Real-Time Analysis of Oxygen Gradient in Oocyte Respiration Using a High-Density Microelectrode Array
Abstract
1. Introduction
2. Materials and Methods
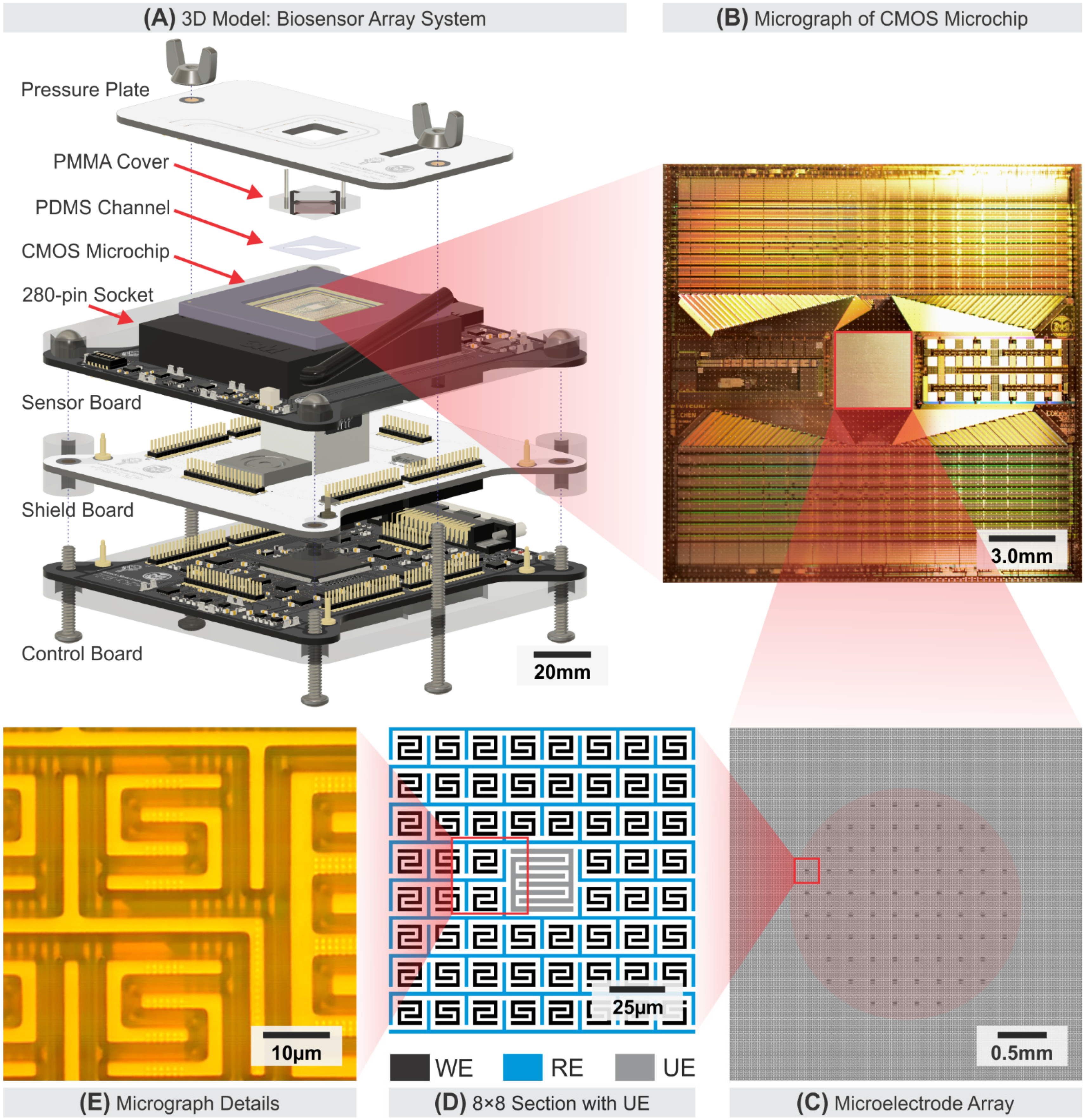
2.1. Sensor System Overview
2.2. CMOS Microchip with Microelectrode Array
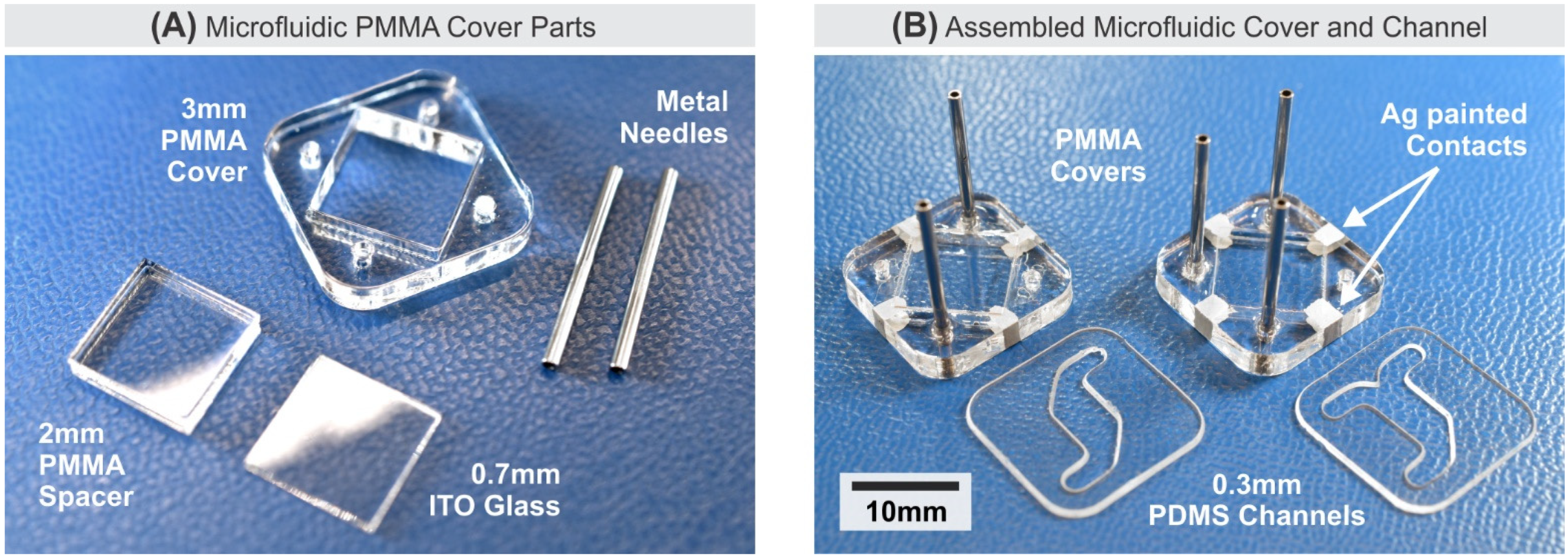
2.3. Microfluidics System and Fabrication
2.4. COC Preparation and Handling
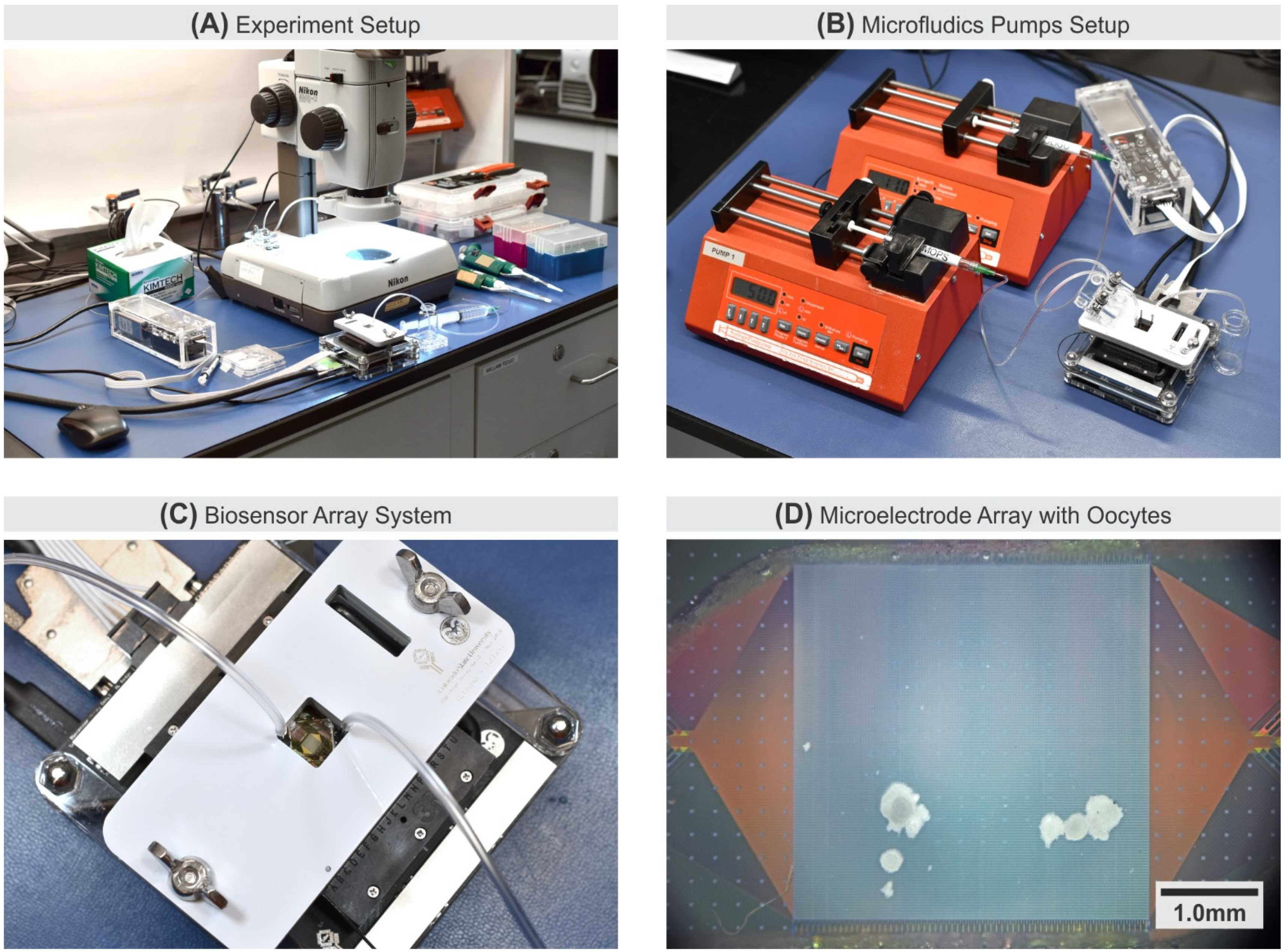
2.5. Experiment Setups
3. Results and Discussion
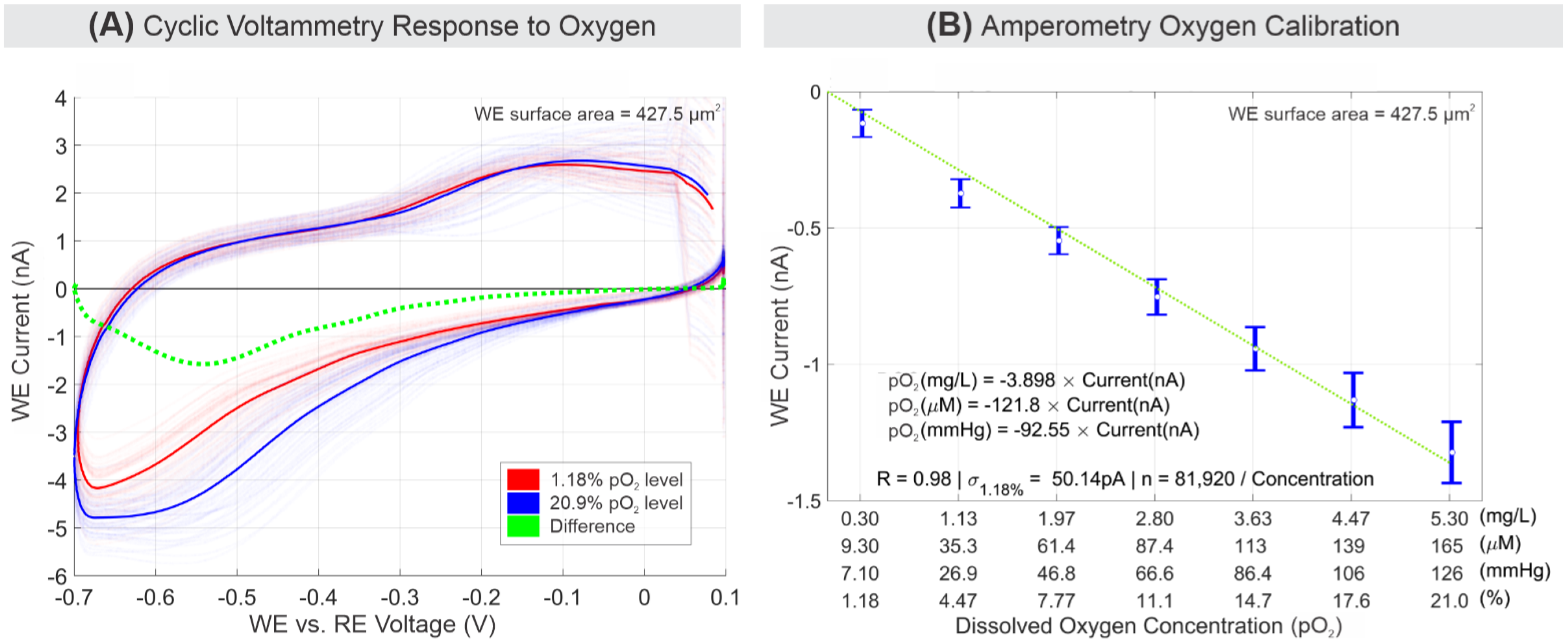
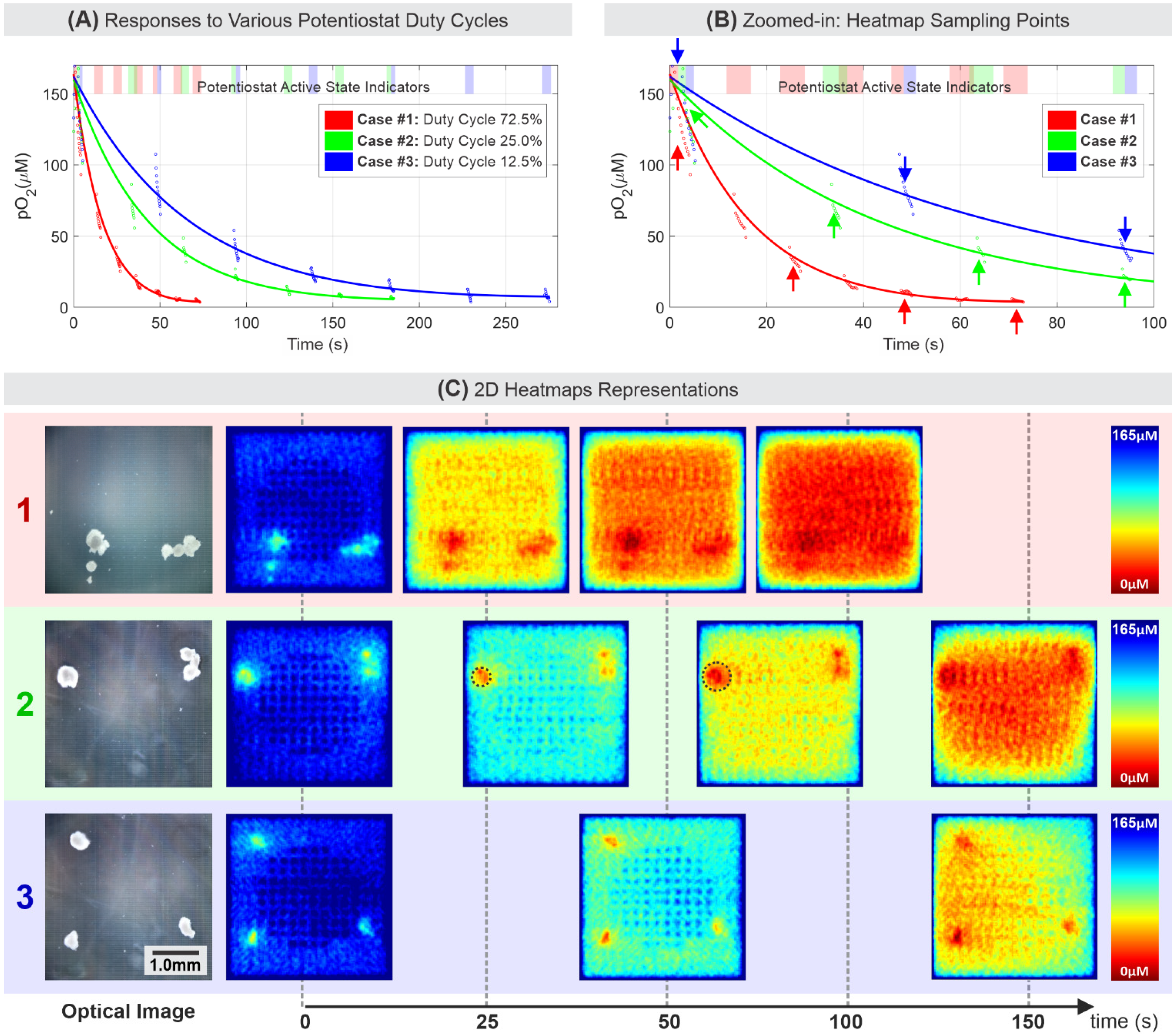
3.1. Electrochemical Oxygen Response
3.2. COC Basal Respiration and MEA Oxygen Reduction
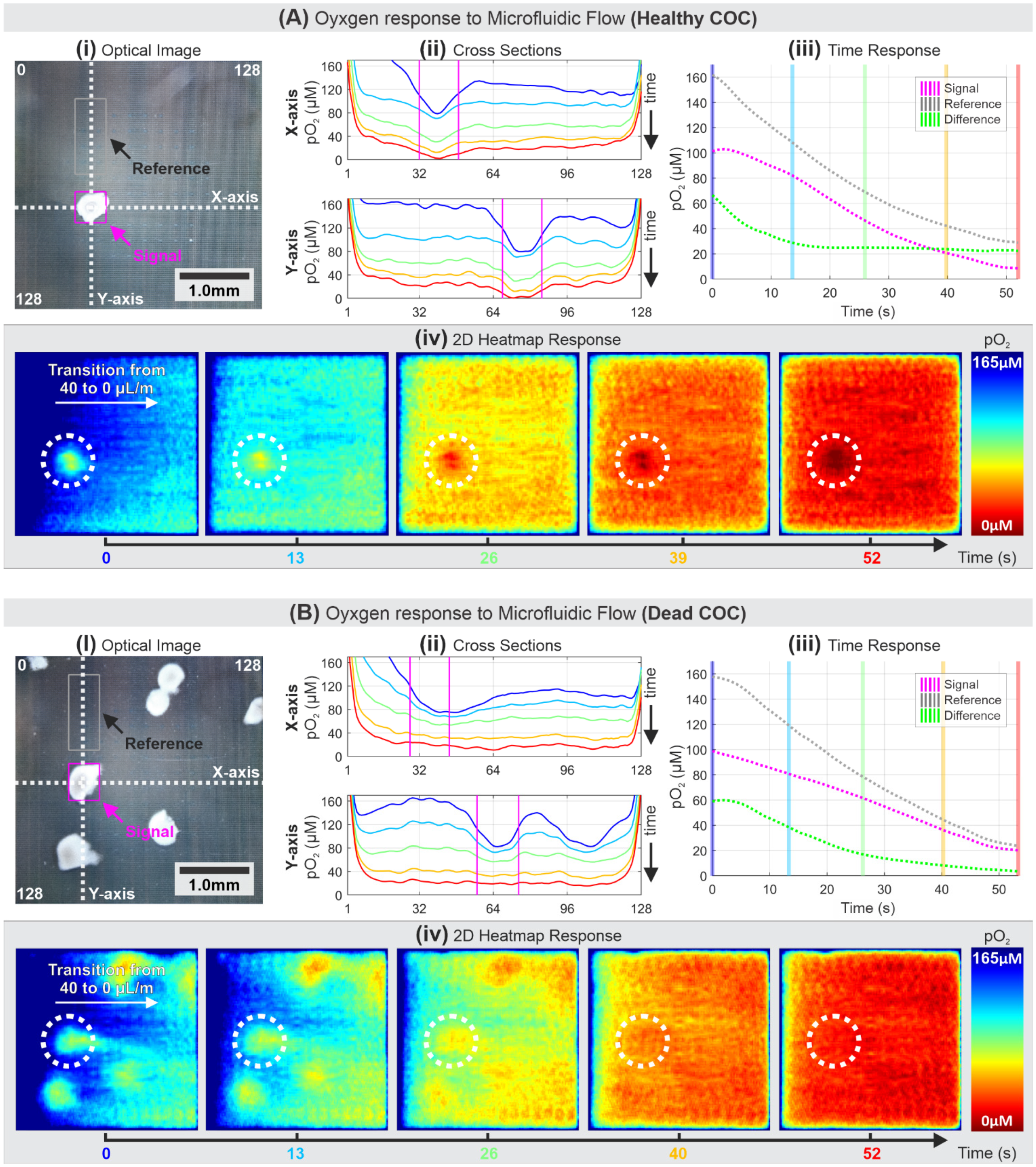
3.3. Comparison of Healthy and Dead COC
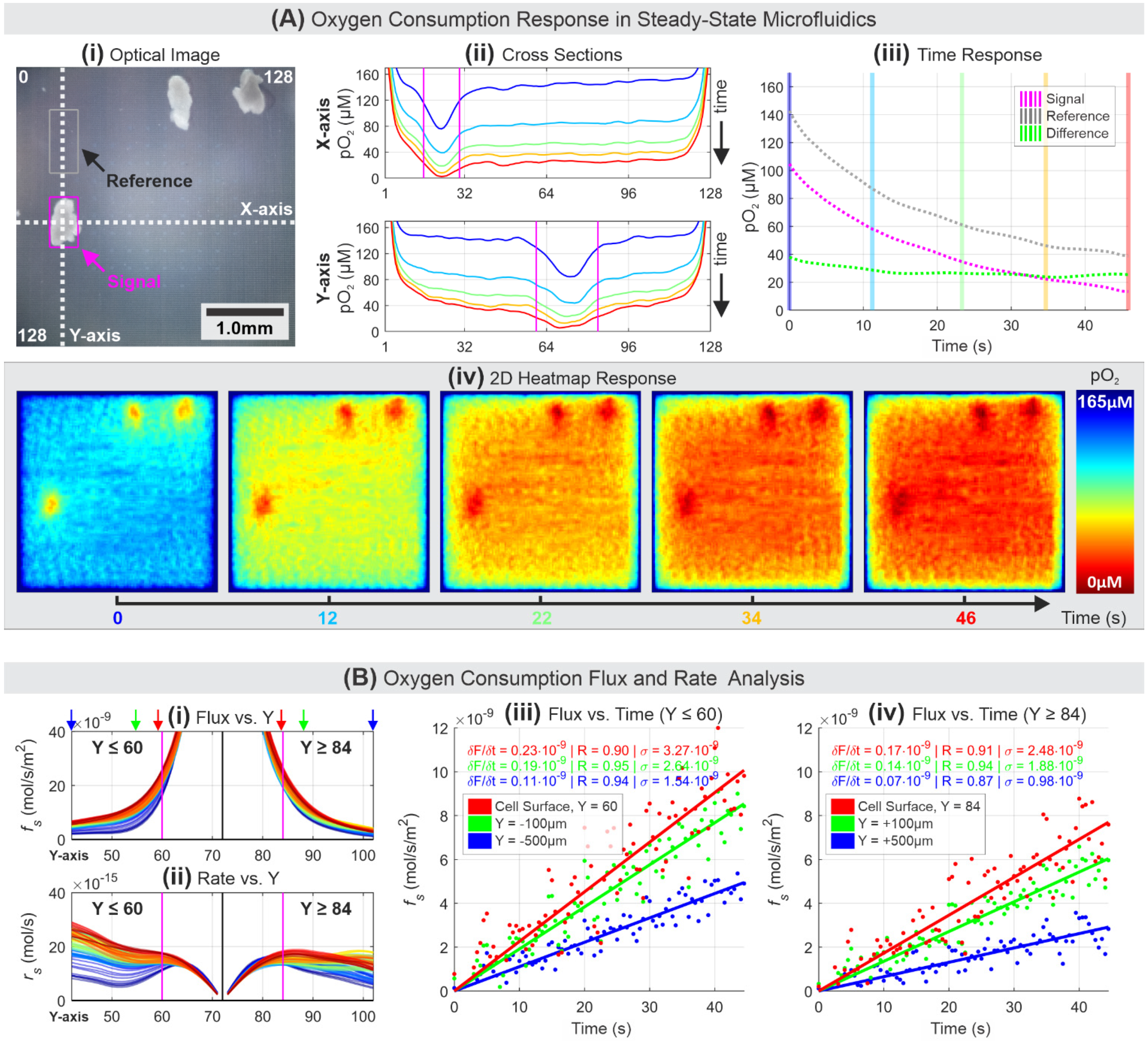
3.4. Oxygen Flux and Consumption Rate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Leng, X.; He, M.; Wang, J.; Cheng, S.; Wu, H. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch. Virol. 2017, 162, 603–610. [Google Scholar] [CrossRef]
- Solaini, G.; Baracca, A.; Lenaz, G.; Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. Biochim. Biophys. Acta Bioenergy 2010, 1797, 1171–1177. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef]
- Mirabello, V.; Cortezon-Tamarit, F.; Pascu, S.I. Corrigendum: Oxygen Sensing, Hypoxia Tracing and in Vivo Imaging with Functional Metalloprobes for the Early Detection of Non-communicable Diseases. Front. Chem. 2018, 6, 27. [Google Scholar] [CrossRef]
- Eales, K.L.; Hollinshead, K.E.R.; Tennant, D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Thompson, C.B. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009, 23, 537–548. [Google Scholar] [CrossRef]
- Dunwoodie, S.L. The Role of Hypoxia in Development of the Mammalian Embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef]
- Scott, L.; Berntsen, J.; Davies, D.; Gundersen, J.; Hill, J.; Ramsing, N. Human oocyte respiration-rate measurement Potential to improve oocyte and embryo selection? Reprod. Biomed. Online 2008, 17, 461–469. [Google Scholar] [CrossRef]
- Donnay, I. Metabolic Markers of Embryo Viability. In Assessment of Mammalian Embryo Quality: Invasive and Non-Invasive Techniques; Van Soom, A., Boerjan, M., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 57–94. [Google Scholar]
