Optical Imaging of Beta-Amyloid Plaques in Alzheimer’s Disease
Abstract
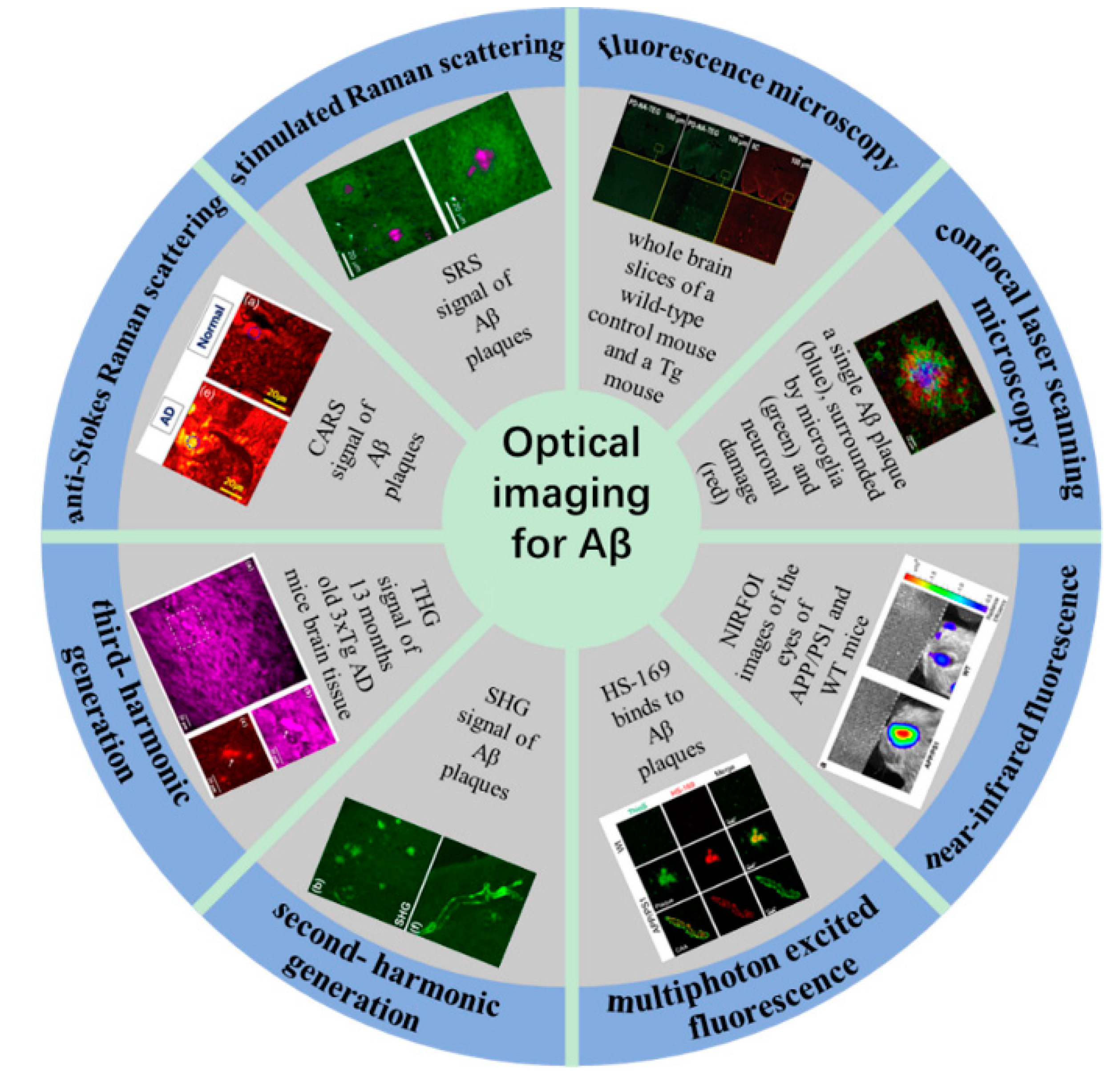
1. Introduction
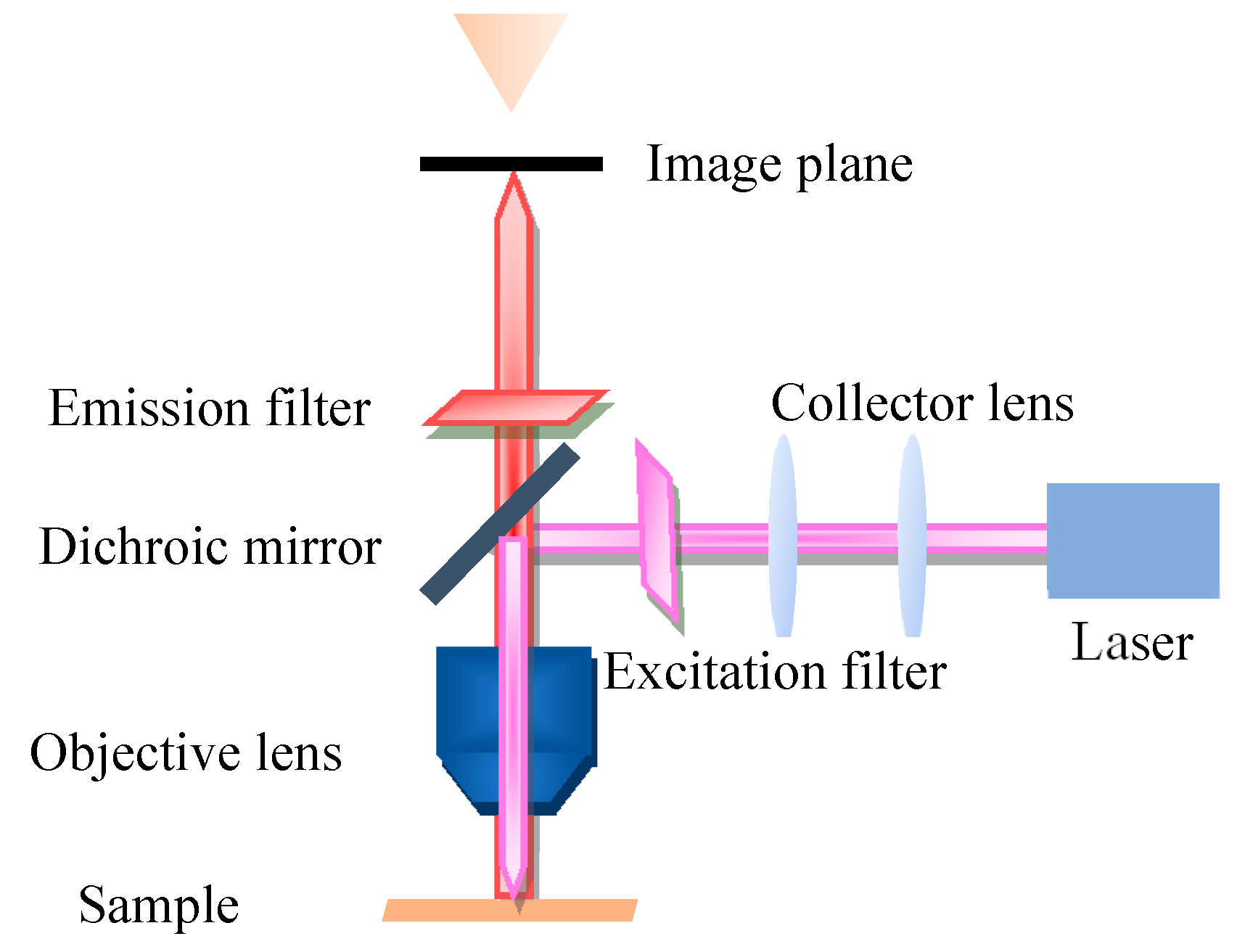
2. Conventional Fluorescence Microscopy Imaging
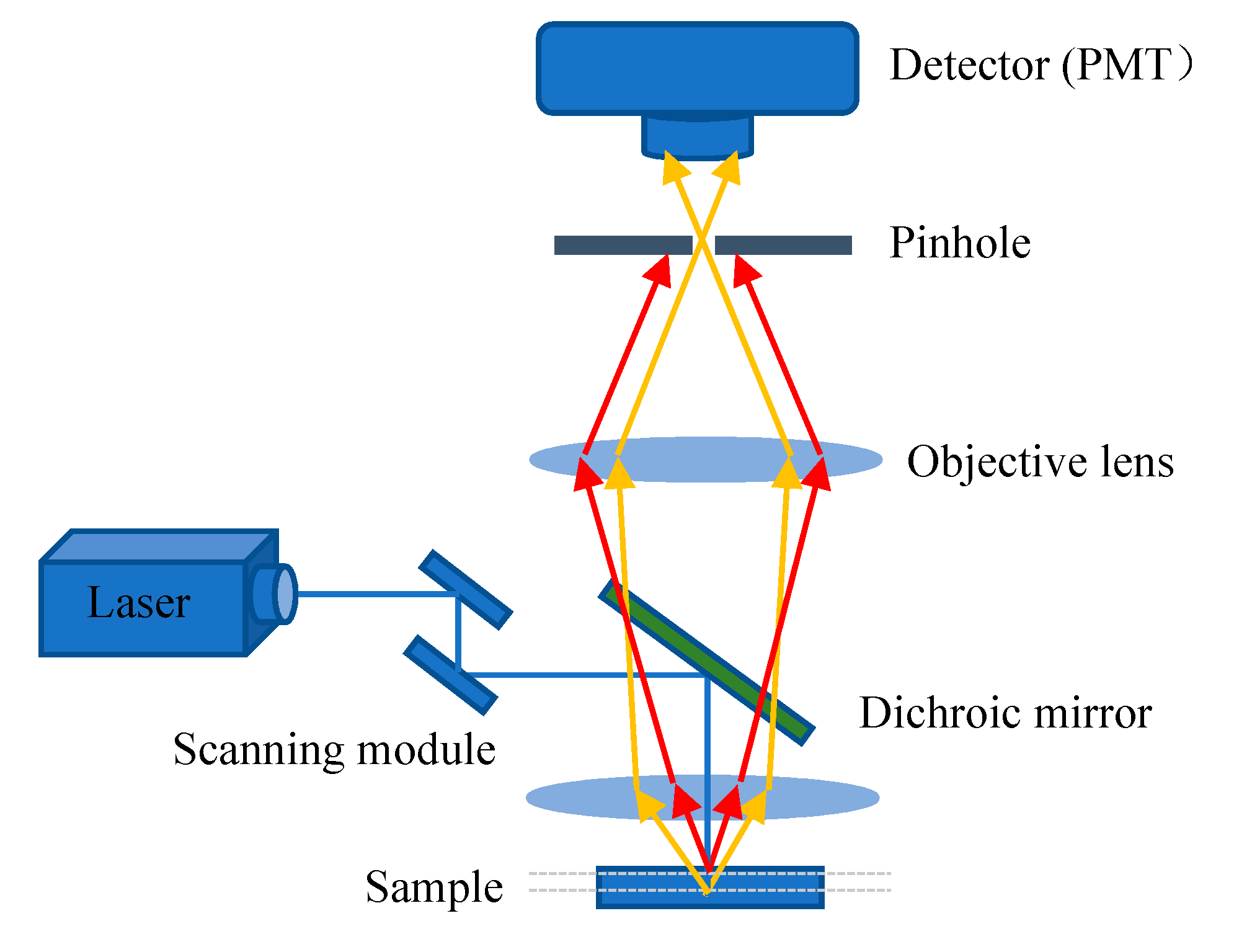
3. Confocal Laser Scanning Microscopy Imaging
4. Near-Infrared Fluorescence Imaging
5. Nonlinear Optical Microscopic Imaging
5.1. Multiphoton Excited Fluorescence Microscopy
5.2. Second- and Third-Harmonic Generation Microscopy
5.3. Coherent Raman Scattering Microscopy
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Selkoe, D.J. Alzheimer’s Disease: Genes, Proteins, and Therapy. Phys. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, D.M.; Montagna, D.R.; Seghers, M.C.; Wolfe, M.S.; Selkoe, D.J. The amyloid-beta forming tripeptide cleavage mechanism of gamma-secretase. eLife 2016, 5. [Google Scholar] [CrossRef]
- Bushman, D.M.; Kaeser, G.E.; Siddoway, B.; Westra, J.W.; Rivera, R.R.; Rehen, S.K.; Yung, Y.C.; Chun, J. Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer’s disease brains. eLlife 2015, 4, e05116. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.C.; Overk, C.R.; Sijben, J.W.; Masliah, E. Meta-analysis of synaptic pathology in Alzheimer’s disease reveals selective molecular vesicular machinery vulnerability. Alzheimers Dement. 2016, 12, 633–644. [Google Scholar] [CrossRef]
- Patterson, C. World Alzheimer Report 2018; Alzheimer’s Disease International (ADI): London, UK, 2018. [Google Scholar]
- Kelly, L.; Seifi, M.; Ma, R.; Mitchell, S.J.; Rudolph, U.; Viola, K.L.; Klein, W.L.; Lambert, J.J.; Swinny, J.D. Identification of intraneuronal amyloid beta oligomers in locus coeruleus neurons of Alzheimer’s patients and their potential impact on inhibitory neurotransmitter receptors and neuronal excitability. Neuropathol. Appl. Neurobiol. 2020, 47, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, E.; Sanchez-Gomez, M.V.; Cavaliere, F.; Perez-Samartin, A.; Zugaza, J.L.; Trullas, R.; Domercq, M.; Matute, C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010, 47, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Weber, G.A.; Zheng, J.; Gendelman, H.E.; Ikezu, T. C1q–calreticulin induced oxidative neurotoxicity: Relevance for the neuropathogenesis of Alzheimer’s disease. J. Neuroimmunol. 2003, 135, 62–71. [Google Scholar] [CrossRef]
- Murakami, K.; Shimizu, T.; Irie, K. Formation of the 42-mer Amyloid beta Radical and the Therapeutic Role of Superoxide Dismutase in Alzheimer’s Disease. J. Amino. Acids. 2011, 2011, 654207. [Google Scholar] [CrossRef][Green Version]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Bruggink, K.A.; Jongbloed, W.; Biemans, E.A.; Veerhuis, R.; Claassen, J.A.; Kuiperij, H.B.; Verbeek, M.M. Amyloid-beta oligomer detection by ELISA in cerebrospinal fluid and brain tissue. Anal. Biochem. 2013, 433, 112–120. [Google Scholar] [CrossRef]
- Damian, A.D.L.F.A.; Castellano, G.; Savio, E.; Buccino, P.; Aguiar, B.; Quagliata, A.; Cano, F.; Gonzalez, M.; Dansilio, S.; Kmaid, A. Resveratrol in Alzheimer’s disease: A PIB positron emission tomography/computed tomography study. Eur. J. Nucl. Med. Mol. Imaging. 2015, 42, S135. [Google Scholar]
- Yao, Z.; Zhang, Y.; Lin, L.; Zhou, Y.; Xu, C.; Jiang, T.; Alzheimer’s Disease Neuroimaging Initiative. Abnormal cortical networks in mild cognitive impairment and Alzheimer’s disease. PLoS Comput. Biol. 2010, 6, e1001006. [Google Scholar] [CrossRef]
- Zhu, X.; Smith, M.A.; Honda, K.; Aliev, G.; Moreira, P.I.; Nunomura, A.; Casadesus, G.; Harris, P.L.; Siedlak, S.L.; Perry, G. Vascular oxidative stress in Alzheimer disease. J. Neurol. Sci. 2007, 257, 240–246. [Google Scholar] [CrossRef]
- He, Y. Advances in neuroimaging studies of Alzheimer’s disease. Prog. Biochem. Biophys. 2012, 39, 811–815. [Google Scholar] [CrossRef]
