AC and DC Differential Bridge Structure Suitable for Electrochemical Interfacial Capacitance Biosensing Applications
Abstract
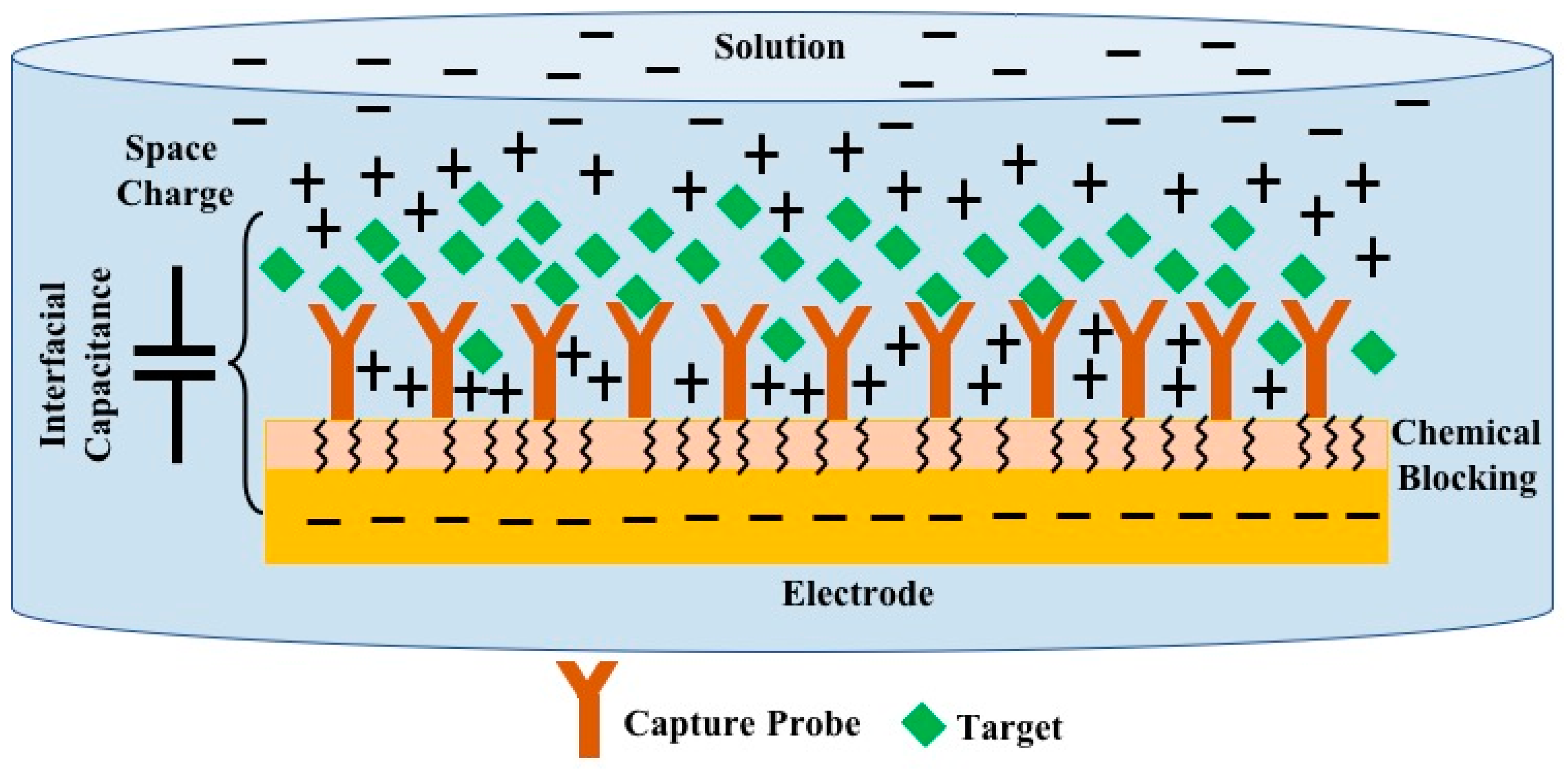
1. Introduction
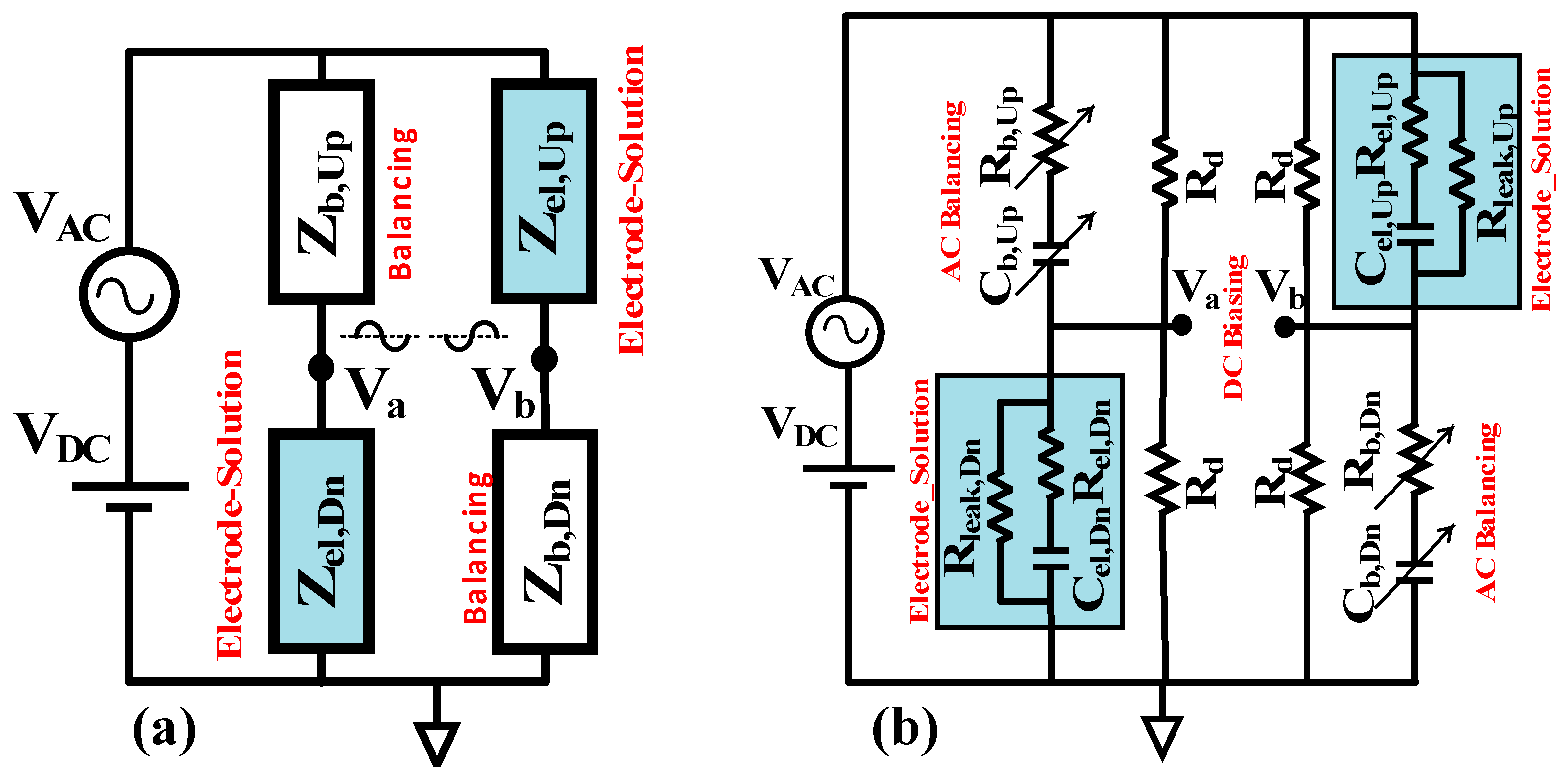
2. Ideal AC/DC Bridge Structure and Analysis
3. Balancing and Mismatch Analysis
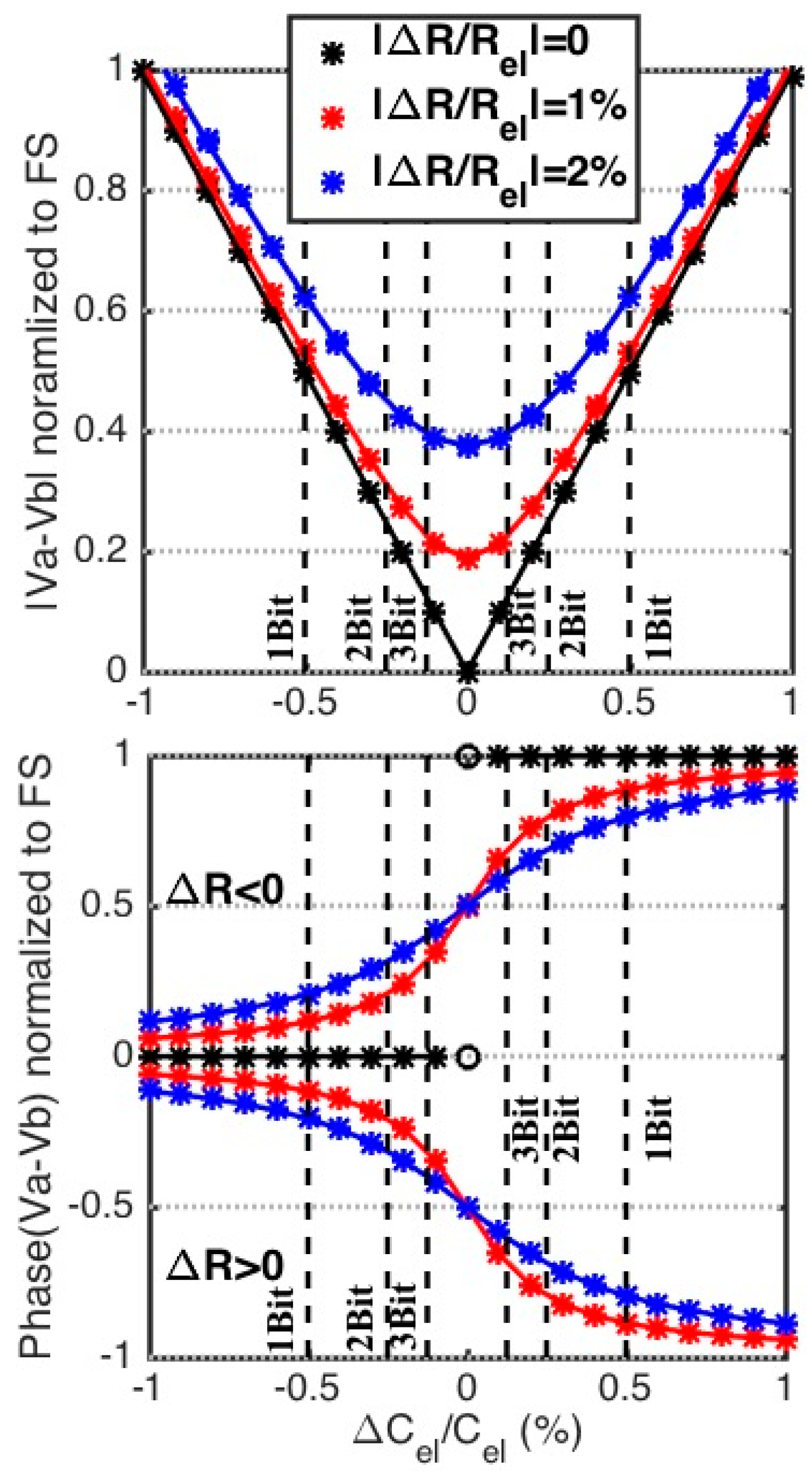
3.1. Capacitive Mismatch
3.2. Resistive Mismatch
3.3. Balancing
3.4. Capacitance Data Extraction
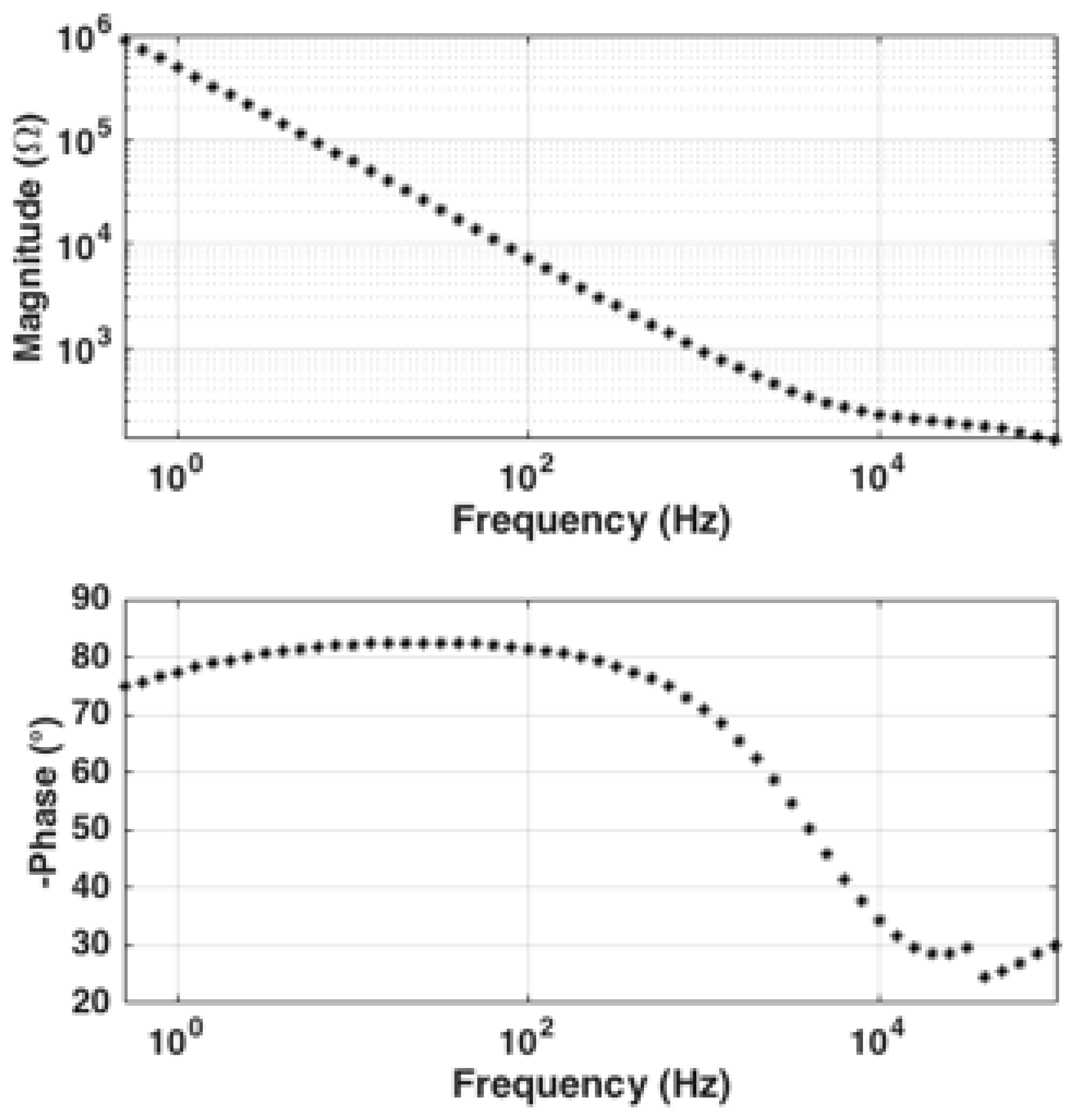
4. Implementation, Setup, and Characterization
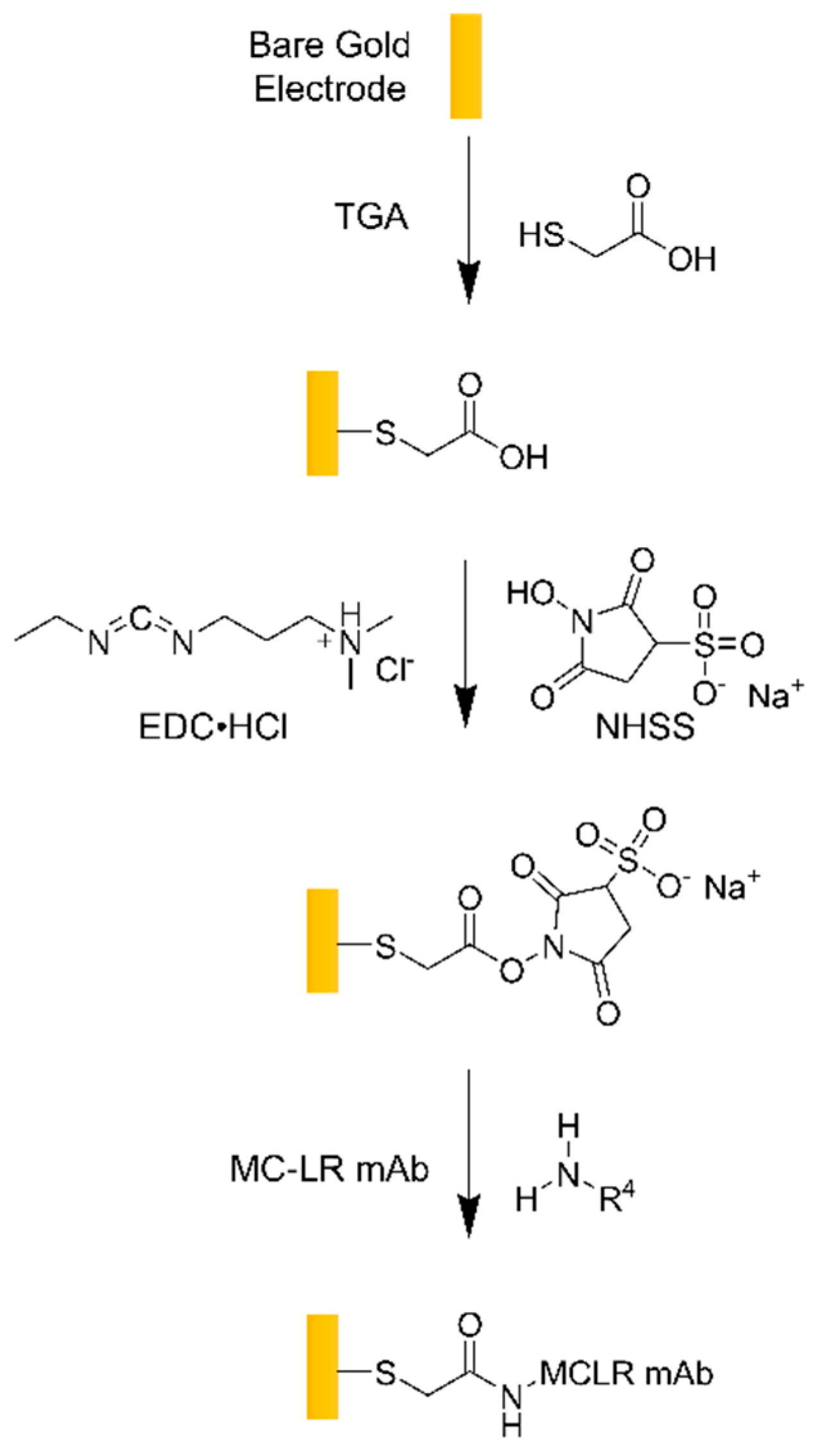
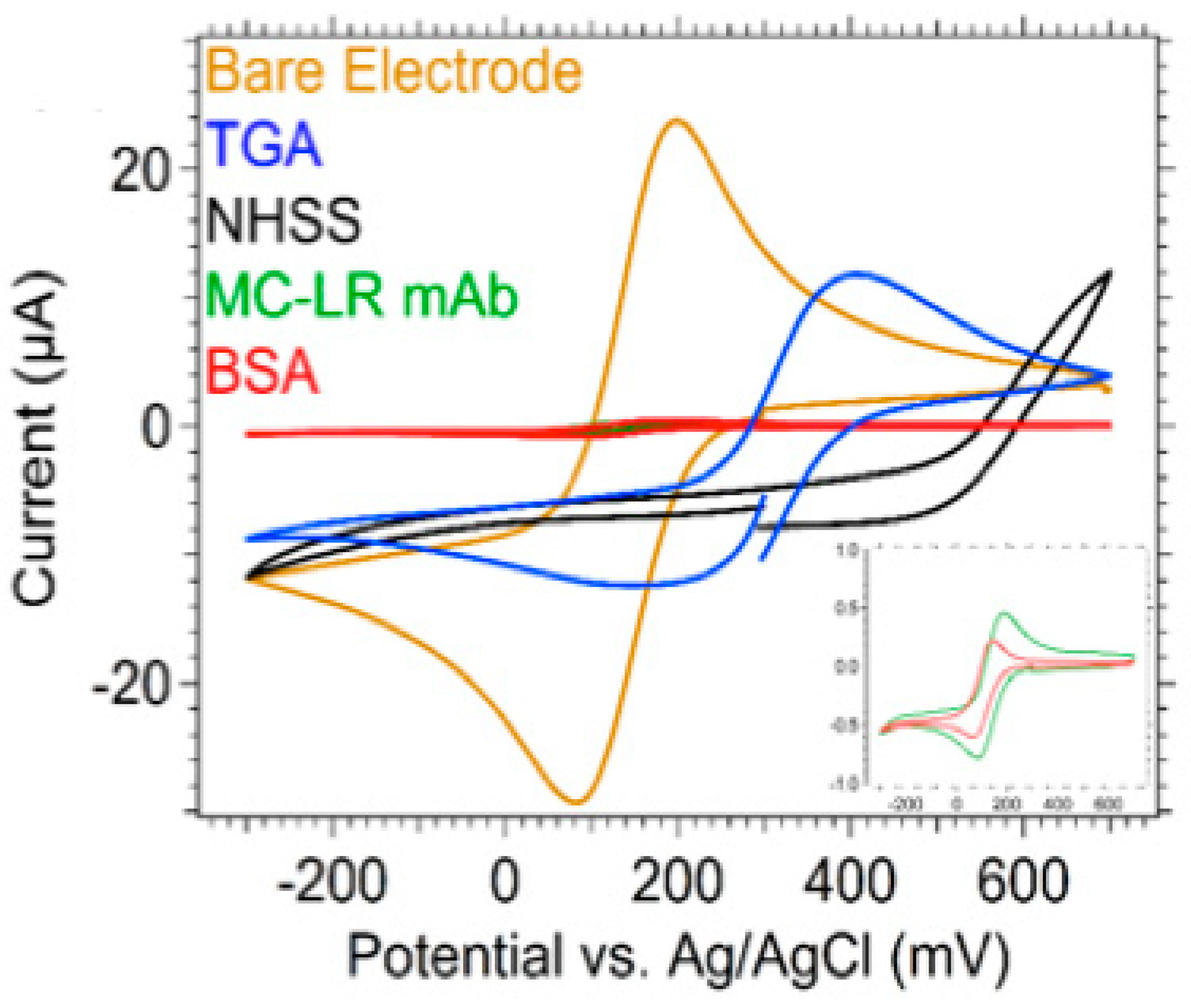
4.1. Transducer Functionalization And Characterization
4.2. Bridge Implementation and Transducer Placement
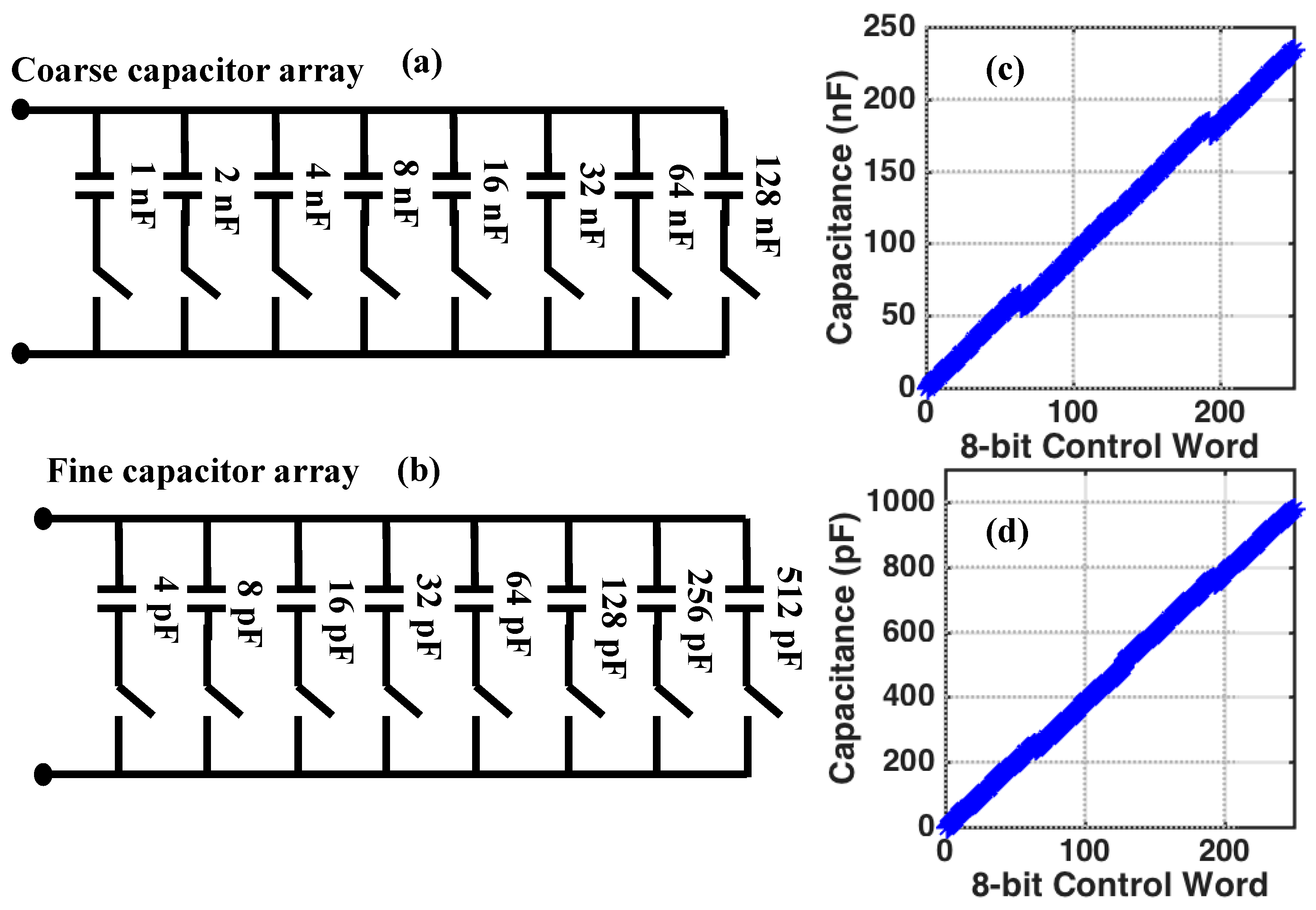
4.2.1. Capacitive Tuning Network
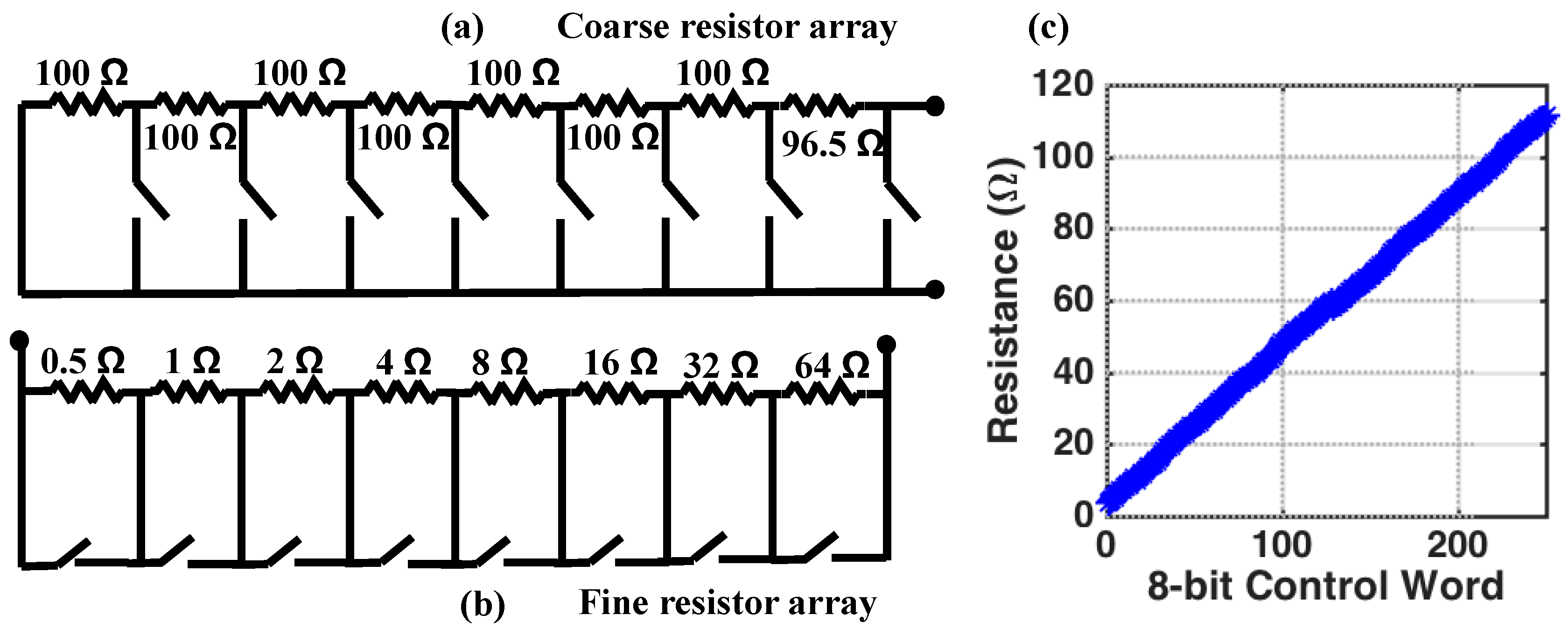
4.2.2. Resistive Tuning Network
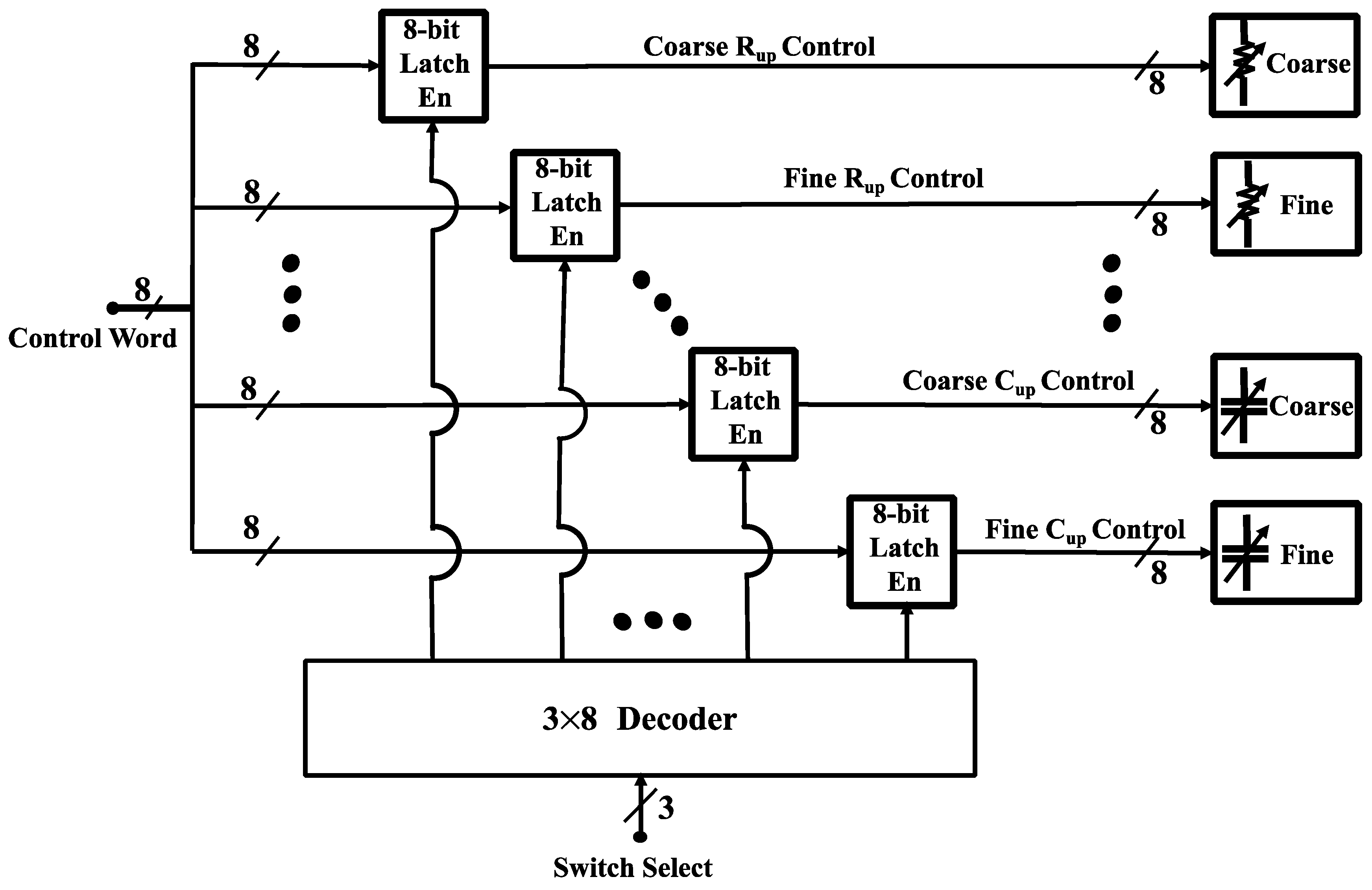
4.2.3. Digital Control Circuitry
5. Experimental Procedure And Real-time Measurement Results
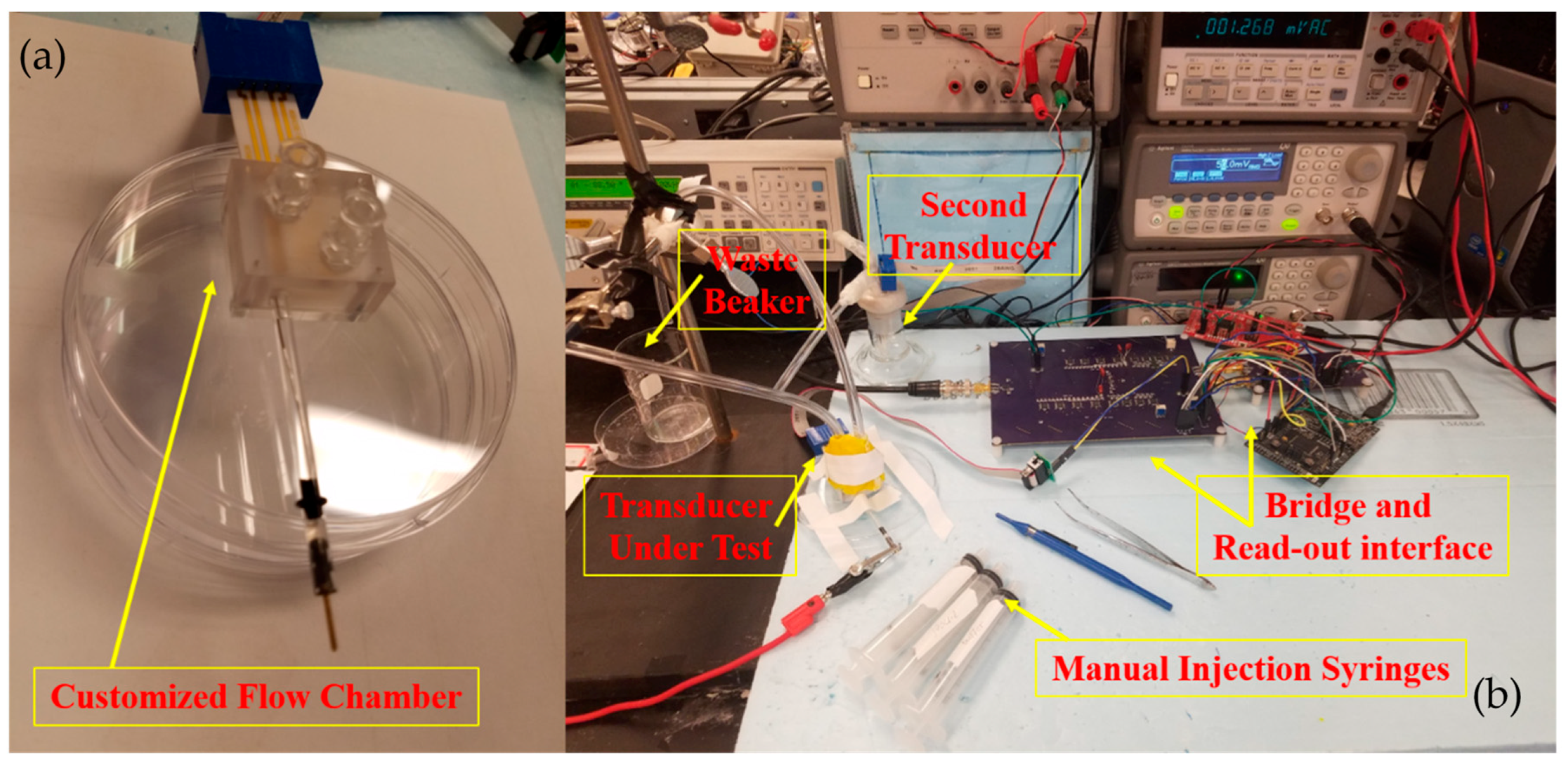
