Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications
Abstract
1. Introduction
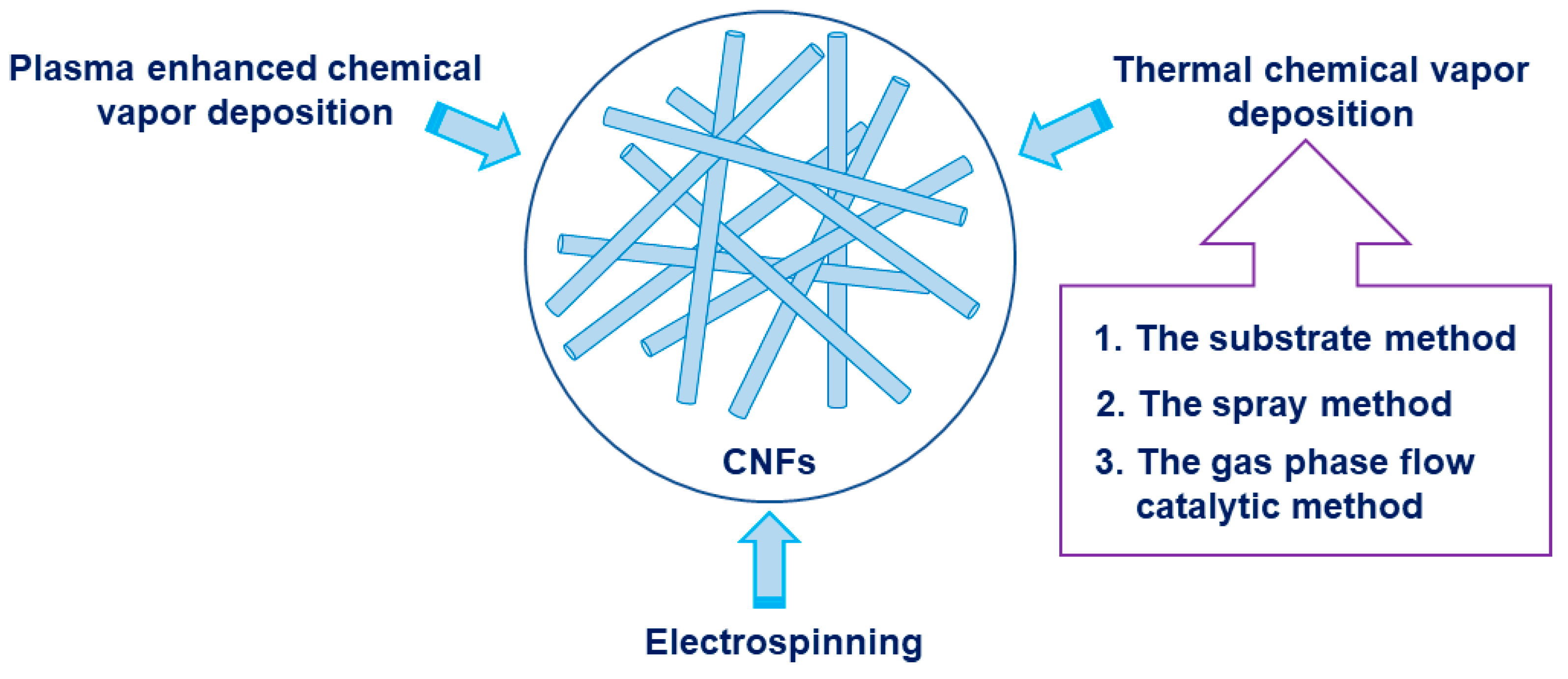
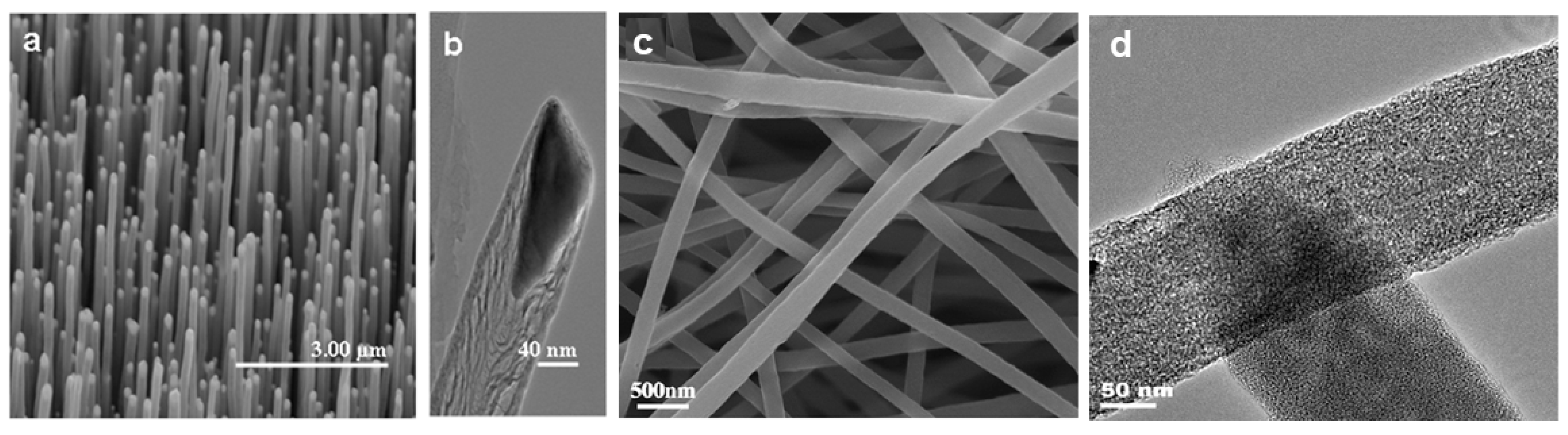
2. Synthesis of Carbon Nanofibers (CNFs)
2.1. Thermal Chemical Vapor Deposition
2.1.1. The Substrate Method
2.1.2. The Spray Method
2.1.3. The Gas Phase Flow Catalytic Method
2.2. Plasma-Enhanced Chemical Vapor Deposition (PECVD)
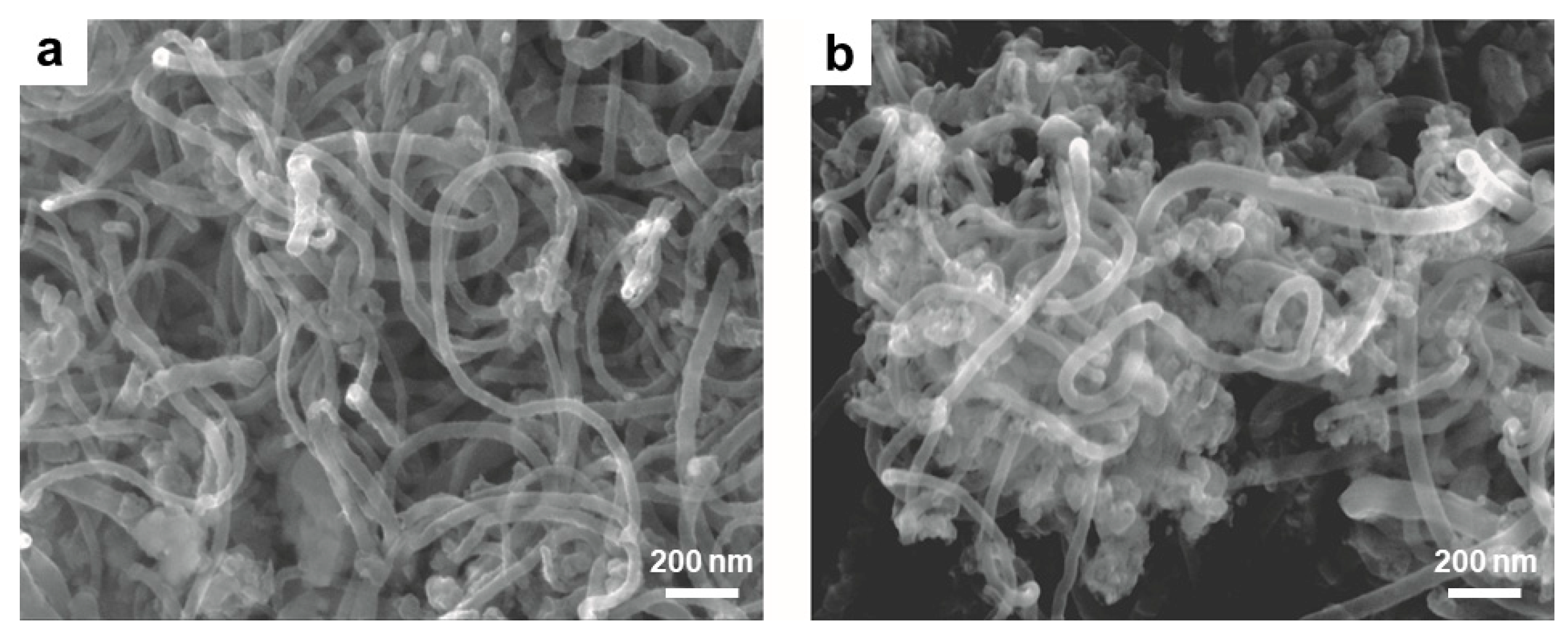
2.3. Electrospinning
3. Design and Synthesis of CNF-Based Nanomaterials
3.1. Pure CNFs
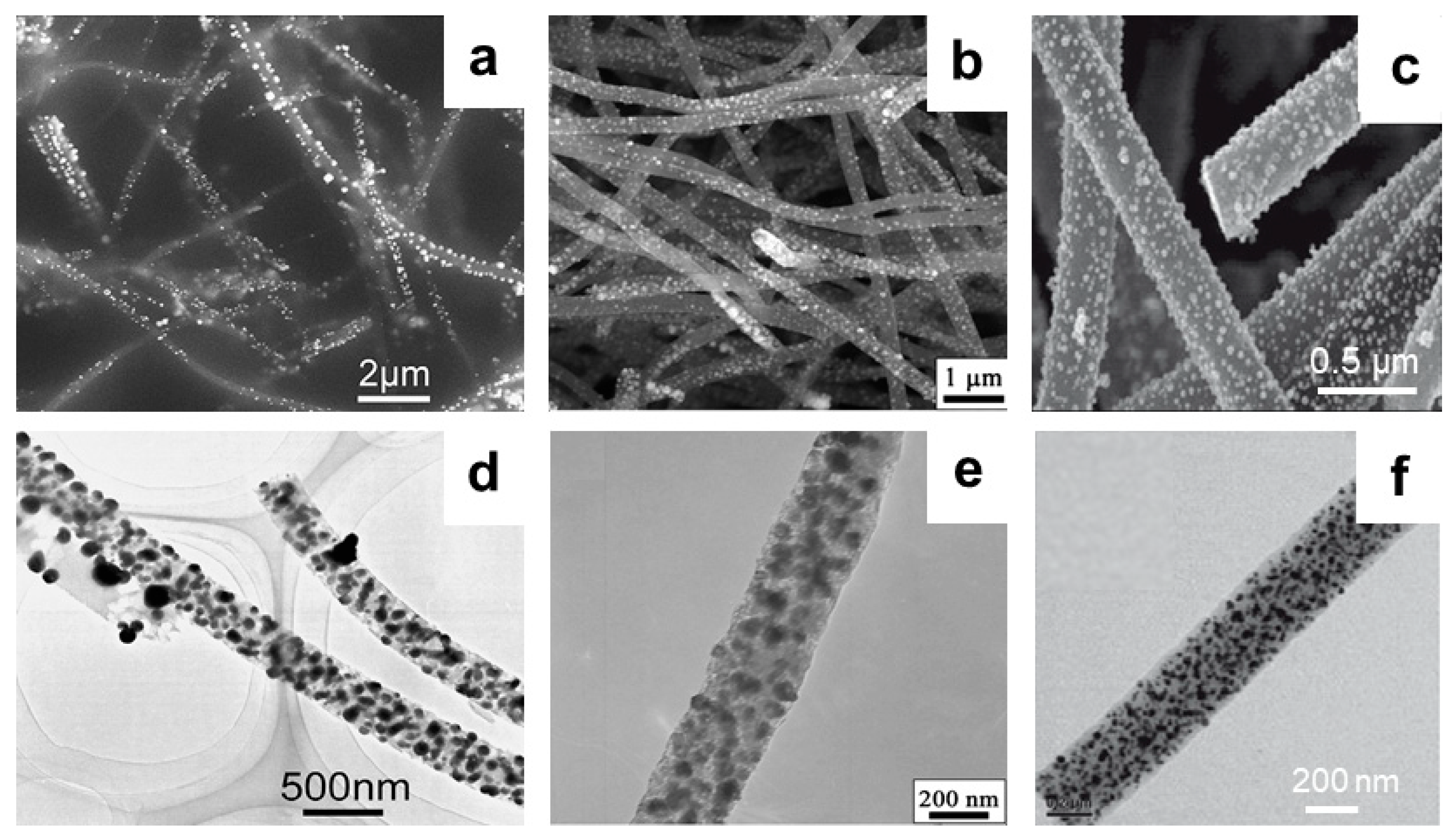
3.2. CNFs Modified with Metal NPs
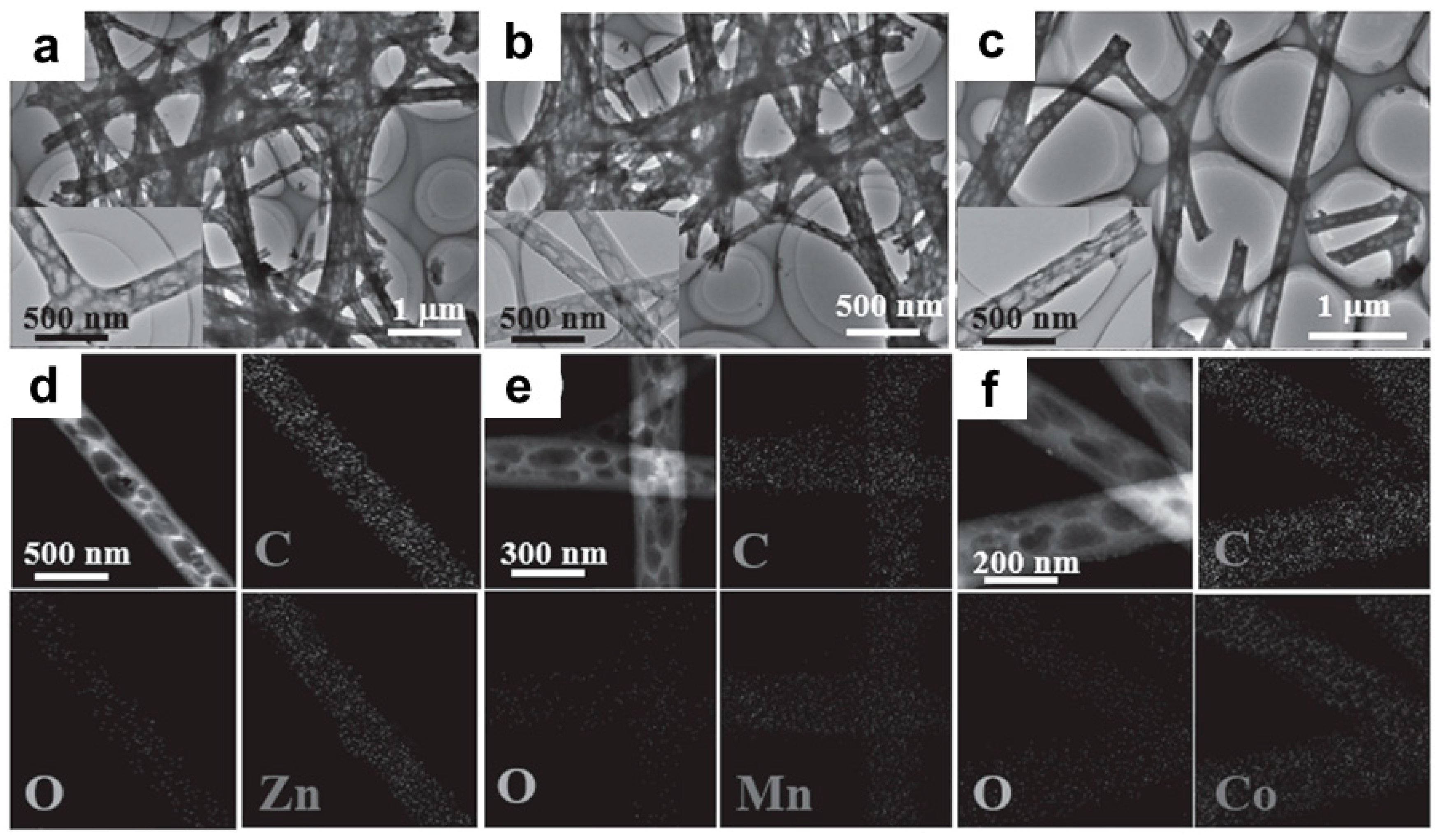
3.3. CNFs Modified with Metal Oxides
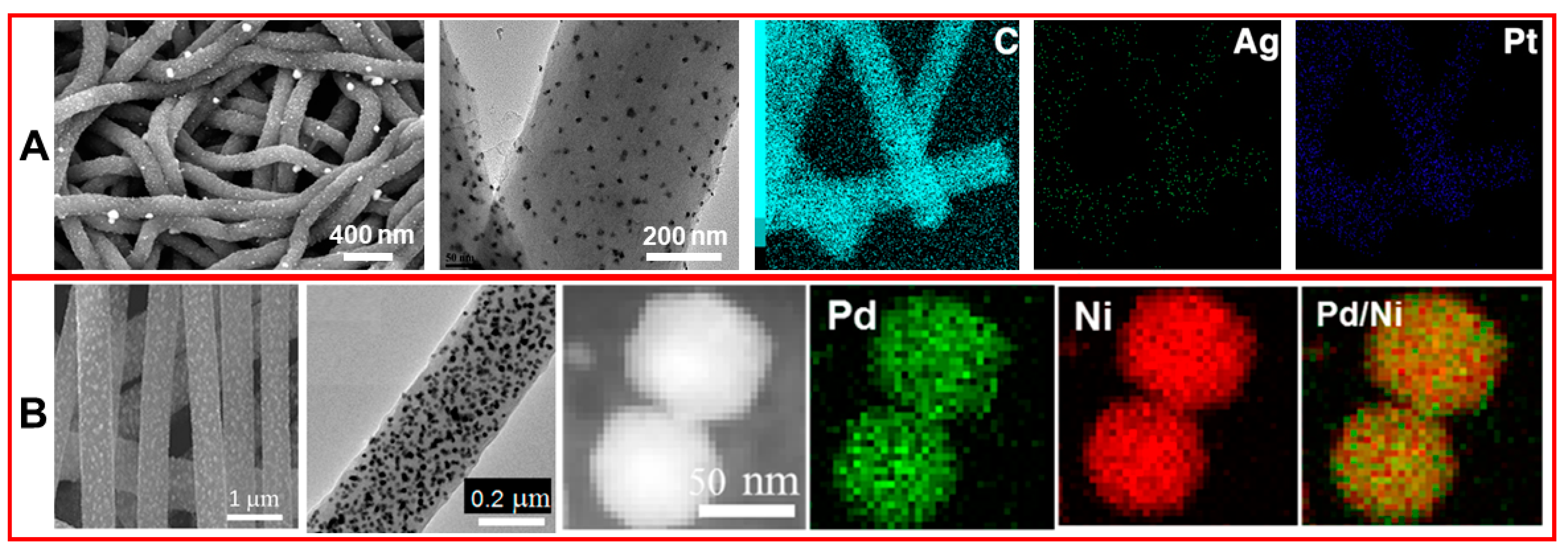
3.4. CNFs Modified with Alloys
3.5. CNFs Modified with Silica and Polymers
4. Sensor Applications of CNF-Based Nanomaterials
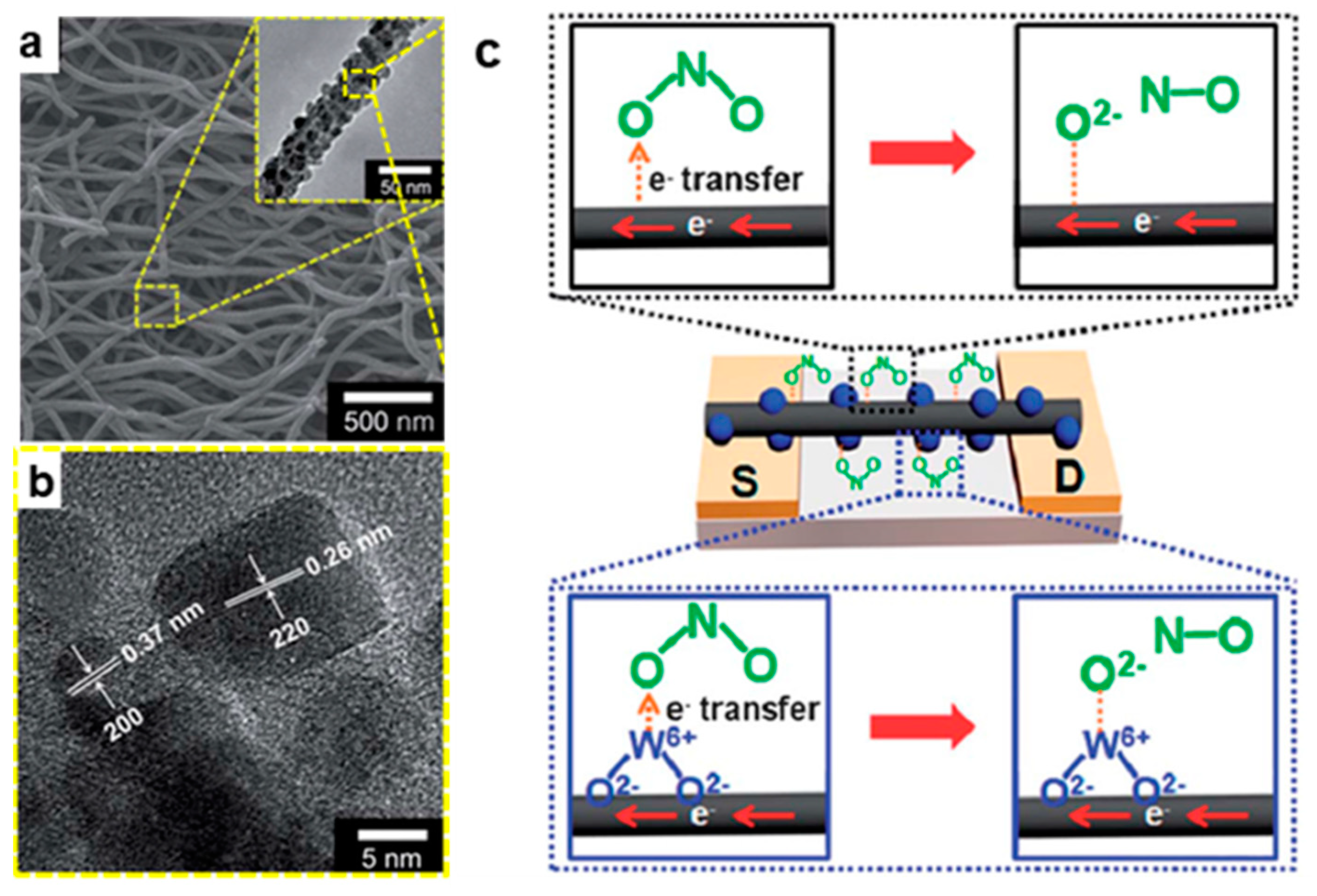
4.1. Gas Sensors
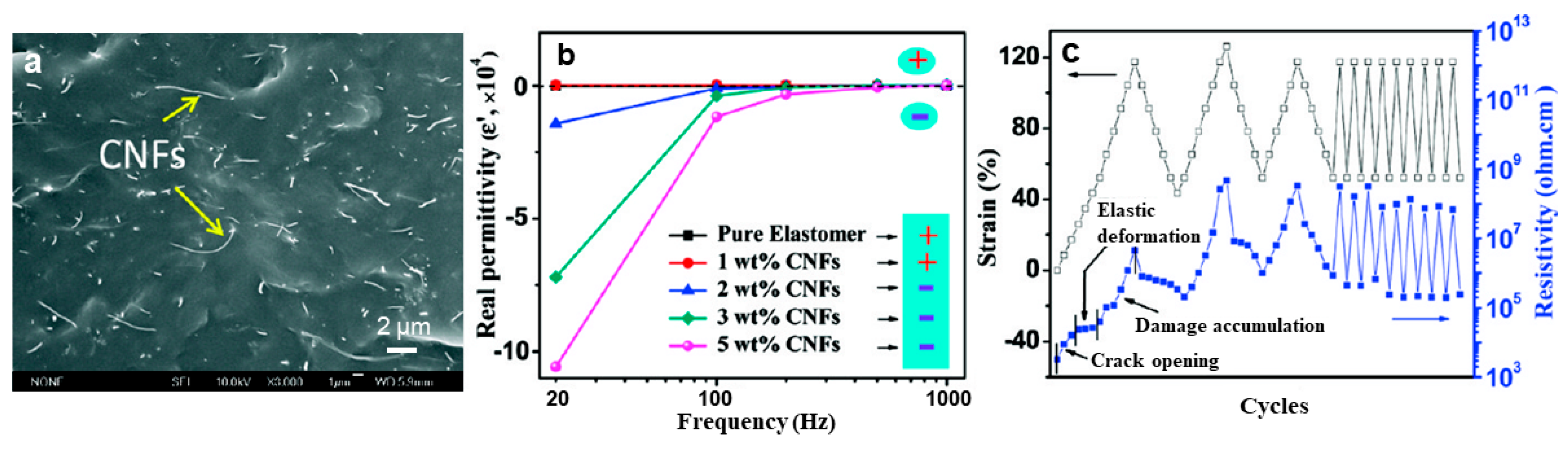
4.2. Strain/Pressure Sensors.
4.3. Sensors of Small Molecules
4.4. Sensors of Biomacromolecules
