Electrochemical Fingerprint Biosensor for Natural Indigo Dye Yielding Plants Analysis
Abstract
1. Introduction
2. Materials and Methods
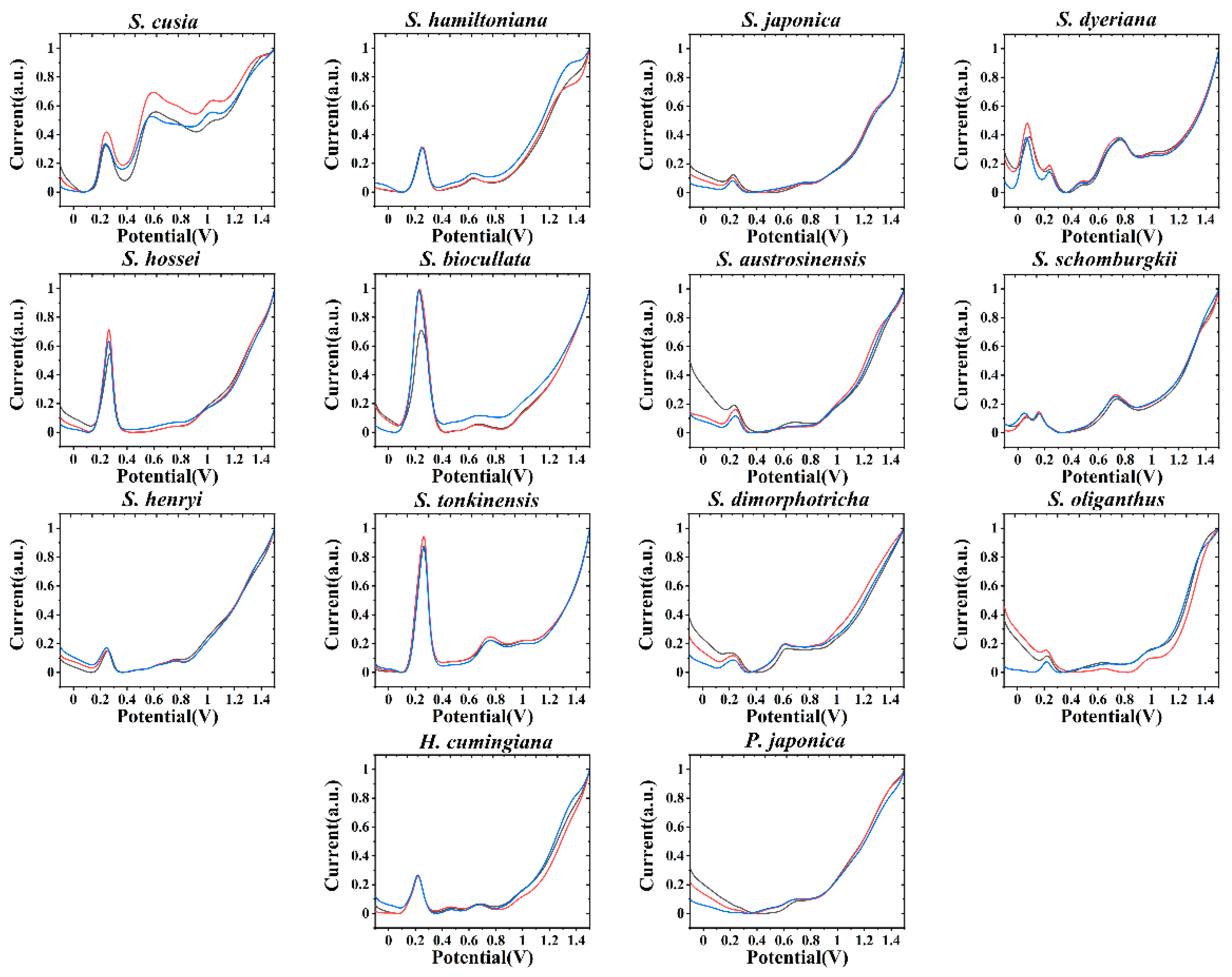
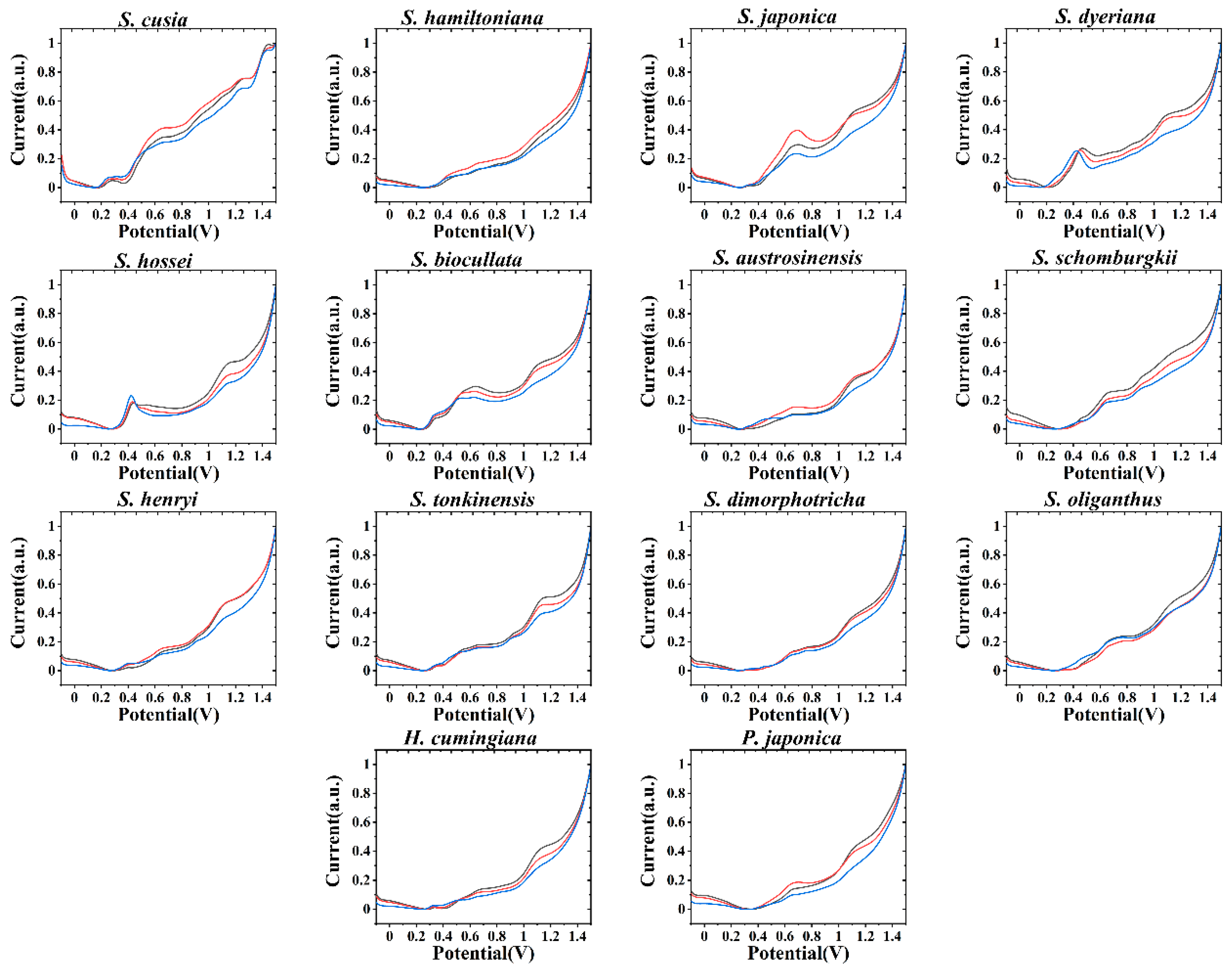
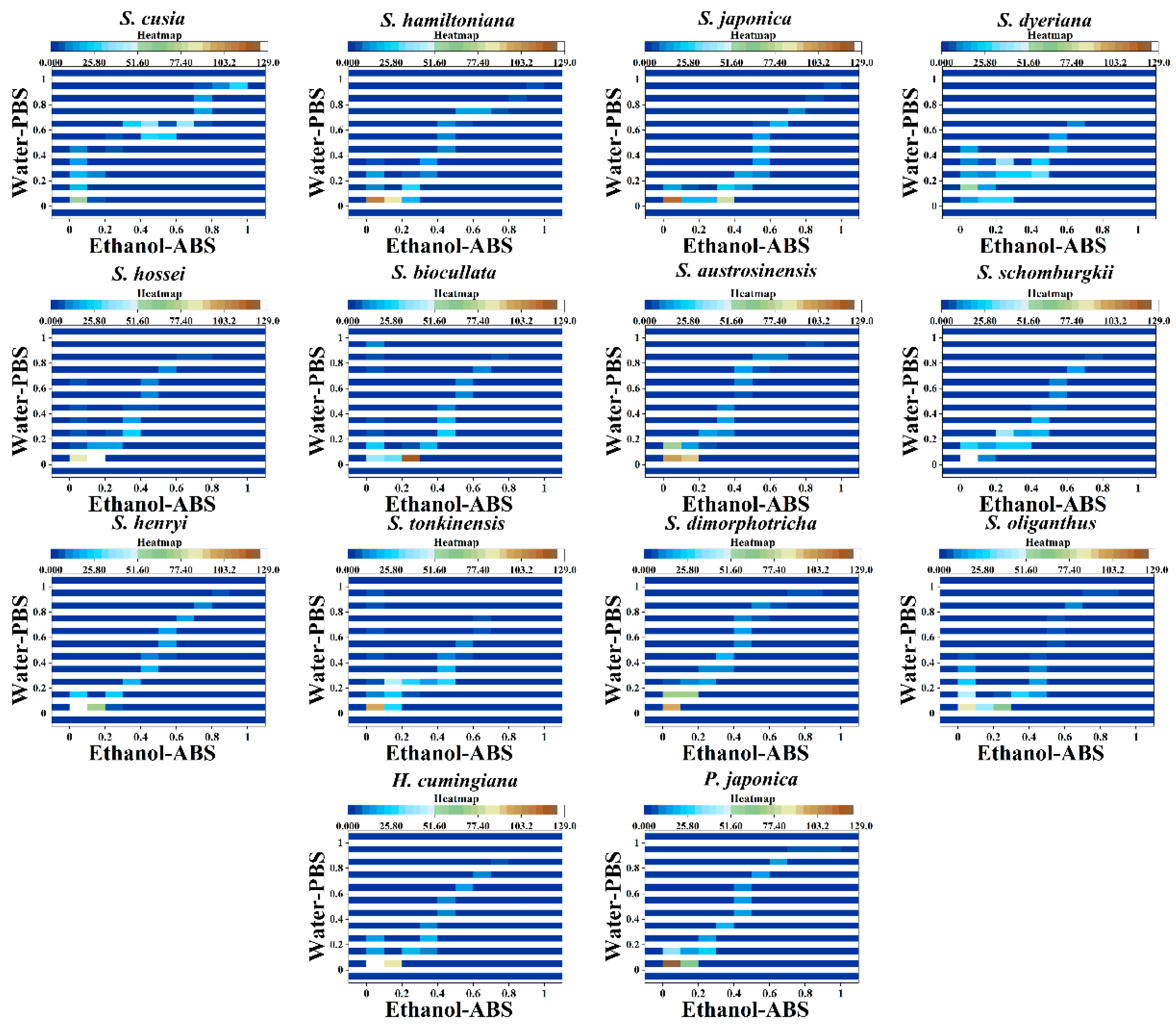
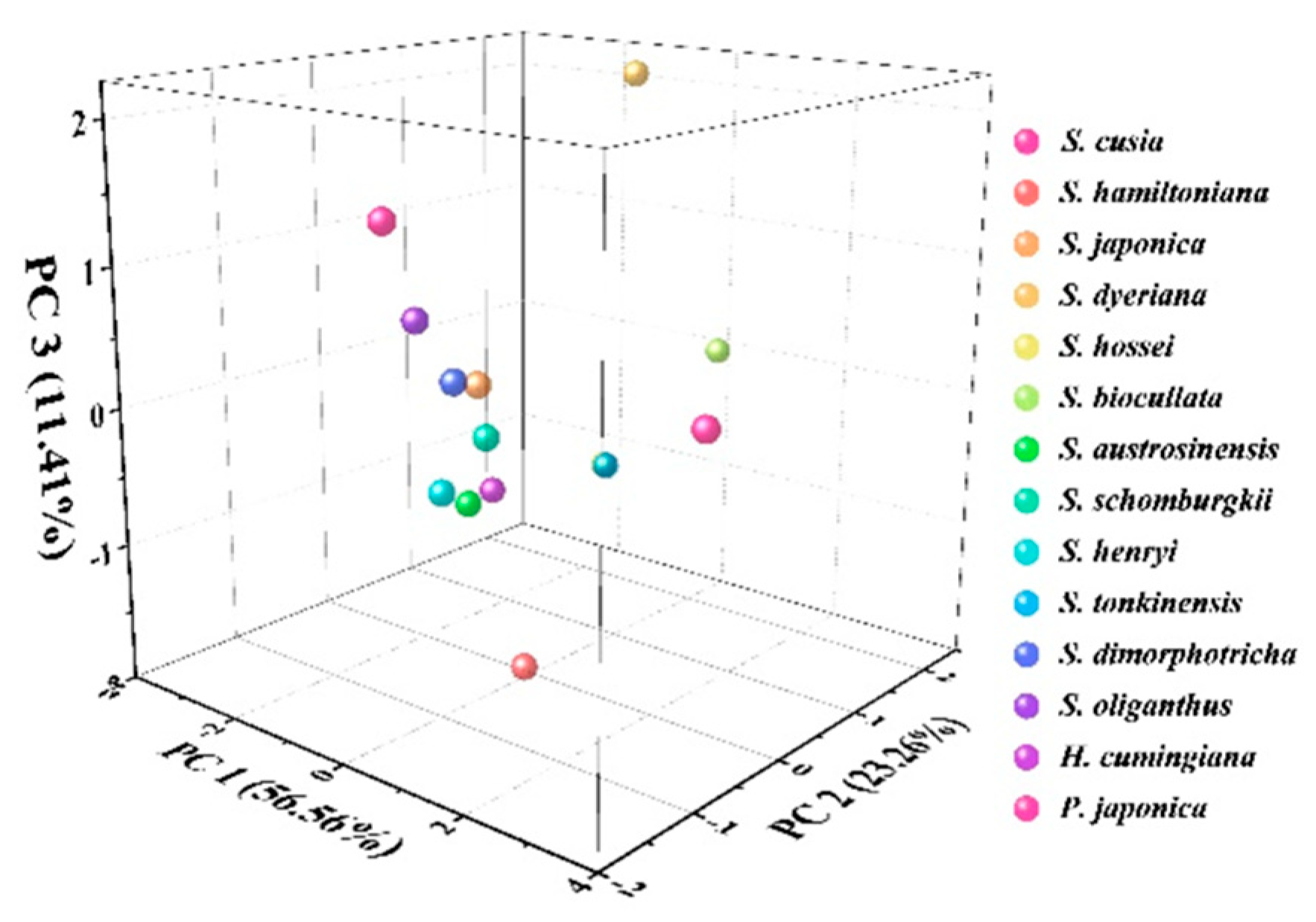
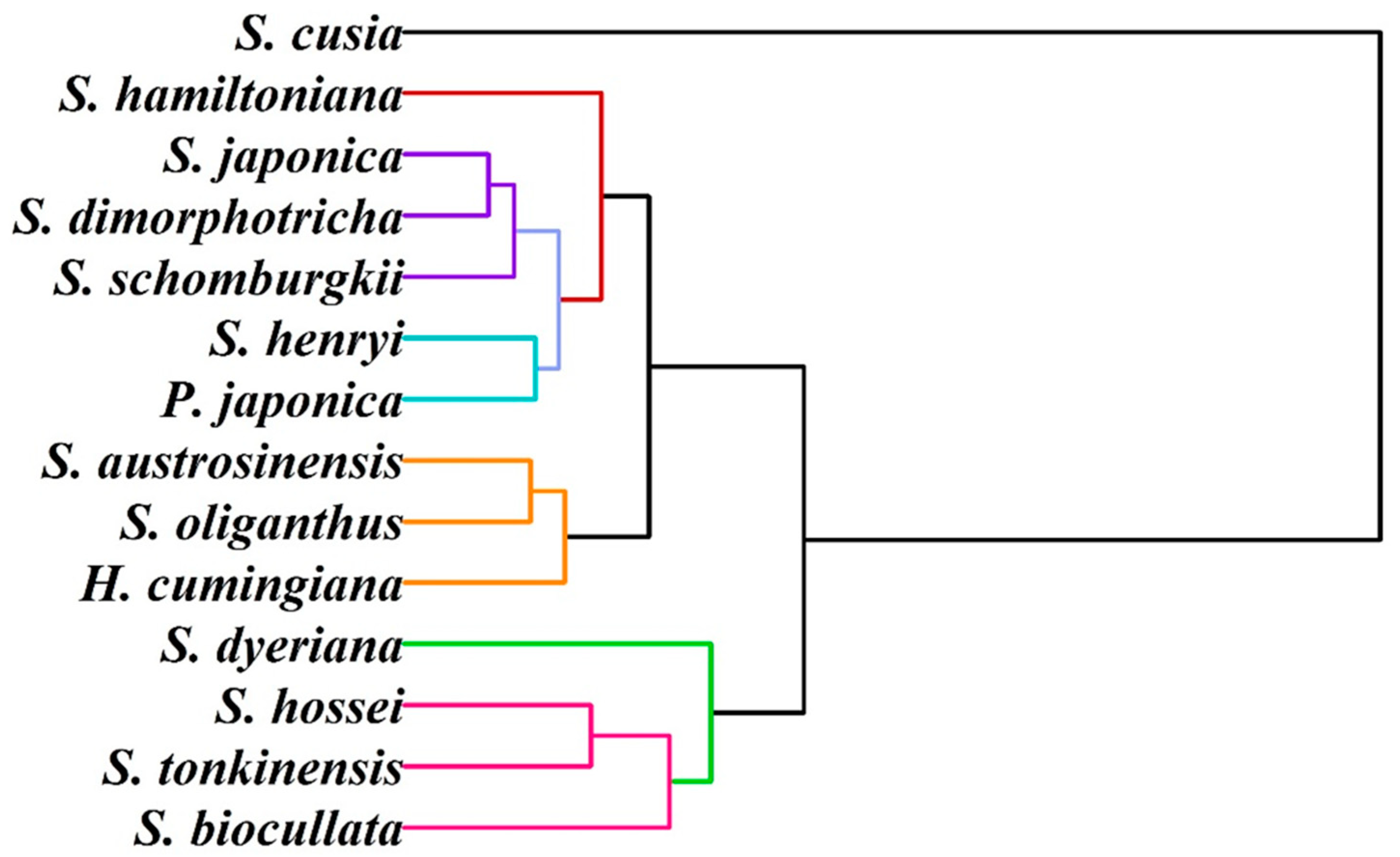
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabara, A.N.; Fraaije, M.W. An overview of microbial indigo-forming enzymes. Appl. Microbiol. Biotechnol. 2020, 104, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Roychoudhary, S.; Sarangi, B.K. Effect of different physico-chemical parameters for natural indigo production during fermentation of Indigofera plant biomass. 3 Biotech. 2017, 7, 322. [Google Scholar] [CrossRef] [PubMed]
- Khoramdel, S.; Rezvani, P.; Hooshmand, M.; Moalem, F. Effects of cow manure levels and plant densities on yield and seed yield components, leaf and indigo yields of true indigo. J. Plant. Prod. Res. 2017, 23, 117–143. [Google Scholar]
- Prabha, C.; Sharma, S. Extraction, Characterization and Tissue Culture of Plant Derived Indigo From Indigofera tinctoria-Preliminary Studies. Int. J. Agrochem. 2018, 4, 53–58. [Google Scholar]
- Li, S.; Cunningham, A.B.; Fan, R.; Wang, Y. Identity blues: The ethnobotany of the indigo dyeing by Landian Yao (Iu Mien) in Yunnan, Southwest China. J. Ethnobiol. Ethnomedicine 2019, 15, 13. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Cunningham, A.B.; Shi, Y.; Wang, Y. Island blues: Indigenous knowledge of indigo-yielding plant species used by Hainan Miao and Li dyers on Hainan Island, China. J. Ethnobiol. Ethnomedicine 2019, 15, 31. [Google Scholar] [CrossRef]
