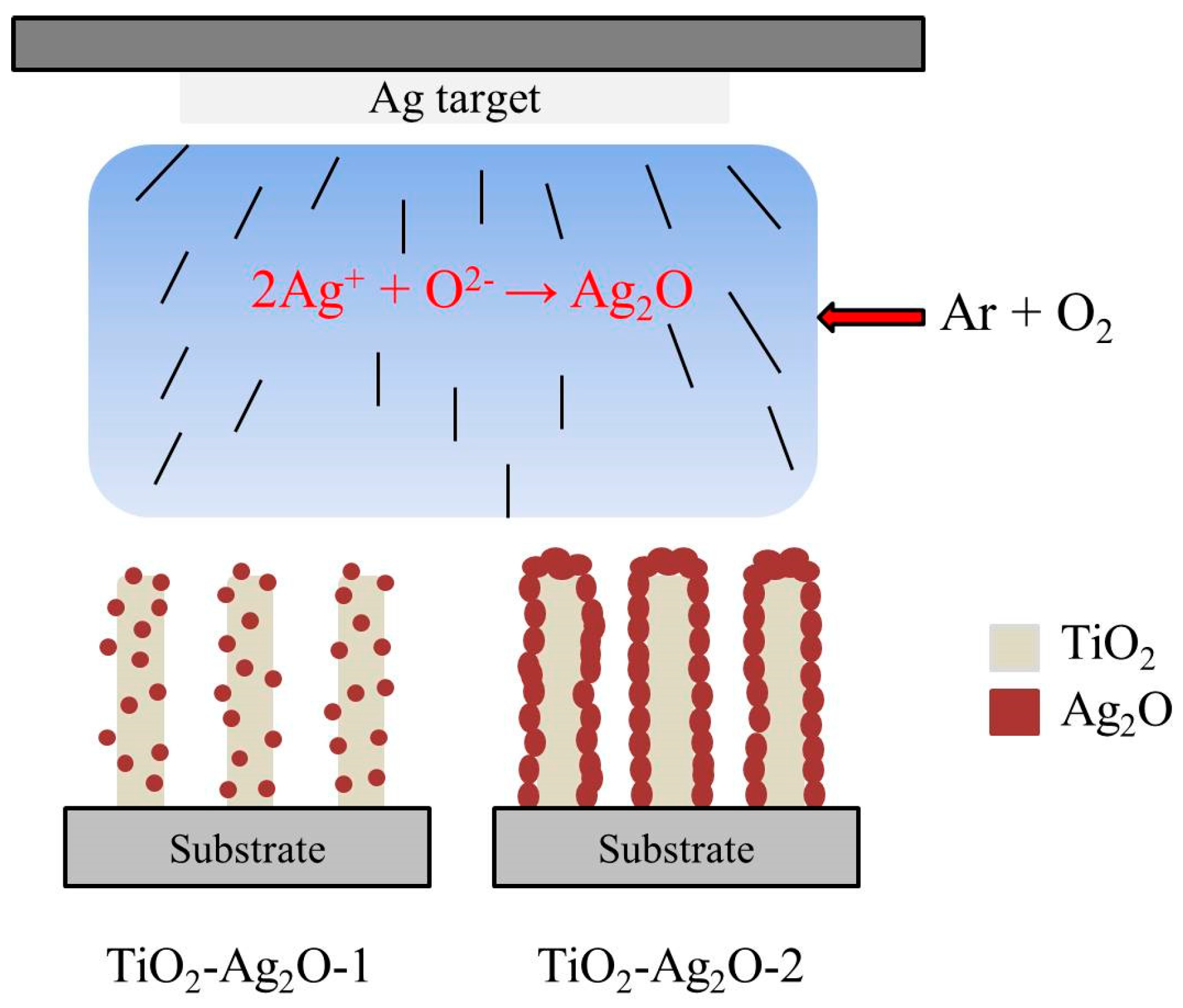
3. Results and Discussion
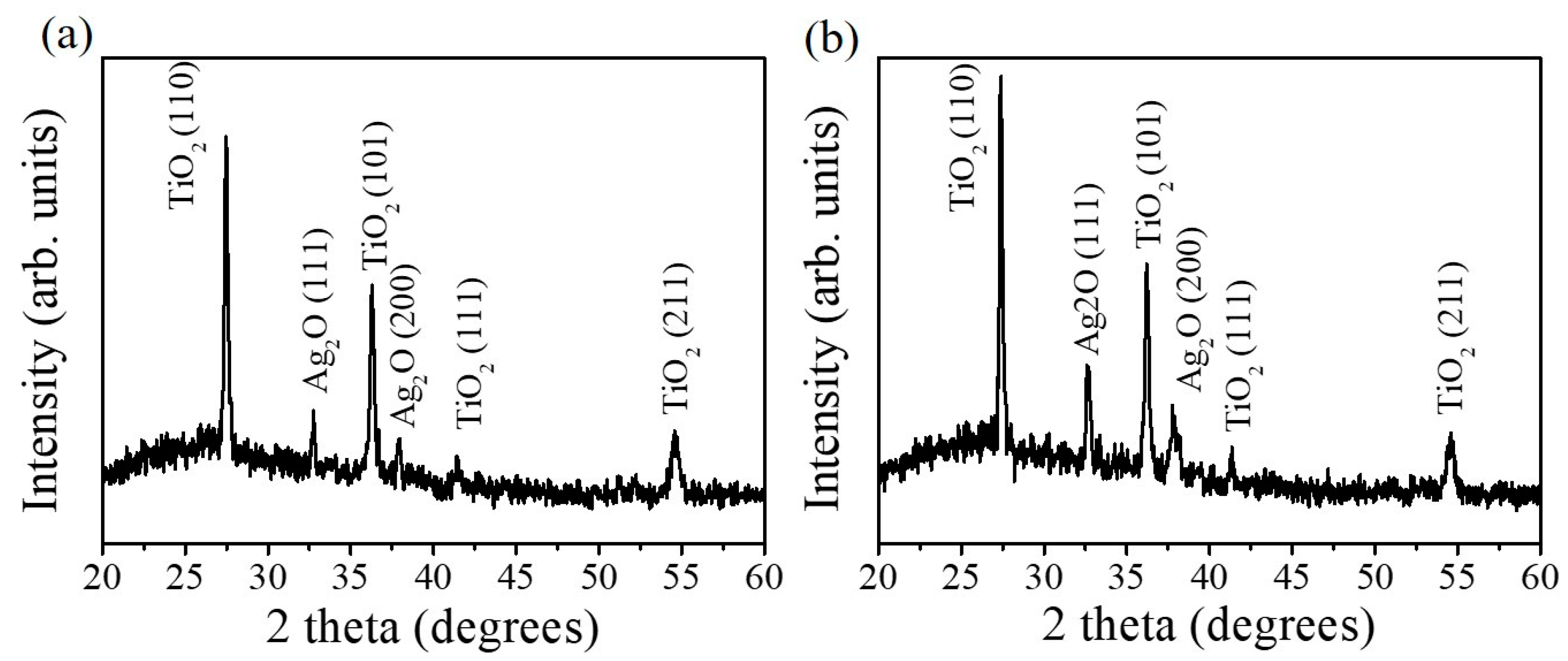
X-ray diffractometer (XRD) patterns of TiO
2–Ag
2O composite nanorods with various Ag
2O thin-film sputtering durations are shown in
Figure 2. The distinct Bragg reflections centered at 27.46°, 36.05°, 41.22°, and 54.33° correspond to the crystallographic planes (110), (101), (111), and (211) of the rutile TiO
2 phase, respectively (JCPDS no. 00-004-0551). Moreover, the Bragg reflections centered at 32.72 ° and 37.98 ° are assigned to the crystallographic planes of cubic Ag
2O (111) and (200), respectively (JCPDS no. 00-012-0793). The XRD results reveal that highly crystalline rutile TiO
2-cubic Ag
2O composite nanorods were formed through the sputtering deposition of Ag
2O thin films onto the surfaces of the TiO
2 nanorods, and no other impurity peak was observed. As expected, the intensity of the Ag
2O Bragg reflections peaks increased with the increase of the Ag
2O thin-film sputtering duration, revealing an increased Ag
2O phase content in the composite nanorods.
