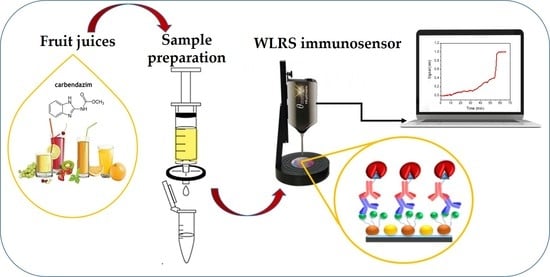
Fast and Sensitive Determination of the Fungicide Carbendazim in Fruit Juices with an Immunosensor Based on White Light Reflectance Spectroscopy †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Carbendazim Standard Solutions and Fruit Juice Samples
2.3. Carbendazim-ELISA Assay
2.4. Evaluation of Primary Antibody—Specificity with the Carbendazim-ELISA Assay
2.5. WLRS Instrumentation
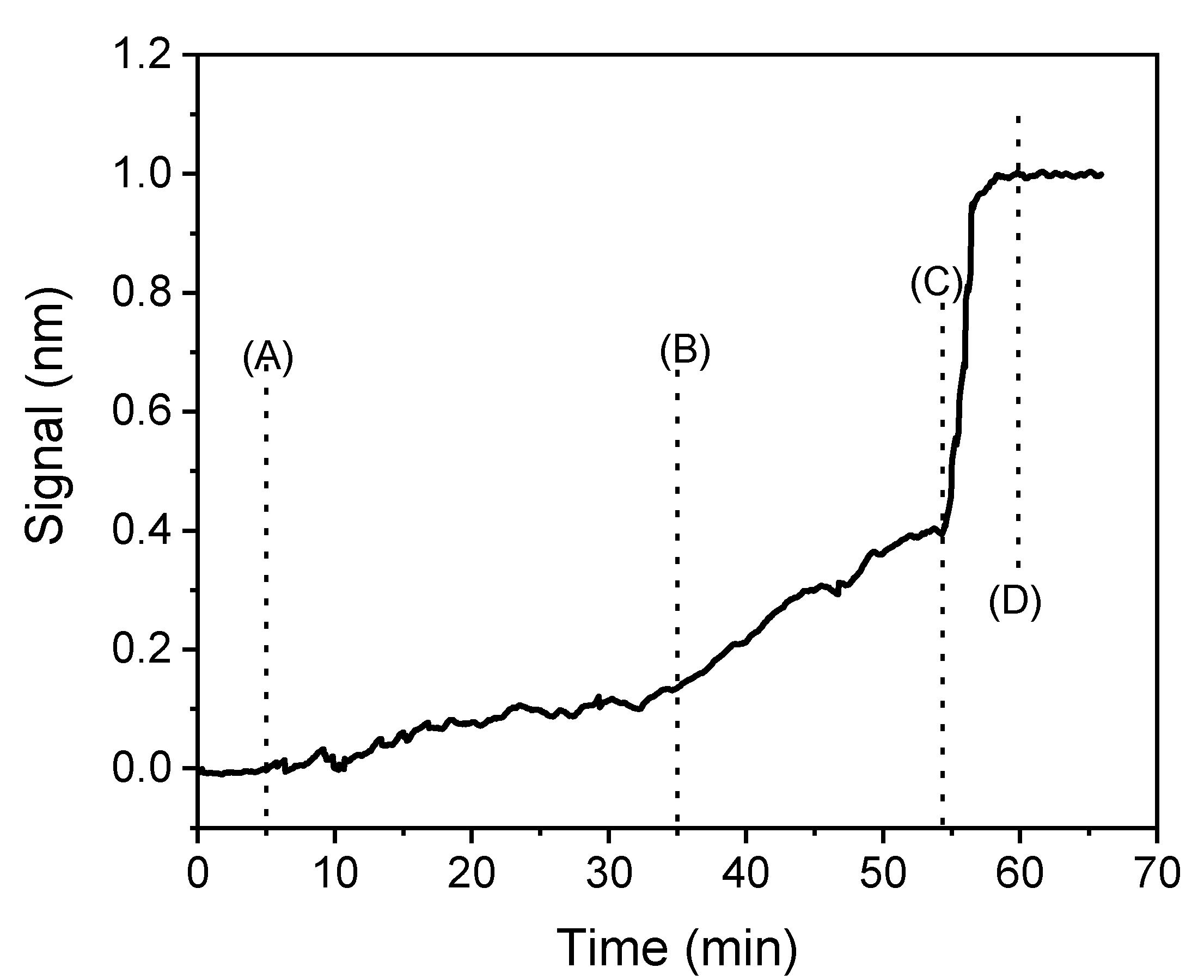
2.6. Biochip Preparation and Biosensor Assay Performance
3. Results and Discussion
3.1. ELISA Assay for Carbendazim
