1. Introduction
Lanthanide-doped upconversion nanoparticles (UCNPs) are of particular attraction as a kind of luminescent nanomaterials because of their significant advantages over other luminescent probes, such as long lifetime, sharp-band emission, and tunable emissions [
1]. Due to their unique photon upconversion ability and ultrahigh photostability, UCNPs attract much attention in the realm of biophotonics and nanophotonic, especially for biological imaging in cells and deep tissues [
2,
3]. As one of the most commonly used activators in upconversion luminescence systems, Tm
3+ ion features typical step-by-step upconversion excitation characteristics and rich cross-relaxation (CR) processes, giving birth to multicolor emissions. Therefore, its optical properties can be easily regulated by optical control and chemical synthesis methods, holding great potential in applications such as stimulated emission depletion (STED) super-resolution imaging [
4], optogenetics [
5,
6], and early stage tumor therenostics [
7] in nanomedicine [
8].
According to recent studies, Tm
3+ ions can directly absorb photon in near-infrared-II spectral range (1000–1400 nm) without the aid of sensitizers for upconversion luminescence, which is of great significance for application in deep tissue imaging [
9,
10,
11]. Previously, some works have reported upconversion mechanism for Tm
3+-doped materials under NIR-II wavelengths [
12,
13], e.g., the energy-looping upconversion nanoparticles under 1064 nm excitation employed for deep tissue imaging [
14]. However, these works only concentrated on emissions originating from lower-lying states (e.g., the two-photon 808 nm emission), but ignored the significance of higher-order emissions. On the other hand, for most of commercially available detectors, the sensitivity for 808 nm emission band is not pretty good as well as exert the advantages of multiphoton imaging.
In this work, the upconversion emission mechanisms of Tm3+-doped nanoparticles under excitations of different NIR-II lasers were systematically investigated and compared. The 1064 nm, 1150 nm, and 1208 nm lasers are proposed to be three NIR-II excitation strategies that can generate different emission spectra from Tm3+ ions. In addition, we excavated the mechanism of energy quenching and further optimized the structure of upconversion nanoparticles to alleviate the luminescence quenching. The high-brightness NIR-II-excitation Tm3+-doped nanoparticles enable great performance upconversion laser scanning microscopic imaging for nanoparticles and cancer cells.
4. Results
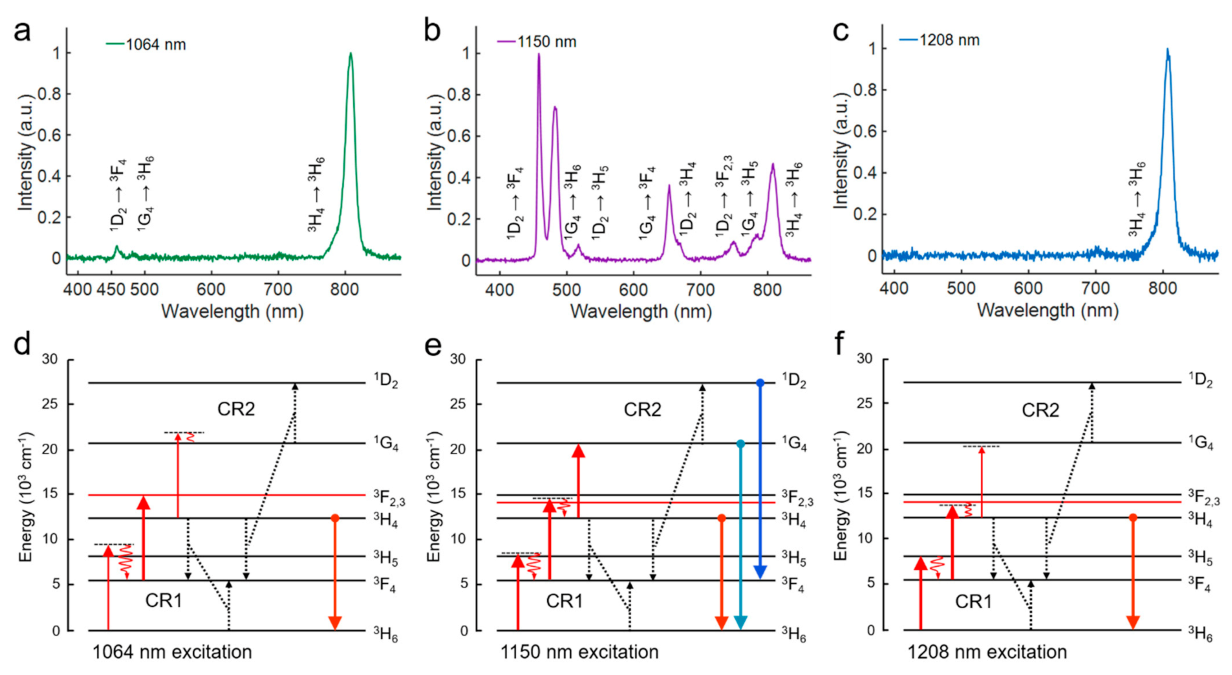