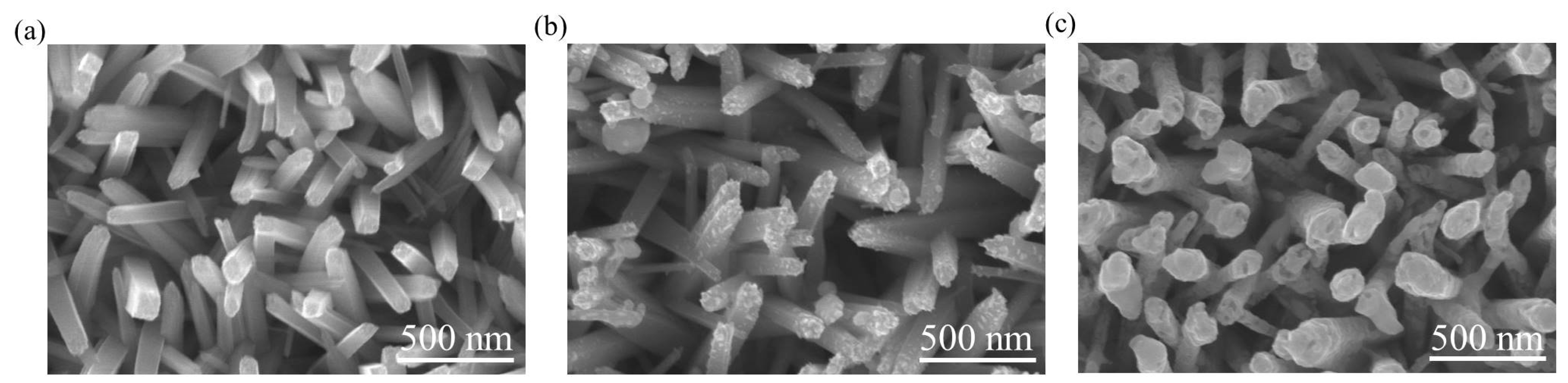
Figure 3a shows the scanning electron microscopy (SEM) image of the as-synthesized TiO
2 nanorods. The TiO
2 nanorods had a rectangular cross-section crystal feature with an average diameter of approximately 100 nm; the side facets of the nanorods were smooth.
Figure 3b presents the SEM image of the TiO
2–Ag
2O-1 composite nanorods. The surface morphology of the composite nanorods reveals that a small amount of nanoparticle-like crystals was decorated onto the surfaces of the TiO
2 nanorods. The nanoparticle-like crystals dispersed separately on the surfaces of the TiO
2 nanorods.
Figure 3c shows the SEM image of the TiO
2–Ag
2O-2 composite nanorods. The TiO
2 nanorods were homogeneously encapsulated by the aggregation of massive Ag
2O nanoparticles, resulting in the irregular-shaped cross-section crystal feature of the composite nanorods. Detailed TEM analyses were performed to further confirm the morphology change of the TiO
2–Ag
2O composite nanorods prepared at various Ag
2O sputtering durations.