- Tsuji, H.; Kondo, M.; Odani, W.; Takino, T.; Takeda, R.; Sakai, T. Treatment with indigo plant (Polygonum tinctorium Lour) improves serum lipid profiles in Wistar rats fed a high-fat diet. J. Med Investig. 2020, 67, 158–162. [Google Scholar] [CrossRef]
- Pattanaik, L.; Duraivadivel, P.; Hariprasad, P.; Naik, S.N. Utilization and re-use of solid and liquid waste generated from the natural indigo dye production process—A zero waste approach. Bioresour. Technol. 2020, 301, 122721. [Google Scholar] [CrossRef]
- Nakai, A.; Tanaka, A.; Yoshihara, H.; Murai, K.; Watanabe, T.; Miyawaki, K. Blue LED light promotes indican accumulation and flowering in indigo plant, Polygonum tinctorium. Ind. Crop. Prod. 2020, 155, 112774. [Google Scholar] [CrossRef]
- Begum, K.; Motobayashi, T.; Hasan, N.; Appiah, K.S.; Shammi, M.; Fujii, Y. Indigo as a Plant Growth Inhibitory Chemical from the Fruit Pulp of Couroupita guianensis Aubl. Agronomy 2020, 10, 1388. [Google Scholar] [CrossRef]
- Prasad, R. Indigo—The Crop that Created History and then Itself Became History. Indian J. Hist. Sci. 2018, 53, 296–301. [Google Scholar] [CrossRef]
- Pattanaik, L.; Padhi, S.K.; Hariprasad, P.; Naik, S.N. Life cycle cost analysis of natural indigo dye production from Indigofera tinctoria L. plant biomass: A case study of India. Clean Technol. Environ. Policy 2020, 22, 1639–1654. [Google Scholar] [CrossRef]
