, D.; Uber, D.; Wierzbicka, M.; Wiczk, W.; Chmurzynski, L. Binding of Cu(II) ions to peptides studied by fluorescence spectroscopy and isothermal titration calorimetry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 451. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Y.; He, Y.; Lin, Q.; Ren, J.; Cao, D.; Zhang, L. A label-free fluorescent peptide probe for sensitive and selective determination of copper and sulfide ions in aqueous systems. RSC Adv. 2021, 11, 7426. [Google Scholar] [CrossRef]
- Żamojć, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L.; Makowska, J. A pentapeptide with tyrosine moiety as fluorescent chemosensor for selective nanomolar-level detection of copper(II) ions. Int. J. Mol. Sci. 2020, 21, 743. [Google Scholar] [CrossRef]
- White, B.R.; Holcombe, J.A. Fluorescent peptide sensor for the selective detection of Cu2+. Talanta 2007, 71, 2015. [Google Scholar] [CrossRef]
- Pang, X.; Gao, L.; Feng, H.; Li, X.; Kong, J.; Li, L. A peptide-based multifunctional fluorescent probe for Cu2+, Hg2+ and biothiols. New J. Chem. 2018, 42, 15770. [Google Scholar] [CrossRef]
- Pang, X.; Wang, L.; Gao, L.; Feng, H.; Kong, J.; Li, L. Multifunctional peptide-based fluorescent chemosensor for detection of Hg2+, Cu2+ and S2− ions. Luminescence 2019, 34, 585. [Google Scholar] [CrossRef]
- Wang, P.; Liu, L.; Zhou, P.; Wu, W.; Wu, J.; Liu, W.; Tang, Y. A peptide-based fluorescent chemosensor for multianalyte detection. Biosens. Bioelectron. 2015, 72, 80. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.; Su, P.; Xu, C.; Ge, Y.; Liu, D.; Liu, W.; Tang, Y. Fluorescence “on-off-on” peptide-based chemosensor for the selective detection of Cu2+ and S2− and its application in living cell bioimaging. Dalton Trans. 2016, 45, 16246. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J. Highly selective and sensitive detection of Zn(II) and Cu(II) ions using a novel peptide fluorescent probe by two different mechanisms and its application in live cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 208, 140. [Google Scholar] [CrossRef]
- An, Y.; Wang, P.; Yue, Z. A sequential and reversibility fluorescent pentapeptide probe for Cu(II) ions and hydrogen sulfide detections and its application in two different living cells imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 216, 319. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.; Zhao, C. A water-soluble peptide fluorescent chemosensor for detection of cadmium(II) and copper(II) by two different response modes and its application in living LNcap cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 226, 117600. [Google Scholar] [CrossRef]
- Wang, P.; Xue, S.; Yang, X. A novel peptide-based fluorescent chemosensor for detection of zinc(II) and copper(II) through differential response and application in logic gate and bioimaging. Microchem. J. 2020, 158, 105147. [Google Scholar] [CrossRef]
- Hu, Y.; Quan, S.; Zhao, C.; Li, J.; Sun, X.; Xiao, J. An “on–off–on” fluorescent peptide probe for the specific detection of Cu2+ and S2− in living cells and zebrafish. New J. Chem. 2022, 46, 7663. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, X.; Orbulescu, J.; Konka, V.; Andreopoulos, F.M.; Pham, S.M.; Leblanc, R.M. Peptidyl fluorescent chemosensors for the detection of divalent copper. Anal. Chem. 2003, 75, 1706. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Wei, G.; Su, Z. One-pot, in-situ synthesis of 8-armed poly(ethylene glycol)-coated Ag nanoclusters as a fluorescent sensor for selective detection of Cu2+. Biosensors 2020, 10, 131. [Google Scholar] [CrossRef]