To systematically study the upconversion mechanism of the three NIR-II excitation routes for Tm
3+ ions, we firstly analyzed and compared the emission characteristics. As shown in
Figure 1a–c, a series of upconversion emission bands of Tm
3+ ions were observed under all the three NIR-II excitations. Interestingly, their spectra are quite different from each other, indicating there are different mechanisms involved in these NIR-II excitation processes. When the nanoparticles were excited by the 1064 nm laser, the emission band at 808 nm (
3 H
4 →
3 H
6) was dominant in the upconversion spectrum, while the higher-order blue emission at 475 nm (
1 G
4 →
3 H
6) and 455 nm (
1 D
2 →
3 F
4) were extremely weak. The ratio between 808 nm and 475 nm was about 147, implying the non-resonant excitation of 1064 nm laser to the higher-lying states
1 G
4 and
1 D
2. Similarly, for 1208 nm excitation, the upcoversion spectrum was also characteristic with a single strong emission band centered at 808 nm, but with a lower intensity than that generated by 1064 nm excitation, and the 455 nm emission could not be observed. Interestingly, for the case of 1150 nm excitation, besides the 808 nm emission band, three main emission bands at 475 nm, 455 nm, and 650 nm (
1 G
4 →
3 F
4) together with several weak emission bands at 750 nm (
1 D
2 →
3 F
2,3) and 785 nm (
1 G
4 →
3 H
5) were generated. Moreover, the blue emission at 475 nm from higher-lying states was 1.6 times stronger than that at 808 nm. The intensities of the high-order blue emission at 475 nm and 455 nm under 1150 nm excitation were 100 times and 15 times stronger than that under the 1064 nm excitation. These results evidenced the superiority of the 1150 nm excitation for the Tm
3+-doped nanoparticles.
The different intensity ratios between higher-order and lower-order upconversion emission bands under different excitation routes are correlated to the energy distribution discrepancy among the energy states, which are mainly caused by the CR process. To determine the upconversion mechanism, it is necessary to analyze the energy transfer characteristic of CR involved in the NIR-II excitation system. Under 1064 nm excitation, the strong 808 nm emission generated by 1064 nm laser can be mainly attributed to an energy-looping process [
14], consisting of an excited state absorption (ESA) process (
3 F
4 →
3 F
2) and a CR process (CR1,
3 H
4 +
3 H
6 →
3 F
4 +
3 F
4) (
Figure 1d). In this process, since the non-resonant GSA process (
3 H
6 →
3 H
5) could only provide few electrons for the subsequent ESA, the CR1 process here plays an important role in lifting the electrons from the ground state to the intermediate state.