- Caroli, A.; Testa, C.; Geroldi, C.; Nobili, F.; Barnden, L.R.; Guerra, U.P.; Bonetti, M.; Frisoni, G.B. Cerebral perfusion correlates of conversion to Alzheimer’s disease in amnestic mild cognitive impairment. J. Neurol. 2007, 254, 1698–1707. [Google Scholar] [CrossRef]
- Ortiz, A.; Górriz, J.M.; Ramírez, J.; Martínez-Murcia, F.J. LVQ-SVM based CAD tool applied to structural MRI for the diagnosis of the Alzheimer’s disease. Pattern Recognit. Lett. 2013, 34, 1725–1733. [Google Scholar] [CrossRef]
- Pennanen, C.; Kivipelto, M.; Tuomainen, S.; Hartikainen, P.; Hänninen, T.; Laakso, M.P.; Hallikainen, M.; Vanhanen, M.; Nissinen, A.; Helkala, E.-L.; et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol. Aging. 2004, 25, 303–310. [Google Scholar] [CrossRef]
- Johnson, S.C.; Saykin, A.J.; Baxter, L.C.; Flashman, L.A.; Santulli, R.B.; McAllister, T.W.; Mamourian, A.C. The relationship between fMRI activation and cerebral atrophy: Comparison of normal aging and alzheimer disease. NeuroImage 2000, 11, 179–187. [Google Scholar] [CrossRef]
- Koepp, M.J.; Woermann, F.G. Imaging structure and function in refractory focal epilepsy. Lancet Neurol. 2005, 4, 42–53. [Google Scholar] [CrossRef]
- Gustavsson, T.; Syvanen, S.; O’Callaghan, P.; Sehlin, D. SPECT imaging of distribution and retention of a brain-penetrating bispecific amyloid-beta antibody in a mouse model of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Song, I.U.; Im, J.J.; Jeong, H.; Na, S.H.; Chung, Y.A. Possible neuroprotective effects of rasagiline in Alzheimer’s disease: A SPECT study. Acta Radiol. 2020, 6, 784–790. [Google Scholar] [CrossRef]
- Zeng, H.M.; Han, H.B.; Zhang, Q.F.; Bai, H. Application of modern neuroimaging technology in the diagnosis and study of Alzheimer’s disease. Neural Regen. Res. 2021, 16, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Nesterov, E.E.; Skoch, J.; Hyman, B.T.; Klunk, W.E.; Bacskai, B.J.; Swager, T.M. In Vivo Optical Imaging of Amyloid Aggregates in Brain: Design of Fluorescent Markers. Angew. Chem. 2005, 117, 5588–5592. [Google Scholar] [CrossRef]
- Raymond, S.B.; Skoch, J.; Hills, I.D.; Nesterov, E.E.; Swager, T.M.; Bacskai, B.J. Smart optical probes for near-infrared fluorescence imaging of Alzheimer’s disease pathology. Eur. J. Nucl. Med. Mol. Imaging. 2008, 35 (Suppl. S1), S93–S98. [Google Scholar] [CrossRef]
- Skoch, J.; Dunn, A.; Hyman, B.T.; Bacskai, B.J. Development of an optical approach for noninvasive imaging of Alzheimer’s disease pathology. J. Biomed. Opt. 2005, 10, 11007. [Google Scholar] [CrossRef] [PubMed]
- Jarvet, J.; Gräslund, A.; Tiiman, A.; Vukoevic, V. Monitoring of Alzheimer’s Amyloid-β Peptide Aggregation via Fluorescence Correlation Spectroscopy and Total Internal Reflection Microscopy. Biophys. J. 2018, 114, 222a–223a. [Google Scholar] [CrossRef]
- Navarro, A.; del Valle, E.; Martinez, E.; Ordonez, C.; Perez, C.; Tolivia, J. Highly selective and fast diagnosis of Alzheimer’s disease hallmark lesions using Congo Red in isopropyl alcoholic solution. J. Alzheimers Dis. 2013, 35, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Rembe, C.; Dräbenstedt, A. Laser-scanning confocal vibrometer microscope: Theory and experiments. Rev. Sci. Instrum. 2006, 77, 083702. [Google Scholar] [CrossRef]
- Wu, J.S.; Chung, Y.C.; Chien, J.J.; Chou, C. Improved axial point spread function in a two-frequency laser scanning confocal fluorescence microscope. J. Biomed. Opt. 2018, 23, 1–4. [Google Scholar] [CrossRef]
- Zhang, Y.; Leng, J.; Hu, W. A multiscale study on photophysical properties of a novel fluorescent probe for imaging amyloid-β in Alzheimer’s disease. Int. J. Quantum Chem. 2020, 120, e26344. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Li, Y.; Xu, Y.; Ran, C. Near-infrared Fluorescence Ocular Imaging (NIRFOI) of Alzheimer’s Disease. Mol. Imaging Biol. 2019, 21, 35–43. [Google Scholar] [CrossRef]
- Cheng, X.R.; Sze Hung, V.W.; Scarano, S.; Mascini, M.; Minunni, M.; Kerman, K. Label-free methods for probing the interaction of clioquinol with amyloid-β. Anal. Methods. 2012, 4, 2228–2232. [Google Scholar] [CrossRef]