5. Conclusions and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, S.M.; Koneswaran, M.; Narayanaswamy, R. A review on fluorescent inorganic nanoparticles for optical sensing applications. RSC Adv. 2016, 6, 21624–21661. [Google Scholar] [CrossRef]
- Namdari, P.; Daraee, H.; Eatemadi, A. Recent advances in silicon nanowire biosensors: Synthesis methods, properties, and applications. Nanoscale Res. Lett. 2016, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, A.G.; Wei, G. Graphene-based aptasensors: From molecule-interface interactions to sensor design and biomedical diagnostics. Analyst 2018, 143, 1526–1543. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.J.; Wu, A.G.; Wei, G. Designed graphene-peptide nanocomposites for biosensor applications: A review. Anal. Chim. Acta 2017, 985, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, R.; Chen, R.S.; Wang, J.; Xiang, L. 3D architectured graphene/metal oxide hybrids for gas sensors: A review. Sensors 2018, 18, 1456. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.I. Fundamental properties of one-dimensional zinc oxide nanomaterials and implementations in various detection modes of enhanced biosensing. Annu. Rev. Phys. Chem. 2016, 67, 691–717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.X.; Zhao, M.M.; Qiu, J.Q.; Lai, W.Y.; Pang, H.; Huang, W. One dimensional silver-based nanomaterials: Preparations and electrochemical applications. Small 2017, 13, 1701091. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Dou, Y.Q.; Wu, Z.X.; Liu, H.J.; Qian, X.F.; Gu, D.; Xia, Y.Y.; Tu, B.; Zhao, D.Y. A self-template strategy for the synthesis of mesoporous carbon nanofibers as advanced supercapacitor electrodes. Adv. Energy Mater. 2011, 1, 382–386. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon nanotube-based chemical sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef]
- Chen, L.F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Ning, P.G.; Duan, X.C.; Ju, X.K.; Lin, X.P.; Tong, X.B.; Pan, X.; Wang, T.H.; Li, Q.H. Facile synthesis of carbon nanofibers/MnO2 nanosheets as high-performance electrodes for asymmetric supercapacitors. Electrochim. Acta 2016, 210, 754–761. [Google Scholar] [CrossRef]