- Qian, D.; Wang, Z.; Xiao, Z.; Fang, C.-J. A fluorescent probe for the detection of Cu(II) in water and tumor cells. Inorg. Chem. Commun. 2021, 126, 108471. [Google Scholar] [CrossRef]
- Zhuang, H.; Jiang, X.; Wu, S.; Wang, S.; Pang, Y.; Huang, Y.; Yan, H. A novel polypeptidemodified fluorescent gold nanoclusters for copper ion detection. Sci. Rep. 2022, 12, 6624. [Google Scholar] [CrossRef]
- Falcone, E.; Gonzalez, P.; Lorusso, L.; Seneque, O.; Faller, P.; Raibaut, L. A terbium(III) luminescent ATCUN-based peptide sensor for selective and reversible detection of copper(II) in biological media. Chem. Commun. 2020, 56, 4797. [Google Scholar] [CrossRef]
- Falcone, E.; Vileno, B.; Hoang, M.; Raibaut, L.; Faller, P. A luminescent ATCUN peptide variant with enhanced properties for copper(II) sensing in biological media. J. Inorg. Biochem. 2021, 221, 111478. [Google Scholar] [CrossRef]
- Jung, K.H.; Oh, E.T.; Park, H.J.; Lee, K.H. Development of new peptide-based receptor of fluorescent probe with femtomolar affinity for Cu+ and detection of Cu+ in Golgi apparatus. Biosens. Bioelectron. 2016, 85, 437. [Google Scholar] [CrossRef]
- Li, Y.; Di, C.; Wu, J.; Si, J.; Chen, Y.; Zhang, H.; Ge, Y.; Liu, D.; Liu, W. A peptide-based fluorescent sensor for selective imaging of glutathione in living cells and zebrafish. Anal. Bioanal. Chem. 2020, 412, 481. [Google Scholar] [CrossRef]
- Wang, P.; Xue, S.; Zhou, D.; Guo, Z.; Wang, Q.; Guo, B.; Yang, X.; Wu, J. Peptide-based colorimetric and fluorescent dual-functional probe for sequential detection of copper(II) and cyanide ions and its application in real water samples, test strips and living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 276, 121222. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.; Di, C.; Zhou, R.; Zhang, H.; Su, P.; Xu, C.; Zhou, P.; Ge, Y.; Liu, D.; et al. A novel peptide-based fluorescence chemosensor for selective imaging of hydrogen sulfide both in living cells and zebrafish. Biosens. Bioelectron. 2017, 92, 602. [Google Scholar] [CrossRef]
- Wang, P.; Sun, L.; Wu, J.; Yang, X.; Lin, P.; Wang, M. A dual-functional colorimetric and fluorescent peptide-based probe for sequential detection of Cu2+ and S2− in 100% aqueous buffered solutions and living cells. J. Hazard. Mater. 2021, 407, 124388. [Google Scholar] [CrossRef]
- Wang, P.; Xue, S.; Yang, X. Highly selective and sensitive detection of hydrogen sulfide in aqueous medium and live cells using peptide-based bioprobe to mimic the binding sites of the ceruloplasmin for Cu(II) ions. Biosens. Bioelectron. 2020, 163, 112283. [Google Scholar] [CrossRef]
- Hao, C.; Guo, X.; Lai, Q.; Li, Y.; Fan, B.; Zeng, G.; He, Z.; Wu, J. Peptide-based fluorescent chemical sensors for the specific detection of Cu2+ and S2−. Inorg. Chim. Acta 2020, 513, 119943. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, K.H. Efficient ensemble system based on the copper binding motif for highly sensitive and selective detection of cyanide ions in 100% aqueous solutions by fluorescent and colorimetric changes. Anal. Chem. 2015, 87, 9308. [Google Scholar] [CrossRef]
