A Surface Dielectric Barrier Discharge Plasma for Preparing Cotton-Fabric-Supported Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
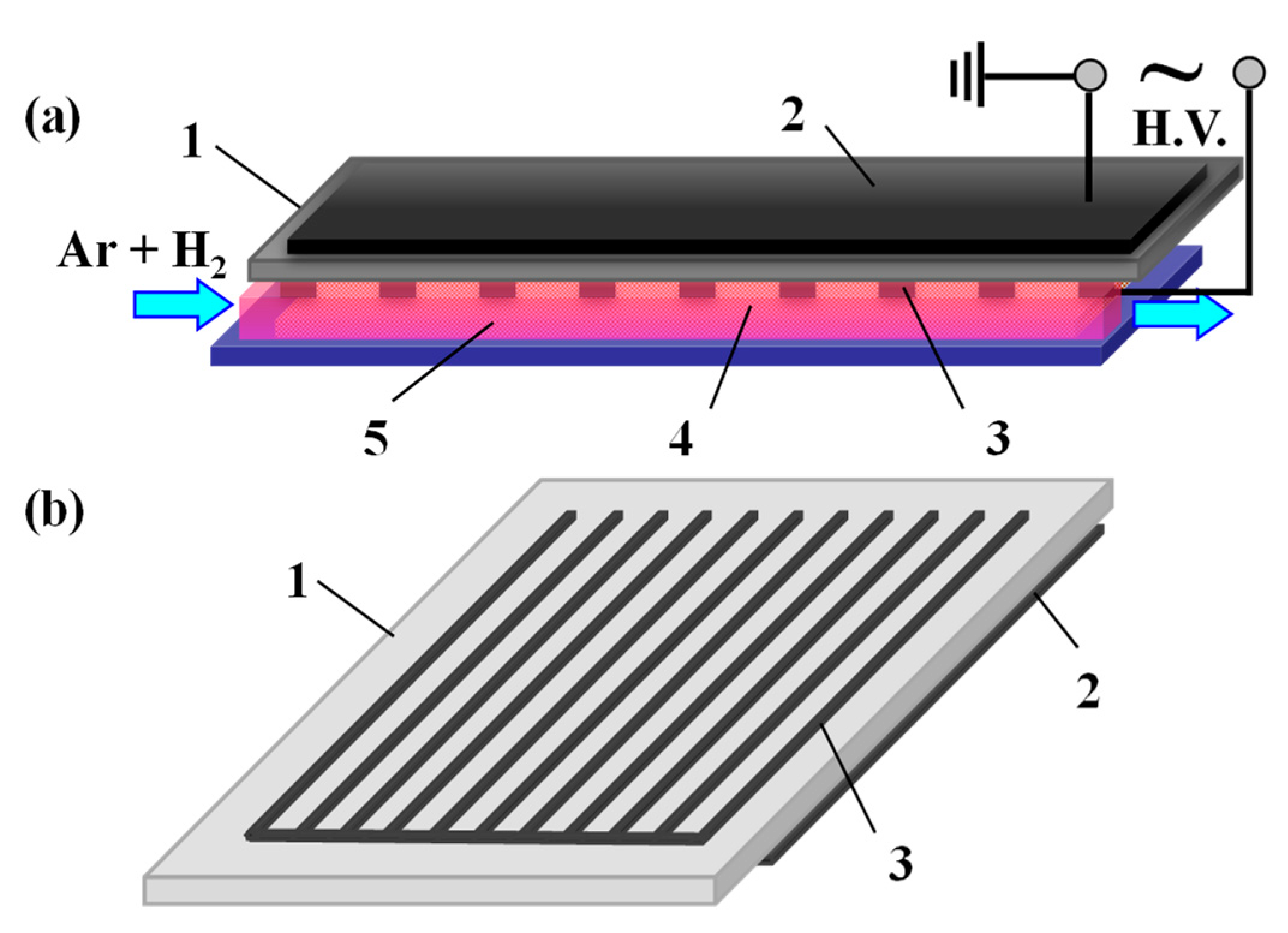
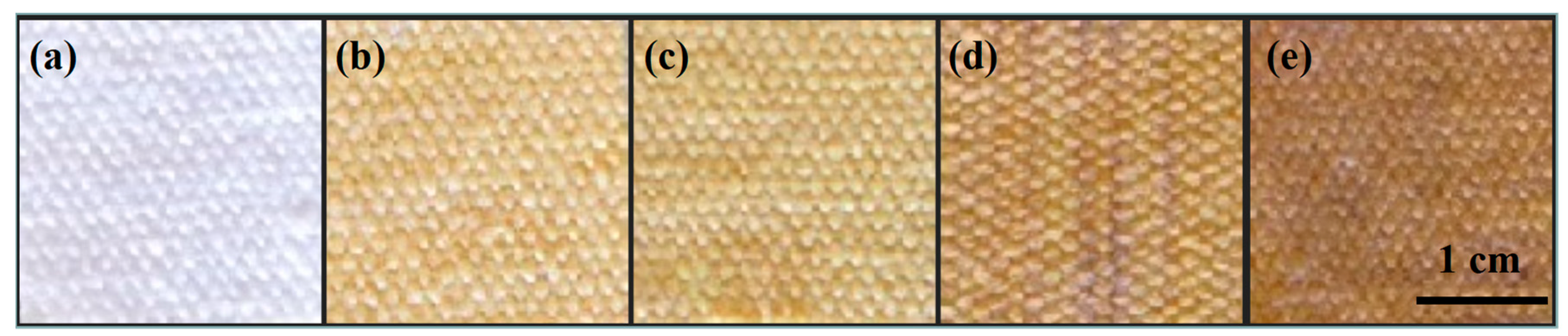
2.1. Preparation of the Ag/Cotton Samples
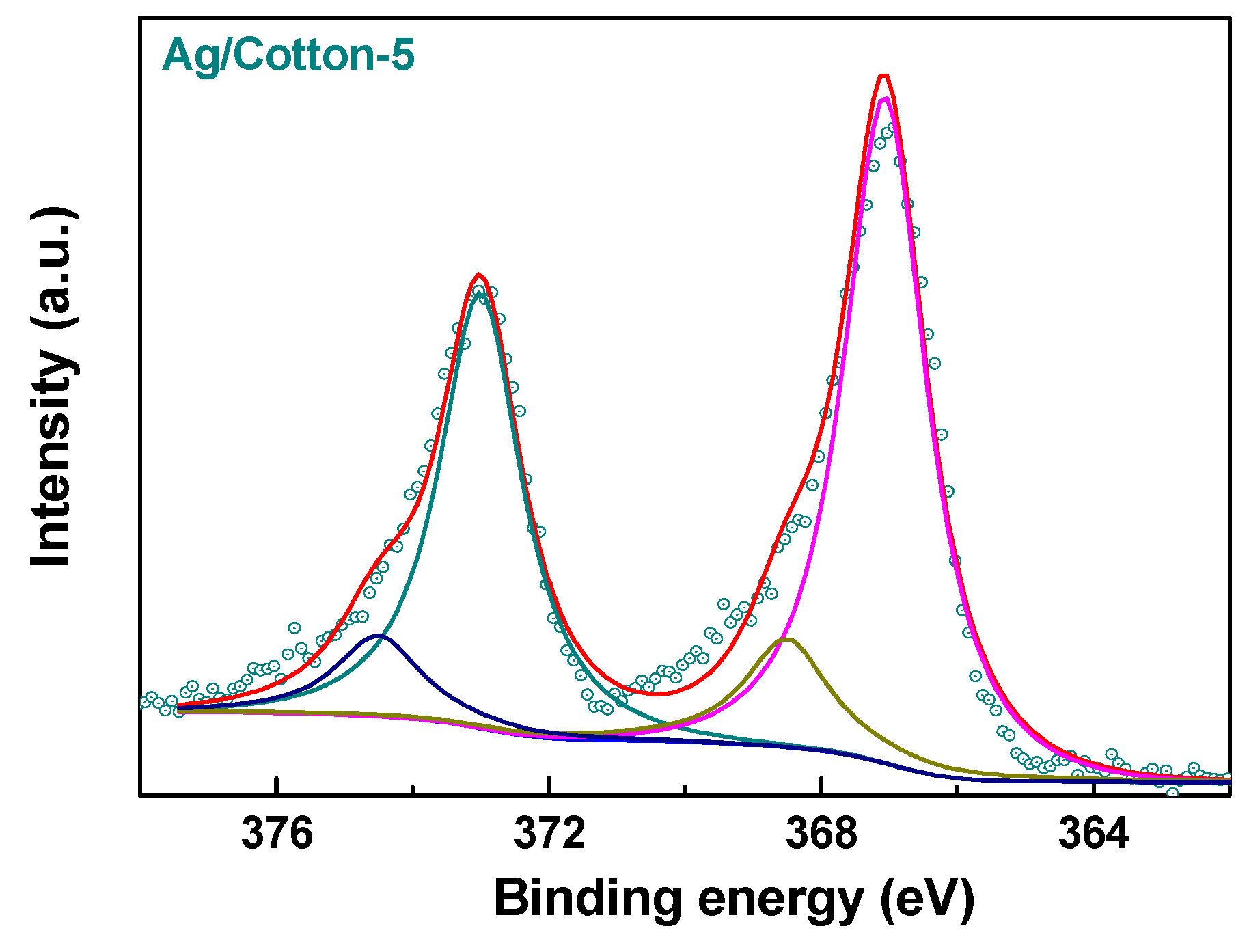
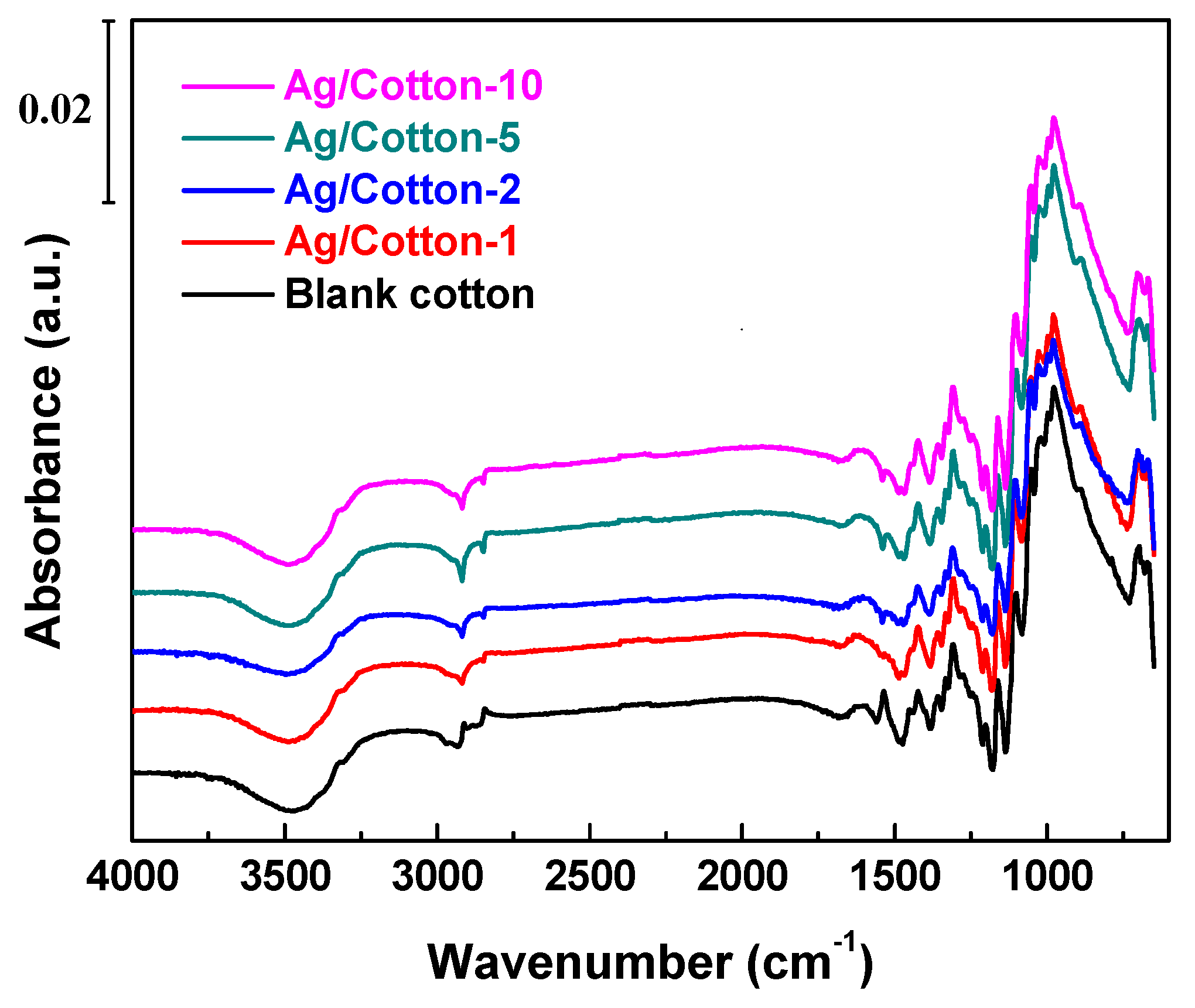
2.2. Characterization
2.3. Wash Fastness
2.4. Antibacterial Activity
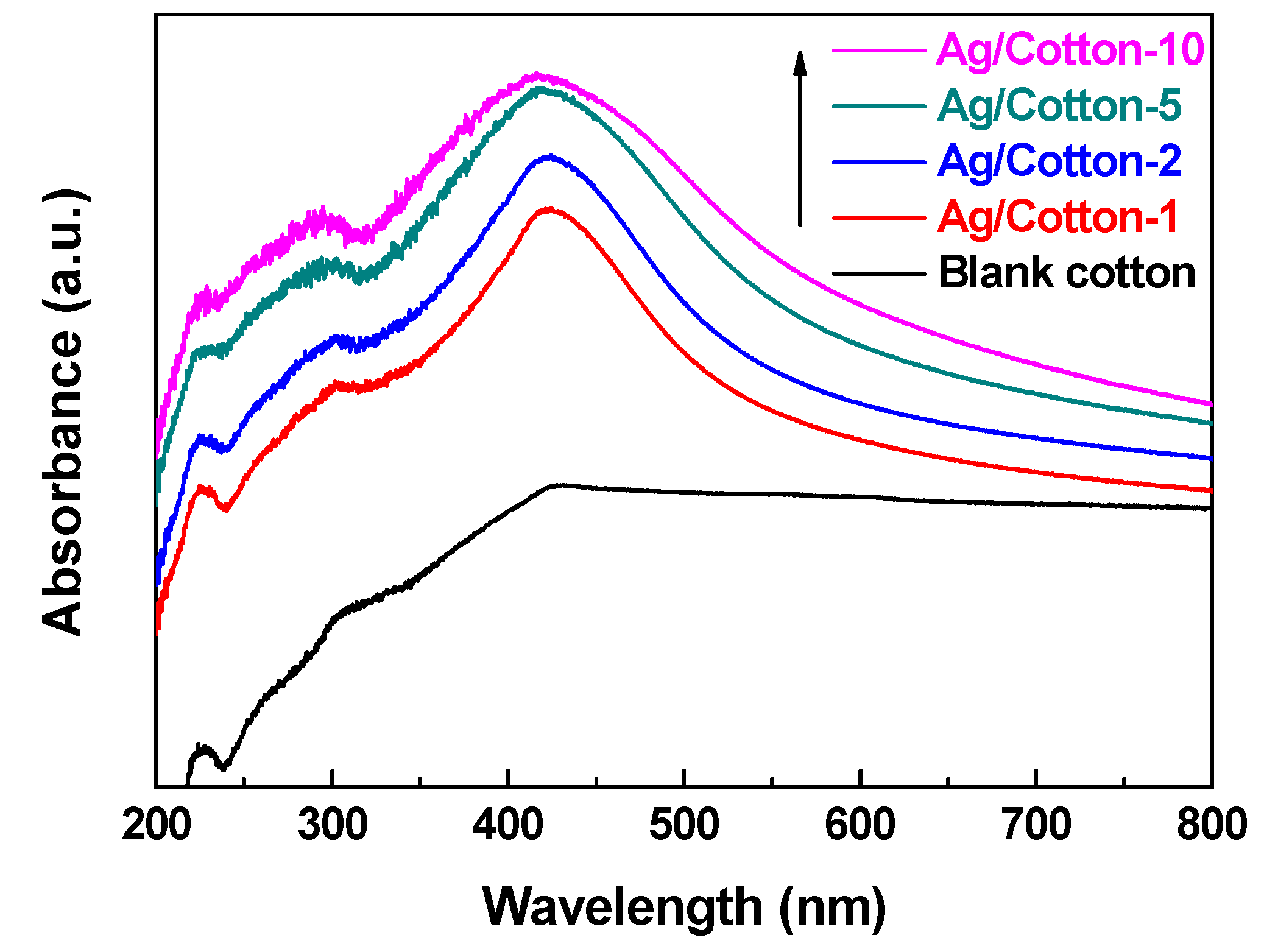
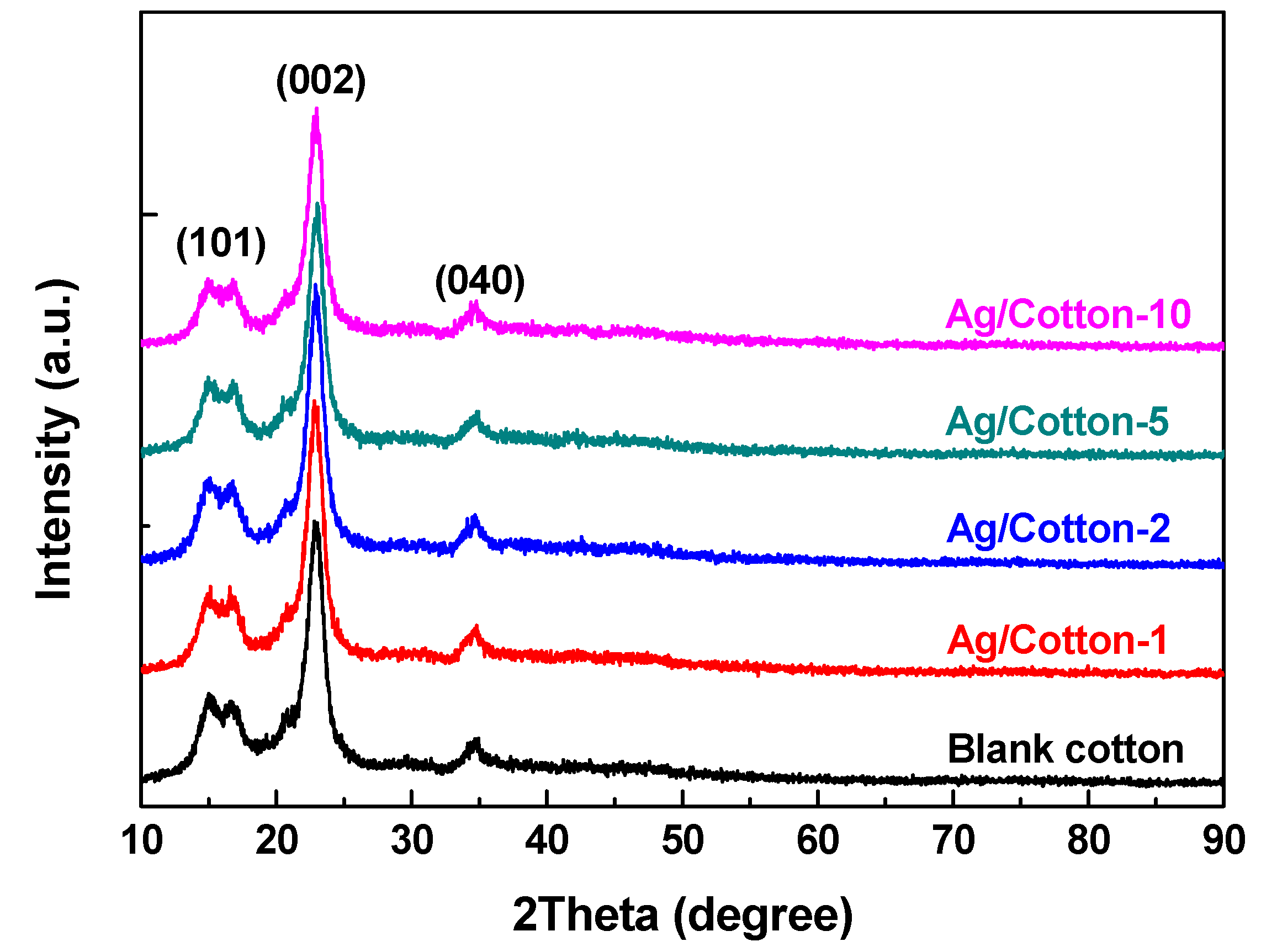
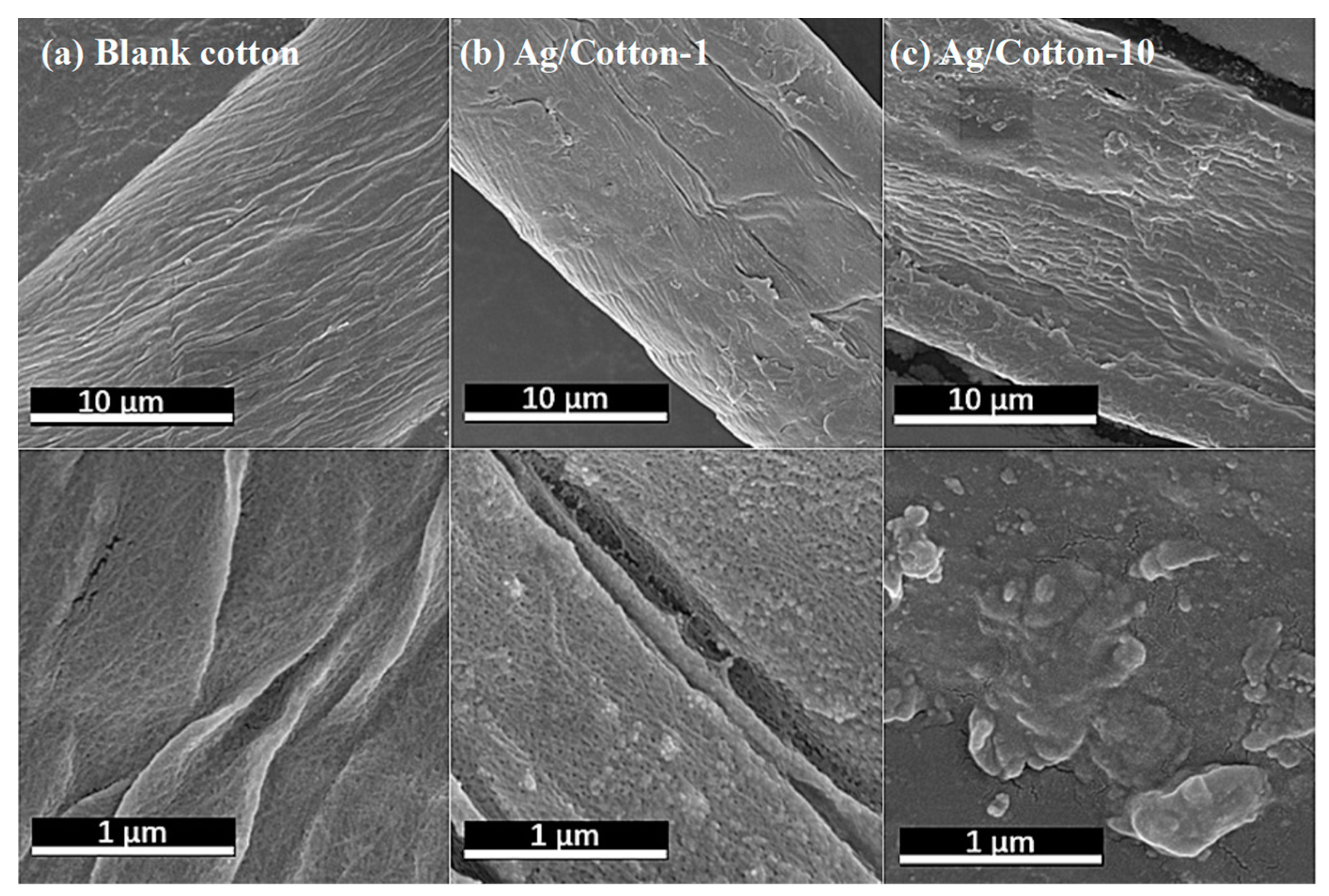
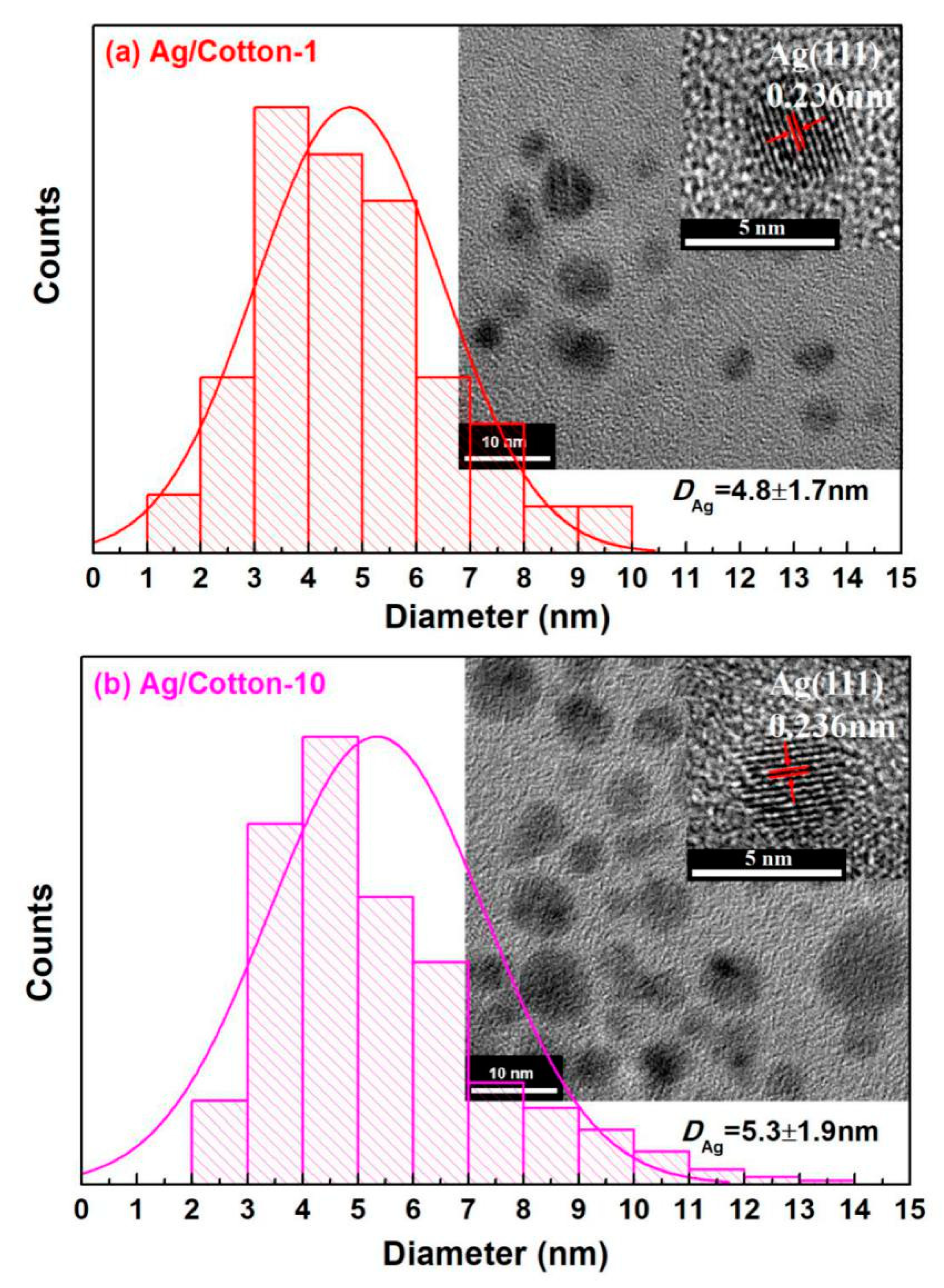
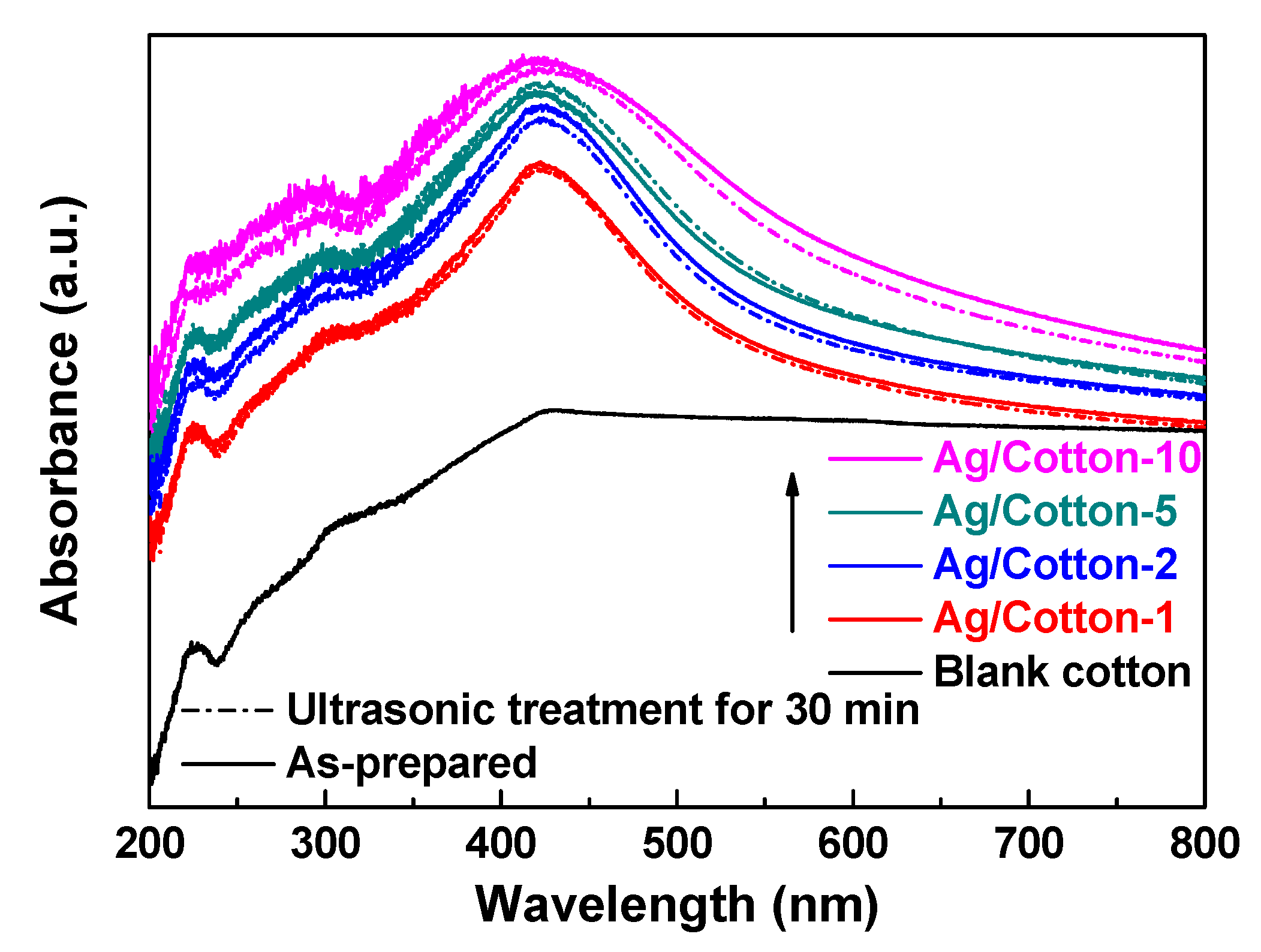
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nikiforov, A.; Deng, X.; Xiong, Q.; Cvelbar, U.; DeGeyter, N.; Morent, R.; Leys, C. Non-thermal plasma technology for the development of antimicrobial surfaces: A review. J. Phys. D Appl. Phys. 2016, 49, 204002. [Google Scholar] [CrossRef]
- Cheong, Y.K.; Calvo-Castro, J.; Ciric, L.; Edirisinghe, M.; Cloutman-Green, E.; Illangakoon, U.E.; Kang, Q.; Mahalingam, S.; Matharu, R.K.; Wilson, R.M.; et al. Characterisation of the chemical composition and structural features of novel antimicrobial nanoparticles. Nanomaterials 2017, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Altun, E.; Aydogdu, M.O.; Koc, F.; Crabbe-Mann, M.; Brako, F.; Kaur-Matharu, R.