- Obeidat, Y.M.; Evans, A.J.; Tedjo, W.; Chicco, A.J.; Carnevale, E.; Chen, T.W. Monitoring oocyte/embryo respiration using electrochemical-based oxygen sensors. Sens. Actuators B Chem. 2018, 276, 72–81. [Google Scholar] [CrossRef]
- Obeidat, Y.; Catandi, G.; Carnevale, E.; Chicco, A.J.; DeMann, A.; Field, S.; Chen, T. A multi-sensor system for measuring bovine embryo metabolism. Biosens. Bioelectron. 2019, 126, 615–623. [Google Scholar] [CrossRef]
- Vaupel, P.; Höckel, M.; Mayer, A. Detection and Characterization of Tumor Hypoxia Using pO2 Histography. Antioxid. Redox Signal. 2007, 9, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef]
- Tejera, A.; Herrero, J.; De Los Santos, M.J.; Garrido, N.; Ramsing, N.; Meseguer, M. Oxygen consumption is a quality marker for human oocyte competence conditioned by ovarian stimulation regimens. Fertil. Steril. 2011, 96, 618–623.e2. [Google Scholar] [CrossRef] [PubMed]
- Ottosen, L.D.M.; Hindkjær, J.; Lindenberg, S.; Ingerslev, H.J. Murine pre-embryo oxygen consumption and developmental competence. J. Assist. Reprod. Genet. 2007, 24, 359–365. [Google Scholar] [CrossRef]
- Elas, M.; Williams, B.B.; Parasca, A.; Mailer, C.; Pelizzari, C.A.; Lewis, M.A.; River, J.N.; Karczmar, G.S.; Barth, E.D.; Halpern, H.J. Quantitative tumor oxymetric images from 4D electron paramagnetic resonance imaging (EPRI): Methodology and comparison with blood oxygen level-dependent (BOLD) MRI. Magn. Reson. Med. 2003, 49, 682–691. [Google Scholar] [CrossRef]
- Elas, M.; Ahn, K.H.; Parasca, A.; Barth, E.D.; Lee, D.; Haney, C.; Halpern, H.J. Electron paramagnetic resonance oxygen images correlate spatially and quantitatively with Oxylite oxygen measurements. Clin. Cancer Res. 2006, 12, 4209–4217. [Google Scholar] [CrossRef]
- Kotecha, M.; Epel, B.; Ravindran, S.; Dorcemus, D.; Nukavarapu, S.; Halpern, H. Noninvasive Absolute Electron Paramagnetic Resonance Oxygen Imaging for the Assessment of Tissue Graft Oxygenation. Tissue Eng. Part C Methods 2017, 24, 14–19. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2018, 92, 20180642. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. BioEssays 2015, 37, 921–928. [Google Scholar] [CrossRef]