- Lee, C.-L.; Wang, C.-M.; Hu, H.-C.; Yen, H.-R.; Song, Y.-C.; Yu, S.-J.; Chen, C.-J.; Li, W.-C.; Wu, Y.-C. Indole alkaloids indigodoles A–C from aerial parts of Strobilanthes cusia in the traditional Chinese medicine Qing Dai have anti-IL-17 properties. Phytochemistry 2019, 162, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, T.; Ran, Q.; Huang, Q.; Wang, J. Strobilanthes cusia (Nees) Kuntze, a multifunctional traditional Chinese medicinal plant, and its herbal medicines: A comprehensive review. J. Ethnopharmacol. 2021, 265, 113325. [Google Scholar] [CrossRef]
- Barré, P.; Stöver, B.C.; Müller, K.F.; Steinhage, V. LeafNet: A computer vision system for automatic plant species identification. Ecol. Inform. 2017, 40, 50–56. [Google Scholar] [CrossRef]
- Wäldchen, J.; Rzanny, M.; Seeland, M.; Mäder, P. Automated plant species identification—Trends and future directions. Plos Comput. Biol. 2018, 14, e1005993. [Google Scholar] [CrossRef]
- Wäldchen, J.; Mäder, P. Machine learning for image based species identification. Methods Ecol. Evol. 2018, 9, 2216–2225. [Google Scholar] [CrossRef]
- Depciuch, J.; Kasprzyk, I.; Drzymała, E.; Parlinska-Wojtan, M. Identification of birch pollen species using FTIR spectroscopy. Aerobiologia 2018, 34, 525–538. [Google Scholar] [CrossRef]
- Kenđel, A.; Zimmermann, B. Chemical analysis of pollen by FT-Raman and FTIR spectroscopies. Front. Plant Sci. 2020, 11, 352. [Google Scholar] [CrossRef]