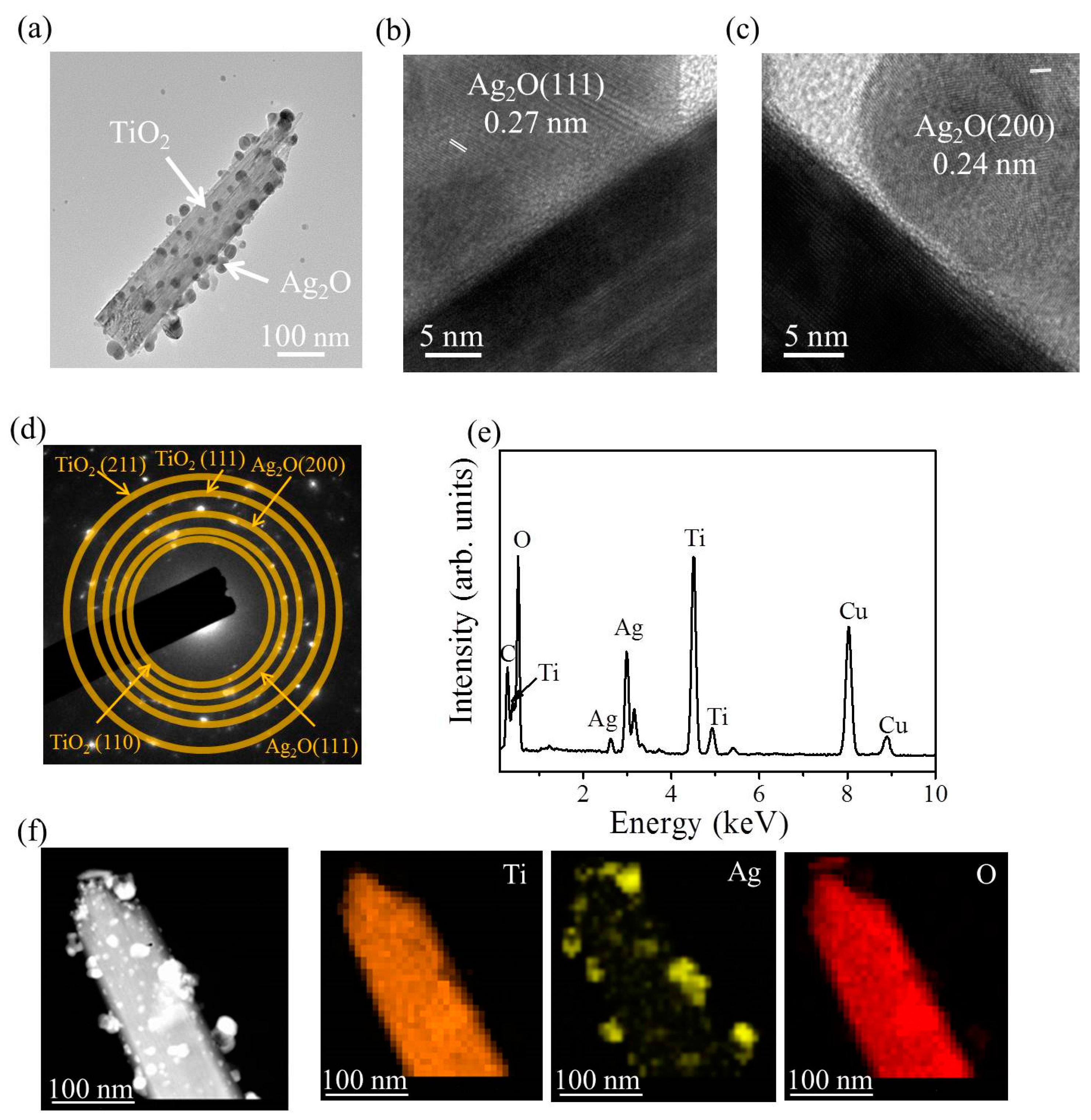
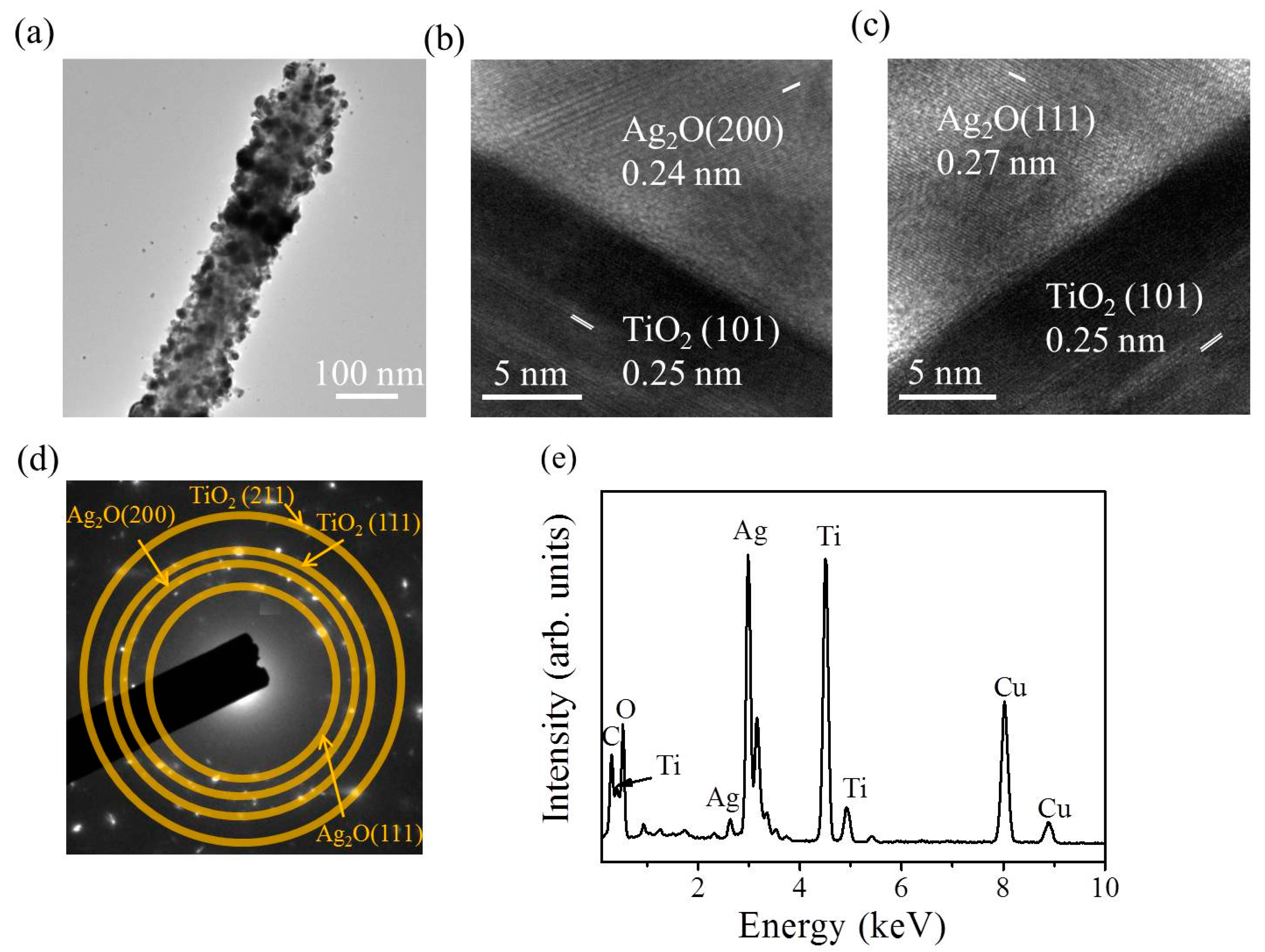
Figure 4a shows the low-magnification transmission electron microscopy (TEM) image of a TiO
2–Ag
2O-1 composite nanorod. A small amount of Ag
2O particles was dispersedly decorated on the surface of the TiO
2 nanorod via the sputtering growth of the Ag
2O. The high-resolution TEM images shown in
Figure 4b,c indicate distinct lattice fringes in the Ag
2O particle. Moreover, the lattice fringe distance of approximately 0.24 nm was assigned to the crystallographic plane spacing of cubic Ag
2O (200).