- Geng, J.; Qu, K.; Ren, J.; Qu, X. Rapid and efficient screening of Alzheimer’s disease beta-amyloid inhibitors using label-free gold nanoparticles. Mol. Biosyst. 2010, 6, 2389–2391. [Google Scholar] [CrossRef]
- Chen, L.-W.; Zhou, Y.; Wu, M.-X.; Hong, M.-H. Remote-mode microsphere nano-imaging: New boundaries for optical microscopes. Opto-Electron. Adv. 2018, 1, 17000101. [Google Scholar] [CrossRef]
- Horneber, A.; Braun, K.; Rogalski, J.; Leiderer, P.; Meixner, A.J.; Zhang, D. Nonlinear optical imaging of single plasmonic nanoparticles with 30 nm resolution. Phys. Chem. Chem. Phys. 2015, 17, 21288–21293. [Google Scholar] [CrossRef]
- Le, T.T.; Langohr, I.M.; Locker, M.J.; Sturek, M.; Cheng, J.X. Label-free molecular imaging of atherosclerotic lesions using multimodal nonlinear optical microscopy. J. Biomed. Opt. 2007, 12, 054007. [Google Scholar] [CrossRef]
- Li, L.; Jiang, L.; Chen, Z.; Kang, D.; Yang, Z.; Liu, X.; Jiang, W.; Zhuo, S.; Guan, G.; Zhou, Y.; et al. Nonlinear optical microscopy for label-free detection of gastrointestinal neuroendocrine tumors. Lasers Med. Sci. 2016, 31, 1285–1291. [Google Scholar] [CrossRef]
- Schlickriede, C.; Waterman, N.; Reineke, B.; Georgi, P.; Li, G.; Zhang, S.; Zentgraf, T. Imaging through Nonlinear Metalens Using Second Harmonic Generation. Adv. Mater. 2018, 30, 1703843. [Google Scholar] [CrossRef]
- Yazdanfar, S.; Chen, Y.Y.; So, P.T.; Laiho, L.H. Multifunctional imaging of endogenous contrast by simultaneous nonlinear and optical coherence microscopy of thick tissues. Microsc. Res. Tech. 2007, 70, 628–633. [Google Scholar] [CrossRef]
- Kachynski, A.V.P.A.; Kuzmin, A.N.; Ohulchanskyy, T.Y.; Baev, A.; Qu, J.; Prasad, P.N. Photodynamic therapy by in situ nonlinear photon conversion. Nat. Photonics 2014, 8, 455–461. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Ren, S.; Li, Z.; Zhao, Y.; Yang, Z.; Hu, R.; Qu, J. Recent advances in nonlinear optics for bio-imaging applications. Opto-Electron. Adv. 2020, 3, 200003. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.H.; Song, W.K.; Oh, M.K.; Ko, D.K. Label-free imaging and quantitative chemical analysis of Alzheimer’s disease brain samples with multimodal multiphoton nonlinear optical microspectroscopy. J. Biomed. Opt. 2015, 20, 56013. [Google Scholar] [CrossRef] [PubMed]
- Kiskis, J.; Fink, H.; Nyberg, L.; Thyr, J.; Li, J.Y.; Enejder, A. Plaque-associated lipids in Alzheimer’s diseased brain tissue visualized by nonlinear microscopy. Sci. Rep. 2015, 5, 13489. [Google Scholar] [CrossRef]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage 2011, 54 (Suppl. S1), S204–S217. [Google Scholar] [CrossRef]
- Ziv, Y.; Avidan, H.; Pluchino, S.; Martino, G.; Schwartz, M. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc. Natl. Acad. Sci. USA 2006, 103, 13174–13179. [Google Scholar] [CrossRef]
- Frost, S.; Kanagasingam, Y.; Sohrabi, H.; Vignarajan, J.; Bourgeat, P.; Salvado, O.; Villemagne, V.; Rowe, C.C.; Macaulay, S.L.; Szoeke, C.; et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl. Psychiatry. 2013, 3, e233. [Google Scholar] [CrossRef]
- Tes, D.; Kratkiewicz, K.; Aber, A.; Horton, L.; Zafar, M.; Arafat, N.; Fatima, A.; Avanaki, M.R. Development and Optimization of a Fluorescent Imaging System to Detect Amyloid-beta Proteins: Phantom Study. Biomed. Eng. Comput. Biol. 2018, 9, 1179597218781081. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Buckley, C.J.; Abrahamson, E.E.; Kofler, J.K.; Mathis, C.A.; Klunk, W.E.; Farrar, G. Post-mortem analyses of PiB and flutemetamol in diffuse and cored amyloid-beta plaques in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 463–476. [Google Scholar] [CrossRef]
- Hovis, D.B.; Heuer, A.H. The use of laser scanning confocal microscopy (LSCM) in materials science. J. Microsc. 2010, 240, 173–180. [Google Scholar] [CrossRef]