- Teradal, N.L.; Jelinek, R. Carbon nanomaterials in biological studies and biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, A.M.; El Backly, R.M.; Taha, N.A.; El-Maghraby, A.; Kandil, S.H. Preparation and characterization of carbon nanofibrous/hydroxyapatite sheets for bone tissue engineering. Mater. Sci. Eng. C 2017, 76, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, M.F.; Zhang, X.P.; Xie, G.C.; Su, Z.Q.; Wei, G. Nanoporous carbon nanofibers decorated with platinum nanoparticles for non-enzymatic electrochemical sensing of H2O2. Nanomaterials 2015, 5, 1891–1905. [Google Scholar] [CrossRef]
- Magana, J.R.; Kolen’ko, Y.V.; Deepak, F.L.; Solans, C.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Shrestha, L.K.; Rodriguez-Abreu, C. From chromonic self-assembly to hollow carbon nanofibers: Efficient materials in supercapacitor and vapor-sensing applications. ACS Appl. Mater. Interfaces 2016, 8, 31231–31238. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Feng, Y.; Liang, H.W.; Wu, Z.Y.; Yu, S.H. Macroscopic-scale three-dimensional carbon nanofiber architectures for electrochemical energy storage devices. Adv. Energy Mater. 2017, 7, 1700826. [Google Scholar] [CrossRef]