Figure 4d exhibits the selected area electron diffraction (SAED) pattern of several TiO
2–Ag
2O-1 composite nanorods. Several clear diffraction rings associated with (111) and (200) planes of the Ag
2O and (110), (111), and (211) planes of the rutile TiO
2 were observed in the SAED pattern. This demonstrates the good crystallinity of the composite nanorods and indicates that these composite nanorods have a polycrystalline nature.
Figure 4e presents the EDS spectrum of a TiO
2–Ag
2O-1 nanorod. In addition to carbon and copper signals originating from the TEM grid, Ti, Ag, and O elements were detected in the selected heterostructure and no other impurity atom was detected. The EDS elemental mapping images taken from the TiO
2–Ag
2O-1 nanorod are presented in
Figure 4f. The Ti signals were homogeneously distributed over the region of the nanorod template. By contrast, the Ag signals were mainly distributed on the outer region of the composite nanorod; the distribution of Ag signals was discrete and randomly decorated on the TiO
2 surface. A good Ag
2O particle-decorated TiO
2 nanorod with a dispersive particle decoration feature was obtained in the TiO
2–Ag
2O-1 composite nanorods.
Figure 5a shows the low-magnification TEM image of the TiO
2–Ag
2O-2 composite nanorod. In comparison with
Figure 4a, the distribution density of the Ag
2O particles on the surface of the TiO
2 nanorod was denser and many particles were clustered, resulting in a rugged surface feature of the composite nanorod. The tiny Ag
2O particles aggregated together and fully encapsulated the surface of the TiO
2 nanorod. A clear heterointerface was observed between the TiO
2 and Ag
2O (
Figure 5b,c). The distinct lattice fringes in the inner and outer regions of the composite nanorod in
Figure 5b,c demonstrated a good crystallinity of the composite nanorod. The SAED pattern in
Figure 5d supports the good crystallinity of the composite nanorods, as revealed in the high-resolution TEM images of the selected composite nanorod, and is also in agreement with the XRD result.
Figure 5e shows the corresponding EDS spectrum of the TiO
2–Ag
2O-2 composite nanorod. Besides the carbon and copper signals and the elements of Ag, Ti, and O, no other impurity atom was detected from the selected composite nanorod. Notably, the relative intensity of the Ag signal was more intense than that of the TiO
2–Ag
2O-1 nanorod in
Figure 4e, revealing a higher Ag content in TiO
2–Ag
2O-2 due to the prolonged sputtering duration of Ag
2O.
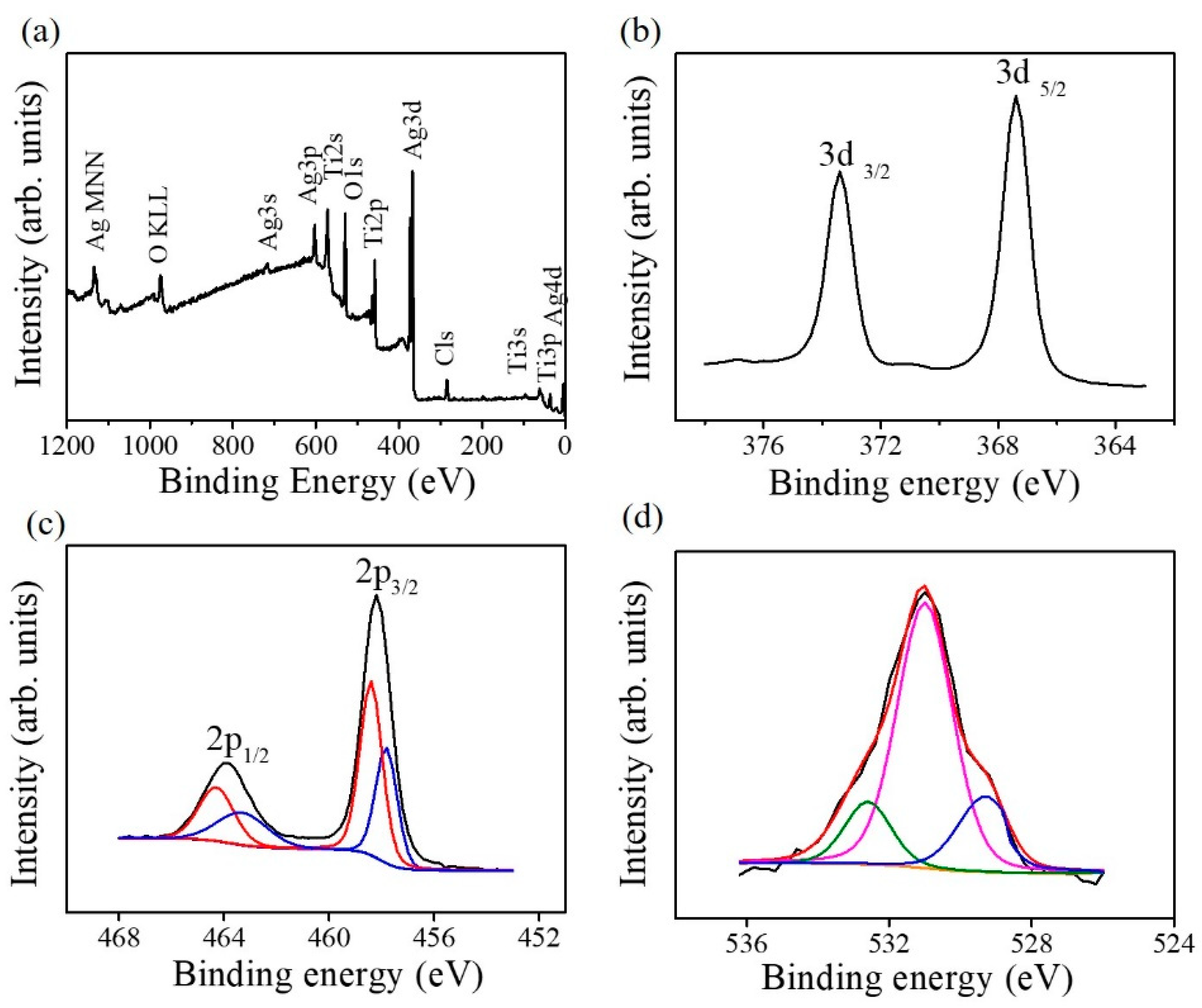
Figure 6a shows the X-ray photoelectron spectroscopy (XPS) survey scan spectrum of the TiO
2–Ag
2O-1 composite nanorods. The primary features include the Ti, Ag, and O peaks that originated from the TiO
2–AgO composites. The trace carbon contamination on the surface of the nanorod sample originated from exposure to ambient air. No impurity atoms were detected in the nanorod sample.