- Hao, C.; Li, Y.; Fan, B.; Zeng, G.; Zhang, D.; Bian, Z.; Wu, J. A new peptide-based chemosensor for selective imaging of copper ion and hydrogen sulfide in living cells. Microchem. J. 2020, 154, 104658. [Google Scholar] [CrossRef]
- Deng, D.; Hao, Y.; Yang, P.; Xia, N.; Yu, W.; Liu, X.; Liu, L. Single-labeled peptide substrates for detection of protease activity based on the inherent fluorescence quenching ability of Cu2+. Anal. Methods 2019, 11, 1248. [Google Scholar] [CrossRef]
- Song, N.; Zhang, Z.; Liu, P.; Yang, Y.-W.; Wang, L.; Wang, D.; Tang, B.Z. Nanomaterials with supramolecular assembly based on AIE luminogens for theranostic applications. Adv. Mater. 2020, 32, 2004208. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Tang, B.Z. Fluorescent sensors based on aggregation-induced emission: Recent advances and perspectives. ACS Sens. 2017, 2, 1382. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ji, S.; Li, H.; Hong, L.; Kong, D.; Qi, X.; Ding, D. Cellular membrane-anchored fluorescent probe with aggregation-induced emission characteristics for selective detection of Cu2+ ions. Faraday Discuss. 2017, 196, 377. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Kshtriya, V.; Gupta, S.; Koshti, B.; Singh, R.; Patel, D.; Joshi, K.B. Synthesis and aggregation studies of a pyridothiazole-based AIEE probe and its application in sensing amyloid fibrillation. ACS Appl. Bio. Mater. 2019, 2, 4442. [Google Scholar] [CrossRef]
- Cai, Y.; Shi, Y.; Wang, H.; Wang, J.; Ding, D.; Wang, L.; Yang, Z. Environment-sensitive fluorescent supramolecular nanofibers for imaging applications. Anal. Chem. 2014, 86, 2193. [Google Scholar] [CrossRef]
- Kim, I.; Jeong, H.H.; Kim, Y.J.; Lee, N.E.; Huh, K.M.; Lee, C.S.; Kim, G.H.; Lee, E. A “Light-up” 1D supramolecular nanoprobe for silver ions based on assembly of pyrene-labeled peptide amphiphiles: Cell-imaging and antimicrobial activity. J. Mater. Chem. B 2014, 2, 6478. [Google Scholar] [CrossRef]
- Xia, N.; Wang, X.; Yu, J.; Wu, Y.; Cheng, S.; Xing, Y.; Liu, L. Design of electrochemical biosensors with peptide probes as the receptors of targets and the inducers of gold nanoparticles assembly on electrode surface. Sens. Actuat. B Chem. 2017, 239, 834. [Google Scholar] [CrossRef]
- Korkmaz, N.; Hwang, C.; Kessler, K.K.; Silina, Y.E.; Müller, L.; Park, J. A novel copper (II) binding peptide for a colorimetric biosensor system design. Talanta 2021, 232, 122439. [Google Scholar] [CrossRef]
- Wang, C.; Wang, K.; Wang, Z. Development of gold nanoparticle based colorimetric method for quantitatively studying the inhibitors of Cu2+/Zn2+ induced beta-amyloid peptide assembly. Anal. Chim. Acta 2015, 858, 42. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, H.; Liu, L.; Xu, M. Simple colorimetric setection of amyloid beta-peptide (1–40) based on aggregation of gold nanoparticles in the presence of copper ions. Small 2015, 11, 2144. [Google Scholar] [CrossRef]
- Pelin, J.N.B.D.; Edwards-Gayle, C.J.C.; Martinho, H.; Gerbelli, B.B.; Castelletto, V.; Hamley, I.W.; Alves, W.A. Self-assembled gold nanoparticles and amphiphile peptides: A colorimetric probe for copper(II) ion detection. Dalton Trans. 2020, 49, 16226. [Google Scholar] [CrossRef] [PubMed]
