EMG-Centered Multisensory Based Technologies for Pattern Recognition in Rehabilitation: State of the Art and Challenges
Abstract
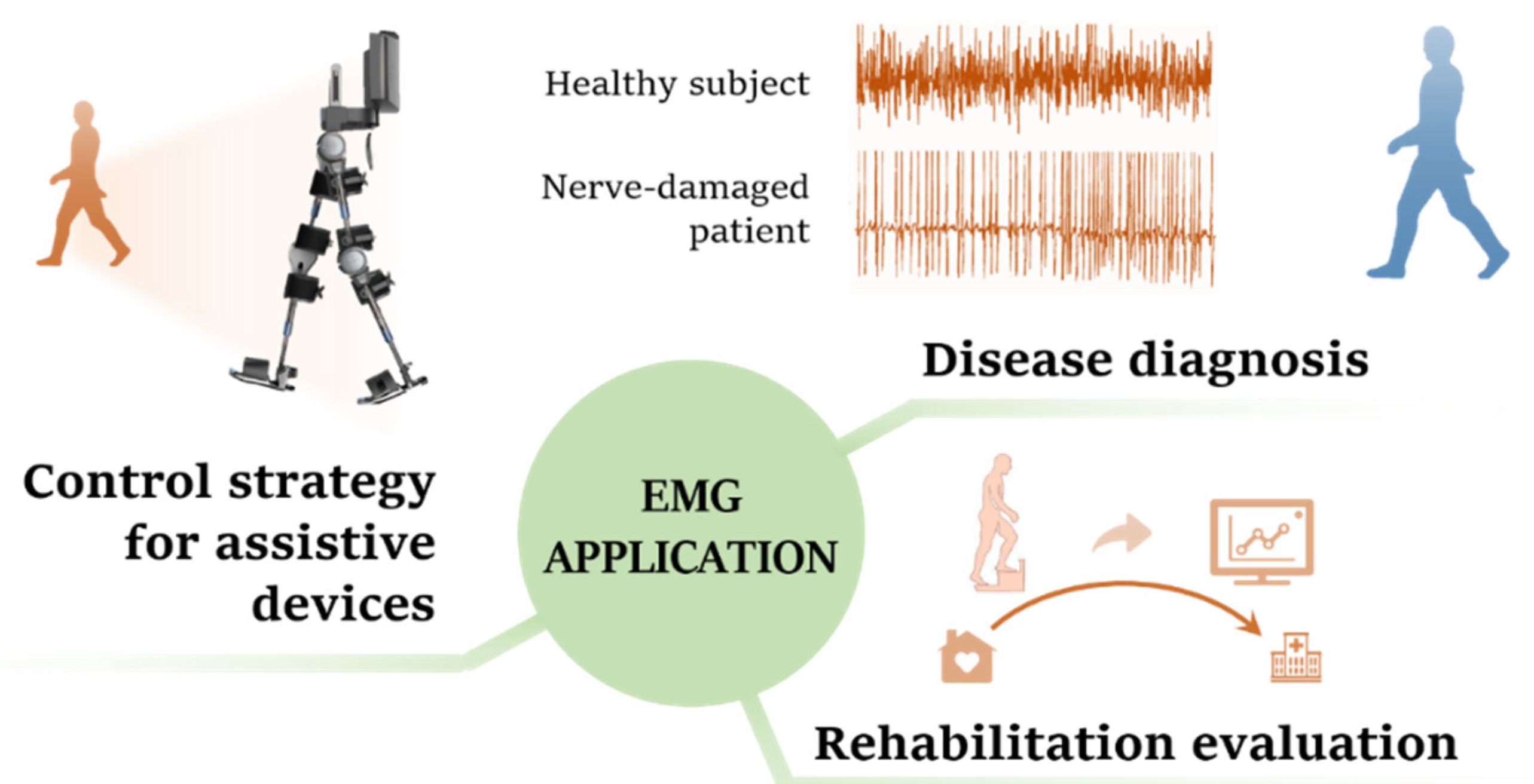
1. Introduction
2. Physiology Background
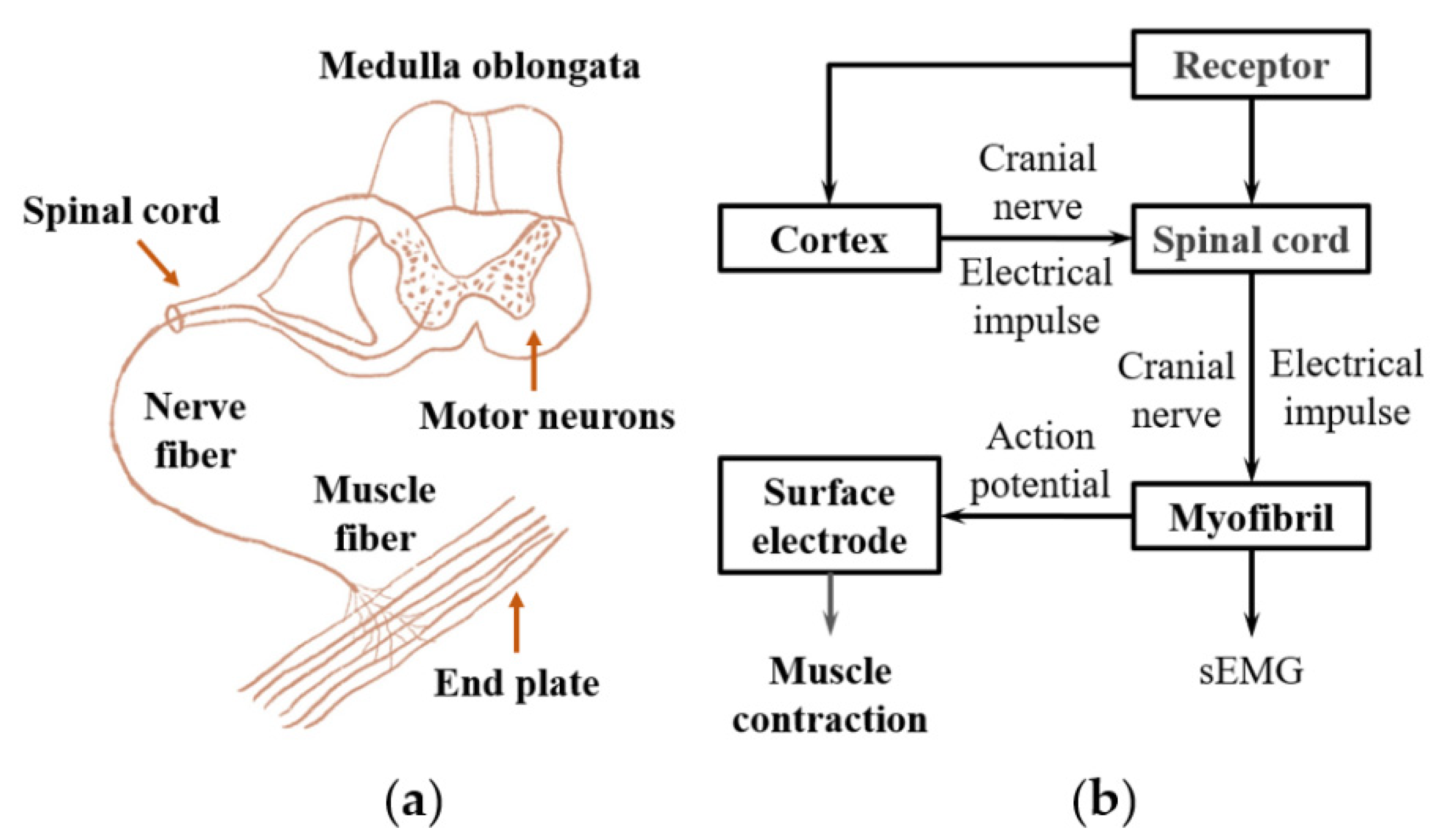
2.1. EMG Signal Overview
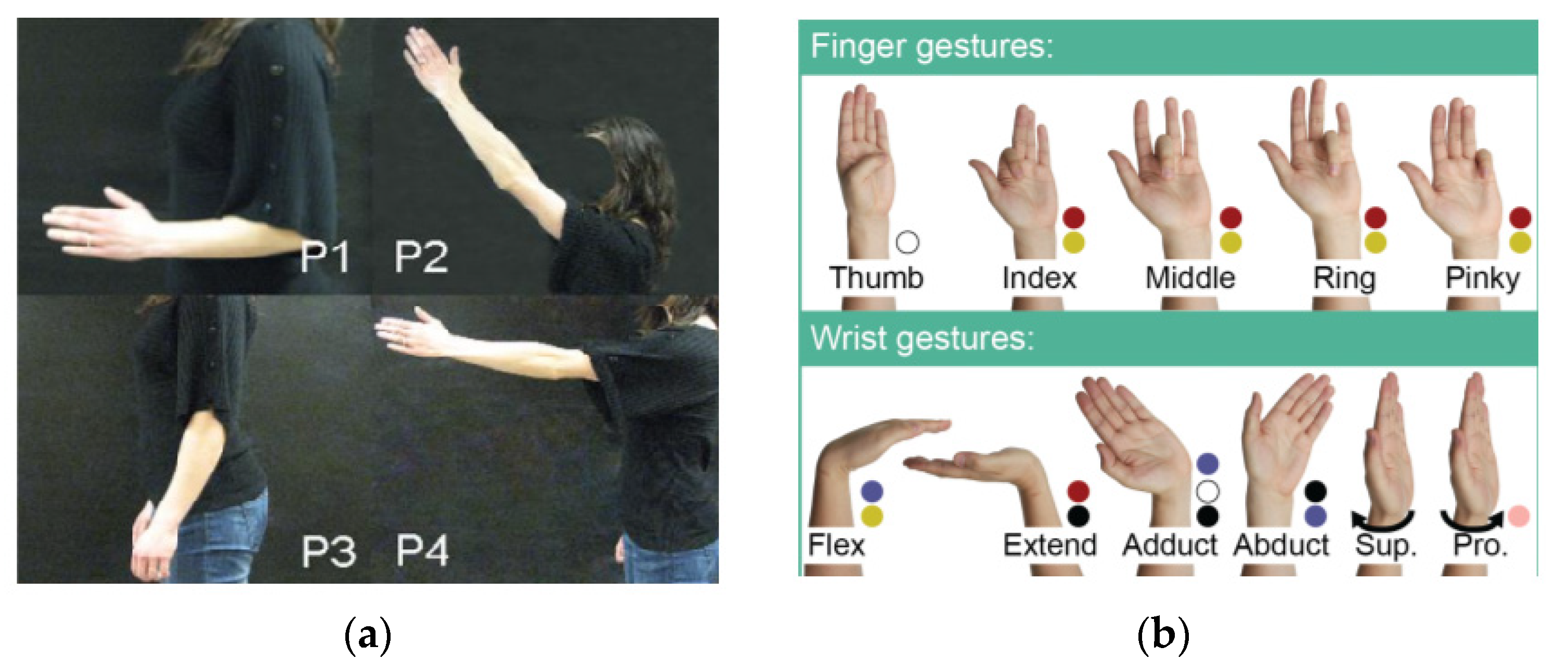
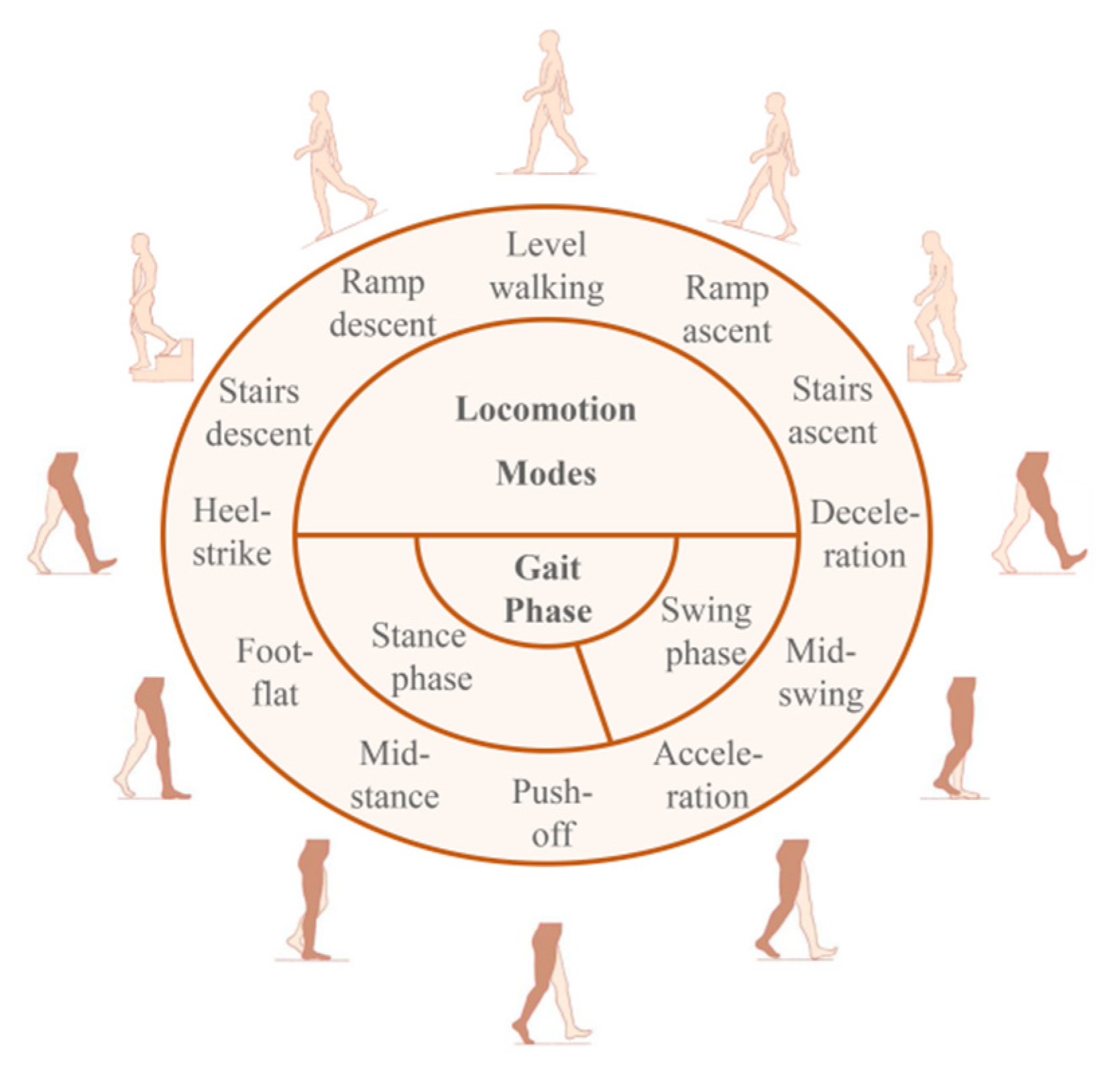
2.2. Human Movement Patterns
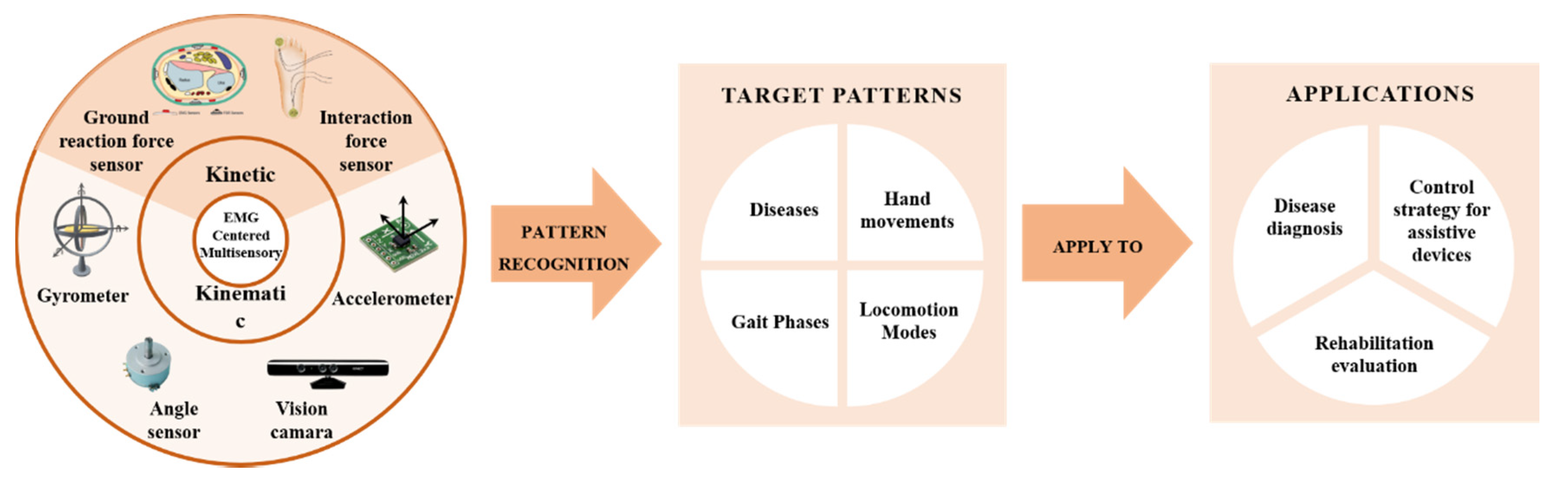
3. EMG Pattern Recognition Pipeline
- Data acquisition
- Signal preprocessing
- Feature extraction and reduction
- Classification
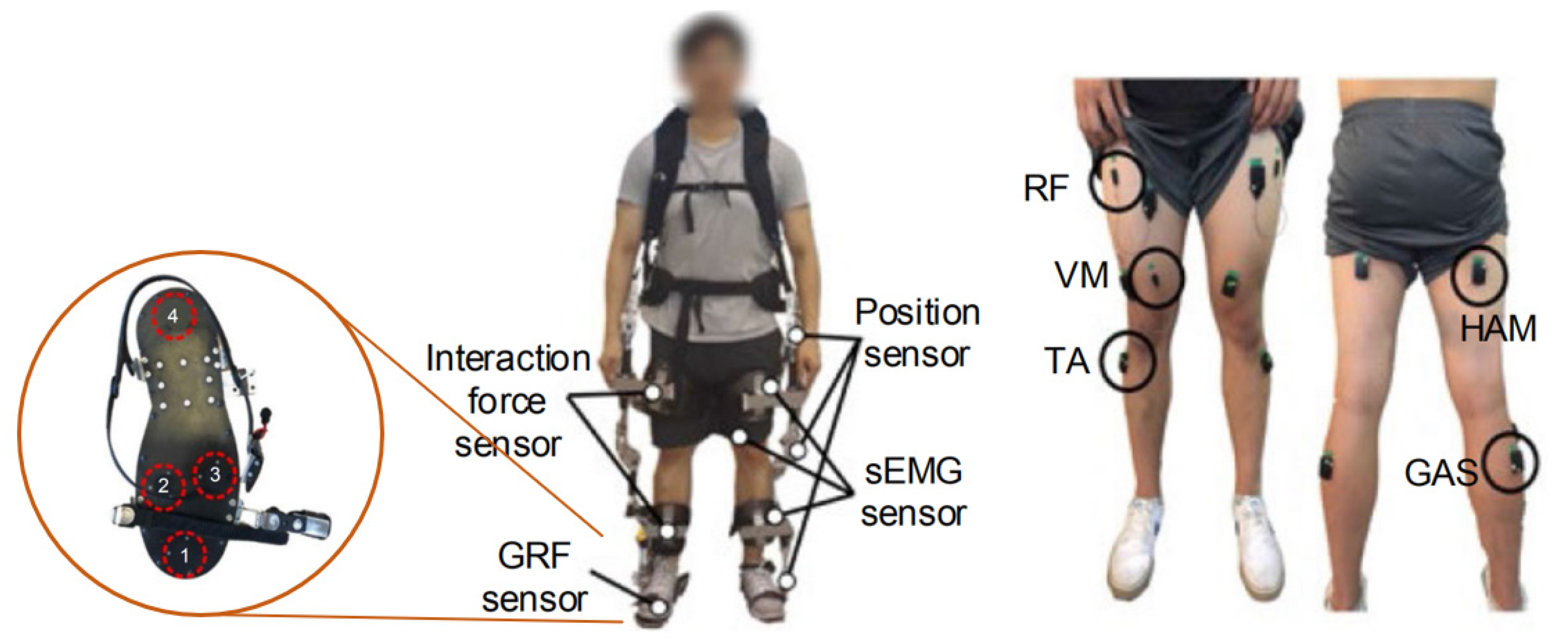
3.1. Data Acquisition
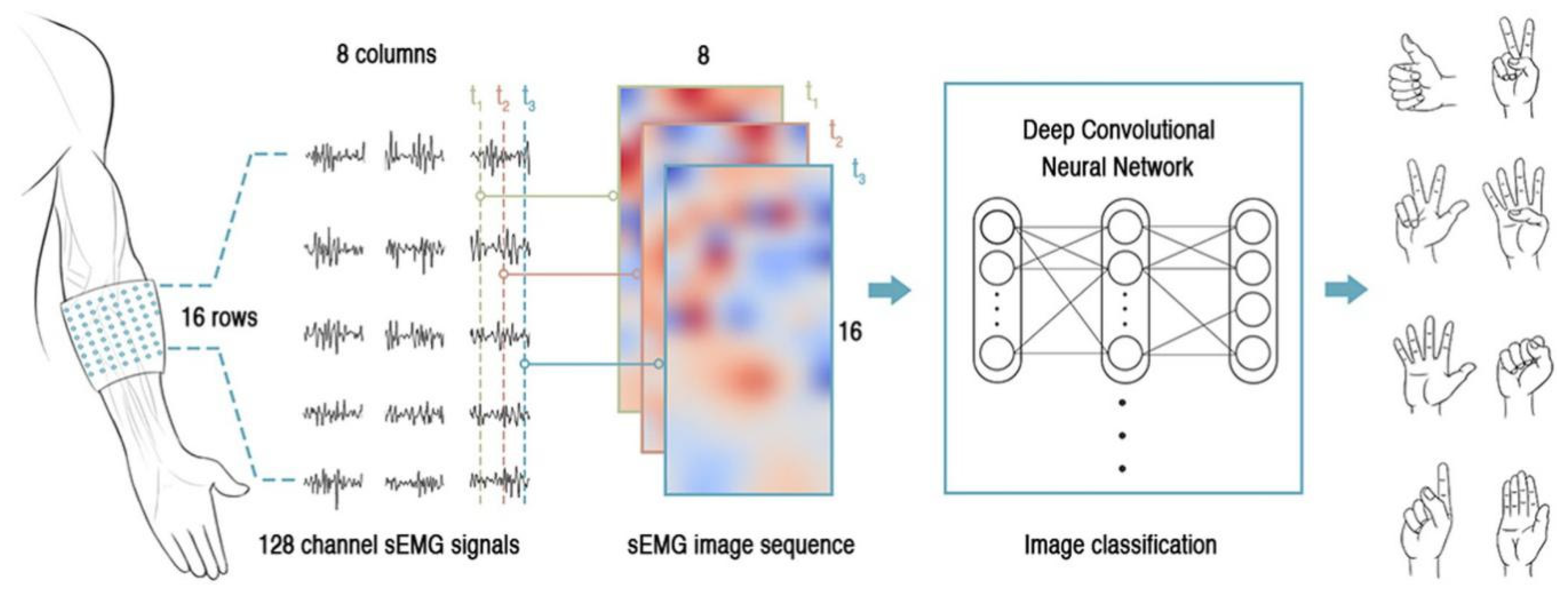
3.1.1. EMG Sensing System
3.1.2. Muscle Site Selection
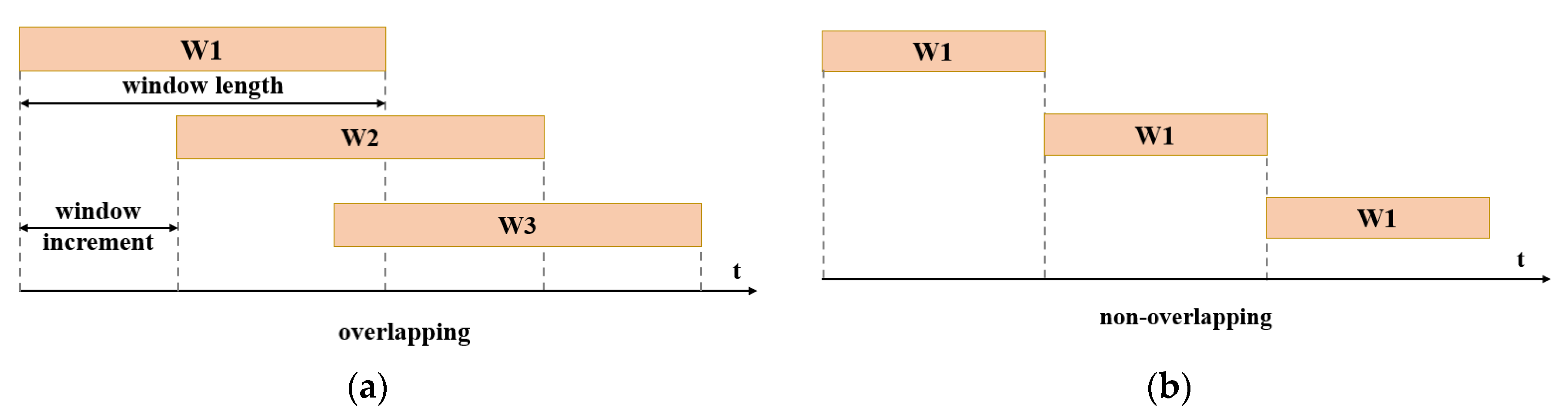
3.2. Signal Preprocessing
3.3. Feature Extraction
3.4. Dimensionality Reduction
3.4.1. Feature Projection
3.4.2. Feature Selection
3.5. Classification Algorithms
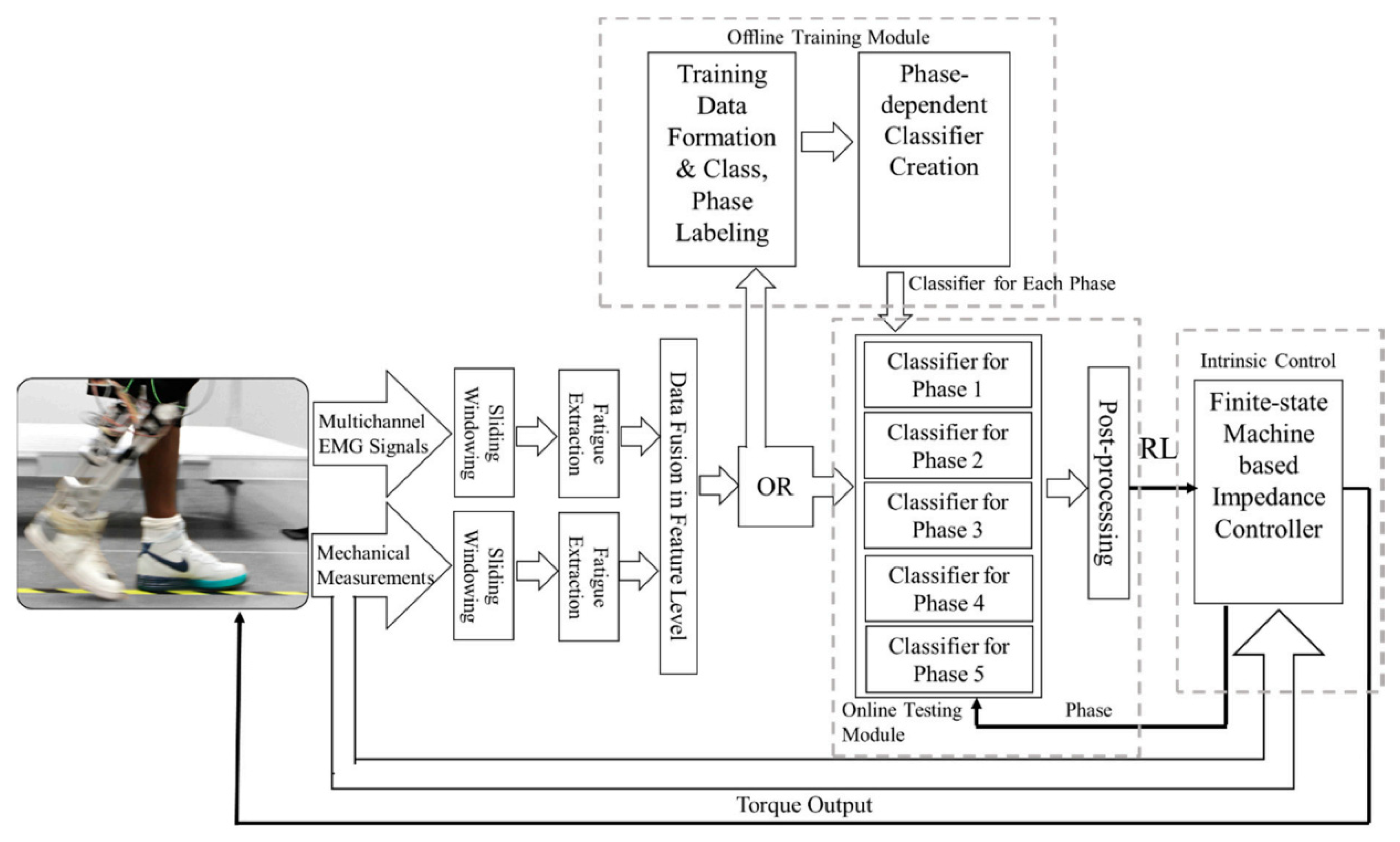
4. Multisensory Fusion
4.1. Fusion with Kinematic Sensors
4.2. Fusion with Kinetic Sensors
4.3. Fusion with Both Kinematics and Kinetic Sensors
5. Challenges and Future Development
5.1. Low Data Quality
5.2. Inadequate and Undisclosed Data
5.3. Discrete Interpretation of Continuous Movements
5.4. Future Analysis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bisio, I.; Delfino, A.; Lavagetto, F.; Sciarrone, A. Enabling IoT for in-home rehabilitation: Accelerometer signals classification methods for activity and movement recognition. IEEE Internet Things J. 2016, 4, 135–146. [Google Scholar] [CrossRef]
- Moore, Z.; Sifferman, C.; Tullis, S.; Ma, M.; Proffitt, R.; Skubic, M. Depth Sensor-Based In-Home Daily Activity Recognition and Assessment System for Stroke Rehabilitation. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 1051–1056. [Google Scholar]
- Nazmi, N.; Rahman, M.A.A.; Yamamoto, S.I.; Ahmad, S.A. Walking gait event detection based on electromyography signals using artificial neural network. Biomed. Signal. Process. Control. 2019, 47, 334–343. [Google Scholar] [CrossRef]
- Joshi, D.; Hahn, M.E. Terrain and Direction Classification of Locomotion Transitions Using Neuromuscular and Mechanical Input. Ann. Biomed. Eng. 2016, 44, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait Analysis Using Wearable Sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, U.; Mahmood, I.; Dehghani-Sanij, A.A. Simultaneous Bayesian recognition of locomotion and gait phases with wearable sensors. IEEE Sens. J. 2017, 18, 1282–1290. [Google Scholar] [CrossRef]
- Young, A.J.; Ferris, D.P. State of the Art and Future Directions for Lower Limb Robotic Exoskeletons. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 171–182. [Google Scholar] [CrossRef]
- Long, Y.; Du, Z.-J.; Wang, W.-D.; Zhao, G.-Y.; Xu, G.-Q.; He, L.; Mao, X.-W.; Dong, W. PSO-SVM-based online locomotion mode identification for rehabilitation robotic exoskeletons. Sensors 2016, 16, 1408. [Google Scholar] [CrossRef]
- Kang, I.; Kunapuli, P.; Hsu, H.; Young, A.J. Electromyography (EMG) Signal Contributions in Speed and Slope Estimation Using Robotic Exoskeletons. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 548–553. [Google Scholar]
- Ziegier, J.; Gattringer, H.; Mueller, A. Classification of gait phases based on bilateral emg data using support vector machines. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 978–983. [Google Scholar]
- Gui, K.; Liu, H.; Zhang, D. A Practical and Adaptive Method to Achieve EMG-Based Torque Estimation for a Robotic Exoskeleton. IEEE Asme Trans. Mechatron. 2019, 24, 483–494. [Google Scholar] [CrossRef]
- Setiawan, J.D.; Ariyanto, M.; Munadi, M.; Mutoha, M.; Glowacz, A.; Caesarendra, W. Grasp Posture Control of Wearable Extra Robotic Fingers with Flex Sensors Based on Neural Network. Electronics 2020, 9, 905. [Google Scholar] [CrossRef]
- Kańtoch, E. Recognition of sedentary behavior by machine learning analysis of wearable sensors during activities of daily living for telemedical assessment of cardiovascular risk. Sensors 2018, 18, 3219. [Google Scholar] [CrossRef]
- Jin, D.; Yang, J.; Zhang, R.; Wang, R.; Zhang, J. Terrain identification for prosthetic knees based on electromyographic signal features. Tsinghua Sci. Technol. 2006, 11, 74–79. [Google Scholar] [CrossRef]