Figure 6b exhibits the Ag 3d core-level doublet spectrum originating from the Ag
2O decorated via sputtering; two distinct features centered at approximately 367.7 and 373.7 eV respectively correspond to the Ag 3d
5/2 and Ag 3d
3/2 binding energies. These binding energies are consistent with the Ag–O binding values reported for the Ag
2O phase [
17]. This indicates that the silver exists in the Ag
+ valence state in the sputtered Ag
2O nanoparticles on the composite nanorods studied herein.
Figure 6c displays the Ti 2p core-level doublet spectrum associated with the TiO
2 nanorod template. The distinct two features were deconvoluted into four subpeaks. The subpeaks centered at 458.7 and 464.3 eV correspond to Ti 2p
3/2 and Ti 2p
1/2 peaks of the Ti
4+ valance state, respectively. By contrast, the subpeaks with a relatively weak intensity centered at 457.6 and 463.3 eV correspond to Ti 2p
3/2 for Ti 2p
1/2 peaks of the Ti
3+ valence state [
5]. The presence of the mixed Ti
4+/Ti
3+ valance state indicates the possible presence of oxygen vacancies in the surfaces of the as-synthesized TiO
2 nanorods [
5,
8]. The O1s spectrum of the composite nanorods is shown in
Figure 6d. The asymmetric O1s spectrum was deconvoluted into three subpeaks centered at 532.5, 531.2, and 529.2 eV. Notably, the subpeaks centered at 529.2 and 531.2 eV are ascribed to the lattice oxygen in Ag
2O and TiO
2, respectively [
18,
19]. Moreover, the external absorbed −OH groups or water molecules on the surfaces of the composite nanorods are reflected by a subpeak at approximately 532.5 eV [
20].
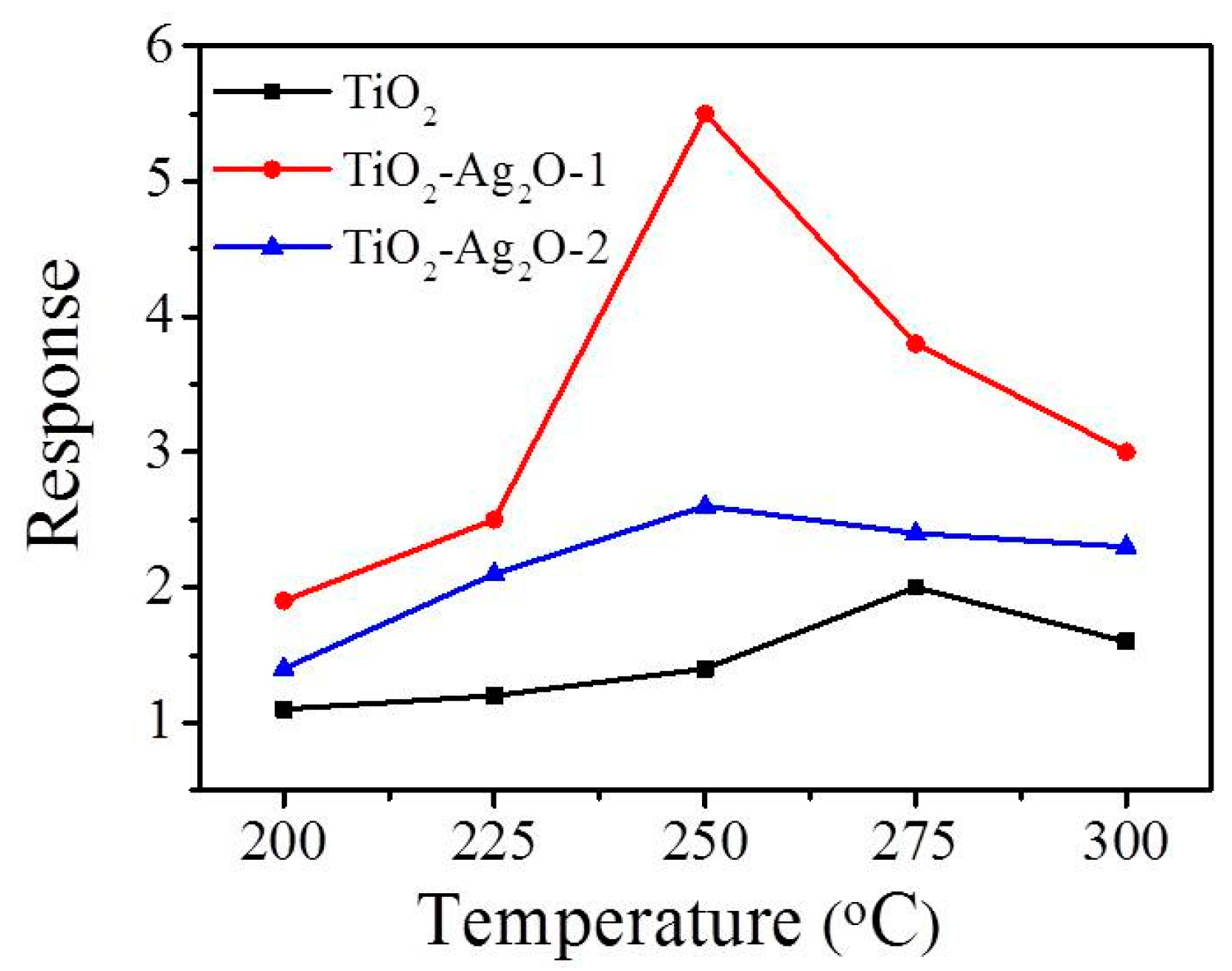
Figure 7 shows the temperature-dependent gas-sensing responses to NO
2 (1.5 ppm) of gas sensors made from TiO
2, TiO
2–Ag
2O-1, and TiO
2–Ag
2O-2 composite nanorods. For the NO
2 target gas, the n-type gas-sensing response of nanorod-based sensors is defined as Rg/Ra and the p-type gas-sensing response of nanorod-based sensors is defined as Ra/Rg, where Rg is the sensor resistance under target gas exposure and Ra is the sensor resistance with the removal of the target gas. The optimal operating temperature of oxide sensors to obtain the highest gas-sensing response is highly associated with the balance between the chemical reactions and the gas diffusion rate of the oxide surfaces [
5]. The maximum responses of the TiO
2–Ag
2O-1 and TiO
2–Ag
2O-2 sensors to NO
2 were obtained at the operating temperature of 250 °C in this study. Meanwhile, a relatively high operating temperature of 275 °C was needed for the TiO
2 nanorods to obtain the maximum gas-sensing response under similar gas-sensing test conditions. Notably, the gas-sensing response versus operating temperature curve of TiO
2–Ag
2O-1 showed a distinct summit at 250 °C, differing substantially from the curves of the TiO
2 and TiO
2–Ag
2O-2 nanorod sensors. This result might be a sign of different gas-detecting mechanisms operating among the various nanorod-based sensors. Therefore, the optimal gas-sensing temperature of the fabricated composite nanorod sensors toward NO
2 was chosen as 250 °C in this study.