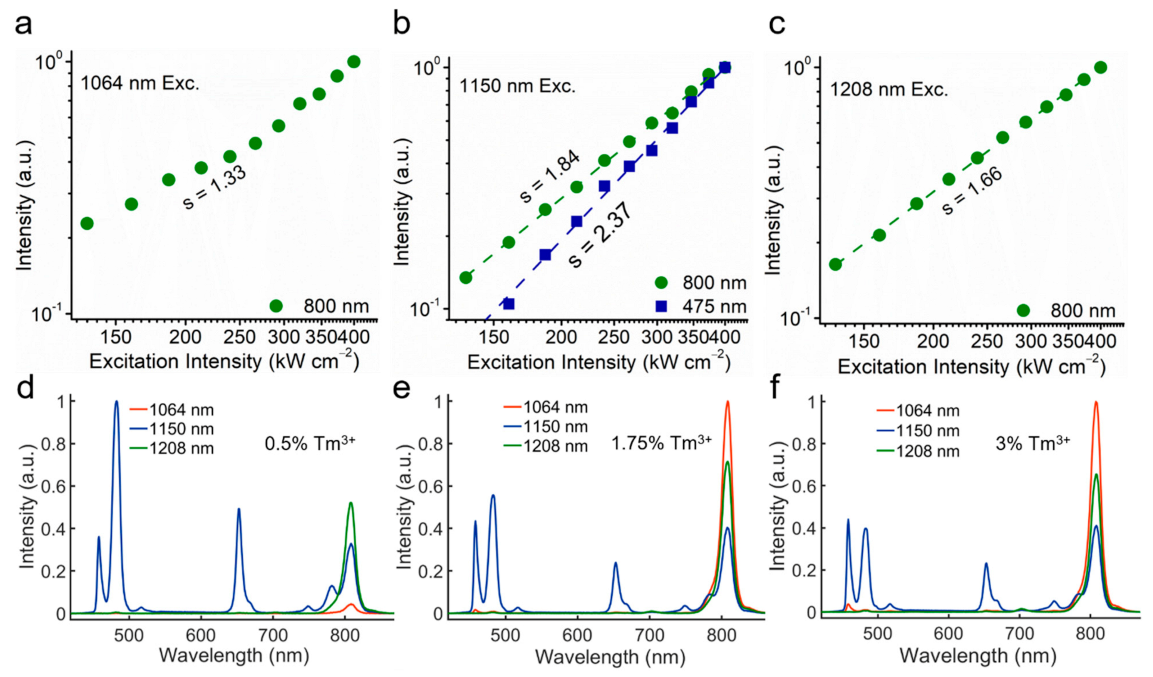
The spectra results (
Figure 2d–f) of different concentrations of Tm
3+ ions at different emission wavelengths show that, when the Tm
3+ concentration decreases to 0.5%, the CR1 process is less effective, and the originally strongest 808 nm emission excited by 1064 nm becomes even weaker than that excited by 1150 nm and 1208 nm. This phenomenon reveals that the excitation processes of 1150 nm and 1208 nm are less dependent on the CR1 because of the better matching between the ground state absorption (GSA) transition and the incident photon energy. In addition, the blue emissions excited by 1150 nm and by 1064 nm also show a dependence on the Tm
3+ concentration, first increasing and then decreasing. The CR process (CR2,
1 G
4 +
3 H
4 →
1 D
2 +
3 F
4) (
Figure 1e) is still the major route for the population of
1 D
2 state rather than a direct ESA process. Indeed, although the 1150 nm excitation process shows less dependence on the CR1, the effect of CR1 would still contribute to the enhancement of 808 nm emission and trap electrons in the lower-lying states as the case of 1064 nm excitation, which lowers the ratio between the higher- and lower-order emissions. Thereby, choosing a moderate concentration of Tm (1.75%) can avoid excessive CR effect of the higher concentration of activator to maintain high emission intensity.
To determine the photon number absorbed in these NIR-II excited upconversion processes, we then measured the dependence curves of major emission bands on the excitation intensity. As shown in
Figure 2a–c, when excited by three different NIR-II wavelengths, the slopes of the 808 nm emission band are 1.33, 1.84, and 1.66, respectively. Due to the possible saturated excitation processes, the measured slope would be always lower than the expected number of pump photon. It can be determined that the
3 H
4 state is excited by similar two-photon processes for all these three NIR-II excitation cases. In the case of 1150 nm excitation, the 475 nm emission with the slope at 2.37 indicates that there are three-photon processes involved in the excitation of the
1 G
4 states.
Based on the above experimental observation and analyses, now we can distinguish the excitation mechanisms of these three NIR-II wavelengths. As depicted in
Figure 1d–f, the GSA process:
3 H
6 →
3 H
5 and the following two-steps ESA processes:
3 F
4 →
3 F
2,3 and
3 H
4 →
1 G
4 are the common transition pathways for all three excitation strategies. The reason for the difference in emissions is mainly the energy mismatching between the energy gaps of these three upconversion transitions and the incident photon energy. Specifically, the 1064 nm laser matches well with the energy gap between
3 F
4 and
3 F
2, but there are relatively large mismatches in the other two transitions. Thus the 1064 nm excitation is a CR-based energy-looping process and is highly dependent on the doping concentration. The 1208 nm laser can directly pump the electrons from ground state
3 H
6 to
3 H
5. However, as for the second step, the relatively lower photon energy of 1208 nm cannot make the electrons at
3 F
4 state suitably reach the
3 F
3 state (note that the 1064 nm pumps the electrons to the
3 F
2 state), which results in the lower intensity of 808 nm emission than that of 1064 nm. Similarly, the 1208 nm laser cannot generate strong higher-order blue emissions, also due to the mismatch in the transition
3 H
4 →
1 G
4. In contrast to the above two wavelengths, the excitation of 1150 nm could be regarded as a compromise method to simultaneously match the first two transitions. More importantly, the 1150 nm laser is the only one matching the energy gap between
3 H
4 and
1 G
4, which means that the electrons at
3 H
4 can be further lifted to the higher-lying states for generating the higher-order blue emissions. Moreover, different from the traditional energy transfer upconversion (ETU) system where energy mismatching can be overcome with the assistance of phonon energy, the perfectly matched transition
3 H
4 →
1 G
4 here has an extra effect on regulating the distribution of electrons. Under this strong ESA process, the electrons were forced to transfer to the higher-lying states and tend to contribute to the higher-order emissions. Beyond that, the CR1 and CR2 processes mentioned above are involved in every NIR-II upconversion system, and the fourth-step upconversion to the
1 D
2 state is mainly achieved by the CR2. In conclusion, among these three wavelengths, the 1150 nm upconversion system is the unique one that can combine the advantages of NIR-II wavelength and the higher-order nonlinear excitation, which make it an ideal UCNPs-based excitation strategy for the following researches of nanoparticles and cancer cell imaging.