5.1. Experimental Setup
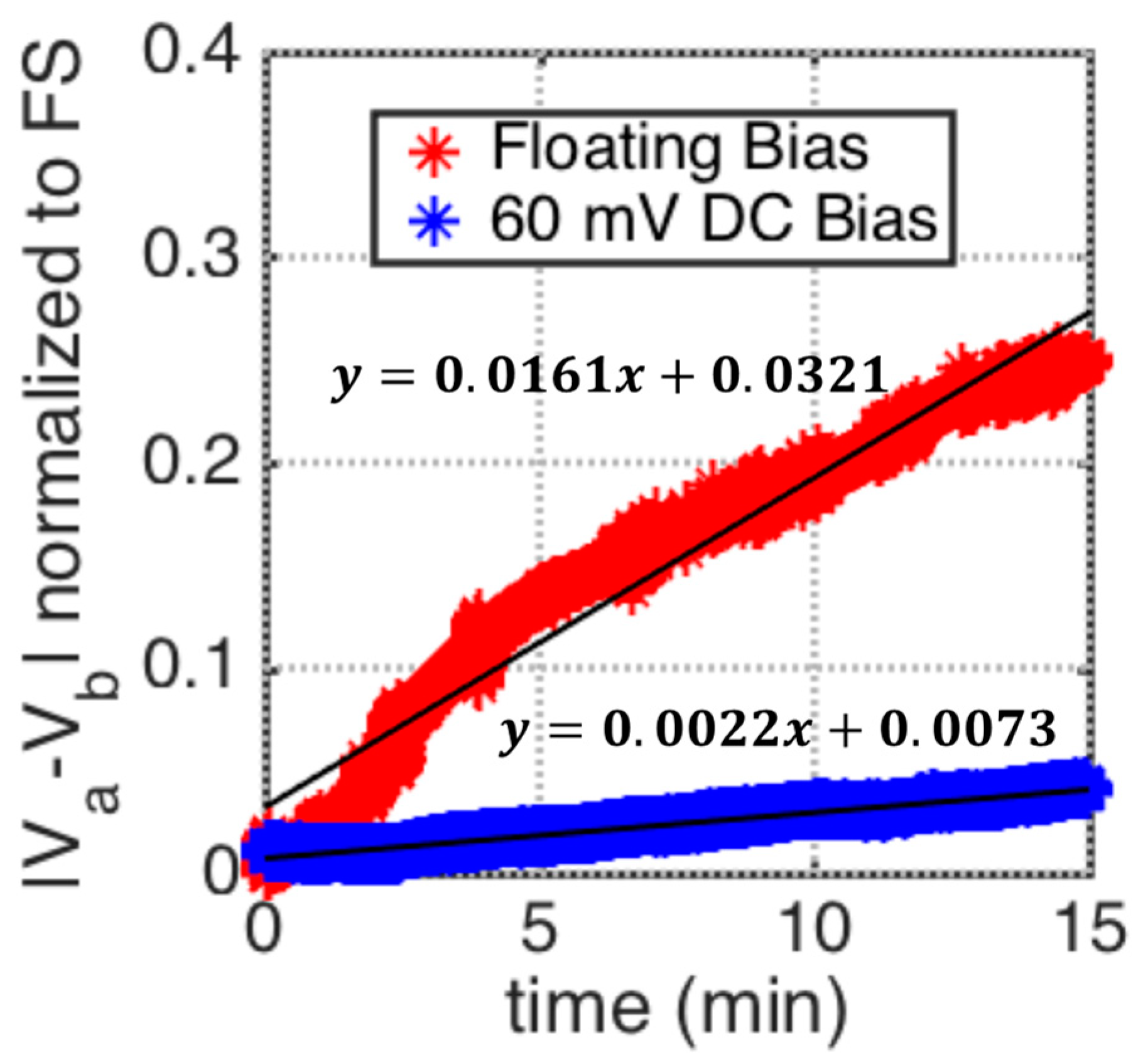
5.2. Drift Rate Control
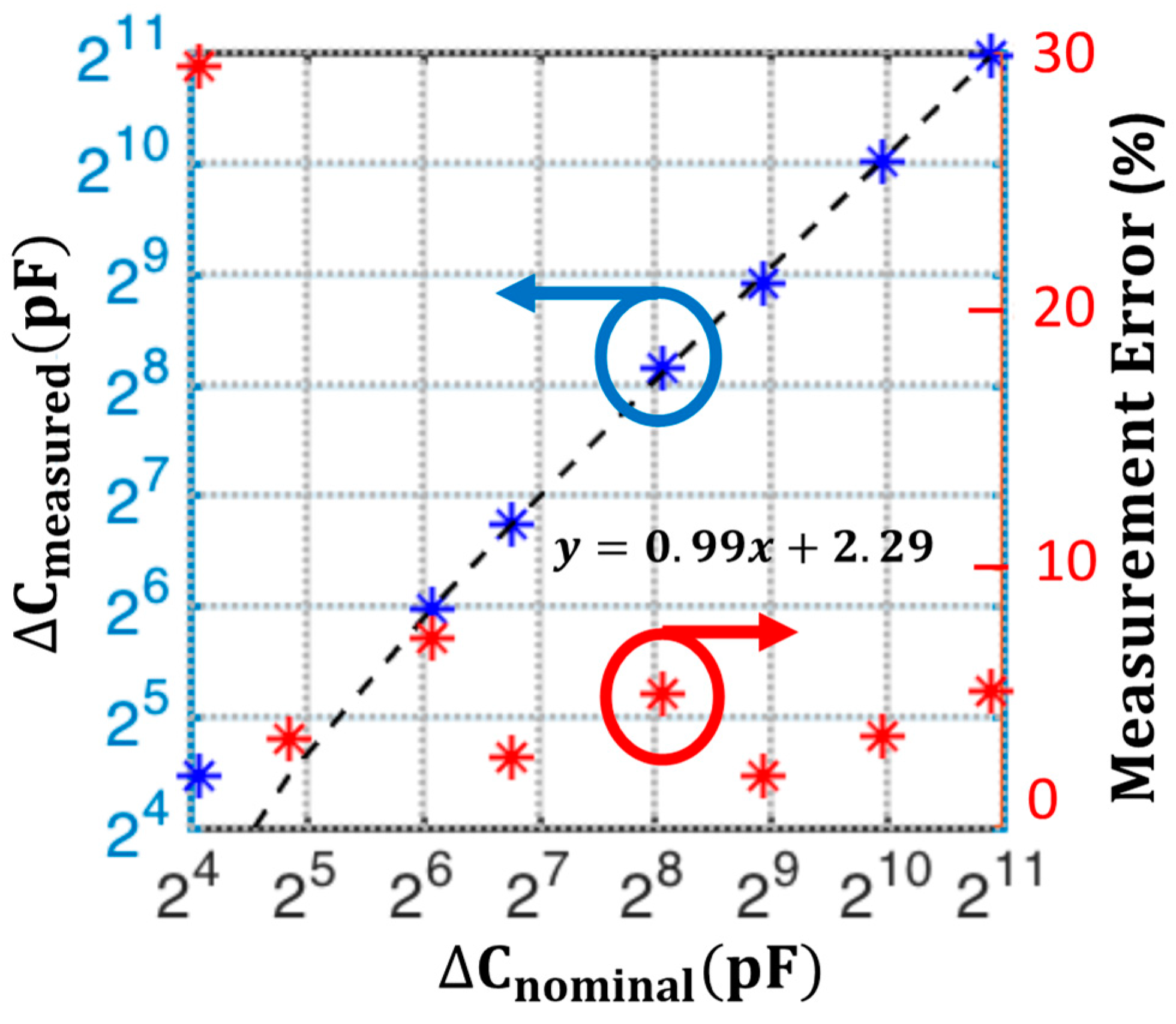
5.3. Sensitivity Characterization
5.4. Real-time Microcystin-(Leucine-Arginine) Measurement
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Kim, Y.M.; Oh, S.W.; Jeong, S.Y.; Pyo, D.J.; Choi, E.Y. Development of an ultrarapid one-step fluorescence immunochromatographic assay system for the quantification of microcystins. Environ. Sci. Technol. 2003, 37, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Carminati, M.; Turolla, A.; Mezzera, L.; Mauro, M.D.; Tizzoni, M.; Pani, G.; Zanetto, F.; Foschi, J.; Antonelli, M. A self-powered wireless water quality sensing network enabling smart monitoring of biological and chemical stability in supply systems. Sensors 2020, 20, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Loyprasert, S.; Thavarungkul, P.; Asawatreratanakul, P.; Wongkittisuksa, B.; Limsakul, C.; Kanatharana, P. Label-free capacitive immunosensor for microcystin-LR using self-assembled thiourea monolayer incorporated with Ag nanoparticles on gold electrode. Biosens. Bioelectron. 