- Sithara, N.; Komathi, S.; Rajalakshmi, G. Identification of bioactive compounds using different solvents through FTIR studies and GCMS analysis. J. Med. Plants Stud. 2017, 5, 192–194. [Google Scholar]
- Zheng, Y.; Zhu, J.; Fu, L.; Liu, Q. Phylogenetic Investigation of Yellow Camellias Based on Electrochemical Voltammetric Fingerprints. Int. J. Electrochem. Sci 2020, 15, 9622–9630. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, R.; Li, Z.; Zhang, M.; Wang, Q.; Xu, Y.; Fu, L.; Du, J.; Zheng, Y.; Zhu, J. Electroanalytical study of infrageneric relationship of Lagerstroemia using glassy carbon electrode recorded voltammograms. Rev. Mex. De Ing. Química 2020, 19, 281–291. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Wu, M.; Zhang, H.; Wang, A.; Su, W.; Chen, F.; Yu, J.; et al. An electrochemical method for plant species determination and classification based on fingerprinting petal tissue. Bioelectrochemistry 2019, 129, 199–205. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Y.; Zhang, J.; Karimi-Maleh, H.; Xu, Y.; Zhou, Q.; Fu, L.; Wu, W. Characterization of the Electrochemical Profiles of Lycoris Seeds for Species Identification and Infrageneric Relationships. Anal. Lett. 2020, 53, 2517–2528. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Xu, Y.; Zhou, J.; Zhang, H.; Karimi-Maleh, H.; Lai, G.; Zhao, S.; et al. Development of an electrochemical biosensor for phylogenetic analysis of Amaryllidaceae based on the enhanced electrochemical fingerprint recorded from plant tissue. Biosens. Bioelectron. 