- Shen, Y.; Li, L.; Xiao, K.J.; Xi, J.Y. Constructing three-dimensional hierarchical architectures by integrating carbon nanofibers into graphite felts for water purification. ACS Sustain. Chem. Eng. 2016, 4, 2351–2358. [Google Scholar] [CrossRef]
- Zhang, L.F.; Aboagye, A.; Kelkar, A.; Lai, C.L.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Feng, L.C.; Xie, N.; Zhong, J. Carbon nanofibers and their composites: A review of synthesizing, properties and applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef]
- Zhang, B.A.; Kang, F.Y.; Tarascon, J.M.; Kim, J.K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Duan, X.Z.; Ji, J.; Qian, G.; Zhou, X.G.; Chen, D. Recent advances in synthesis of reshaped fe and Ni particles at the tips of carbon nanofibers and their catalytic applications. Catal. Today 2015, 249, 2–11. [Google Scholar] [CrossRef]
- De Jong, K.P.; Geus, J.W. Carbon nanofibers: Catalytic synthesis and applications. Catal. Rev. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, M.H.; Sundararaj, U. Review of the mechanical properties of carbon nanofiber/polymer composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2126–2142. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, H.Y.; Jiang, C.; Zhang, T.H.; Zhan, R.; Li, X.W.; Li, J.F.; Tian, J.H.; Yang, R.Z. NiCo alloy nanoparticles decorated on N-doped carbon nanofibers as highly active and durable oxygen electrocatalyst. Adv. Funct. Mater. 2018, 28, 1705094. [Google Scholar] [CrossRef]
- Sharma, C.S.; Katepalli, H.; Sharma, A.; Madou, M. Fabrication and electrical conductivity of suspended carbon nanofiber arrays. Carbon 2011, 49, 1727–1732. [Google Scholar] [CrossRef]
- Zahid, M.U.; Pervaiz, E.; Hussain, A.; Shahzad, M.I.; Niazi, M.B.K. Synthesis of carbon nanomaterials from different pyrolysis techniques: A review. Mater. Res. Express 2018, 5, 052002. [Google Scholar] [CrossRef]
- Garcia-Bordeje, E.; Kvande, I.; Chen, D.; Ronning, M. Synthesis of composite materials of carbon nanofibres and ceramic monoliths with uniform and tuneable nanofibre layer thickness. Carbon 2007, 45, 1828–1838. [Google Scholar] [CrossRef]