- Young, A.; Kuiken, T.; Hargrove, L. Analysis of using EMG and mechanical sensors to enhance intent recognition in powered lower limb prostheses. J. Neural Eng. 2014, 11, 056021. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Y.; Yin, Y.H. Improving the Transparency of an Exoskeleton Knee Joint Based on the Understanding of Motor Intent Using Energy Kernel Method of EMG. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Kilicarslan, A.; Prasad, S.; Grossman, R.G.; Contreras-Vidal, J.L. High accuracy decoding of user intentions using EEG to control a lower-body exoskeleton. Proceedings of THE 2013 35th annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5606–5609. [Google Scholar]
- Scheme, E.; Englehart, K. Electromyogram pattern recognition for control of powered upper-limb prostheses: State of the art and challenges for clinical use. J. Rehabil. Res. Dev. 2011, 48, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Samuel, O.W.; Asogbon, M.G.; Geng, Y.; Al-Timemy, A.H.; Pirbhulal, S.; Ji, N.; Chen, S.; Fang, P.; Li, G. Intelligent EMG pattern recognition control method for upper-limb multifunctional prostheses: Advances, current challenges, and future prospects. IEEE Access 2019, 7, 10150–10165. [Google Scholar] [CrossRef]
- Kawamoto, H.; Taal, S.; Niniss, H.; Hayashi, T.; Kamibayashi, K.; Eguchi, K.; Sankai, Y. Voluntary Motion Support Control of Robot Suit HAL Triggered by Bioelectrical Signal for Hemiplegia. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 31 August–4 September 2010; IEEE: New York, NY, USA, 2010; pp. 462–466. [Google Scholar] [CrossRef]
- Kawamoto, H.; Lee, S.; Kanbe, S.; Sankai, Y. Power assist method for HAL-3 using EMG-based feedback controller. In Proceedings of the 2003 IEEE International Conference on Systems, Man and Cybernetics, Washington, DC, USA, 5–8 October 2003; pp. 1648–1653. [Google Scholar]
- Solomonow, M.; Baratta, R.; Bernardi, M.; Zhou, B.; Lu, Y.; Zhu, M.; Acierno, S. Surface and wire EMG crosstalk in neighbouring muscles. J. Electromyogr. Kinesiol. 1994, 4, 131–142. [Google Scholar] [CrossRef]
- Al-Timemy, A.H.; Khushaba, R.N.; Bugmann, G.; Escudero, J. Improving the performance against force variation of EMG controlled multifunctional upper-limb prostheses for transradial amputees. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 650–661. [Google Scholar] [CrossRef]
- Sadikoglu, F.; Kavalcioglu, C.; Dagman, B. Electromyogram (EMG) signal detection, classification of EMG signals and diagnosis of neuropathy muscle disease. Procedia Comput. Sci. 2017, 120, 422–429. [Google Scholar] [CrossRef]
- Scheme, E.; Fougner, A.; Stavdahl, Ø.; Chan, A.D.; Englehart, K. Examining the adverse effects of limb position on pattern recognition based myoelectric control. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6337–6340. [Google Scholar]
- Arozi, M.; Caesarendra, W.; Ariyanto, M.; Munadi, M.; Setiawan, J.D.; Glowacz, A. Pattern Recognition of Single-Channel sEMG Signal Using PCA and ANN Method to Classify Nine Hand Movements. Symmetry 2020, 12, 541. [Google Scholar] [CrossRef]
- McIntosh, J.; McNeill, C.; Fraser, M.; Kerber, F.; Löchtefeld, M.; Krüger, A. EMPress: Practical hand gesture classification with wrist-mounted EMG and pressure sensing. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems, San Jose, CA, USA, 7–12 May 2016; pp. 2332–2342. [Google Scholar]
- Shultz, A.H.; Goldfarb, M. A Unified Controller for Walking on Even and Uneven Terrain With a Powered Ankle Prosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 788–797. [Google Scholar] [CrossRef]
- Huang, H.; Kuiken, T.A.; Lipschutz, R.D. A strategy for identifying locomotion modes using surface electromyography. IEEE Trans. Biomed. Eng. 2008, 56, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gavin, J.P.; Immins, T.; Wainwright, T. Stair negotiation as a rehabilitation intervention for enhancing recovery following total hip and knee replacement surgery. Int. J. Orthop. Trauma Nurs. 2017, 25, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, S.M.; Kankaanpää, M.; Meigal, A.; Tarvainen, M.P.; Nuutinen, J.; Tarkka, I.M.; Airaksinen, O.; Karjalainen, P.A. Surface EMG and acceleration signals in Parkinson’s disease: Feature extraction and cluster analysis. Med. Biol. Eng. Comput. 2008, 46, 849–858. [Google Scholar] [CrossRef]
- Jenkins, M.; Almeida, Q.; Spaulding, S.; Van Oostveen, R.; Holmes, J.; Johnson, A.M.; Perry, S. Plantar cutaneous sensory stimulation improves single-limb support time, and EMG activation patterns among individuals with Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, H.; Li, G.; Zhu, X. A multichannel surface EMG system for hand motion recognition. Int. J. Hum. Robot. 2015, 12, 1550011. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Mikawa, M.; Tanaka, K. Real-time hand motion estimation using EMG signals with support vector machines. In Proceedings of the 2006 SICE-ICASE International Joint Conference, Busan, Korea, 18–21 October 2006; pp. 593–598. [Google Scholar]