2008, 24, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Li, L.; Mu, X.; Genov, R.; Mason, A.J. CMOS electrochemical instrumentation for biosensor microsystems: A review. Sensors 2017, 17, 74–99. [Google Scholar] [CrossRef] [PubMed]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Carminati, M.; Gervasoni, G.; Sampietro, M.; Ferrari, G. Note: Differential configurations for the mitigation of slow fluctuations limiting the resolution of digital lock-in amplifiers. Rev. Sci. Instrum. 2016, 87, 0261021–0261023. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.S.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Mitra, A.; Das, S. Characterization of electrode/electrolyte interface of ECIS devices. Electroanalysis 2012, 24, 2405–2414. [Google Scholar] [CrossRef]
- Franks, W.; Schenker, I.; Schmutz, P.; Hierlemann, A. Impedance characterization and modeling of electrodes for biomedical applications. IEEE Trans. Biomed. Eng. 2005, 52, 1295–1302. [Google Scholar] [CrossRef]
- Sanjurio, J.P.; Prefasi, E.; Buffa, C.; Gaggl, R. A capacitance-to-digital converter for MEMS sensors for smart applications. Sensors 2017, 17, 1312–1328. [Google Scholar] [CrossRef] [PubMed]
- Heidary, A. A Low-Cost Universal Integrated Interface for Capacitive Sensors. PhD Thesis, Electrical Engineering, Mathematics and Computer Science, Delft University of Technology, Delft, The Netherlands, 2010. [Google Scholar]
- Ceylan, O.; Mishra, G.K.; Yazici, M.; Cakmakci, R.C.; Niazi, J.H.; Qureshi, A.; Gurbuz, Y. Development of hand-held point-of-care diagnostic device for detection of multiple cancer and cardiac disease biomarkers. IEEE Int. Symp. Circuits Syestems (ISCAS) 2018, 8351593–8351596. [Google Scholar] [CrossRef]
- Bera, S.C.; Chattopadhyay, S. A modified Schering bridge for measurement of dielectric parameters of a material and capacitance of a capacitive transducer. Measurement 2003, 33, 3–7. [Google Scholar] [CrossRef]