- Ya-li, L. Investigation and comparison of the endocytosis, transport and degradation mechanisms of β-amyloid monomers and oligomers in astrocytes. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2013. [Google Scholar]
- Icke, I.; Zhang, A.Z.; Singh, S.; Dogdas, B.; Mirescu, C.; Kennedy, M.; Bardehle, S.; Tomaszewski, J.E.; Ward, A.D. 3D profiling of amyloid plaque-associated microglia and neuronal damage on confocal fluorescence images to aid drug discovery in Alzheimer’s disease. In Proceedings of the Medical Imaging 2019: Digital Pathology, San Diego, CA, USA, 16–21 February 2019. [Google Scholar]
- Zhang, R.; Zhou, T.; Liu, L.; Ohulchanskyy, T.Y.; Qu, J. Dose–effect relationships for PBM in the treatment of Alzheimer’s disease. J. Phys. D Appl. Phys. 2021, 54, 353001. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Singer, A.C.; Martorell, A.J.; Douglas, J.M.; Abdurrob, F.; Attokaren, M.K.; Tipton, J.; Mathys, H.; Adaikkan, C.; Tsai, L.H. Noninvasive 40-Hz light flicker to recruit microglia and reduce amyloid beta load. Nat. Protoc. 2018, 13, 1850–1868. [Google Scholar] [CrossRef]
- Alsunusi, S.; Kumosani, T.A.; Glabe, C.G.; Huwait, E.A.; Moselhy, S.S. In vitro study of the mechanism of intraneuronal beta-amyloid aggregation in Alzheimer’s disease. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chibhabha, F.; Yang, Y.; Ying, K.; Jia, F.; Zhang, Q.; Ullah, S.; Liang, Z.; Xie, M.; Li, F. Non-invasive optical imaging of retinal Abeta plaques using curcumin loaded polymeric micelles in APPswe/PS1DeltaE9 transgenic mice for the diagnosis of Alzheimer’s disease. J. Mater. Chem. B 2020, 8, 7438–7452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Fan, C.; Xin, B.; Zhang, J.-P.; Luo, T.; Chen, Z.-Q.; Zhou, Q.-Y.; Yu, Q.; Li, X.-N.; Huang, Z.-L.; et al. AIE-based super-resolution imaging probes for β-amyloid plaques in mouse brains. Mater. Chem. Front. 2018, 2, 1554–1562. [Google Scholar] [CrossRef]
- Ni, R.; Chen, Z.; Gerez, J.; Shi, G.; Villois, A.; Zhou, Q.; Riek, R.; Nilsson, K.P.R.; Klohs, J.; Razansky, D. Detection of cerebral tauopathy in P301L mice using high-resolution large-field multifocal illumination fluorescence microscopy. Biomed Opt Express. 2020, 11, 4989–5002. [Google Scholar] [CrossRef]
- Long, B.; Li, X.; Zhang, J.; Chen, S.; Li, W.; Zhong, Q.; Li, A.; Gong, H.; Luo, Q. Three-dimensional quantitative analysis of amyloid plaques in the whole brain with high voxel resolution. Sci. Sin. (Vitae) 2019, 49, 10. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Tian, Y.; Zhang, C.; Tian, X.; Ross, A.W.; Moir, R.D.; Sun, H.; Tanzi, R.E.; Moore, A.; Ran, C. Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 9734–9739. [Google Scholar] [CrossRef]
- Chen, C.; Liang, Z.; Zhou, B.; Ip, N.; Qu, J.Y. Deep brain two-photon NIR fluorescence imaging for study of Alzheimer’s disease. Neural Imaging Sens. 2018. [Google Scholar] [CrossRef]
- Ralph-Weissleder, V.N. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-β deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef]
- Raymond, S.B.; Kumar, A.T.; Boas, D.A.; Bacskai, B.J. Optimal parameters for near infrared fluorescence imaging of amyloid plaques in Alzheimer’s disease mouse models. Phys. Med. Biol. 2009, 54, 6201–6216. [Google Scholar] [CrossRef][Green Version]
- Schmidt, A.; Pahnke, J. Efficient near-infrared in vivo imaging of amyoid-beta deposits in Alzheimer’s disease mouse models. J. Alzheimers Dis. 2012, 30, 651–664. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Miller, D.R.; Jarrett, J.W.; Hassan, A.M.; Dunn, A.K. Deep Tissue Imaging with Multiphoton Fluorescence Microscopy. Curr. Opin. Biomed. Eng. 2017, 4, 32–39. [Google Scholar] [CrossRef]
- Schenke-Layland, K.; Riemann, I.; Damour, O.; Stock, U.A.; Konig, K. Two-photon microscopes and in vivo multiphoton tomographs--powerful diagnostic tools for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 878–896. [Google Scholar] [CrossRef]