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Gunduz, O.; et al. Novel making of bacterial cellulose blended polymeric fiber bandages. Macromol. Mater. Eng. 2018, 303, 1700607. [Google Scholar] [CrossRef]
- Matharu, R.K.; Porwal, H.; Ciric, L.; Edirisinghe, M. The effect of graphene–poly (methyl methacrylate) fibres on microbial growth. Interface Focus 2018, 8, 20170058. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial activity of tellurium-loaded polymeric fiber meshes. J. Appl. Polym. Sci. 2018, 135, 46368. [Google Scholar] [CrossRef]
- Simončič, B.; Klemenčič, D. Preparation and performance of silver as an antimicrobial agent for textiles: A review. Text. Res. J. 2016, 86, 210–223. [Google Scholar] [CrossRef]
- Cheng, F.; Betts, J.W.; Kelly, S.M.; Schaller, J.; Heinze, T. Synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using aminocellulose as a combined reducing and capping reagent. Green Chem. 2013, 15, 989–998. [Google Scholar] [CrossRef]
- Wu, M.; Ma, B.; Pan, T.; Chen, S.; Sun, J. Silver-nanoparticle-colored cotton fabrics with tunable colors and durable antibacterial and self-healing superhydrophobic properties. Adv. Funct. Mater. 2016, 26, 569–576. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, W.; Duan, P.; Chen, J.; Zhang, Y.; Fu, F.; Liu, X. One-pot fabrication of durable antibacterial cotton fabric coated with silver nanoparticles via carboxymethyl chitosan as a binder and stabilizer. Carbohyd. Polym. 2019, 204, 42–49. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Mahalingam, S.; Wang, K.; Cheong, Y.K.; Canales, E.; Ren, G.G.; Cloutman-Green, E.; Edirisinghe, M.; Ciric, L. Gyrospun antimicrobial nanoparticle loaded fibrous polymeric filters. Mater. Sci. Eng. C Mater. 2017, 74, 315–324. [Google Scholar] [CrossRef]
- Xu, Z.; Mahalingam, S.; Rohn, J.L.; Ren, G.; Edirisinghe, M. Physio-chemical and antibacterial characteristics of pressure spun nylon nanofibres embedded with functional silver nanoparticles. Mater. Sci. Eng. C Mater. 2015, 56, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Heseltine, P.L.; Ahmed, J.; Edirisinghe, M. Developments in pressurized gyration for the mass production of polymeric fibers. Macromol. Mater. Eng. 2018, 303, 1800218. [Google Scholar] [CrossRef]
- Bacciarelli-Ulacha, A.; Rybicki, E.; Matyjas-Zgondek, E.; Pawlaczyk, A.; Szynkowska, M.I. A new method of finishing of cotton fabric by in situ synthesis of silver nanoparticles. Ind. Eng. Chem. Res. 2014, 53, 4147–4155. [Google Scholar] [CrossRef]