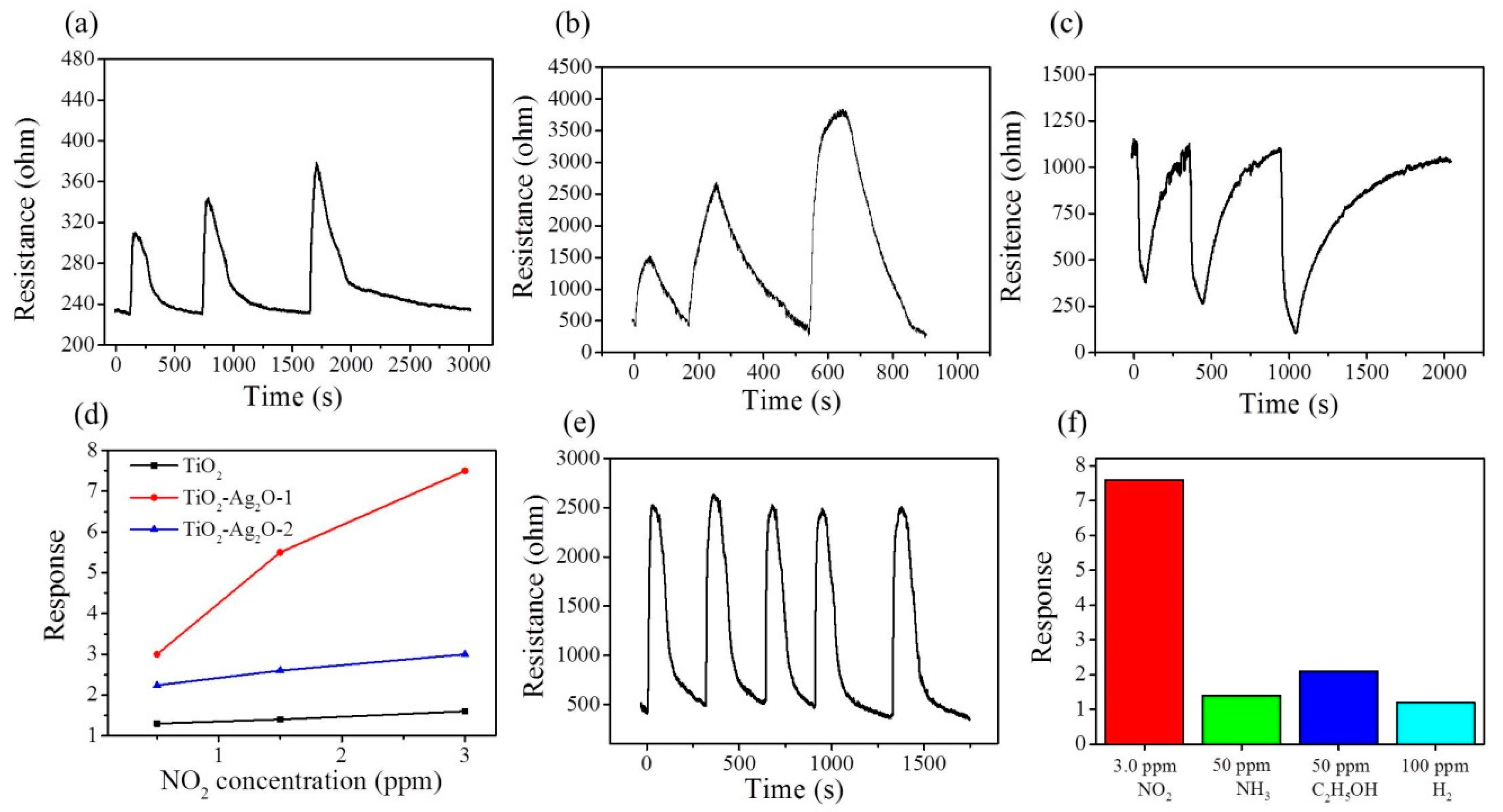
Figure 8a–c shows the dynamic NO
2 gas-sensing response curves of the TiO
2, TiO
2–Ag
2O-1, and TiO
2–Ag
2O-2 sensors, respectively, upon exposure to 0.5–3.0 ppm NO
2. A sharp increase in sensor resistance was observed for the TiO
2 and TiO
2–Ag
2O-1 nanorod sensors upon exposure to NO
2; moreover, the sensor resistance decreased with the removal of the NO
2 target gas (
Figure 8a,b). By contrast, the TiO
2–Ag
2O-2 showed an opposite sensor resistance variation upon exposure to NO
2 gas (
Figure 8c). This indicates that the TiO
2 and TiO
2–Ag
2O-1 sensors showed an n-type conduction nature and the TiO
2–Ag
2O-2 sensor demonstrated a p-type conduction nature during the NO
2 gas-sensing tests. The aforementioned structural results reveal that the TiO
2–Ag
2O-1 sensor exhibited a morphology in which the Ag
2O particles were dispersedly distributed on the surfaces of the TiO
2 nanorods. The incomplete coverage of the Ag
2O particles on the TiO
2 surfaces of the TiO
2–Ag
2O-1 nanorods meant that, upon exposure to the NO
2 target gas, the n-type conduction dominated the material’s gas-sensing behavior. By contrast, the TiO
2–Ag
2O-2 nanorods demonstrated a thick, full-coverage layer of Ag
2O clusters or aggregations on the surfaces of the TiO
2 nanorods. This morphology feature might account for the conduction and chemoresistive variation in the TiO
2–Ag
2O-2 sensor, which was dominated by p-type Ag
2O shell layers of the composite nanorods. A similar conduction type variation due to the p-type crystal coverage effect on the p–n heterogeneous oxides has been demonstrated in a ZnO–Cr
2O
3 system [
11]. Comparatively, the TiO
2–Ag
2O-1 sensor exhibited the largest degree of sensor resistance variation before and after the introduction of the NO
2 gas under the given test conditions. Notably, the pristine TiO
2 sensor demonstrated the lowest sensor resistance variation size upon expose to NO
2 gas. The plot of NO
2 gas-sensing response versus NO
2 concentration for various TiO
2 nanorod-based sensors is shown in
Figure 8d. The NO
2 gas-sensing response of the TiO
2–Ag
2O-1 sensor was approximately 3.1 upon exposure to 0.5 ppm NO
2. Moreover, the gas-sensing response of the TiO
2–Ag
2O-1 sensor increased to 7.6 upon exposure to 3.0 ppm NO
2. An approximate increase of the gas-sensing response by 2.4 times was observed with an increase in NO
2 concentration from 0.5 ppm to 3.0 ppm by the TiO
2–Ag
2O-1 sensor. By contrast, the TiO
2–Ag
2O-2 sensor exhibited gas-sensing responses of approximately 2.2 and 3.1 upon exposure to 0.5 ppm and 3.0 ppm NO
2, respectively; these response values are lower than those of the TiO
2–Ag