3.1.1. Assay Optimization
3.1.2. Evaluation of the Anti-Carbendazim Antibody Specificity
3.2. WLRS Assay
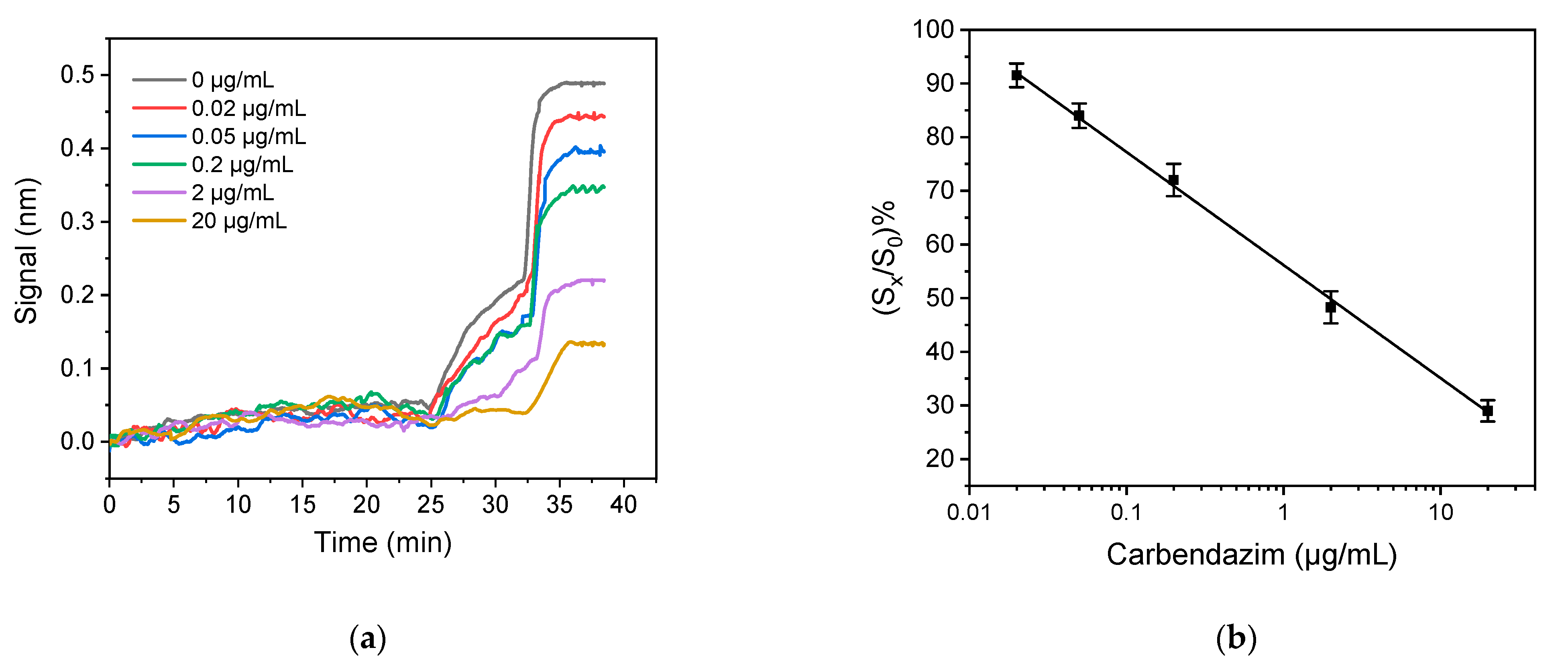
3.2.1. Assay Optimization
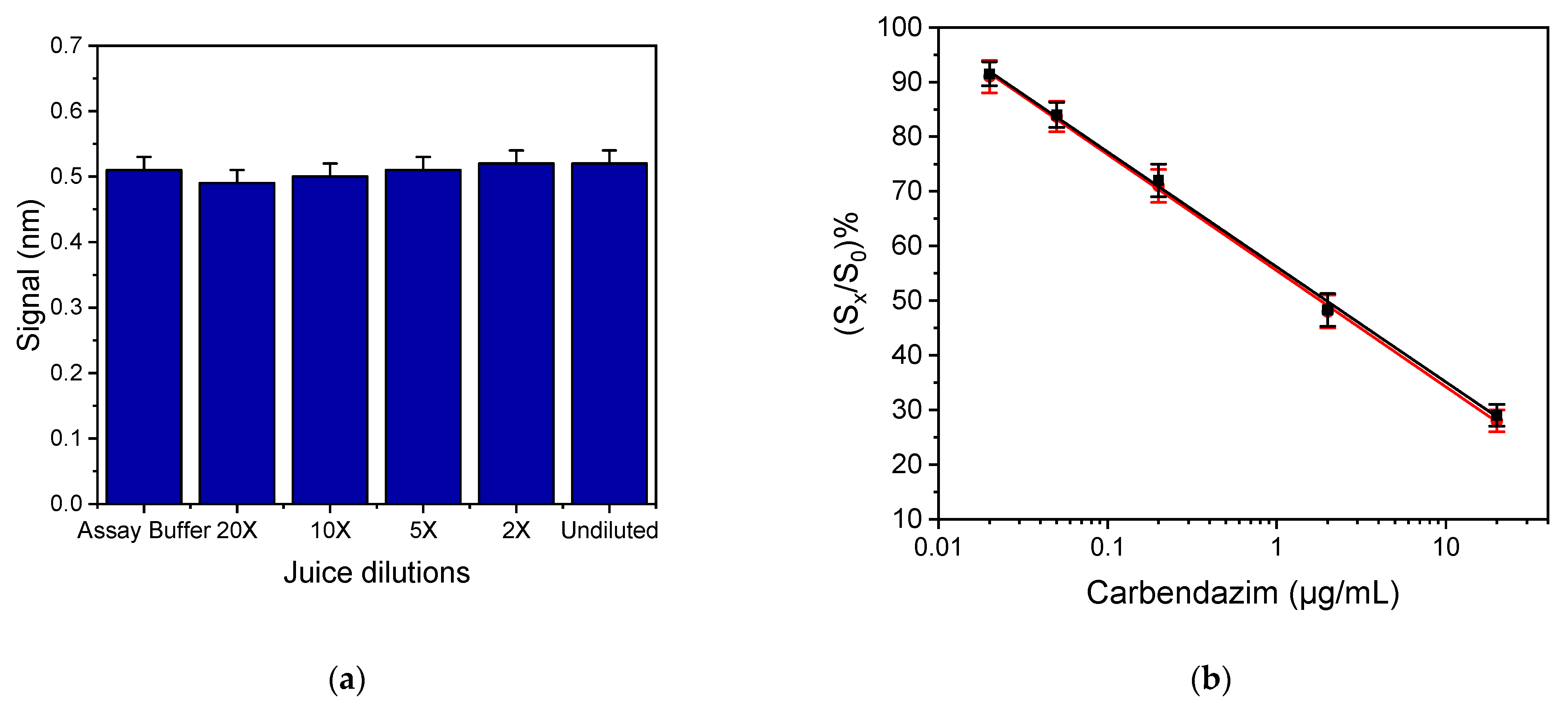
3.2.2. Optimization of Sample Preparation Procedure
3.2.3. Accuracy and Precision of the Developed ELISA and Sensor Assays
3.2.4. Analysis of Commercially Available Fruit Juices
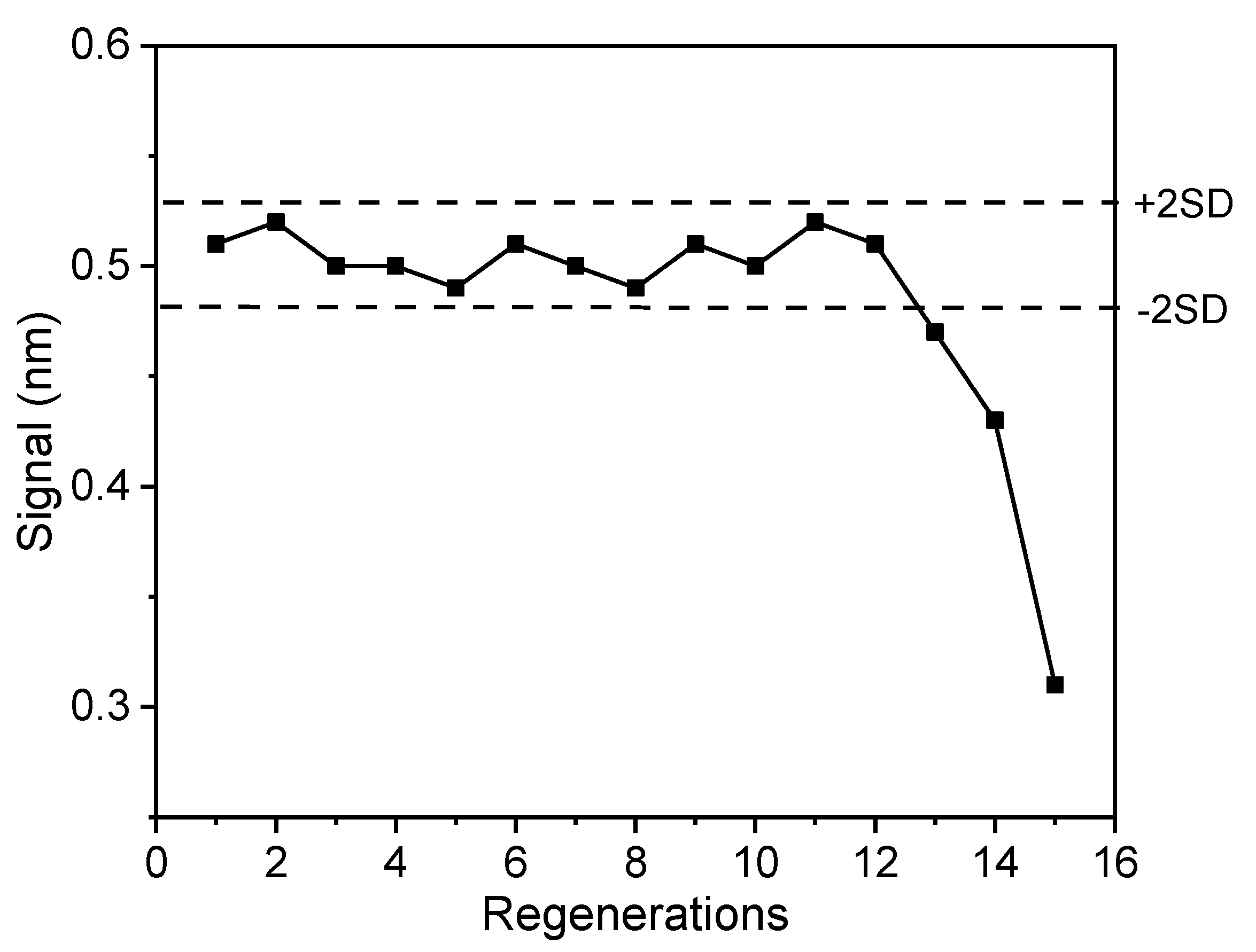
3.2.5. Regeneration of Biochips
3.3. Comparison with Other Biosensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karlsson, I.; Friberg, H.; Steinberg, C.; Persson, P. Fungicide Effects on Fungal Community Composition in the Wheat Phyllosphere. PLoS ONE 2014, 9, e111786. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A.B.; Singh, D.; Singh, K.; Singh, J. Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ. Chem. Lett. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Tortella, G.; Mella-Herrera, R.; Sousa, D.; Rubilar, O.; Briceño, G.; Parra, L.; Diez, M. Carbendazim dissipation in the biomixture of on-farm biopurification systems and its effect on microbial communities. Chemosphere 2013, 93, 1084–1093. [Google Scholar] [CrossRef]
- Goodson, W.H.; Lowe, L.; Carpenter, D.O.; Gilbertson, M.; Ali, A.M.; Salsamendi, A.L.D.C.; Lasfar, A.; Carnero, A.; Azqueta, A.; Amedei, A.; et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: The challenge ahead. Carcinogenesis 2015, 36, S254–S296. [Google Scholar] [CrossRef]
- Stahel, W.R. Reuse Is the Key to the Circular Economy. Available online: http://ec.europa.eu/environment/ecoap/about-eco-innovation/experts-interviews/reuse-is-the-key-to-the-circular-economy_en.htm (accessed on 12 March 2020).