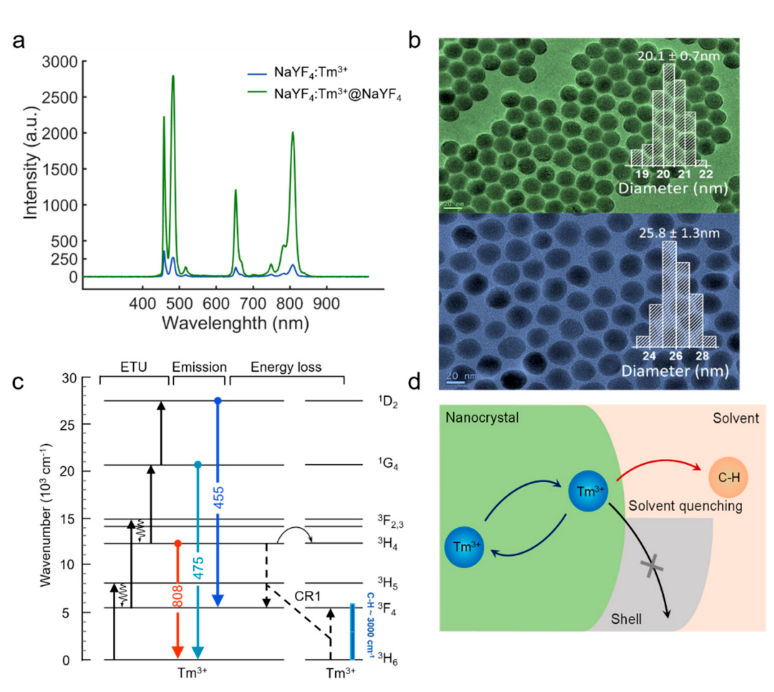
Fluorescence imaging has high brightness requirements for UNCPs. The luminescence properties especially the upconversion efficiency of UCNPs are very sensitive to variations in the surface area-to-volume ratio, crystal structure, and lanthanide doping concentration as well as to the ligand and surrounding medium [
20]. Previous study about Yb
3+ sensitizer-activator system indicates that energy attenuation of the sensitizer caused by surface defects is the primary reason for the decrease of fluorescence intensity. However, the studies about the surface quenching effect on the single-doped Tm
3+ system excites by NIR-II wavelengths is unspecified. By comparing the same dilution concentration of NaYF
4: Tm (1.75%) core-only nanoparticles with the core-shell NaYF
4: Tm (1.75%)@NaYF
4 nanoparticles, the luminescence intensity of the core-shell sample is nearly ten times higher than the former one (
Figure 3a). This phenomenon indicates that severe luminescence quenching effect induced by surface defects still exists in singly doped Tm
3+ UCNPs. Therefore, the mechanism that causes the energy loss of the bare core structure can be inferred.