- Papkovsky, D.B.; Dmitriev, R.I. Imaging of oxygen and hypoxia in cell and tissue samples. Cell. Mol. Life Sci. 2018, 75, 2963–2980. [Google Scholar] [CrossRef]
- Yoshihara, T.; Hirakawa, Y.; Hosaka, M.; Nangaku, M.; Tobita, S. Oxygen imaging of living cells and tissues using luminescent molecular probes. J. Photochem. Photobiol. C Photochem. Rev. 2017, 30, 71–95. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ito, H.; Inoue, M.; Tabata, K.; Sato, Y.; Yamagata, K.; Kizaka-Kondoh, S.; Kadonosono, T.; Yano, S.; Inoue, M.; et al. High resolution imaging of intracellular oxygen concentration by phosphorescence lifetime. Sci. Rep. 2015, 5, 10657. [Google Scholar] [CrossRef]
- Esipova, T.V.; Barrett, M.J.P.; Erlebach, E.; Masunov, A.E.; Weber, B.; Vinogradov, S.A. Oxyphor 2P: A High-Performance Probe for Deep-Tissue Longitudinal Oxygen Imaging. Cell Metab. 2019, 29, 736–744.e7. [Google Scholar] [CrossRef]
- Lesher-Pérez, S.C.; Kim, G.A.; Kuo, C.H.; Leung, B.M.; Mong, S.; Kojima, T.; Moraes, C.; Thouless, M.D.; Luker, G.D.; Takayama, S. Dispersible oxygen microsensors map oxygen gradients in three-dimensional cell cultures. Biomater. Sci. 2017, 5, 2106–2113. [Google Scholar] [CrossRef]
- Icha, J.; Weber, M.; Waters, J.C.; Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays 2017, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Taei, M.; Khayamian, T. A differential pulse voltammetric method for simultaneous determination of ascorbic acid, dopamine, and uric acid using poly (3-(5-chloro-2-hydroxyphenylazo)-4,5-dihydroxynaphthalene-2,7-disulfonic acid) film modified glassy carbon electrode. J. Electroanal. Chem. 2009, 633, 212–220. [Google Scholar] [CrossRef]
- Sun, H.; Chao, J.; Zuo, X.; Su, S.; Liu, X.; Yuwen, L.; Fan, C.; Wang, L. Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2014, 4, 27625. [Google Scholar] [CrossRef]
- Eklund, S.E.; Taylor, D.; Kozlov, E.; Prokop, A.; Cliffel, D.E. A microphysiometer for simultaneous measurement of changes in extracellular glucose, lactate, oxygen, and acidification rate. Anal. Chem. 2004, 76, 519–527. [Google Scholar] [CrossRef]