- Ming, L.; Fan, Z.; Helen, H.H. An Adaptive Classification Strategy for Reliable Locomotion Mode Recognition. Sensors 2017, 17, 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Wang, Q.N.; Wang, L. Adaptive Slope Walking With a Robotic Transtibial Prosthesis Based on Volitional EMG Control. IEEE Asme Trans. Mechatron. 2015, 20, 2146–2157. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Roy, S.; Sarkar, A.D. Active Exoskeleton Hand Exerciser Using Multisensory Feed Back. Int. J. Ind. Eng. Manag. 2017, 2, 33–36. [Google Scholar] [CrossRef]
- Nazmi, N.; Rahman, A.; Azizi, M.; Yamamoto, S.-I.; Ahmad, S.A.; Zamzuri, H.; Mazlan, S.A. A review of classification techniques of EMG signals during isotonic and isometric contractions. Sensors 2016, 16, 1304. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Stegeman, D. Biophysics of the generation of EMG signals. Electromyogr. Physiol. Eng. Noninvasive Appl. 2004, 4, 81–105. [Google Scholar]
- Ibrahim, A.; Gannapathy, V.; Chong, L.; Isa, I. Analysis of electromyography (EMG) signal for human arm muscle: A review. In Advanced Computer and Communication Engineering Technology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 567–575. [Google Scholar]
- David, A.W. The Biomechanics and Motor Control of Human Gait; University of Waterloo Press: Waterloo, ON, Canada, 1988. [Google Scholar]
- Huang, H.; Zhang, F.; Hargrove, L.J.; Dou, Z.; Rogers, D.R.; Englehart, K.B. Continuous locomotion-mode identification for prosthetic legs based on neuromuscular–mechanical fusion. IEEE Trans. Biomed. Eng. 2011, 58, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, Y.; Fang, C.; Xu, L. A Smart Terrain Identification Technique Based on Electromyography, Ground Reaction Force, and Machine Learning for Lower Limb Rehabilitation. Appl. Sci. 2020, 10, 2638. [Google Scholar] [CrossRef]
- Lay, A.N.; Hass, C.J.; Nichols, T.R.; Gregor, R.J. The effects of sloped surfaces on locomotion: An electromyographic analysis. J. Biomech. 2007, 40, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.; Schwameder, H. Effect of sloped walking on lower limb muscle forces. Gait Posture 2016, 47, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Y.; Chen, N.; Song, R.; Li, L. Effect of different terrains on onset timing, duration and amplitude of tibialis anterior activation. Biomed. Signal. Process. Control. 2015, 19, 115–121. [Google Scholar] [CrossRef]
- Li, B.; Gui, Q.; Ali, H.B.; Li, H.; Jin, Z. A wearable sit-to-stand detection system based on angle tracking and lower limb EMG. In Proceedings of the 2016 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 3 December 2016; pp. 1–6. [Google Scholar]
- Pappas, I.P.; Popovic, M.R.; Keller, T.; Dietz, V.; Morari, M. A reliable gait phase detection system. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 113–125. [Google Scholar] [CrossRef]
- Perry, J.; Davids, J.R. Gait analysis: Normal and pathological function. J. Pediatr. Orthop. 1992, 12, 815. [Google Scholar] [CrossRef]
- Jung, J.Y.; Heo, W.; Yang, H.; Park, H. A Neural Network-Based Gait Phase Classification Method using Sensors Equipped on Lower Limb Exoskeleton Robots. Sensors 2015, 15, 27738–27759. [Google Scholar] [CrossRef]
- Abdulhay, E.; Arunkumar, N.; Narasimhan, K.; Vellaiappan, E.; Venkatraman, V. Gait and tremor investigation using machine learning techniques for the diagnosis of Parkinson disease. Future Gener. Comput. Syst. 2018, 83, 366–373. [Google Scholar] [CrossRef]
- Simão, M.; Mendes, N.; Gibaru, O.; Neto, P. A review on electromyography decoding and pattern recognition for human-machine interaction. IEEE Access 2019, 7, 39564–39582. [Google Scholar] [CrossRef]
- Smith, L.H.; Hargrove, L.J. Comparison of surface and intramuscular EMG pattern recognition for simultaneous wrist/hand motion classification. In Proceedings of the 2013 35th annual international conference of the IEEE engineering in medicine and biology society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4223–4226. [Google Scholar]
- Smith, L.H.; Kuiken, T.A.; Hargrove, L.J. Real-time simultaneous and proportional myoelectric control using intramuscular EMG. J. Neural Eng. 2014, 11, 066013. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.T.; Brown, J.S.; Price, T.R.; Queen, S.A.; Mikhailenok, E.L. Comparison of surface electromyography and myotonometric measurements during voluntary isometric contractions. J. Electromyogr. Kinesiol. 2004, 14, 709–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samadani, A.-A.; Kulic, D. Hand gesture recognition based on surface electromyography. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4196–4199. [Google Scholar]