- Fonseca da Silva, M.; Cruz Sera, A. Study of the sensitivity in an automatic capacitance measurement system. IEEE Instrum. and Meas. Conf. 1997, 329–334. [Google Scholar] [CrossRef]
- Holmberg, P. Automatic balancing of AC bridge circuits for capacitive sensor elements. IEEE Trans. Instrum. Meas. 1995, 44, 803–805. [Google Scholar] [CrossRef]
- Yang, W.Q. Aself balancing circuit to measure capacitance and loss conductance for industrial transducer aplications. IEEE Trans. Instrum. Meas. 1996, 45, 955–958. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Manickam, A.; Chevalier, A.; McDermott, M.; Ellington, A.D.; Hassibi, A. A CMOS Electrochemical Impedance Spectroscopy (EIS) Biosensor Array. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 379–390. [Google Scholar] [CrossRef]
- Pine research. Available online: https://pineresearch.com (accessed on 8 March 2020).
- Fischera, L.M.; Tenje, M.; Heiskanen, A.R.; Masuda, N.; Castillo, J.; Bentien, A.; Émneus, J.; Jakobsen, M.H.; Boisen, A. Gold cleaning methods for electrochemical detection applications. Microelectron. Eng. 2009, 86, 1282–1285. [Google Scholar] [CrossRef]