- Pemberton, R.M.; Cox, T.; Tuffin, R.; Drago, G.A.; Griffiths, J.; Pittson, R.; Johnson, G.; Xu, J.; Sage, I.C.; Davies, R.; et al. Fabrication and evaluation of a micro(bio)sensor array chip for multiple parallel measurements of important cell biomarkers. Sensors 2014, 14, 20519–20532. [Google Scholar] [CrossRef]
- Obeidat, Y.; Cheng, M.H.; Catandi, G.; Carnevale, E.; Chicco, A.J.; Chen, T.W. Design of a multi-sensor platform for integrating extracellular acidification rate with multi-metabolite flux measurement for small biological samples. Biosens. Bioelectron. 2019, 133, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Virkar, A.V.; Singhal, S.C.; Dunham, G.C.; Marina, O.A. Design, fabrication and characterization of a miniaturized series-connected potentiometric oxygen sensor. Sens. Actuators B Chem. 2005, 105, 312–321. [Google Scholar] [CrossRef]
- Date, Y.; Takano, S.; Shiku, H.; Ino, K.; Ito-Sasaki, T.; Yokoo, M.; Abe, H.; Matsue, T. Monitoring oxygen consumption of single mouse embryos using an integrated electrochemical microdevice. Biosens. Bioelectron. 2011, 30, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, E.; Ben-Amor, S.; Charlot, S.; Colin, D.; Devin, A.; Rigoulet, M.; Sojic, N.; Belaidi, F.S.; Launay, J.; Temple-Boyer, P.; et al. Development of electrochemical microsensors for the monitoring of mitochondrial activities. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems, Barcelona, Spain, 16–20 June 2013; pp. 1135–1138. [Google Scholar]
- Wu, C.C.; Luk, H.N.; Lin, Y.T.T.; Yuan, C.Y. A Clark-type oxygen chip for in situ estimation of the respiratory activity of adhering cells. Talanta 2010, 81, 228–234. [Google Scholar] [CrossRef]
- Oomen, P.E.; Skolimowski, M.D.; Verpoorte, E. Implementing oxygen control in chip-based cell and tissue culture systems. Lab Chip 2016, 16, 3394–3414. [Google Scholar] [CrossRef]
- Zimmermann, P.; Weltin, A.; Urban, G.A.; Kieninger, J. Active potentiometry for dissolved oxygen monitoring with platinum electrodes. Sensors 2018, 18, 2404. [Google Scholar] [CrossRef]