- Raza, Z.A.; Rehman, A.; Mohsin, M.; Bajwa, S.Z.; Anwar, F.; Naeem, A.; Ahmad, N. Development of antibacterial cellulosic fabric via clean impregnation of silver nanoparticles. J. Clean. Prod. 2015, 101, 377–386. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shaarawy, S.; Hebeish, A.A. Bactericidal finishing of loomstate, scoured and bleached cotton fibres via sustainable in-situ synthesis of silver nanoparticles. Int. J. Biol. Macromol. 2018, 106, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- El-Rafie, M.H.; Shaheen, T.I.; Mohamed, A.A.; Hebeish, A. Bio-synthesis and applications of silver nanoparticles onto cotton fabrics. Carbohyd. Polym. 2012, 90, 915–920. [Google Scholar] [CrossRef]
- Rehan, M.; Barhoum, A.; Van Assche, G.; Dufresne, A.; Gätjen, L.; Wilken, R. Towards multifunctional cellulosic fabric: UV photo-reduction and in-situ synthesis of silver nanoparticles into cellulose fabrics. Int. J. Biol. Macromol. 2017, 98, 877–886. [Google Scholar] [CrossRef]
- Di, L.; Zhang, J.; Zhang, X. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process. Polym. 2018, 15, 1700234. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C.J. Catalyst preparation with plasmas: How does it work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Di, L.; Li, Z.; Zhang, X.; Wang, H.; Fan, Z. Reduction of supported metal ions by a safe atmospheric pressure alcohol cold plasma method. Catal. Today 2019. [Google Scholar] [CrossRef]