As evidenced by the effect of energy resonances between electronic transitions of the lanthanide ions and vibrations of the solvent molecules [
21]. The energy level diagram of the core–shell nanoparticles NaYF
4: Tm (1.75%)@NaYF
4 is shown in
Figure 3c. Here, the solvent is cyclohexane, and the highest-energy vibrational modes in hydrocarbon molecules are C–H stretch modes with energies around 3000 cm
−1. Resonances of the C–H stretch vibrational energy with transitions between the Tm
3+ states will directly affect the strength of solvent quenching [
22]. The near-infrared levels
3 F
4 can be quenched by coupling to the C–H stretch vibrational energy (
Figure 3d) via overtone vibrational transitions, since the gap of the two energy levels (
3 F
4 →
3 H
6) of Tm
3+ (~5577 cm
−1) is nearly 2 times of the highest solvent vibrations (C–H stretch) [
23]. In addition, the effect of CR1 will drive the electrons to tend to concentrate at the
3 F
4. In this context, the Tm
3+ 3 F
4 level would be easily quenched by the solvent, and thereby affects the emission intensity of the nanoparticles NaYF
4: Tm (1.75%). Furthermore, CR as an energy transfer progress by dipole−dipole interaction depends on the distance between a donor center and an acceptor center, in which a luminescent center transfers part of its energy to neighbors, and finally to the surface quenchers [
22,
24]. To address the fluorescence quenching effect, we coated the core NaYF
4: Tm (1.75%) with an inert shell NaYF
4. Core-shell nanostructures are believed to confine energy migration and prevent energy trapping by quenching centers.
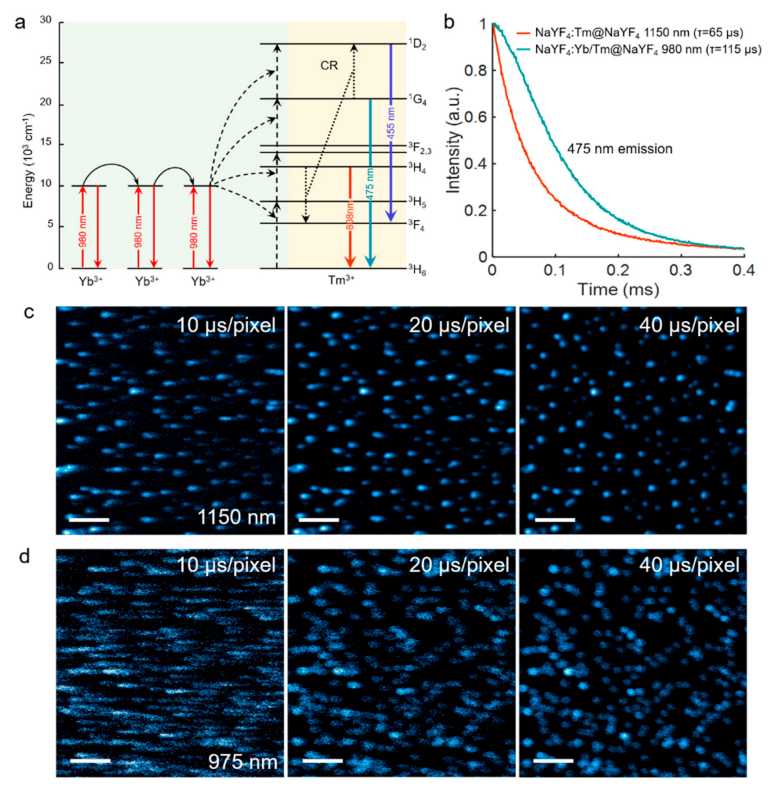
In
Figure 4a, our work also studied the energy transfer path of traditional Yb
3+/Tm
3+ co-doped system. Through successive energy transfer steps from three (or more) Yb
3+ ions to Tm
3+, the conversion process from low-energy near-infrared photons to high-energy visible photons can be realized. In principle, the decay time extracted from the upconversion luminescence decay profile generally cannot be interpreted as the intrinsic lifetime of the upconversion luminescence emitting state [
25]. Instead, it is an overall temporal response of the whole upconversion system to the excitation function, influenced by the sensitizer’s excited-state intrinsic lifetime and the effects of energy transfer [
25]. Since these NIR-II excited upconversion processes do not rely on the Yb
3+ sensitizer with long-lived state, the upconversion luminescence decay would not be influenced by the long excited-state lifetime of the sensitizers and the effect of energy transfer. Therefore, compared to traditional 980 nm excited Yb
3+/Tm
3+ co-doped system, shorter decay lifetimes of three-photon emissions are also expected in this 1150 nm upconversion system. As the decay profiles of 475 nm emissions of different upconversion systems shown in
Figure 4b, it is found that the 475 nm blue emissions aroused by the direct excitation of 1150 nm beam in singly doped Tm
3+ sample exhibit faster kinetics than those by the excitation of 980 nm beam in Yb
3+/Tm
3+ co-doped sample. Specifically, the measured decay lifetime of 475 nm excited by 1150 nm beam (65 μs) is nearly half of that excited by 980 nm beam (115 μs), which can help to improve the imaging scanning speed and hold larger potential for the application of rapid, dynamics life studies [
26]. We then compared the laser scanning imaging results of these two upconversion systems obtained with different imaging speed (
Figure 4c,d). Clearly, when the scanning dwelling time was reduced from 40 μs/pixel to 10 μs/pixel, the 475 nm three-photon fluorescence images excited by 1150 nm beam only show a relatively slight smearing. The position of nanoparticles can still be easily determined from the luminescence spots. In contrast, the longer lifetime of the emission excited by 980 nm beam suffered more severe streaking effect during the imaging. The spots became streaky and would destroy the lateral resolution in the scanning direction. In this case, the imaging speed was restricted. It should be noticed that in traditional photon upconversion of Tm
3+ system, through step-wised ETU processes assisted by Yb
3+ ions, the higher-order blue emissions were always much weaker than the lower-order 808 emission because the electrons tended to populate at lower-lying states [
27].