- Ghodake, G.S.; Shinde, S.K.; Saratale, R.G.; Kadam, A.A.; Saratale, G.D.; Syed, A.; Ameen, F.; Kim, D.Y. Colorimetric detection of Cu(2+) based on the formation of peptide-copper complexes on silver nanoparticle surfaces. Beilstein J. Nanotechnol. 2018, 9, 1414. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chen, C.; Huang, G.; Yang, X. Antibody modified-silver nanoparticles for colorimetric immuno sensing of Aβ(1–40/1–42) based on the interaction between β-amyloid and Cu2+. Sens. Actuat. B Chem. 2016, 234, 63. [Google Scholar] [CrossRef]
- Guarise, C.; Pasquato, L.; Filippis, V.D.; Scrimin, P. Gold nanoparticles-based protease assay. Proc. Natl. Acad. Sci. USA 2006, 103, 3978. [Google Scholar] [CrossRef]
- Liu, L.; Deng, D.; Wang, Y.; Song, K.; Shang, Z.; Wang, Q.; Xia, N.; Zhang, B. A colorimetric strategy for assay of protease activity based on gold nanoparticle growth controlled by ascorbic acid and Cu(II)-coordinated peptide. Sens. Actuat. B Chem. 2018, 266, 246. [Google Scholar] [CrossRef]
- Sousa, C.P.; Coutinho-Neto, M.D.; Liberato, M.S.; Kubota, L.T.; Alves, W.A. Self-assembly of peptide nanostructures onto an electrode surface for nonenzymatic oxygen sensing. J. Phys. Chem. C 2015, 119, 1038–1046. [Google Scholar] [CrossRef]
- Xia, N.; Huang, Y.; Zhao, Y.; Wang, F.; Liu, L.; Sun, Z. Electrochemical biosensors by in situ dissolution of self-assembled nanolabels into small monomers on electrode surface. Sens. Actuat. B Chem. 2020, 325, 128777. [Google Scholar] [CrossRef]
- Mitra, S.; Prakash, D.; Rajabimoghadam, K.; Wawrzak, Z.; Prasad, P.; Wu, T.; Misra, S.K.; Sharp, J.S.; Garcia-Bosch, I.; Chakraborty, S. De novo design of a self-assembled artificial copper peptide that activates and reduces peroxide. ACS Catal. 2021, 11, 10267. [Google Scholar] [CrossRef]
- Sun, T.; Xia, N.; Yuan, F.; Liu, X.; Chang, Y.; Liu, S.; Liu, L. A colorimetric method for determination of the prostate specific antigen based on enzyme-free cascaded signal amplification via peptide-copper(II) nanoparticles. Microchim. Acta 2020, 187, 116. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; Binks, B.P.; He, Z. Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants. Appl. Catal. B Environ. 2019, 254, 452. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Li, X. Supramolecular assemblies of histidine-containing peptides with switchable hydrolase and peroxidase activities through Cu(II) binding and co-assembling. J. Mater. Chem. B 2022, 10, 3716. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chow, E.; Willett, G.D.; Hibbert, D.B.; Gooding, J.J. Exploring the use of the tripeptide Gly-Gly-his as a selective recognition element for the fabrication of electrochemical copper sensors. Analyst 2003, 128, 712. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.R.; Hibbert, D.B.; Zhang, R.; Willett, G.D.; Gooding, J.J. Stepwise synthesis of Gly-Gly-His on gold surfaces modified with mixed self-assembled monolayers. Langmuir 2005, 21, 260. [Google Scholar] [CrossRef]
- Liu, G.; Nguyen, Q.T.; Chow, E.; Böcking, T.; Hibbert, D.B.; Gooding, J.J. Study of factors affecting the performance of voltammetric copper sensors based on Gly-Gly-His modified glassy carbon and gold electrodes. Electroanalysis 2006, 18, 1141. [Google Scholar] [CrossRef]