- Grujic, S.; Radišić, M.; Vasiljevic, T.; Lausevic, M. Determination of carbendazim residues in fruit juices by liquid chromatography-tandem mass spectrometry. Food Addit. Contam. 2005, 22, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- EU Database. Available online: https://eur-lex.europa.eu/eli/reg/2011/559/oj (accessed on 2 November 2020).
- Heyman, M.B.; Abrams, S.A.; Gastroenterology, H.S.O.; Committee on Nutrition. Fruit Juice in Infants, Children, and Adolescents: Current Recommendations. Pediatrics 2017, 139, e20170967. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, M.; De Kok, A. Comprehensive multi-residue method for the target analysis of pesticides in crops using liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2007, 1154, 3–25. [Google Scholar] [CrossRef]
- Chayata, H.; Lassalle, Y.; Nicol, É.; Manolikakes, S.; Souissi, Y.; Bourcier, S.; Gosmini, C.; Bouchonnet, S. Characterization of the ultraviolet–visible photoproducts of thiophanate-methyl using high performance liquid chromatography coupled with high resolution tandem mass spectrometry—Detection in grapes and tomatoes. J. Chromatogr. A 2016, 1441, 75–82. [Google Scholar] [CrossRef]
- Gough, K.C.; Jarvis, S.; Maddison, B.C. Development of competitive immunoassays to hydroxyl containing fungicide metabolites. J. Environ. Sci. Health Part B 2011, 46, 581–589. [Google Scholar] [CrossRef]
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold Nanoparticle-Based Paper Sensor for Simultaneous Detection of 11 Benzimidazoles by One Monoclonal Antibody. Small 2017, 14, 1701782. [Google Scholar] [CrossRef]
- Reynoso, E.C.; Torres, E.; Bettazzi, F.; Palchetti, I. Trends and Perspectives in Immunosensors for Determination of Currently-Used Pesticides: The Case of Glyphosate, Organophosphates, and Neonicotinoids. Biosensor 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Koukouvinos, G.; Petrou, P.; Goustouridis, D.; Misiakos, K.; Kakabakos, S.; Raptis, I. Development and Bioanalytical Applications of a White Light Reflectance Spectroscopy Label-Free Sensing Platform. Biosensors 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, V.; Koukouvinos, G.; Petrou, P.S.; Economou, A.; Dekker, J.; Harjanne, M.; Heimala, P.; Goustouridis, D.; Raptis, I.; Kakabakos, S.E. Multiplexed mycotoxins determination employing white light reflectance spectroscopy and silicon chips with silicon oxide areas of different thickness. Biosens. Bioelectron. 2020, 153, 112035. [Google Scholar] [CrossRef] [PubMed]
- Stavra, E.; Petrou, P.S.; Koukouvinos, G.; Economou, A.; Goustouridis, D.; Misiakos, K.; Raptis, I.; Kakabakos, S.E. Fast, sensitive and selective determination of herbicide glyphosate in water samples with a White Light Reflectance Spectroscopy immunosensor. Talanta 2020, 214, 120854. [Google Scholar] [CrossRef] [PubMed]
- Zikos, C.; Evangelou, A.; Karachaliou, C.-E.; Gourma, G.; Blouchos, P.; Moschopoulou, G.; Yialouris, C.; Griffiths, J.; Johnson, G.; Petrou, P.; et al. Commercially available chemicals as immunizing haptens for the development of a polyclonal antibody recognizing carbendazim and other benzimidazole-type fungicides. Chemosphere 2015, 119, S16–S20. [Google Scholar] [CrossRef]