- Dong, J.; Revilla-Sanchez, R.; Moss, S.; Haydon, P.G. Multiphoton in vivo imaging of amyloid in animal models of Alzheimer’s disease. Neuropharmacology 2010, 59, 268–275. [Google Scholar] [CrossRef]
- Christie, R.H.; Bacskai, B.J.; Zipfel, W.R.; Williams, R.M.; Hyman, B.T. Growth arrest of individual senile plaques in a model of Alzheimer’s disease observed by in vivo multiphoton microscopy. J. Neurosci. 2001, 21, 858–864. [Google Scholar] [CrossRef]
- Bacskai, B.J.; Hyman, B.T. Alzheimer’s disease: What multiphoton microscopy teaches us. Neuroscientist 2002, 8, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Bacskai, B.J.; Skoch, J.; Hickey, G.A.; Allen, R.; Hyman, B.T. Fluorescence resonance energy transfer determinations using multiphoton fluorescence lifetime imaging microscopy to characterize amyloid-beta plaques. J. Biomed. Opt. 2003, 8, 368–375. [Google Scholar] [CrossRef]
- Brian, J.; Bacskai, S.T.K.; Kasischke, K.A.; Christie, R.H.; Webb, W.W.; Zipfel, W.R.; Williams, R.M.; Hyman, B.T. Chronic imaging of amyloid plaques in the live mouse brain using multiphoton microscopy. Proc. SPIE Int. Soc. Opt. Eng. 2001, 4262, 125–133. [Google Scholar]
- Bacskai, B.J.K.; Stephen, T.; Christie, R.H.; Cordelia, C.; Games, D.; Seubert, P.; Schenk, D.; Hyman, B. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat. Med. 2001, 7, 369–372. [Google Scholar] [CrossRef]
- Chen, C.; Liang, Z.; Zhou, B.; Li, X.; Lui, C.; Ip, N.Y.; Qu, J.Y. In Vivo Near-Infrared Two-Photon Imaging of Amyloid Plaques in Deep Brain of Alzheimer’s Disease Mouse Model. ACS Chem. Neurosci. 2018, 9, 3128–3136. [Google Scholar] [CrossRef]
- Yan, P.; Bero, A.W.; Cirrito, J.R.; Xiao, Q.; Hu, X.; Wang, Y.; Gonzales, E.; Holtzman, D.M.; Lee, J.M. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J. Neurosci. 2009, 29, 10706–10714. [Google Scholar] [CrossRef]
- Baik, S.H.; Kang, S.; Son, S.M.; Mook-Jung, I. Microglia contributes to plaque growth by cell death due to uptake of amyloid beta in the brain of Alzheimer’s disease mouse model. Glia 2016, 64, 2274–2290. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; de Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Domnitz, S.B.; Robbins, E.M.; Hoang, A.W.; Alloza, M.G.; Hyman, B.T.; Rebeck, G.W.; Greenberg, S.M.; Bacskai, B.J.; Frosch, M.P. Progression of cerebral amyloid angiopathy in transgenic mouse models of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2005, 64, 588–594. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Dujardin, S.; Shirani, H.; Nilsson, K.P.R.; Bacskai, B.J. In vivo detection of tau fibrils and amyloid beta aggregates with luminescent conjugated oligothiophenes and multiphoton microscopy. Acta Neuropathol. Commun. 2019, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Megan, E.; McLellan, S.T.K.; Bradley, T.; Hyman, B.; Bacskai, J. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J. Neurosci. 2003, 23, 2212–2217. [Google Scholar]
- Hefendehl, J.K.; Wegenast-Braun, B.M.; Liebig, C.; Eicke, D.; Milford, D.; Calhoun, M.E.; Kohsaka, S.; Eichner, M.; Jucker, M. Long-term in vivo imaging of beta-amyloid plaque appearance and growth in a mouse model of cerebral beta-amyloidosis. J. Neurosci. 2011, 31, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Hefendehl, J.K.; Milford, D.; Eicke, D.; Wegenast-Braun, B.M.; Calhoun, M.E.; Grathwohl, S.A.; Jucker, M.; Liebig, C. Repeatable target localization for long-term in vivo imaging of mice with 2-photon microscopy. J. Neurosci. Methods 2012, 205, 357–363. [Google Scholar] [CrossRef]
- Kwan, A.C.; Duff, K.; Gouras, G.K.; Webb, W.W. Optical visualization of Alzheimer’s pathology via multiphoton-excited intrinsic fluorescence and second harmonic generation. Opt. Exp. 2009, 17, 3679–3689. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, B.; Lin, G.; Sun, C.; Lin, R.; Huang, J.; Tao, J.; Wang, X.; Wu, Y.; Chen, L.; et al. Label-free multiphoton imaging of beta-amyloid plaques in Alzheimer’s disease mouse models. Neurophotonics 2019, 6, 045008. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Grange, R.; Pu, Y.; Psaltis, D. Bioconjugation of barium titanate nanocrystals with immunoglobulin G antibody for second harmonic radiation imaging probes. Biomaterials 2010, 31, 2272–2277. [Google Scholar] [CrossRef]