- Wawrzyniak, U.E.; Ciosek, P.; Zaborowski, M.; Liu, G.; Gooding, J.J. Gly-Gly-His immobilized on monolayer modified back-side contact miniaturized sensors for complexation of copper ions. Electroanalysis 2013, 25, 1461. [Google Scholar] [CrossRef]
- Sam, S.S.; Chazalviel, J.N.; Gouget-Laemmel, A.C.; Ozanam, F.F.; Etcheberry, A.A.; Gabouze, N.E. Peptide immobilisation on porous silicon surface for metal ions detection. Nanoscale Res. Lett. 2011, 6, 412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Li, X.; Chen, Y.; Yin, Y.; Li, G. Electrochemical assay of melanoma biomarker in human blood. Electrochem. Commun. 2014, 39, 12. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Liu, X.; Gao, Y.; Ye, Z.; Li, G. Colorimetric copper(II) ion sensor based on the conformational change of peptide immobilized onto the surface of gold nanoparticles. Anal. Methods 2014, 6, 2580. [Google Scholar] [CrossRef]
- Chen, H.; Jia, S.; Zhang, J.; Jang, M.; Chen, X.; Koh, K.; Wang, Z. Sensitive detection of copper(II) ions based on the conformational change of peptides by surface plasmon resonance spectroscopy. Anal. Methods 2015, 7, 8942. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.; Lan, L.; Pan, Y.; Zhu, G.; Miao, P. Gly–Gly–His tripeptide- and silver nanoparticle-assisted electrochemical evaluation of copper(ii) ions in aqueous environment. New J. Chem. 2018, 42, 14733. [Google Scholar] [CrossRef]
- Mervinetsky, E.; Alshanski, I.; Hamo, Y.; Sandonas, L.M.; Dianat, A.; Buchwald, J.; Gutierrez, R.; Cuniberti, G.; Hurevich, M.; Yitzchaik, S. Copper induced conformational changes of tripeptide monolayer based impedimetric biosensor. Sci. Rep. 2017, 7, 9498. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yang, K.-L. Quantitative serine protease assays based on formation of copper(II)–oligopeptide complexes. Analyst 2015, 140, 340. [Google Scholar] [CrossRef] [PubMed]
- Synhaivska, O.; Mermoud, Y.; Baghernejad, M.; Alshanski, I.; Hurevich, M.; Yitzchaik, S.; Wipf, M.; Calame, M. Detection of Cu2+ ions with GGH peptide realized with Si-nanoribbon ISFET. Sensors 2019, 19, 4022. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Liu, L.; Bu, Y.; Liu, X.; Wang, X.; Zhang, B. Electrochemical sensing devices using ATCUN-Cu(II) complexes as electrocatalysts for water oxidation. Sens. Actuat. B Chem. 2018, 269, 189. [Google Scholar] [CrossRef]
- Deng, D.; Hao, Y.; Yang, S.; Han, Q.; Liu, L.; Xiang, Y.; Tu, F.; Xia, N. A signal-on electrochemical biosensor for evaluation of caspase-3 activity and cell apoptosis by the generation of molecular electrocatalysts on graphene electrode surface for water oxidation. Sens. Actuat. B Chem. 2019, 286, 415. [Google Scholar] [CrossRef]
- Xia, N.; Deng, D.; Yang, S.; Hao, Y.; Wang, L.; Liu, Y.; An, C.; Han, Q.; Liu, L. Electrochemical immunosensors with protease as the signal label for the generation of peptide-Cu(II) complexes as the electrocatalysts toward water oxidation. Sens. Actuat. B Chem. 2019, 291, 113. [Google Scholar] [CrossRef]
- Xia, N.; Liu, G.; Zhang, S.; Shang, Z.; Yang, Y.; Li, Y.; Liu, L. Oxidase-mimicking peptide-copper complexes and their applications in sandwich affinity biosensors. Anal. Chim. Acta 2022, 1214, 339965. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, G.; Zhang, C.; Li, J.; Li, Y.; Liu, L. Fluorescent immunoassay with a copper polymer as the signal label for catalytic oxidation of O-phenylenediamine. Molecules 2022, 27, 3675. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, G.; Zhang, F.; Liu, J.; La, M.; Xia, N. A general, label-free and homogeneous electrochemical strategy for probing of protease activity and screening of inhibitor. Micromachines 2022, 13, 803. [Google Scholar] [CrossRef]