- Krommenhoek, E.E.; van Leeuwen, M.; Gardeniers, H.; van Gulik, W.M.; van den Berg, A.; Li, X.; Ottens, M.; van der Wielen, L.A.M.; Heijnen, J.J. Lab-scale fermentation tests of microchip with integrated electrochemical sensors for pH, temperature, dissolved oxygen and viable biomass concentration. Biotechnol. Bioeng. 2008, 99, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Mitrovski, S.M.; Nuzzo, R.G. An electrochemically driven poly(dimethylsiloxane) microfluidic actuator: Oxygen sensing and programmable flows and pH gradients. Lab Chip 2005, 5, 634–645. [Google Scholar] [CrossRef]
- Park, J.; Pak, Y.K.; Pak, J.J. A microfabricated reservoir-type oxygen sensor for measuring the real-time cellular oxygen consumption rate at various conditions. Sens. Actuators B Chem. 2010, 147, 263–269. [Google Scholar] [CrossRef]
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef]
- Hiramoto, K.; Yasumi, M.; Ushio, H.; Shunori, A.; Ino, K.; Shiku, H.; Matsue, T. Development of Oxygen Consumption Analysis with an on-Chip Electrochemical Device and Simulation. Anal. Chem. 2017, 89, 10303–10310. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.Y.; Matsudaira, M.; Nakano, M.; Ino, K.; Sakamoto, C.; Kanno, Y.; Kubo, R.; Kunikata, R.; Kira, A.; Suda, A.; et al. Advanced LSI-based amperometric sensor array with light-shielding structure for effective removal of photocurrent and mode selectable function for individual operation of 400 electrodes. Lab Chip 2015, 15, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Pettine, W.; Jibson, M.; Chen, T.; Tobet, S.; Nikkel, P.; Henry, C.S. Characterization of novel microelectrode geometries for detection of neurotransmitters. IEEE Sens. J. 2012, 12, 1187–1192. [Google Scholar] [CrossRef]
- Wydallis, J.B.; Feeny, R.M.; Wilson, W.; Kern, T.; Chen, T.; Tobet, S.; Reynolds, M.M.; Henry, C.S. Spatiotemporal norepinephrine mapping using a high-density CMOS microelectrode array. Lab Chip 2015, 15, 4075–4082. [Google Scholar] [CrossRef]
- Tedjo, W.; Nejad, J.E.; Feeny, R.; Yang, L.; Henry, C.S.; Tobet, S.; Chen, T. Electrochemical biosensor system using a CMOS microelectrode array provides high spatially and temporally resolved images. Biosens. Bioelectron. 2018, 114, 78–88. [Google Scholar] [CrossRef]