- Dao, V.D.; Choi, Y.; Yong, K.; Larina, L.L.; Shevaleevskiy, O.; Choi, H.S. A facile synthesis of bimetallic AuPt nanoparticles as a new transparent counter electrode for quantum-dot-sensitized solar cells. J. Power. Sources 2015, 274, 831–838. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Chu, W.; Xu, J.; Hong, J.; Lin, T.; Khodakov, A. Design of efficient Fischer Tropsch cobalt catalysts via plasma enhancement: Reducibility and performance. Catal. Today 2015, 256, 41–48. [Google Scholar] [CrossRef]
- Di, L.; Zhang, J.; Ma, C.; Tu, X.; Zhang, X. Atmospheric-pressure dielectric barrier discharge cold plasma for synthesizing high performance Pd/C formic acid dehydrogenation catalyst. Catal. Today 2019. [Google Scholar] [CrossRef]
- Li, Z.; Meng, J.; Wang, W.; Wang, Z.; Li, M.; Chen, T.; Liu, C.J. The room temperature electron reduction for the preparation of silver nanoparticles on cotton with high antimicrobial activity. Carbohyd. Polym. 2017, 161, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Gallon, H.J.; Twigg, M.V.; Gorry, P.A.; Whitehead, J.C. Dry reforming of methane over a Ni/Al2O3 catalyst in a coaxial dielectric barrier discharge reactor. J. Phys. D Appl. Phys. 2011, 44, 274007. [Google Scholar] [CrossRef]