- Zavelani-Rossi, M.; Celebrano, M.; Biagioni, P.; Polli, D.; Finazzi, M.; Duò, L.; Cerullo, G.; Labardi, M.; Allegrini, M.; Grand, J.; et al. Near-field second-harmonic generation in single gold nanoparticles. Appl. Phys. Lett. 2008, 92, 093119. [Google Scholar] [CrossRef]
- Lippitz, M.; van Dijk, M.A.; Orrit, M. Third-harmonic generation from single gold nanoparticles. Nano Lett. 2005, 5, 799–802. [Google Scholar] [CrossRef]
- Cheng, J.X.; Jia, Y.K.; Zheng, G.F.; Xie, X.S. Laser-scanning coherent anti-Stokes Raman scattering microscopy and applications to cell biology. Biophys. J. 2002, 83, 502–509. [Google Scholar] [CrossRef]
- Liu, T.-M.; Tai, S.-P.; Yu, C.-H.; Wen, Y.-C.; Chu, S.-W.; Chen, L.-J.; Prasad, M.R.; Lin, K.-J.; Sun, C.-K. Measuring plasmon-resonance enhanced third-harmonic χ(3) of Ag nanoparticles. Appl. Phys. Lett. 2006, 89, 043122. [Google Scholar] [CrossRef]
- Hanczyc, P.; Samoc, M.; Norden, B. Multiphoton absorption in amyloid protein fibres. Nat. Photonics. 2013, 7, 969–972. [Google Scholar] [CrossRef]
- Heo, C.H.K.; Kim, H.J.; Baik, S.H.; Song, H.; Kim, Y.S.; Lee, J.; Mook-jung, I.; Kim, H.M. A two-photon fluorescent probe for amyloid-β plaques in living mice. Chem. Commun. 2013, 49, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.K.; Koelsch, P. Label-free imaging of amyloids using their intrinsic linear and nonlinear optical properties. Biomed Opt. Express. 2017, 8, 743–756. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Loew, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 2003, 21, 1356–1360. [Google Scholar] [CrossRef]
- Williams, R.M.; Zipfel, W.R.; Webb, W.W. Interpreting second-harmonic generation images of collagen I fibrils. Biophys. J. 2005, 88, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Wu, P.-C.; Chen, S.-T.; Chiu, M.-J.; Sun, C.-K.; Shaked, N.T.; Hayden, O. Assessment of neuropathology of Alzheimer’s disease brain with high-resolution, label-free multi-harmonic generation microscopy. In Proceedings of the Label-free Biomedical Imaging and Sensing (LBIS) 2020, Symposium on Label-Free Biomedical Imaging and Sensing (LBIS), San Francisco, CA, USA, 1–4 February 2020. [Google Scholar]
- Chakraborty, S.; Chen, S.T.; Hsiao, Y.T.; Chiu, M.J.; Sun, C.K. Additive-color multi-harmonic generation microscopy for simultaneous label-free differentiation of plaques, tangles, and neuronal axons. Biomed. Opt. Express. 2020, 11, 571–585. [Google Scholar] [CrossRef]
- Kiefer, W. Recent advances in linear and nonlinear Raman spectroscopy II. J. Raman Spectrosc. 2008, 39, 1710–1725. [Google Scholar] [CrossRef]
- Nafie, L.A. Recent advances in linear and non-linear Raman spectroscopy. Part XI. J. Raman Spectrosc. 2017, 48, 1692–1717. [Google Scholar] [CrossRef]
- Opilik, L.; Schmid, T.; Zenobi, R. Modern Raman imaging: Vibrational spectroscopy on the micrometer and nanometer scales. Annu. Rev. Anal. Chem. 2013, 6, 379–398. [Google Scholar] [CrossRef]
- Müller, M.S.J.M. Imaging the thermodynamic state of lipid membranes with multiplex CARS microscopy. J. Phys. Chem. B 2002, 106, 3715–3723. [Google Scholar] [CrossRef]
- Volkmer, A.; Cheng, J.-X.; Sunney Xie, X. Vibrational Imaging with High Sensitivity via Epidetected Coherent Anti-Stokes Raman Scattering Microscopy. Phys. Rev. Lett. 2001, 87. [Google Scholar] [CrossRef]
- Zumbusch, A.; Holtom, G.R.; Xie, X. Three-dimensional vibrational imaging by coherent anti-Stokes Raman scattering. Phys. Rev. Lett. 1999, 82, 4142–4145. [Google Scholar] [CrossRef]