2020, 159, 112212. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Q.; Zhang, M.; Zheng, Y.; Wu, M.; Lan, Z.; Pu, J.; Zhang, H.; Chen, F.; Su, W. Electrochemical sex determination of dioecious plants using polydopamine-functionalized graphene sheets. Front. Chem. 2020, 8, 92. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Zhang, P.; Wang, Y.; Zheng, Y.; Fu, L.; Zhang, H.; Lin, C.-T.; Yu, A. Infrageneric phylogenetics investigation of Chimonanthus based on electroactive compound profiles. Bioelectrochemistry 2020, 133, 107455. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, B.; Wang, Y.; Du, X.; Fu, L.; Zheng, Y.; Chen, F.; Wu, W.; Zhou, Q.; Ding, S. Recording the Electrochemical Profile of Pueraria Leaves for Polyphyly Analysis. ChemistrySelect 2020, 5, 5035–5040. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Duan, J.; Li, N.; Li, B.; Song, T.; Sardar, M.F.; Lv, X.; Zhu, C. Electrochemical disinfection of secondary effluent from a wastewater treatment plant: Removal efficiency of ARGs and variation of antibiotic resistance in surviving bacteria. Chem. Eng. J. 2020, 392, 123674. [Google Scholar] [CrossRef]
- Durán, F.E.; de Araújo, D.M.; do Nascimento Brito, C.; Santos, E.V.; Ganiyu, S.O.; Martínez-Huitle, C.A. Electrochemical technology for the treatment of real washing machine effluent at pre-pilot plant scale by using active and non-active anodes. J. Electroanal. Chem. 2018, 818, 216–222. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.G.; Hosseini, M.; Safari, R.; Moshrefi, R.; Mohammad Shiri, H. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Ligaj, M.; Kobus-Cisowska, J.; Maciejewska, P.; Tichoniuk, M.; Szulc, P. Application for novel electrochemical screening of antioxidant potential and phytochemicals in Cornus mas extracts. Cyta-J. Food 2019, 17, 781–789. [Google Scholar] [CrossRef]
- Marsoul, A.; Ijjaali, M.; Elhajjaji, F.; Taleb, M.; Salim, R.; Boukir, A. Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L): Evaluation of the inhibitory effect in acidic medium 1 M HCl. Mater. Today Proc. 2020, 27, 3193–3198. [Google Scholar] [CrossRef]
- Reddy, Y.M.; Kumar, S.; Saritha, K.; Gopal, P.; Reddy, T.M.; Simal-Gandara, J. Phytochemical Profiling of Methanolic Fruit Extract of Gardenia latifolia Ait. by LC-MS/MS Analysis and Evaluation of Its Antioxidant and Antimicrobial Activity. Plants 2021, 10, 545. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.; Shi, H.; Zhang, P.Z.H.; Fu, L. Feasibility of electrochemical fingerprinting for plant phylogeography study: A case of Chimonanthus praecox. Int. J. Electrochem. Sci. 2020, 15, 758–764. [Google Scholar] [CrossRef]
- Vijay, A.; Chhabra, M.; Vincent, T. Microbial community modulates electrochemical performance and denitrification rate in a biocathodic autotrophic and heterotrophic denitrifying microbial fuel cell. Bioresour. Technol. 