- Hu, S.; Li, F.; Fan, Z.; Gui, J. Improved photocatalytic hydrogen production property over Ni/NiO/N–TiO2−x heterojunction nanocomposite prepared by NH3 plasma treatment. J. Power Sources 2014, 250, 30–39. [Google Scholar] [CrossRef]
- Di, L.; Li, Z.; Lee, B.; Park, D.W. An alternative atmospheric-pressure cold plasma method for synthesizing Pd/P25 catalysts with the assistance of ethanol. Int. J. Hydrog. Energy 2017, 42, 11372–11378. [Google Scholar] [CrossRef]
- Audier, P.; Fénot, M.; Bénard, N.; Moreau, E. Flow control of an elongated jet in cross-flow: Film cooling effectiveness enhancement using surface dielectric barrier discharge plasma actuator. Appl. Phys. Lett. 2016, 108, 084103. [Google Scholar] [CrossRef]
- Park, Y.; Oh, K.S.; Oh, J.; Seok, D.C.; Kim, S.B.; Yoo, S.J.; Lee, M.J. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process Polym. 2018, 15, 1600056. [Google Scholar] [CrossRef]
- Di, L.B.; Li, X.S.; Shi, C.; Xu, Y.; Zhao, D.Z.; Zhu, A.M. Atmospheric-pressure plasma CVD of TiO2 photocatalytic films using surface dielectric barrier discharge. J. Phys. D Appl. Phys. 2009, 42, 032001. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Ishijima, T.; Seto, T.; Osawa, N.; Wedaa, H.; Otani, Y. Characterization of surface dielectric barrier discharge influenced by intermediate frequency for ozone production. Plasma Sources Sci. Teachnol. 2016, 25, 035012. [Google Scholar] [CrossRef]
- Jeon, J.; Rosentreter, T.M.; Li, Y.; Isbary, G.; Thomas, H.M.; Zimmermann, J.L.; Shimizu, T. Bactericidal Agents Produced by Surface Micro-Discharge (SMD) Plasma by Controlling Gas Compositions. Plasma Process Polym. 2014, 11, 426–436. [Google Scholar] [CrossRef]
- Qi, B.; Di, L.; Xu, W.; Zhang, X. Dry plasma reduction to prepare a high performance Pd/C catalyst at atmospheric pressure for CO oxidation. J. Mater. Chem. A 2014, 2, 11885–11890. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Montazer, M.; Keshvari, A.; Kahali, P. Tragacanth gum/nano silver hydrogel on cotton fabric: In-situ synthesis and antibacterial properties. Carbohyd. Polym. 2016, 154, 257–266. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.D. Internally dispersed synthesis of uniform silver nanoparticles via in situ reduction of [Ag(NH3)2]+ along natural microfibrillar substructures of cotton fiber. Cellulose 2014, 21, 2963–2972. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, Z.; Cao, D.; Liu, C.J. Covalent organic polymer supported palladium catalysts for CO oxidation. Chem. Commun. 2013, 49, 5633–5635. [Google Scholar] [CrossRef]