- Papasarantos, I.; Klimentzou, P.; Koutrafouri, V.; Anagnostouli, M.; Zikos, C.; Paravatou-Petsotas, M.; Livaniou, E. Solid-Phase Synthesis of a Biotin Derivative and its Application to the Development of Anti-Biotin Antibodies. Appl. Biochem. Biotechnol. 2009, 162, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Zalis, M.G.; Wilchek, M. 3-(N-maleimido-propionyl) biocytin: A versatile thiol-specific biotinylating reagent. Anal. Biochem. 1985, 149, 529–536. [Google Scholar] [CrossRef]
- Ortelli, D.; Edder, P.; Corvi, C. Pesticide residues survey in citrus fruits. Food Addit. Contam. 2005, 22, 423–428. [Google Scholar] [CrossRef]
- De Souza, D.; Machado, S. Electrochemical detection of the herbicide paraquat in natural water and citric fruit juices using microelectrodes. Anal. Chim. Acta 2005, 546, 85–91. [Google Scholar] [CrossRef]
- Zhao, B.; Feng, S.; Hu, Y.; Wang, S.; Lu, X. Rapid determination of atrazine in apple juice using molecularly imprinted polymers coupled with gold nanoparticles-colorimetric/SERS dual chemosensor. Food Chem. 2019, 276, 366–375. [Google Scholar] [CrossRef]
- Skerritt, J.H.; Rani, B.E.A. Detection and removal of sample matrix effects in agrochemical immunoassays. In Immunoassays for Residue Analysis; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1996; Volume 621, pp. 29–43. [Google Scholar]
- Moreno, M.-J.; Plana, E.; Montoya, A.; Caputo, P.; Manclús, J.J. Application of a monoclonal-based immunoassay for the determination of imazalil in fruit juices. Food Addit. Contam. 2007, 24, 704–712. [Google Scholar] [CrossRef]
- Feng, S.; Li, Y.; Zhang, R.; Li, Y. A novel electrochemical sensor based on molecularly imprinted polymer modified hollow N, S-Mo2C/C spheres for highly sensitive and selective carbendazim determination. Biosens. Bioelectron. 2019, 142, 111491. [Google Scholar] [CrossRef] [PubMed]
- Barboza, A.D.M.; Da Silva, A.B.; Da Silva, E.M.; De Souza, W.P.; Soares, M.A.; De Vasconcelos, L.G.; Terezo, A.J.; Castilho, M. A biosensor based on microbial lipase immobilized on lamellar zinc hydroxide-decorated gold nanoparticles for carbendazim determination. Anal. Methods 2019, 11, 5388–5397. [Google Scholar] [CrossRef]
- Liao, X.; Huang, Z.; Huang, K.; Qiu, M.; Chen, F.; Zhang, Y.; Wen, Y.; Chen, J. Highly Sensitive Detection of Carbendazim and Its Electrochemical Oxidation Mechanism at a Nanohybrid Sensor. J. Electrochem. Soc. 2019, 166, B322–B327. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. Selection and Characterization of DNA Aptamers for Electrochemical Biosensing of Carbendazim. Anal. Chem. 2017, 89, 3138–3145. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, L.; Zhang, L. Simultaneous Electrochemical Detection of Benzimidazole Fungicides Carbendazim and Thiabendazole Using a Novel Nanohybrid Material-Modified Electrode. J. Agric. Food Chem. 2017, 65, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Arruda, G.J.; De Lima, F.; Cardoso, C.A.L. Ultrasensitive determination of carbendazim in water and orange juice using a carbon paste electrode. J. Environ. Sci. Health Part B 2016, 51, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Razzino, C.A.; Sgobbi, L.F.; Canevari, T.C.; Cancino, J.; Machado, S.A. Sensitive determination of carbendazim in orange juice by electrode modified with hybrid material. Food Chem. 2015, 170, 360–365. [Google Scholar] [CrossRef]