- Du, Y.-C.; Lin, C.-H.; Shyu, L.-Y.; Chen, T. Portable hand motion classifier for multi-channel surface electromyography recognition using grey relational analysis. Expert Syst. Appl. 2010, 37, 4283–4291. [Google Scholar] [CrossRef]
- Farrell, T.R. A comparison of the effects of electrode implantation and targeting on pattern classification accuracy for prosthesis control. IEEE Trans. Biomed. Eng. 2008, 55, 2198–2211. [Google Scholar] [CrossRef]
- Martelloni, C.; Carpaneto, J.; Micera, S. Classification of upper arm EMG signals during object-specific grasp. In Proceedings of the Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 21–24 August 2008; pp. 5061–5064. [Google Scholar]
- Fang, C.; Wang, Y.; Gao, S. Flexible and Wearable GRF and EMG Sensors Enabled Locomotion Mode Recognition for IoHT Based In-Home Rehabilitation. Available online: https://www.techrxiv.org/articles/Flexible_and_Wearable_GRF_and_EMG_Sensors_Enabled_Locomotion_Mode_Recognition_for_IoHT_Based_In-home_Rehabilitation/11987994/1 (accessed on 21 March 2020).
- Li, L.; Ogden, L.L. Muscular activity characteristics associated with preparation for gait transition. J. Sport Health Sci. 2012, 1, 27–35. [Google Scholar] [CrossRef]
- Yousif, A.E.; Aziz, M.Y. Biomechanical analysis of the human femur bone during going upstairs and sitting down. In Proceedings of the 2012 First National Conference for Engineering Sciences (FNCES 2012), Baghdad, Iraq, 7–8 November 2012; pp. 1–7. [Google Scholar]
- Pati, S.; Joshi, D.; Mishra, A. Locomotion classification using EMG signal. In Proceedings of the 2010 International Conference on Information and Emerging Technologies, Karachi, Pakistan, 14–16 June 2010; pp. 1–6. [Google Scholar]
- Chiu, M.-C.; Wang, M.-J. The effect of gait speed and gender on perceived exertion, muscle activity, joint motion of lower extremity, ground reaction force and heart rate during normal walking. Gait Posture 2007, 25, 385–392. [Google Scholar] [CrossRef]
- Komi, P.V.; Tesch, P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur. J. Appl. Physiol. Occup. Physiol. 1979, 42, 41–50. [Google Scholar] [CrossRef]
- Larsson, B.; Månsson, B.; Karlberg, C.; Syvertsson, P.; Elert, J.; Gerdle, B. Reproducibility of surface EMG variables and peak torque during three sets of ten dynamic contractions. J. Electromyogr. Kinesiol. 1999, 9, 351–357. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Bronlund, J.E. Surface EMG signal amplification and filtering. Int. J. Comput. Appl. 2013, 82, 15–22. [Google Scholar] [CrossRef]
- Barzilay, O.; Wolf, A. A fast implementation for EMG signal linear envelope computation. J. Electromyogr. Kinesiol. 2011, 21, 678–682. [Google Scholar] [CrossRef]
- Dutta, A.; Khattar, B.; Banerjee, A. Nonlinear Analysis of Electromyogram Following Neuromuscular Electrical Stimulation-Assisted Gait Training in Stroke Survivors. In Converging Clinical and Engineering Research on Neurorehabilitation; Springer: Berlin/Heidelberg, Germany, 2013; pp. 53–57. [Google Scholar]
- Barsakcioglu, D.Y.; Farina, D. A real-time surface emg decomposition system for non-invasive human-machine interfaces. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OI, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Hudgins, B.; Parker, P.; Scott, R.N. A new strategy for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 1993, 40, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Englehart, K.; Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Zardoshti-Kermani, M.; Wheeler, B.C.; Badie, K.; Hashemi, R.M. EMG feature evaluation for movement control of upper extremity prostheses. IEEE Trans. Rehabil. Eng. 1995, 3, 324–333. [Google Scholar] [CrossRef]
- Englehart, K.; Hugdins, B.; Parker, P. Multifunction Control of Prostheses Using the Myoelectric Signal; CRC Press: New York, NY, USA, 2000; pp. 153–208. [Google Scholar]
- Afzal, T.; Iqbal, K.; White, G.; Wright, A.B. A method for locomotion mode identification using muscle synergies. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 25, 608–617. [Google Scholar] [CrossRef]
- Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. Feature reduction and selection for EMG signal classification. Expert Syst. Appl. 2012, 39, 7420–7431. [Google Scholar] [CrossRef]
- Englehart, K.; Hudgins, B.; Parker, P.A.; Stevenson, M. Classification of the myoelectric signal using time-frequency based representations. Med. Eng. Phys. 1999, 21, 431–438. [Google Scholar] [CrossRef]