- Wang, W.; Yang, M.; Wang, Z.; Yan, J.; Liu, C. Silver nanoparticle aggregates by room temperature electron reduction: Preparation and characterization. RSC Adv. 2014, 4, 63079–63084. [Google Scholar] [CrossRef]
- Cölfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Di, L.; Zhang, X.; Wang, H. A Surface Dielectric Barrier Discharge Plasma for Preparing Cotton-Fabric-Supported Silver Nanoparticles. Nanomaterials 2019, 9, 961. https://doi.org/10.3390/nano9070961
Fan Z, Di L, Zhang X, Wang H. A Surface Dielectric Barrier Discharge Plasma for Preparing Cotton-Fabric-Supported Silver Nanoparticles. Nanomaterials. 2019; 9(7):961. https://doi.org/10.3390/nano9070961
Chicago/Turabian StyleFan, Zhiyuan, Lanbo Di, Xiuling Zhang, and Hongyang Wang. 2019. "A Surface Dielectric Barrier Discharge Plasma for Preparing Cotton-Fabric-Supported Silver Nanoparticles" Nanomaterials 9, no. 7: 961. https://doi.org/10.3390/nano9070961
APA StyleFan, Z., Di, L., Zhang, X., & Wang, H. (2019). A Surface Dielectric Barrier Discharge Plasma for Preparing Cotton-Fabric-Supported Silver Nanoparticles. Nanomaterials, 9(7), 961. https://doi.org/10.3390/nano9070961