- Strickland, A.D.; Batt, C.A. Detection of Carbendazim by Surface-Enhanced Raman Scattering Using Cyclodextrin Inclusion Complexes on Gold Nanorods. Anal. Chem. 2009, 81, 2895–2903. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO2 luminescent resonance energy transfer biosensor. Food Chem. 2021, 349, 129157. [Google Scholar] [CrossRef]
- Li, Q.; Dou, X.; Zhao, X.; Zhang, L.; Luo, J.; Xing, X.; Yang, M. A gold/Fe3O4 nanocomposite for use in a surface plasmon resonance immunosensor for carbendazim. Microchim. Acta 2019, 186, 313. [Google Scholar] [CrossRef] [PubMed]



| Juice Sample | Spiked Level (ng/mL) | Determined Concentration (ng/mL) | % Recovery | ||
|---|---|---|---|---|---|
| WLRS | ELISA | WLRS | ELISA | ||
| Orange | 1000 | 1052 ± 53 | 1097 ± 41 | 105 | 110 |
| 500 | 522 ± 32 | 540 ± 22 | 104 | 108 | |
| 100 | 98 ± 4 | 106 ± 3 | 98.0 | 106 | |
| Orange–apple–carrot | 1000 | 973 ± 57 | 1010 ± 37 | 97.3 | 101 |
| 500 | 547 ± 25 | 451 ± 26 | 109 | 90.2 | |
| 100 | 89 ± 25 | 91 ± 4 | 89.0 | 91.0 | |
| Lemon | 1000 | 951 ± 61 | 896 ± 32 | 95.1 | 89.6 |
| 500 | 487 ± 28 | 496 ± 18 | 97.4 | 99.2 | |
| 100 | 110 ± 7 | 113 ± 6 | 110 | 113 | |
| Sample Number/Name (Storage) | Amount Detected (ng/mL) |
|---|---|
| 1/ Orange juice (Amita, RT) | <LoD |
| 2/ Orange juice (Eviva, RT) | <LoD |
| 3/ Orange juice (Marata, RT) | <LoD |
| 4/ Orange-lemon-carrot (Amita, RT) | <LoD |
| 5/ 9 fruits Motion (Amita, RT) | <LoD |
| 6/ Orange juice (Olympos, 4 °C) | <LoD |
| 7/ Orange juice (Eviva, 4 °C) | <LoD |
| 8/ Orange-apple-carrot (Olympos, 4 °C) | <LoD |
| 9/ 9 Fruits (Olympos, 4 °C) | <LoD |
| 10/ Lemon juice (Eviva, 4 °C) | <LoD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukouvinos, G.; Karachaliou, C.-E.; Raptis, I.; Petrou, P.; Livaniou, E.; Kakabakos, S. Fast and Sensitive Determination of the Fungicide Carbendazim in Fruit Juices with an Immunosensor Based on White Light Reflectance Spectroscopy . Biosensors 2021, 11, 153. https://doi.org/10.3390/bios11050153
Koukouvinos G, Karachaliou C-E, Raptis I, Petrou P, Livaniou E, Kakabakos S. Fast and Sensitive Determination of the Fungicide Carbendazim in Fruit Juices with an Immunosensor Based on White Light Reflectance Spectroscopy . Biosensors. 2021; 11(5):153. https://doi.org/10.3390/bios11050153
Chicago/Turabian StyleKoukouvinos, Georgios, Chrysoula-Evangelia Karachaliou, Ioannis Raptis, Panagiota Petrou, Evangelia Livaniou, and Sotirios Kakabakos. 2021. "Fast and Sensitive Determination of the Fungicide Carbendazim in Fruit Juices with an Immunosensor Based on White Light Reflectance Spectroscopy " Biosensors 11, no. 5: 153. https://doi.org/10.3390/bios11050153
APA StyleKoukouvinos, G., Karachaliou, C.-E., Raptis, I., Petrou, P., Livaniou, E., & Kakabakos, S. (2021). Fast and Sensitive Determination of the Fungicide Carbendazim in Fruit Juices with an Immunosensor Based on White Light Reflectance Spectroscopy . Biosensors, 11(5), 153. https://doi.org/10.3390/bios11050153