| Probe | Analyte | Detection Limit (nM) | Ref. |
|---|---|---|---|
| Dansyl-Gly-Gly-Asp-Gly-Gly-Asp-Gly-Gly-Asp-Gly-Gly-Asp-Gly-Gly-Trp-CONH2 | Cu2+ | 500 | [25] |
| Dansyl-His-Pro-Gly-Trp-NH2 | Cu2+, Hg2+ | 105, 37 | [26] |
| Dansyl-Gly-His-Gly-Gly-Trp-COOH | Cu2+, Hg2+, S2− | 85, 25 | [27] |
| Dansyl-His-Lys-His- Dansyl | Cu2+, Zn2+, S2− | 78, 82 | [28] |
| Dansyl-Ser-Pro-Gly-His-NH2 | Cu2+, S2− | 88, 75 | [29] |
| Dansyl-His-Pro-Gly-Glu-NH2 | Cu2+, Zn2+ | 15, 4.9 | [30] |
| Dansyl-Ser-Pro-Gly-His-Gly-NH2 | Cu2+, H2S | 23.5, 17.2 | [31] |
| Dansyl-Gly-Cys-NH2 | Cu2+, Cd2+ | 26.3, 14.5 | [32] |
| Dansyl-Ser-Pro-Gly-His-NH2 | Cu2+, Zn2+ | 4.1, 13.2 | [33] |
| Dansyl-Asp-Asp-Gly-Glu-Glu-NH2 | Cu2+, S2− | 47, 57 | [34] |
| Dansyl-Leu-Leu-Cys | Cu+, Cu2+, Zn2+ | Not reported | [41] |
| FITC-Ahx-His-Lys-His-NH2 | Cu2+, CN− | 1.5, 12.7 | [43] |
| FITC-Ahx-Ser-Pro-Gly-His-NH2 | Cu2+, H2S | 31 | [44] |
| FITC-Ahx-Ser-Ser-His-NH2 | Cu2+, S2− | 76.7, 27.2 | [45] |
| FITC-Ahx-Gly-His-Lys-NH2 | Cu2+, H2S | 14.7 | [46] |
| FITC-Ahx-His-Glu-Phe-Cys-NH2 | Cu2+, S2− | 32, 53 | [47] |
| FITC-Ahx-His-Glu-Phe-His-NH2 | Cu2+, S2− | 54, 68 | [49] |
| NBD-Ser-Ser-His | Cu2+, CN− | Not reported | [48] |
| Signal Reporters | Analyte | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|
| AuNPs | Aβ(1–40) | Not reported | 0.6 nM | [60] |
| AuNPs | Cu2+ | Not reported | 190 nM | [61] |
| AuNPs | Cu2+ | 0.08~1.44 μM | 0.16 nM | [62] |
| Ab-AgNPs | Cu2+ | 0.00025~0.125 μM | 0.086 nM | [63] |
| AuNPs | beta-secretase | 1~75 nM | 1 nM | [65] |
| Cu–PNPs | PSA | 0.001–1 ng/mL | 1 pg/mL | [69] |
| CH–Cu | epinephrine | Not reported | 0.31 μg/mL | [70] |
| Signal Tags | Analyte | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|
| AuNPs/DNA/DCH–Cu2+ | DNA | 0.1~2.5 pM | 0.1 pM | [84] |
| ATCUN–Cu2+ | caspase-3 | 0.0005~2 ng/mL | 2 pg/mL | [85] |
| Ab2-CNTs-trypsin | PSA | 0.01~2 ng/mL | 10 pg/mL | [86] |
| Ab2/AuNP/peptide | PSA | 0.001~0.5 ng/mL | 0.4 pg/mL | [87] |
| Ab2-biotin-poly-(KH–Cu)20 | PSA | 0.01~2 ng/mL | 8 pg/mL | [88] |
| GGH–Cu2+ | ACE | 1~50 mU/mL | 1 mU/mL | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Xia, N.; Tian, L.; Sun, Z.; Liu, L. Progress in the Development of Biosensors Based on Peptide–Copper Coordination Interaction. Biosensors 2022, 12, 809. https://doi.org/10.3390/bios12100809
Liu G, Xia N, Tian L, Sun Z, Liu L. Progress in the Development of Biosensors Based on Peptide–Copper Coordination Interaction. Biosensors. 2022; 12(10):809. https://doi.org/10.3390/bios12100809
Chicago/Turabian StyleLiu, Gang, Ning Xia, Linxu Tian, Zhifang Sun, and Lin Liu. 2022. "Progress in the Development of Biosensors Based on Peptide–Copper Coordination Interaction" Biosensors 12, no. 10: 809. https://doi.org/10.3390/bios12100809
APA StyleLiu, G., Xia, N., Tian, L., Sun, Z., & Liu, L. (2022). Progress in the Development of Biosensors Based on Peptide–Copper Coordination Interaction. Biosensors, 12(10), 809. https://doi.org/10.3390/bios12100809