- Hu, C.R.; Zhang, D.; Slipchenko, M.N.; Cheng, J.X.; Hu, B. Label-free real-time imaging of myelination in the Xenopus laevis tadpole by in vivo stimulated Raman scattering microscopy. J. Biomed. Opt. 2014, 19, 086005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.C.; Min, W.; Freudiger, C.W.; Ruvkun, G.; Xie, X.S. RNAi screening for fat regulatory genes with SRS microscopy. Nat. Methods. 2011, 8, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Pliss, A.; Kuzmin, A.N.; Kachynski, A.V.; Prasad, P.N. Biophotonic probing of macromolecular transformations during apoptosis. Proc. Natl. Acad. Sci. USA 2010, 107, 12771–12776. [Google Scholar] [CrossRef]
- Lim, R.S.; Kratzer, A.; Barry, N.P.; Miyazaki-Anzai, S.; Miyazaki, M.; Mantulin, W.W.; Levi, M.; Potma, E.O.; Tromberg, B.J. Multimodal CARS microscopy determination of the impact of diet on macrophage infiltration and lipid accumulation on plaque formation in ApoE-deficient mice. J. Lipid Res. 2010, 51, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Yi, R.; Liu, L.; Qu, J. Coherent Anti-Stokes Raman Scattering Microscopy and Its Applications. Front. Phys. 2020, 8, 515. [Google Scholar] [CrossRef]
- Annika, E.; Kiskis, J.; Fink, H.; Nyberg, L.; Thyr, J.; Li, J. CARS microscopy of Alzheimer’s diseased brain tissue. In Proceedings of the Multiphoton Microscopy in the Biomedical Sciences XIV, San Francisco, CA, USA, 2–4 February 2014. [Google Scholar]
- Minbiao, J.; Zhang, L.; Freudiger, C.W.; Hou, S.S.; Lin, D.; Yang, X.; Bacskai, B.J.; Xie, X.S. Label-free imaging of amyloid plaques in Alzheimer’s disease with stimulated Raman scattering microscopy. Sci. Adv. 2018, 4, eaat7715. [Google Scholar]
- Gualda, E.J.; Voglis, G.F.G.; Mari, M.; Fotakis, C.; Tavernarakis, N. In vivo imaging of cellular structures in Caenorhabditis elegans by combined TPEF, SHG and THG microscopy. J. Microsc. 2008, 229, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, H.; Hong, J.H.; Lim, Y.-S.; Choi, W. Laser scanning reflection-matrix microscopy for aberration-free imaging through intact mouse skull. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]


| Optical Imaging Method | Probes | Parameters | Imaged Samples | Reference |
|---|---|---|---|---|
| FM | curcumin | λex = 550/25 nm λem = 605/70 nm Resolution: 0.25 μm | retina slices | [45] |
| FM | Cy5, CRANAD-2 | λex = 649 nm, λex = 649 nm λem = 675 nm, λem = 715 nm Resolution: 5 μm laser power: 5~50 mW | agar phantom | [48] |
| FM CLSM | PiB flutemetamol | λex = 330~390 nm λem = 390~450 nm Resolution: 0.10 × 0.108 × 0.11 μm | brain slices | [49] |
| FM super-resolution images | PD-NA, PD-NA-TEG | λex = 405 nm λem = 500~546 nm Resolution: sub-100 nm laser power: 50 mW | brain slices | [58] |
| LMI-FM | HS-169 | λex = 532 nm Resolution: 20 μm | In vivo brain | [59] |
| fMOST | DANIR-8c | Resolution: 0.32 × 0.32 × 2 μm | In vitro brain | [60] |
| CLSM | ThT | λex = 450 nm λem = 482 nm | oAβ42 | [51] |
| CLSM | ThS | 1024 × 1024 pixel | brain slices | [52] |
| CLSM | 12F4 | 1024 × 1024 pixel | brain slices | [55] |
| CLSM | specific monoclonal M78 | pheochromocytoma | [56] | |
| CLSM | curcumin micelles 12F4 | λex = 405 nm λem = 525 nm a laser beam (2 mW) at 514 nm for 6 min. | brain and retinal slices | [57] |
| NIRF | AOI987, NIAD-11, NIAD-16 | λex = 650 nm, λem = 670 nm λex = 545 nm, λem = 690 nm λex = 470 nm, λem = 720 nm | brain slices | [25,65] |
| NIRF | CRANAD-2 | λex = 640 nm, λem = 805 nm laser power: 10 mW/cm2 532 × 256 pixels | In vivo and in vitro brain | [64] |
| NIRF | THK-265 | λex = 665 nm, λem = 725 nm 169 or 84 μm resolution | brain slices | [66] |
| NIRF | CRANAD-102 | λex = 605 nm, λem = 680 nm | brain slices | [32] |
| MPEF | ThS | two-photon fluorescence λex = 750 nm λem = 380~480 nm laser power after the objective: 10 mW, pulse 60–100 fs Resolution: 1 μm depth = 150 μm | In vivo brain | [71] |
| MPEF | methoxy-X04 | two-photon fluorescence λex = 750 nm λem = 435~485 nm depth = 200 μm | In vivo brain | [77] |