- Tedjo, W.; Chen, T. An Integrated Biosensor System with a High-Density Microelectrode Array for Real-Time Electrochemical Imaging. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 20–35. [Google Scholar] [CrossRef]
- Clark, L.C. Monitor and Control of Blood and Tissue Oxygen Tensions. Trans. Am. Soc. Artif. Intern. Organs 1956, 2, 41–48. [Google Scholar]
- Smith, G.D.; Takayama, S. Application of microfluidic technologies to human assisted reproduction. Mol. Hum. Reprod. 2017, 23, 257–268. [Google Scholar] [CrossRef]
- Le Gac, S.; Nordhoff, V. Microfluidics for mammalian embryo culture and selection: Where do we stand now? Mol. Hum. Reprod. 2017, 23, 213–226. [Google Scholar] [CrossRef]
- Olson, S.E.; Seidel, G.E. Reduced oxygen tension and EDTA improve bovine zygote development in a chemically defined medium. J. Anim. Sci. 2000, 78, 152–157. [Google Scholar] [CrossRef][Green Version]
- De La Torre-Sanchez, J.F.; Preis, K.; Seidel, G.E. Metabolic regulation of in-vitro-produced bovine embryos. I. Effects of metabolic regulators at different glucose concentrations with embryos produced by semen from different bulls. Reprod. Fertil. Dev. 2006, 18, 585. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000; ISBN 9780471043720. [Google Scholar]
- Si, F.; Zhang, Y.; Yan, L.; Zhu, J.; Xiao, M.; Liu, C.; Xing, W.; Zhang, J. Electrochemical Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 133–170. [Google Scholar]
- Suzuki, H.; Sugama, A.; Kojima, N. Micromachined Clark oxygen electrode. Sens. Actuators B Chem. 1993, 10, 91–98. [Google Scholar] [CrossRef]
- Wu, C.C.; Saito, T.; Yasukawa, T.; Shiku, H.; Abe, H.; Hoshi, H.; Matsue, T. Microfluidic chip integrated with amperometric detector array for in situ estimating oxygen consumption characteristics of single bovine embryos. Sens. Actuators B Chem. 2007, 125, 680–687. [Google Scholar] [CrossRef]
- Wu, C.C.; Yasukawa, T.; Shiku, H.; Matsue, T. Fabrication of miniature Clark oxygen sensor integrated with microstructure. Sens. Actuators B Chem. 2005, 110, 342–349. [Google Scholar] [CrossRef]