2019, 272, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, S.; Ji, X.; Jiang, B.; Xue, L.; Li, M.; Tan, L. Performance and microbial community dynamics of electricity-assisted sequencing batch reactor (SBR) for treatment of saline petrochemical wastewater. Environ. Sci. Pollut. Res. 2017, 24, 17556–17565. [Google Scholar] [CrossRef]
- Wood, J.; Scotland, R. New and little-known species of Strobilanthes (Acanthaceae) from India and South East Asia. Kew Bull. 2009, 64, 3–47. [Google Scholar] [CrossRef]
- Somprasong, W.; Vjarodaya, S.; Chayamarit, K. Taxonomic Study of the Family Acanthaceae used as traditional medicinal plants for ethnic groups in North, Central and Northeastern Thailand. Thai Agric. Res. J. 2014, 32, 77–88. [Google Scholar]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. J. Clean. Prod. 2021, 291, 125880. [Google Scholar] [CrossRef]
- Bremekamp, C.E.B. Materials for a Monograph of the Strobilanthinae (Acanthaceae); NV Noord-Hollandsche Uitgevers Maatschappij: Amsterdam, The Netherlands, 1944. [Google Scholar]
- Terao, H. Taxonomic study of the genus Strobilanthes Bl. (Acanthaceae): Generic delimitation and infrageneric classification. Ph.D. Thesis, Kyoto University, Kyoto, Japan, 1983. [Google Scholar]
- Carine, M.A.; Scotland, R.W. Classification of Strobilanthinae (Acanthaceae): Trying to classify the unclassifiable? Taxon 2002, 51, 259–279. [Google Scholar] [CrossRef]
- Wood, J. Notes on Strobilanthes (Acanthaceae) for the flora of Ceylon. Kew Bull. 1995, 50, 1–24. [Google Scholar] [CrossRef]