- Samuel, O.W.; Zhou, H.; Li, X.; Wang, H.; Zhang, H.; Sangaiah, A.K.; Li, G. Pattern recognition of electromyography signals based on novel time domain features for amputees’ limb motion classification. Comput. Electr. Eng. 2018, 67, 646–655. [Google Scholar] [CrossRef]
- Hargrove, L.J.; Li, G.; Englehart, K.B.; Hudgins, B.S. Principal components analysis preprocessing for improved classification accuracies in pattern-recognition-based myoelectric control. IEEE Trans. Biomed. Eng. 2008, 56, 1407–1414. [Google Scholar] [CrossRef]
- Naik, G.R.; Nguyen, H.T. Nonnegative matrix factorization for the identification of EMG finger movements: Evaluation using matrix analysis. IEEE J. Biomed. Health Inform. 2014, 19, 478–485. [Google Scholar] [CrossRef]
- Naik, G.R.; Kumar, D.K.; Palaniswami, M. Surface EMG based hand gesture identification using semi blind ICA: Validation of ICA matrix analysis. Electromyogr. Clin. Neurophysiol. 2008, 48, 169–180. [Google Scholar]
- Gallant, P.; Morin, E.; Peppard, L. Feature-based classification of myoelectric signals using artificial neural networks. Med. Biol. Eng. Comput. 1998, 36, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.R.; Selvan, S.E.; Gobbo, M.; Acharyya, A.; Nguyen, H.T. Principal component analysis applied to surface electromyography: A comprehensive review. IEEE Access 2016, 4, 4025–4037. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, P. A novel framework based on FastICA for high density surface EMG decomposition. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Seung, H.S. Algorithms for non-negative matrix factorization. In Proceedings of the Advances in neural information processing systems, Vancouver, BC, Canada, 3–8 December 2001; pp. 556–562. [Google Scholar]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: Emerging avenues and challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, P. A novel myoelectric pattern recognition strategy for hand function restoration after incomplete cervical spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 21, 96–103. [Google Scholar] [CrossRef]
- Zhou, P.; Lowery, M.M.; Englehart, K.B.; Huang, H.; Li, G.; Hargrove, L.; Dewald, J.P.; Kuiken, T.A. Decoding a new neural–machine interface for control of artificial limbs. J. Neurophysiol. 2007, 98, 2974–2982. [Google Scholar] [CrossRef]
- Nazarpour, K.; Sharafat, A.; Firoozabadi, S. Surface EMG signal classification using a selective mix of higher order statistics. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 1–4 September 2005; pp. 4208–4211. [Google Scholar]
- Chowdhury, R.H.; Reaz, M.B.I.; Ali, M.A.B.; Bakar, A.A.A.; Chellappan, K.; Chang, T.G. Surface Electromyography Signal Processing and Classification Techniques. Sensors 2013, 13, 12431–12466. [Google Scholar] [CrossRef]
- Al-Angari, H.M.; Kanitz, G.; Tarantino, S.; Cipriani, C. Distance and mutual information methods for EMG feature and channel subset selection for classification of hand movements. Biomed. Signal. Process. Control. 2016, 27, 24–31. [Google Scholar] [CrossRef]
- Boostani, R.; Moradi, M.H. Evaluation of the forearm EMG signal features for the control of a prosthetic hand. Physiol. Meas. 2003, 24, 309. [Google Scholar] [CrossRef]
- Too, J.; Abdullah, A.R.; Mohd Saad, N.; Tee, W. Emg feature selection and classification using a pbest-guide binary particle swarm optimization. Computation 2019, 7, 12. [Google Scholar] [CrossRef]
- Phinyomark, A.; Hirunviriya, S.; Limsakul, C.; Phukpattaranont, P. Evaluation of EMG feature extraction for hand movement recognition based on Euclidean distance and standard deviation. In Proceedings of the ECTI-CON2010: The 2010 ECTI International Confernce on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Chiang Mai, Thailand, 19–21 May 2010; pp. 856–860. [Google Scholar]
- Huang, H.; Xie, H.-B.; Guo, J.-Y.; Chen, H.-J. Ant colony optimization-based feature selection method for surface electromyography signals classification. Comput. Biol. Med. 2012, 42, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Li, G.; Zhou, P. EMG feature assessment for myoelectric pattern recognition and channel selection: A study with incomplete spinal cord injury. Med. Eng. Phys. 2014, 36, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Benazzouz, A.; Guilal, R.; Amirouche, F.; Slimane, Z.E.H. EMG Feature Selection for Diagnosis of Neuromuscular Disorders. In Proceedings of the 2019 International Conference on Networking and Advanced Systems (ICNAS), Annaba, Algeria, 26–27 June 2019; pp. 1–5. [Google Scholar]
- Gupta, R.; Agarwal, R. Single channel EMG-based continuous terrain identification with simple classifier for lower limb prosthesis. Biocybern. Biomed. Eng. 2019, 39, 775–788. [Google Scholar] [CrossRef]