- Nguyen, K.C. Quantitative analysis of COOH-terminated alkanethiol SAMs on gold nanoparticle surfaces. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 045008–0450013. [Google Scholar] [CrossRef]
- Vashist, S.K. Comparison of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Based Strategies to Crosslink Antibodies on Amine-Functionalized Platforms for Immunodiagnostic Applications. Diagnostic 2012, 2, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Xing, Y.; Wang, G.; Feng, Q.; Chen, Q.; Feng, H.; Sun, X.; Liu, L. Probing of EDC/NHSS-Mediated Covalent Coupling Reaction by the Immobilization of Electrochemically Active Biomolecules. Int. J. Electrochem. Sci. 2013, 8, 2459–2467. [Google Scholar]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E.D. Spontaneously Organized Molecular Assemblies. 4. Structural Characterization of n-Alkyl Thiol Monolayers on Gold by Optical Ellipsometry, Infrared Spectroscopy, and Electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- Lebogang, L.; Mattiasson, B.; Hedstrom, M. Capacitive sensing of microcystin variants of Microcystis aeruginosa using a gold immunoelectrode modified with antibodies, gold nanoparticles and polytyramine. Microchim Acta. 2014, 181, 1009–1017. [Google Scholar] [CrossRef]
- Dalimia, A.; Liu, C.C.; Savinell, R.F. Electrochemical behavior of gold electrodes modified with self-assembled monolayers with an acidic end group for selective detection of dopamine. J. Electroanal. Chem. 1997, 430, 205–214. [Google Scholar] [CrossRef]
- Analog Devices Inc. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/ADG811_812.pdf (accessed on 17 March 2020).





| PBS | 0.1 μg/L | 1 μg/L | 10 μg/L | |
|---|---|---|---|---|
| (pF) | 82.38 | 411.9 | 1592.68 | 3679.64 |
| (Ω) | 0.042 | 2.01 | 4.97 | 9.86 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neshani, S.; Nyamekye, C.K.A.; Melvin, S.; Smith, E.A.; Chen, D.J.; Neihart, N.M. AC and DC Differential Bridge Structure Suitable for Electrochemical Interfacial Capacitance Biosensing Applications. Biosensors 2020, 10, 28. https://doi.org/10.3390/bios10030028
Neshani S, Nyamekye CKA, Melvin S, Smith EA, Chen DJ, Neihart NM. AC and DC Differential Bridge Structure Suitable for Electrochemical Interfacial Capacitance Biosensing Applications. Biosensors. 2020; 10(3):28. https://doi.org/10.3390/bios10030028
Chicago/Turabian StyleNeshani, Sara, Charles K. A. Nyamekye, Scott Melvin, Emily A. Smith, Degang J. Chen, and Nathan M. Neihart. 2020. "AC and DC Differential Bridge Structure Suitable for Electrochemical Interfacial Capacitance Biosensing Applications" Biosensors 10, no. 3: 28. https://doi.org/10.3390/bios10030028
APA StyleNeshani, S., Nyamekye, C. K. A., Melvin, S., Smith, E. A., Chen, D. J., & Neihart, N. M. (2020). AC and DC Differential Bridge Structure Suitable for Electrochemical Interfacial Capacitance Biosensing Applications. Biosensors, 10(3), 28. https://doi.org/10.3390/bios10030028