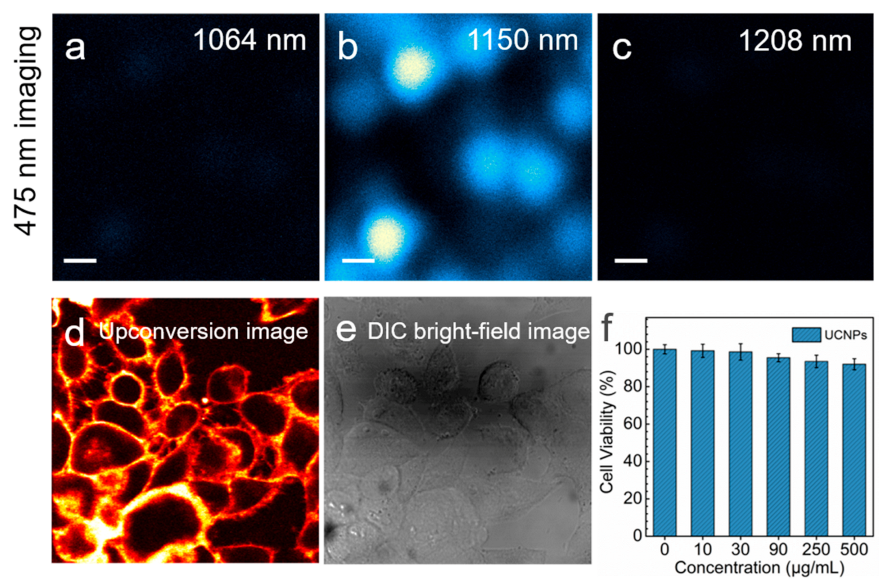
As mentioned above, 808 nm is in a luminescence band where the detector cannot respond sensitively. From the combination of excitation mechanisms and spectrogram results, 1150 nm is the most favorable wavelength for blue emission, which is more conducive to subsequent luminescence decay lifetime, nanoparticles, and cancer cell imaging research. The excellent performance of 1150 nm excitation strategy was also demonstrated through multiphoton scanning microscopic imaging. The bright blue 475 nm luminescence excited by 1150 nm laser was successfully observed from the nanoparticles. In contrast, the 1064 nm and 1208 nm excitation can only use the 808 nm emission for imaging (
Figure 5a–c). Hela cells labeled with the hydrophilic and surface functionalization UCNPs (20 nm diameter; 1.75% Tm
3+) with 5 nm inert shell have been manipulated with 1150 nm lasers as cancer cell fluorescence imaging (
Figure 5d,e). For upconversion imaging, excitation at NIR-II wavelengths and anti-Stokes NIR emission suggests the possibility of imaging with little or no phototoxicity or autofluorescence background. We sought to demonstrate that UCNPs could be deployed and unambiguously visualized inside cells with 1150 nm excitation. We also assessed the cytotoxicity of the UCNPs by CCK-8 assay. As shown in
Figure 5f, the cell viability was calculated to be larger than 92% even at a concentration as high as 500 μg mL
−1 PAA-coupled NaYF
4: Tm (1.75%)@NaYF
4 UCNPs, which indicated the UCNPs exhibited good biocompatibility and nearly nontoxic. The cytotoxicity and bioimaging results illustrated the UCNPs as biological labels can achieve a well presentation of the cellular morphology and contour. The bright-field morphology corresponding to the fluorescence labeled cells is shown in
Figure 5e.