2O-1 sensor under similar test conditions. A concentration-dependent increment of the gas-sensing response for a low concentration range of 0.5–3.0 ppm NO
2 was less visible for the TiO
2–Ag
2O-2 sensor. Notably, the gas-sensing response of the pristine TiO
2 sensor at the same operating temperature did not show a response value larger than 2.0, revealing that the decoration of discrete or layered Ag
2O particles or aggregations on the surfaces of TiO
2 nanorods to form a p–n heterogeneous system is beneficial to the enhancement of the NO
2 gas-sensing response of TiO
2 nanorods. The gas-sensing response time of the nanorod-based sensors is defined as the duration required for an occurrence of a 90% change in sensor resistance upon exposure to the target gas, while the recovery time is the duration in which the sensor resistance drops by 90% from the maximal steady-state value, following the removal of the target gas. The response times for the TiO
2, TiO
2–Ag
2O-1, and TiO
2–Ag
2O-2 nanorod sensors upon exposure to 0.5–3.0 ppm NO
2 gas ranged from 85 to 93 s. No substantial difference in the response times of various nanorod sensors exposed to different concentrations of NO
2 gas was observed. By contrast, a marked improvement in the recovery time of the TiO
2 nanorods sputtered with a coating of Ag
2O particles was visibly demonstrated. The recovery times of the pristine TiO
2 nanorod sensor ranged from 405 to 820 s after exposure to 0.5 to 3.0 ppm NO
2. Decreased recovery times were shown in the TiO
2–Ag
2O-2 nanorod sensor, which ranged from 191 to 280 s after exposure to 0.5 to 3.0 ppm NO
2. Notably, the TiO
2–Ag
2O-1 nanorod sensor exhibited a substantial decrease in the recovery time upon the removal of NO
2 gas; the recovery times ranged from 97 to 136 s in the NO
2 concentration range of 0.5 to 3.0 ppm. The size of the Ag
2O particles (or clusters) and their dispersibility are vital factors affecting gas-sensing performance, which lead to the highly effective desorption of surface-adsorbed ions with the removal of the target gas at elevated temperatures [
21]. The TiO
2–Ag
2O-1 nanorod sensor exhibited the superior gas-sensing performance among the various nanorod sensors in this study. The cycling gas-sensing tests of the TiO
2–Ag
2O-1 nanorod sensor exposed to 1.5 ppm NO
2 at 250 °C are shown in
Figure 8e. The result indicates that the TiO
2–Ag
2O-1 nanorod sensor had good reproducibility during multiple cycles of response and recovery.
Figure 8f shows the across selectivity profiles of the TiO
2–Ag
2O-1 sensor upon exposure to 100 ppm H
2, 50 ppm C
2H
5OH, and 50 ppm NH
3 gases, as well as 3.0 ppm NO
2. The TiO
2–Ag
2O-1 sensor exhibited a highly selective gas-sensing response toward the low-concentration NO
2 gas as compared to the other various target gases.