- Pires, R.; Falcari, T.; Campo, A.B.; Pulcineli, B.C.; Hamill, J.; Ervilha, U.F. Using a Support Vector Machine Algorithm to Classify Lower-Extremity EMG Signals During Running Shod/Unshod With Different Foot Strike Patterns. J. Appl. Biomech. 2019, 35, 87–90. [Google Scholar] [CrossRef]
- Li, H.-T.; Han, S.-L.; Pan, M.-C. Lower-limb motion classification for hemiparetic patients through IMU and EMG signal processing. In Proceedings of the 2016 International Conference on Biomedical Engineering (BME-HUST), Hanoi, Vietnam, 4–6 October 2016; pp. 113–118. [Google Scholar]
- Mesbah, S.; Gonnelli, F.; El-baz, A.; Angeli, C.; Harkema, S.; Rejc, E. Spectral analysis of lower limb EMG activity in individuals with motor complete SCI during standing with epidural stimulation. In Proceedings of the 2018 IEEE International Symposium on Signal Processing and Information Technology (ISSPIT), Louisville, KY, USA, 6–8 December 2018; pp. 1–5. [Google Scholar]
- Prasad, C.; Balakandan, V.K.; Moorthy, P.; Kochuvila, S. Classification of sEMG Signals for Controlling of a Prosthetic foot using SVM and KNN. In Proceedings of the 2019 International Conference on Intelligent Computing and Control Systems (ICCS), Madurai, India, 15–17 May 2019; pp. 454–458. [Google Scholar]
- Xie, H.-B.; Guo, T.; Bai, S.; Dokos, S. Hybrid soft computing systems for electromyographic signals analysis: A review. Biomed. Eng. Online 2014, 13, 8. [Google Scholar] [CrossRef]
- Meng, M.; Luo, Z.; She, Q.; Ma, Y. Automatic recognition of gait mode from EMG signals of lower limb. In Proceedings of the 2010 The 2nd International Conference on Industrial Mechatronics and Automation, HongKong, China, 30 May 2010; pp. 282–285. [Google Scholar]
- Morbidoni, C.; Principi, L.; Mascia, G.; Strazza, A.; Verdini, F.; Cucchiarelli, A.; Di Nardo, F. Gait phase classification from surface EMG signals using Neural Networks. In Mediterranean Conference on Medical and Biological Engineering and Computing; Springer: Cham, Switzerland, 2019; pp. 75–82. [Google Scholar]
- Lendaro, E.; Mastinu, E.; Håkansson, B.; Ortiz-Catalan, M. Real-time classification of non-weight bearing lower-limb movements using EMG to facilitate phantom motor execution: Engineering and case study application on phantom limb pain. Front. Neurol. 2017, 8, 470. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Yang, M.; Chen, S. PSO-SVM-based gait phase classification during human walking on unstructured terrains: Application in lower-limb exoskeleton. Proc. Inst. Mech. Eng. Part. C J. Mech. Eng. Sci. 2019, 233, 7144–7154. [Google Scholar] [CrossRef]
- Bellingegni, A.D.; Gruppioni, E.; Colazzo, G.; Davalli, A.; Sacchetti, R.; Guglielmelli, E.; Zollo, L. NLR, MLP, SVM, and LDA: A comparative analysis on EMG data from people with trans-radial amputation. J. Neuroeng. Rehabil. 2017, 14, 82. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Cheng, Y.; Xi, N. Surface EMG based continuous estimation of human lower limb joint angles by using deep belief networks. Biomed. Signal. Process. Control. 2018, 40, 335–342. [Google Scholar] [CrossRef]
- Afzal, T.; White, G.; Wright, A.B.; Iqbal, K. Locomotion mode identification for lower limbs using neuromuscular and joint kinematic signals. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 27–31 August 2014; pp. 4071–4074. [Google Scholar]
- Blana, D.; Kyriacou, T.; Lambrecht, J.M.; Chadwick, E.K. Feasibility of using combined EMG and kinematic signals for prosthesis control: A simulation study using a virtual reality environment. J. Electromyogr. Kinesiol. 2016, 29, 21–27. [Google Scholar] [CrossRef]
- Kundu, A.S.; Mazumder, O.; Lenka, P.K.; Bhaumik, S. Hand gesture recognition based omnidirectional wheelchair control using IMU and EMG sensors. J. Intell. Robot. Syst. 2018, 91, 529–541. [Google Scholar] [CrossRef]
- Ceolini, E.; Taverni, G.; Khacef, L.; Payvand, M.; Donati, E. Sensor fusion using EMG and vision for hand gesture classification in mobile applications. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; pp. 1–4. [Google Scholar]
- Pedotti, A.; Assente, R.; Fusi, G.; De Rossi, D.; Dario, P.; Domenici, C. Multisensor piezoelectric polymer insole for pedobarography. Ferroelectrics 1984, 60, 163–174. [Google Scholar] [CrossRef]
- Hidler, J. Robotic-assessment of walking in individuals with gait disorders. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–4 September 2004; pp. 4829–4831. [Google Scholar]