| MPEF | methoxy-X04 | two-photon fluorescence λex = 850 nm λem = 460 nm laser power < 35 mW | In vivo brain | [78] |
| MPEF | methoxy-X04 | two-photon fluorescence λex = 800 nm λem = 380~480 nm Resolution: 150 × 150 × 1 μm | In vivo brain | [79] |
| MPEF | ThS | two-photon fluorescence λex = 750 nm λem = 380~480 nm depth = 200 μm Resolution: 615 × 615 μm | In vitro brain | [80] |
| MPEF | HS-84, HS-169 | λex = ~375 nm and ~535 nm (double excitation peaks), λem = ~ 665 nm resolution of 512 × 512 pixels depth = ~200 μm | brain slices | [81] |
| MPEF | ThS | two-photon fluorescence λex = 750 or 800 nm λem = 380~480 nm depth = 200 μm Resolution: 615 × 615 μm | In vivo brain | [82] |
| MPEF, SHG | Label-free | two-photon fluorescence λex = 810 nm SHG signals λem = 395~415 nm TPEF signals λem = 430~690 nm laser power: 5~10 mW 1024 × 1024 pixel | brain slices | [85] |
| MPEF, SHG | Label-free | MPEF λex = 830 nm SHG signals λem = 387 nm TPEF signals λem = 400~550 nm laser power: 25 mW | brain slices | [86] |
| CLSM, MPEF, SHG | Label-free | CLSM λex = 405 nm, λem > 420 nm MPEF λex = 910 nm SHG signals λem = 420~460 nm TPEF signals λem = 495~540 nm laser power: 680 mW pixel sizes < 200 nm | brain slices | [94] |
| THG | Label-free | MPEF λex = 1262 nm, λem > 430 nm laser power: 20 mW 1024 × 1024 pixels | brain slices | [97,98] |
| CARS | ThS Cy2 | Stokes λex = 1064 nm Pump λex = 817 nm the CH2 stretch vibration: 2845 cm−1 ThS signal: short-pass filters (600SP and 2 × 750SP, Ealing) Cy2 signal: band-pass filter (525/50 nm, Chroma) average laser power: 25 mW | brain slices | [44,111] |
| SRS | ThS | Stokes λex = 1064 nm Pump λex = 720~990 nm maximum brightness of the plaque images: 1670 cm−1 the CH2 stretch vibration at 2845 cm−1 resolution: ~8 cm−1 | brain slices | [112] |
| CARS TPEF SHG | Label-free | Stokes λex = 1064 nm Pump λex = 800 nm CARS: HQ650/20 m, Chroma, TPEF: FF01-550/88, SHG: FF01-390/18, Semrock resolution: ~5 cm−1 laser power1: 20 mW laser power2: 3 mW | brain slices | [43] |
| Optical Imaging Method | Advantages | Disadvantage | Applications in Biology |
|---|---|---|---|
| FM | Easy to operate, low cost | Low resolution and low contrast | Thin biological samples, slice |
| CLSM | High resolution, high contrast | Expensive, damage to living cells, time-consuming | Thick biological samples |
| NIRF | Fast imaging speed, high penetration, non-destructive, | Poor sensitivity, vulnerable to interference | In vivo imaging |
| MPEF | High penetration depth, low phototoxicity | High cost, complex system | In vivo imaging |
| SHG | No photobleaching, label-free | The signal is weak and difficult to collect | Occurs only in an asymmetric medium (e.g., collagen) |
| THG | No photobleaching, label-free | The signal is weak and difficult to collect | Can occur in any medium (whether symmetric or not) |
| CARS | Good chemical specificity, small light damage, high sensitivity, high spatial resolution, fast scanning speed | Strong non-resonant background | In vivo imaging |
| SRS | Low background noise, fast scanning speed | Expensive, complex system | In vivo imaging |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Xu, H.; Liu, L.; Ohulchanskyy, T.Y.; Qu, J. Optical Imaging of Beta-Amyloid Plaques in Alzheimer’s Disease. Biosensors 2021, 11, 255. https://doi.org/10.3390/bios11080255
Luo Z, Xu H, Liu L, Ohulchanskyy TY, Qu J. Optical Imaging of Beta-Amyloid Plaques in Alzheimer’s Disease. Biosensors. 2021; 11(8):255. https://doi.org/10.3390/bios11080255
Chicago/Turabian StyleLuo, Ziyi, Hao Xu, Liwei Liu, Tymish Y. Ohulchanskyy, and Junle Qu. 2021. "Optical Imaging of Beta-Amyloid Plaques in Alzheimer’s Disease" Biosensors 11, no. 8: 255. https://doi.org/10.3390/bios11080255
APA StyleLuo, Z., Xu, H., Liu, L., Ohulchanskyy, T. Y., & Qu, J. (2021). Optical Imaging of Beta-Amyloid Plaques in Alzheimer’s Disease. Biosensors, 11(8), 255. https://doi.org/10.3390/bios11080255