The NO
2 gas-sensing performances of the sensors based on several TiO
2-based composite oxides are summarized in
Table 1. Compared to previous works [
22,
23,
24,
25], the TiO
2–Ag
2O-1 nanorod sensor herein showed superior NO
2 gas-sensing performance under similar test conditions. The gas-sensing test results herein demonstrated that the TiO
2–Ag
2O composite nanorods decorated with discrete Ag
2O particles have potential for applications as NO
2 gas sensors at low concentrations. The possible surface chemisorption reactions occurring during the gas-sensing process of the TiO
2–Ag
2O composite nanorods upon exposure to NO
2 gas are described below:
The NO
2 molecules capture electrons from the oxide surface to form NO
2− ions; this engenders the electron density variation of the oxides. By contrast, the surface-adsorbed NO
2− ions are desorbed with the removal of the NO
2 gas and, consequently, in this process the recovery of the initial conditions takes place. Notably, the contact of the TiO
2–Ag
2O oxides form p–n junctions at the hetero-interfacial regions. This additionally formed potential barrier in the TiO
2–Ag
2O composite nanorods explains the superior gas-sensing responses of the composite nanorods compared to that of the pristine TiO
2 nanorods. A similar formation of heterogeneous p–n junctions improves the gas-sensing responses of composite nanorods, as has been demonstrated in ZnO–ZnCr
2O
4, ZnO–Mn
3O
4, and ZnO–Cr
2O
3 p–n composite structures [
9,
10,
11]. Furthermore, the reasons for the NO
2 gas-sensing response of the TiO
2–Ag
2O-1 sensor being higher than that of the TiO
2–Ag
2O-2 sensor at the given test conditions are explained by the schematic mechanisms exhibited in
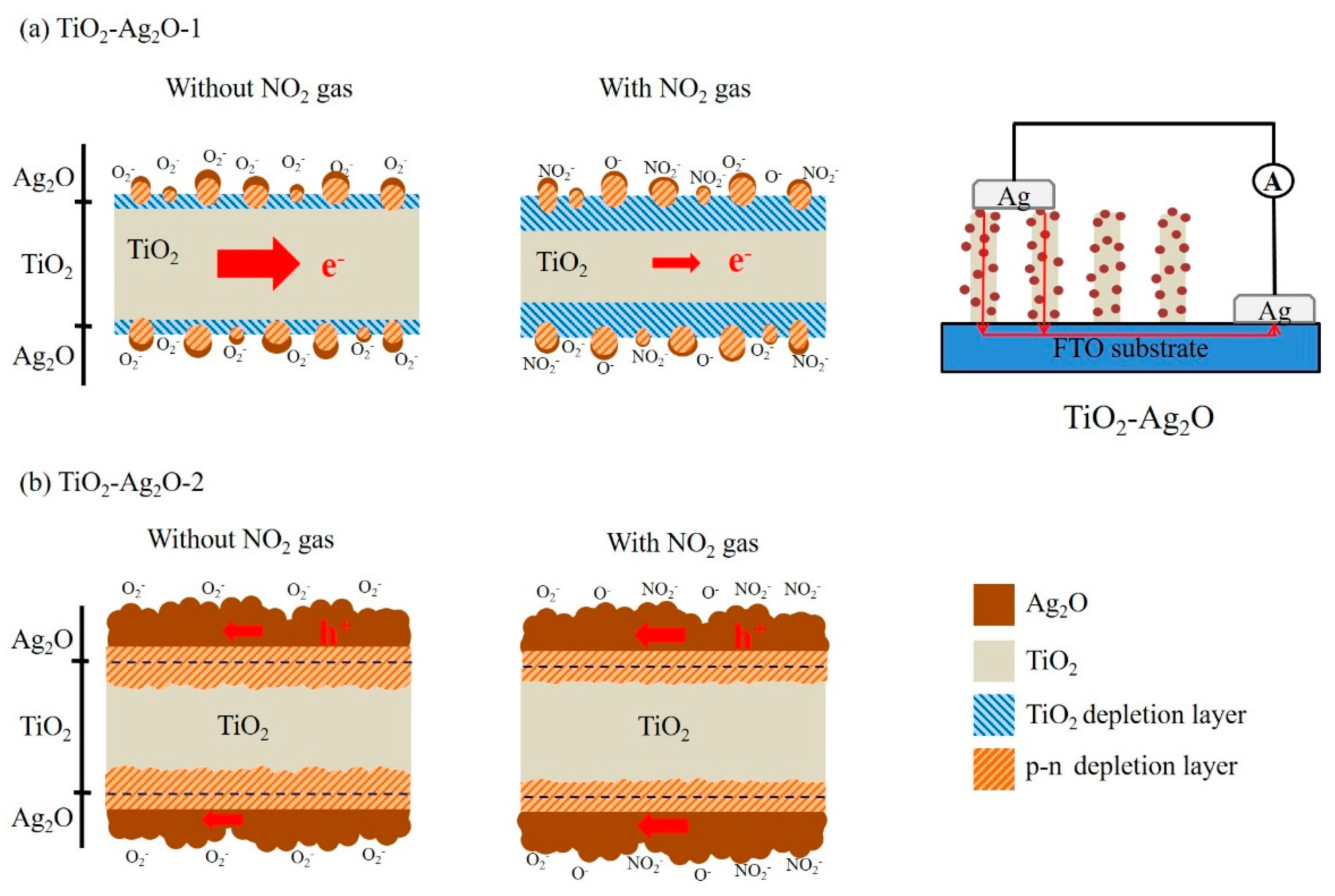
Figure 9. The schematic of the gas sensor device is also shown in
Figure 9a. When the Ag
2O particles are coated on the surfaces of the TiO
2 nanorods in a discrete configuration, the randomly distribution of the depletion region at the interface of the p-Ag
2O and n-type TiO
2 will initially narrow the space of the conducting channel along the radial direction of the TiO
2 (
Figure 9a). Moreover, the exposure of the free surfaces of the Ag
2O particles and TiO
2 rods in ambient air also initially lead to a surface hole accumulation layer and depletion layer, respectively. Furthermore, following the decoration of the Ag
2O particles in a continuous layer configuration on the surfaces of the TiO
2 nanorods, the conducting channel in the TiO
2 will also be narrowed (
Figure 9b). After introducing NO
2 gas into the test chamber, the depletion region size at the TiO
2–Ag
2O hetero-interfacial region of the TiO
2–Ag
2O-1 sensor varies due to the surface-adsorbed NO
2⁻ ions. Moreover, the surface depletion region of the TiO
2 nanorods is also thickened. The variation of the depletion size at different regions, further narrowing the conduction channel size of the TiO
2 nanorods, results in the increased sensor resistance of the TiO
2–Ag
2O-1 nanorod sensor. By contrast, the TiO
2–Ag
2O-2 nanorod sensor exhibits a p-type conduction gas-sensing behavior in this study. This reveals that the conduction channel size in the TiO
2 nanorod of the composite nanorod no longer plays a vital role affecting the chemoresistive variation upon exposure to NO
2 gas. It has also been shown that in core–shell ZnO–ZnMn
2O
4 and ZnO–Cr
2O
3 nanostructures, the p–n contact regions at the hetero-interfaces no longer play significant roles in the gas-sensing reaction [
10,
11]. The conduction path in the Ag
2O layer, by contrast, dominates the gas-sensing response of the TiO
2–Ag
2O-2 nanorod sensor. Notably, when the TiO
2–Ag
2O-2 nanorod is exposed to NO
2 gas, the accumulation layer in the Ag
2O layer thickens. This increases the carrier number in the p-type Ag
2O layer; therefore, a decreased sensor resistance is expected. However, the surface Ag
2O layer-dominated chemoresistive variation size of the TiO
2–Ag
2O-2 nanorod sensor is expected to be lower than that of the TiO
2–Ag
2O-1 nanorod sensor, which is dominated by the rugged conduction channel size in the TiO
2 core region upon exposure to NO
2 gas. Therefore, the superior NO
2 gas-sensing performance was obtained by the TiO
2–Ag
2O-1 nanorod sensor in this study.