- Moylan, E.C.; Bennett, J.R.; Carine, M.A.; Olmstead, R.G.; Scotland, R.W. Phylogenetic relationships among Strobilanthes s.l. (Acanthaceae): Evidence from ITS nrDNA, trnL-F cpDNA, and morphology. Am. J. Bot. 2004, 91, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Wood, J. Notes relating to the flora of Bhutan: XXIX. Acanthaceae, with special reference to Strobilanthes. Edinb. J. Bot. 1994, 51, 175–273. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.; Wang, Q.; Wu, W.; Zhou, Q.; Li, D.; Xu, Z.; Fu, L.; Zhu, J.; Karimi-Maleh, H.; Lin, C.-T. Electrochemical Fingerprint Biosensor for Natural Indigo Dye Yielding Plants Analysis. Biosensors 2021, 11, 155. https://doi.org/10.3390/bios11050155
Fan B, Wang Q, Wu W, Zhou Q, Li D, Xu Z, Fu L, Zhu J, Karimi-Maleh H, Lin C-T. Electrochemical Fingerprint Biosensor for Natural Indigo Dye Yielding Plants Analysis. Biosensors. 2021; 11(5):155. https://doi.org/10.3390/bios11050155
Chicago/Turabian StyleFan, Boyuan, Qiong Wang, Weihong Wu, Qinwei Zhou, Dongling Li, Zenglai Xu, Li Fu, Jiangwei Zhu, Hassan Karimi-Maleh, and Cheng-Te Lin. 2021. "Electrochemical Fingerprint Biosensor for Natural Indigo Dye Yielding Plants Analysis" Biosensors 11, no. 5: 155. https://doi.org/10.3390/bios11050155
APA StyleFan, B., Wang, Q., Wu, W., Zhou, Q., Li, D., Xu, Z., Fu, L., Zhu, J., Karimi-Maleh, H., & Lin, C.-T. (2021). Electrochemical Fingerprint Biosensor for Natural Indigo Dye Yielding Plants Analysis. Biosensors, 11(5), 155. https://doi.org/10.3390/bios11050155