- Riener, R.; Rabuffetti, M.; Frigo, C.; Quintern, J.; Schmidt, G. Instrumented staircase for ground reaction measurement. Med. Biol. Eng. Comput. 1999, 37, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Faivre, A.; Dahan, M.; Parratte, B.; Monnier, G. Instrumented shoes for pathological gait assessment. Mech. Res. Commun. 2004, 31, 627–632. [Google Scholar] [CrossRef]
- Lacković, I.; Bilas, V.; Šantić, A.; Nikolić, V. Measurement of gait parameters from free moving subjects. Measurement 2000, 27, 121–131. [Google Scholar] [CrossRef]
- Šantić, A.; Bilas, V.; Lacković, I. A system for measurement forces in feet and crutches during normal na pathological gait. Period. Biol. 2002, 104, 305–310. [Google Scholar]
- Hennig, E.M.; Staats, A.; Rosenbaum, D. Plantar pressure distribution patterns of young school children in comparison to adults. Foot Ankle Int. 1994, 15, 35–40. [Google Scholar] [CrossRef]
- Zadravec, M.; Olenšek, A.; Matjačić, Z. Emulation of hill walking and turning on Balance Assessment Robot: A preliminary study. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 7–12. [Google Scholar]
- KYEONG, S.; SHIN, W.; YANG, M.; HEO, U.; FENG, J.-r.; KIM, J. Recognition of walking environments and gait period by surface electromyography. Front. Inf. Technol. Electron. Eng. 2019, 20, 342–352. [Google Scholar] [CrossRef]
- Galli, M.; Cimolin, V.; Crugnola, V.; Priano, L.; Menegoni, F.; Trotti, C.; Milano, E.; Mauro, A. Gait pattern in myotonic dystrophy (Steinert disease): A kinematic, kinetic and EMG evaluation using 3D gait analysis. J. Neurol. Sci. 2012, 314, 83–87. [Google Scholar] [CrossRef]
- Cipriani, C.; Antfolk, C.; Controzzi, M.; Lundborg, G.; Rosén, B.; Carrozza, M.C.; Sebelius, F. Online myoelectric control of a dexterous hand prosthesis by transradial amputees. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 260–270. [Google Scholar] [CrossRef]
- Young, A.; Hargrove, L. Strategies to reduce myoelectric pattern recognition sensitivity to electrode shift. In Proceedings of the 18th Congress of the International Society of Electrophysiology and Kinesiology, Aalborg, Denmark, 17–19 June 2010; pp. 16–19. [Google Scholar]
- Geng, W.; Du, Y.; Jin, W.; Wei, W.; Hu, Y.; Li, J. Gesture recognition by instantaneous surface EMG images. Sci. Rep. 2016, 6, 36571. [Google Scholar] [CrossRef] [PubMed]
- Amma, C.; Krings, T.; Böer, J.; Schultz, T. Advancing muscle-computer interfaces with high-density electromyography. In Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems, Seoul, Korea, 18–23 April 2015; pp. 929–938. [Google Scholar]
- Winter, D.A.; Yack, H. EMG profiles during normal human walking: Stride-to-stride and inter-subject variability. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Phinyomark, A.; Scheme, E. EMG pattern recognition in the era of big data and deep learning. Big Data Cogn. Comput. 2018, 2, 21. [Google Scholar] [CrossRef]
- Gorgolewski, K.; Margulies, D.S.; Milham, M.P. Making data sharing count: A publication-based solution. Front. Neurosci. 2013, 7, 9. [Google Scholar] [CrossRef]
- Atzori, M.; Gijsberts, A.; Heynen, S.; Hager, A.-G.M.; Deriaz, O.; Van Der Smagt, P.; Castellini, C.; Caputo, B.; Müller, H. Building the Ninapro database: A resource for the biorobotics community. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 1258–1265. [Google Scholar]
- Young, A.J.; Simon, A.M.; Hargrove, L.J. A Training Method for Locomotion Mode Prediction Using Powered Lower Limb Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 671–677. [Google Scholar] [CrossRef]
- Martinez-Hernandez, U.; Rubio-Solis, A.; Panoutsos, G.; Dehghani-Sanij, A.A. A combined adaptive neuro-fuzzy and bayesian strategy for recognition and prediction of gait events using wearable sensors. In Proceedings of the 2017 IEEE International Conference on Fuzzy Systems (FUZZ-IEEE), Naples, Italy, 9–12 July 2017; pp. 1–6. [Google Scholar]
- Blana, D.; Van Den Bogert, A.J.; Murray, W.M.; Ganguly, A.; Krasoulis, A.; Nazarpour, K.; Chadwick, E.K. Model-based control of individual finger movements for prosthetic hand function. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 612–620. [Google Scholar] [CrossRef]

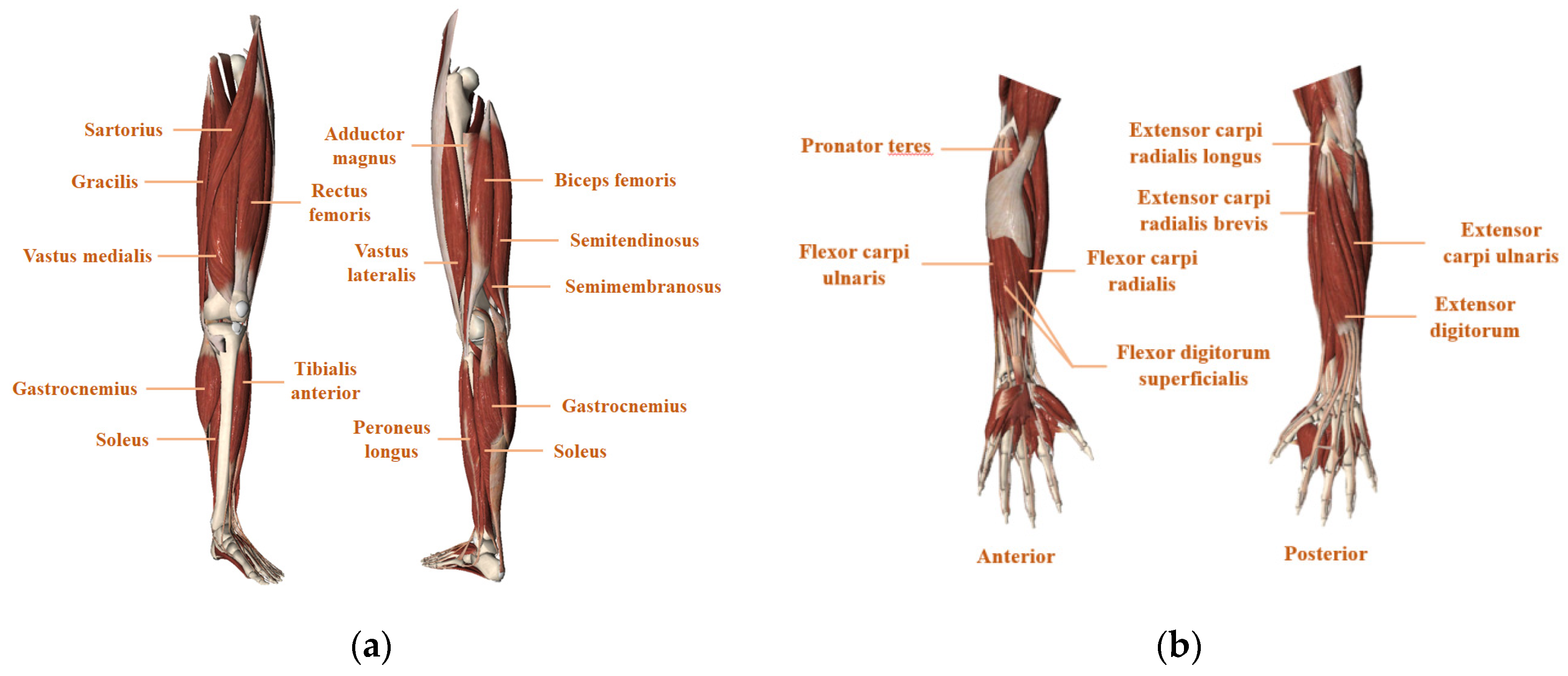
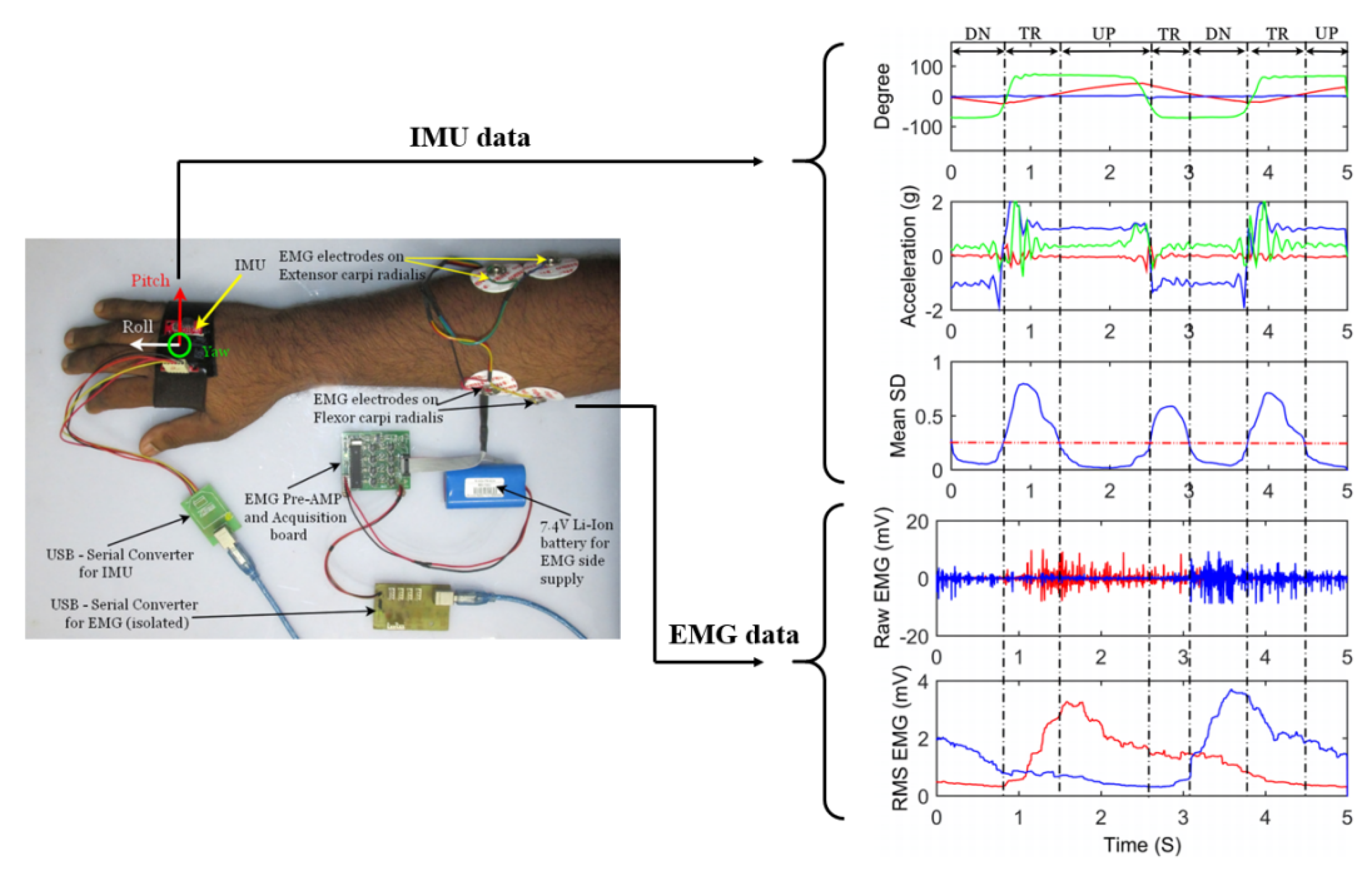
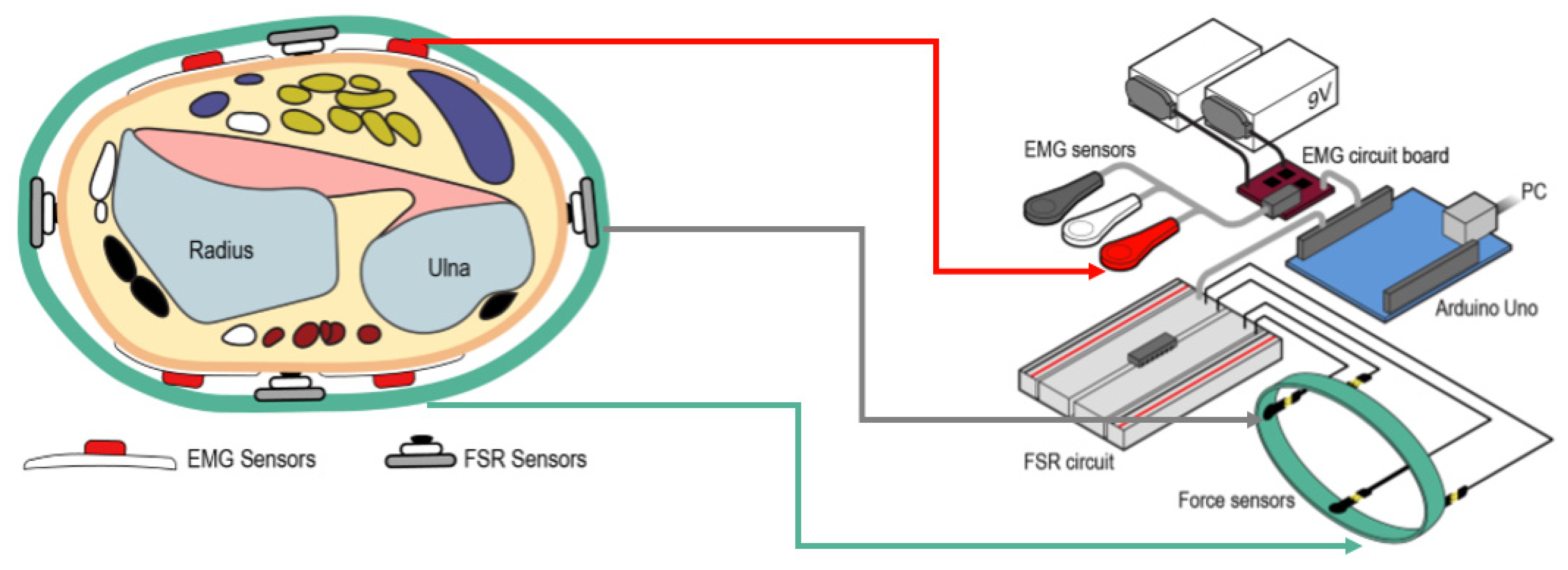
| No. | Proximal Muscle | Distal Muscle | Comments |
|---|---|---|---|
| [42] | SAR, RF, VL, VM, GRA, BFL, SEM, BFS, ADM | / | Gluteal muscles (gluteus maximus and gluteus medius) on the amputated side and the thigh muscles of the residual limb were monitored |
| [35] | RF, VL, VM, BFL, SEM, BTS, TFL | / | The accurate electrodes locations are adjusted according to the able-bodied subjects and transfemoral subjects |