- Suzuki, H.; Hirakawa, T.; Watanabe, I.; Kikuchi, Y. Determination of blood pO2 using a micromachined Clark-type oxygen electrode. Anal. Chim. Acta 2001, 431, 249–259. [Google Scholar] [CrossRef]
- Iffelsberger, C.; Raith, T.; Vatsyayan, P.; Vyskočil, V.; Matysik, F.M. Detection and imaging of reactive oxygen species associated with the electrochemical oxygen evolution by hydrodynamic scanning electrochemical microscopy. Electrochim. Acta 2018, 281, 494–501. [Google Scholar] [CrossRef]
- Giagkoulovits, C.; Cheah, B.C.; Al-Rawhani, M.A.; Accarino, C.; Busche, C.; Grant, J.P.; Cumming, D.R.S. A 16 × 16 CMOS Amperometric Microelectrode Array for Simultaneous Electrochemical Measurements. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Dragas, J.; Viswam, V.; Shadmani, A.; Chen, Y.; Bounik, R.; Stettler, A.; Radivojevic, M.; Geissler, S.; Obien, M.E.J.; Müller, J.; et al. In Vitro Multi-Functional Microelectrode Array Featuring 59760 Electrodes, 2048 Electrophysiology Channels, Stimulation, Impedance Measurement, and Neurotransmitter Detection Channels. IEEE J. Solid State Circuits 2017, 52, 1576–1590. [Google Scholar] [CrossRef]
- Hayes, M.A.; Kristensen, E.W.; Kuhr, W.G. Background-subtraction of fast-scan cyclic staircase voltammetry at protein-modified carbon-fiber electrodes. Biosens. Bioelectron. 1998, 13, 1297–1305. [Google Scholar] [CrossRef]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.A.V.; Wightman, R.M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef]
- Thompson, J.; Lane, M.; Gilchrist, R. Metabolism of the bovine cumulus-oocyte complex and influence on subsequent developmental competence. Reprod. Domest. Rumin. 2011, 6, 179–190. [Google Scholar] [CrossRef]
- Kurosawa, H.; Utsunomiya, H.; Shiga, N.; Takahashi, A.; Ihara, M.; Ishibashi, M.; Nishimoto, M.; Watanabe, Z.; Abe, H.; Kumagai, J.; et al. Development of a new clinically applicable device for embryo evaluation which measures embryo oxygen consumption. Hum. Reprod. 2016, 31, 2321–2330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agung, B.; Otoi, T.; Abe, H.; Hoshi, H.; Murakami, M.; Karja, N.W.K.; Murakami, M.K.; Wongsrikeao, P.; Watari, H.; Suzuki, T. Relationship between oxygen consumption and sex of bovine in vitro fertilized embryos. Reprod. Domest. Anim. 2005, 40, 51–56. [Google Scholar] [CrossRef]