| [29] | SAR, RF, VL, VM, GRA, BFL, SEM, BFS, ADM | / | It should be noted that the locations of EMG electrodes on the distal muscles were approximate |
| [60] | / | TA, SL | Although only two muscles are selected, the classification accuracy is still satisfying |
| [59] | TPA, DPA, PMC, BCL, TBL, FCR, ECR | / | One of the eight signal channels is used for the synchronization of data from the Fastrack while the left seven are utilized to collect muscle activities signal. |
| [27] | / | FDS, FDP, EDC, EIP, EMP | These selected muscles are responsible for controlling all fingers except the thumb. |
| [9] | AM, GM, PRF, VL, VM | / | The proximal hip muscle groups have higher rates of the change in EMG activation with regard to different walking speeds while the distal knee extensor muscle groups show higher rates of change for different waling slopes |
| [61] | GM, RF, VL, BFL | TA, GA, SL | Humans often change gait patterns to prevent overexertion and possible injury to the relatively small dorsiflexor muscles, which are walking close to maximum capacity. |
| [63] | RF, VL, SEM | These three thigh muscles are the most commonly used muscles to classify locomotion modes at different speeds. | |
| [64] | BF, RF | MG, TA | To reflect the effect of gait speed and gender on joint motion of lower extremity more comprehensively, bilateral lumbar erectors spinae are also utilized besides the muscles mentioned before. |
| No. | Applied Sensors | Classes | Feature | Classifier | Accuracy |
|---|---|---|---|---|---|
| [35] | EMG + GRF | Five common locomotion modes (W, RA, RD, SA, SD) and eight task transitions: W->SA, W->SD, W->RA, W->RD, SA->W, SD->W, RA->W and RD->W | EMG data feature: MAV, SL, SSC and ZC, mechanical signals: maximum, minimum, mean value and standard deviation | Entropy-based adaptation (EBA), Learning form testing data (LIFT) and Transductive Support Vector Machine (TSVM) | EBA: 95%, LIFT: 95% and TSVM: 96.25%, vanilla SVM: 87.5% |
| [42] | EMG + GRF | Locomotion modes: LW, SO, SA, SD, RA and RD and related transitions: W->sA, W->RA, W->O, SD->W, RD->W, SA/RA->W, W->SD/RD | EMG time-domain feature: MAV, SSC, WL, ZC, Mechanical signal features: maximum, minimum, mean value of each direction of force and moment | SVM | 99% or higher accuracy in the stance phase and 95% accuracy in the swing phase |
| [122] | Position sensors, GRF, interaction force EMG | Five walking environments: LW, RA, RD, SA, and SD Seven gait periods: LS, MST, TST, PS, IS, MS and TS | GRF feature: four positions in the foot for four time periods, position feature: three joint angles for four time periods. Interaction force feature: two points in the link for four time periods, sEMG feature: MAV, ZC, SSC and WL | BLDA | 96.1%(environment classification accuracy) 97.8%(gait period classification period) |
| [27] | EMG sensor, pressure force sensor | Finger gestures, wrist gestures, and other gestures | Root mean square (RMS), standard deviation (SD) and peak amplitude | SVM | 95.8% |
| [112] | IMU, EMG sensor | Six hand gestures (forward, clockwise, left, backward, anticlockwise, right) | Nine IMU features extracted from wrist Euler angle and six EMG features extracted from EMG RMS signal | DSVM | Real-time recognition accuracy 90.5% |
| [124] | EMG signal acquisition system, data glove | Thumb flexion, finger flexion, thumb opposition, middle/ring/little finger flexion, long fingers flexion, tradigital grasp, lateral grip/key grip | MAV (mean of absolute value) | Locally weighted learning | 79% for amputee and 89% for non-disabled participants |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.; He, B.; Wang, Y.; Cao, J.; Gao, S. EMG-Centered Multisensory Based Technologies for Pattern Recognition in Rehabilitation: State of the Art and Challenges. Biosensors 2020, 10, 85. https://doi.org/10.3390/bios10080085
Fang C, He B, Wang Y, Cao J, Gao S. EMG-Centered Multisensory Based Technologies for Pattern Recognition in Rehabilitation: State of the Art and Challenges. Biosensors. 2020; 10(8):85. https://doi.org/10.3390/bios10080085
Chicago/Turabian StyleFang, Chaoming, Bowei He, Yixuan Wang, Jin Cao, and Shuo Gao. 2020. "EMG-Centered Multisensory Based Technologies for Pattern Recognition in Rehabilitation: State of the Art and Challenges" Biosensors 10, no. 8: 85. https://doi.org/10.3390/bios10080085
APA StyleFang, C., He, B., Wang, Y., Cao, J., & Gao, S. (2020). EMG-Centered Multisensory Based Technologies for Pattern Recognition in Rehabilitation: State of the Art and Challenges. Biosensors, 10(8), 85. https://doi.org/10.3390/bios10080085