- Shiku, H.; Shiraishi, T.; Aoyagi, S.; Utsumi, Y.; Matsudaira, M.; Abe, H.; Hoshi, H.; Kasai, S.; Ohya, H.; Matsue, T. Respiration activity of single bovine embryos entrapped in a cone-shaped microwell monitored by scanning electrochemical microscopy. Anal. Chim. Acta 2004, 522, 51–58. [Google Scholar] [CrossRef]
- Murakawa, H.; Aono, N.; Tanaka, T.; Kikuchi, H.; Yoshida, H.; Yoshida, H.; Yokoo, M.; Abe, H. Morphological Evaluation and Measurement of the Respiration Activity of Cumulus-oocyte Complexes to Assess Oocyte Quality. J. Mamm. Ova Res. 2009, 26, 32–41. [Google Scholar] [CrossRef]
- Muller, B.; Lewis, N.; Adeniyi, T.; Leese, H.J.; Brison, D.R.; Sturmey, R.G. Application of extracellular flux analysis for determining mitochondrial function in mammalian oocytes and early embryos. Sci. Rep. 2019, 9, 16778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sheng, M.; Jiang, X.; Wu, G.; Gao, F. Simultaneous determination of dopamine, serotonin and ascorbic acid at a glassy carbon electrode modified with carbon-spheres. Sensors 2013, 13, 14029–14040. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedjo, W.; Obeidat, Y.; Catandi, G.; Carnevale, E.; Chen, T. Real-Time Analysis of Oxygen Gradient in Oocyte Respiration Using a High-Density Microelectrode Array. Biosensors 2021, 11, 256. https://doi.org/10.3390/bios11080256
Tedjo W, Obeidat Y, Catandi G, Carnevale E, Chen T. Real-Time Analysis of Oxygen Gradient in Oocyte Respiration Using a High-Density Microelectrode Array. Biosensors. 2021; 11(8):256. https://doi.org/10.3390/bios11080256
Chicago/Turabian StyleTedjo, William, Yusra Obeidat, Giovana Catandi, Elaine Carnevale, and Thomas Chen. 2021. "Real-Time Analysis of Oxygen Gradient in Oocyte Respiration Using a High-Density Microelectrode Array" Biosensors 11, no. 8: 256. https://doi.org/10.3390/bios11080256
APA StyleTedjo, W., Obeidat, Y., Catandi, G., Carnevale, E., & Chen, T. (2021). Real-Time Analysis of Oxygen Gradient in Oocyte Respiration Using a High-Density Microelectrode Array. Biosensors, 11(8), 256. https://doi.org/10.3390/bios11080256