- Endo, M.; Takeuchi, K.; Hiraoka, T.; Furuta, T.; Kasai, T.; Sun, X.; Kiang, C.H.; Dresselhaus, M.S. Stacking nature of graphene layers in carbon nanotubes and nanofibres. J. Phys. Chem. Solids 1997, 58, 1707–1712. [Google Scholar] [CrossRef]
- Guan, H.J.; Zhang, J.; Liu, Y.; Zhao, Y.F.; Zhang, B. Rapid quantitative determination of hydrogen peroxide using an electrochemical sensor based on PtNi alloy/CeO2 plates embedded in n-doped carbon nanofibers. Electrochim. Acta 2019, 295, 997–1005. [Google Scholar] [CrossRef]
- Ci, L.J.; Wei, J.Q.; Wei, B.Q.; Liang, J.; Xu, C.L.; Wu, D.H. Carbon nanofibers and single-walled carbon nanotubes prepared by the floating catalyst method. Carbon 2001, 39, 329–335. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Mishakov, I.V.; Rudneva, Y.V.; Plyusnin, P.E.; Shubin, Y.V.; Korneev, D.V.; Vedyagin, A.A. Formation of active sites of carbon nanofibers growth in self-organizing Ni-Pd catalyst during hydrogen-assisted decomposition of 1,2-dichloroethane. Ind. Eng. Chem. Res. 2019, 58, 685–694. [Google Scholar] [CrossRef]
- Saidin, M.A.R.; Ismail, A.F.; Sanip, S.M.; Goh, P.S.; Aziz, M.; Tanemura, M. Controlled growth of carbon nanofibers using plasma enhanced chemical vapor deposition: Effect of catalyst thickness and gas ratio. Thin Solid Films 2012, 520, 2575–2581. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Synthesis of vertically aligned carbon nanofibers using inductively coupled plasma-enhanced chemical vapor deposition. Electr. Eng. 2018, 100, 997–1002. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, S.C. Modelling the effects of nitrogen doping on the carbon nanofiber growth via catalytic plasma-enhanced chemical vapour deposition process. Contrib. Plasma Phys. 2019, 59, 72–85. [Google Scholar] [CrossRef]
- Siddiqui, S.; Arumugam, P.U.; Chen, H.; Li, J.; Meyyappan, M. Characterization of carbon nanofiber electrode arrays using electrochemical impedance spectroscopy: Effect of scaling down electrode size. ACS Nano 2010, 4, 955–961. [Google Scholar] [CrossRef]
- Maitra, T.; Sharma, S.; Srivastava, A.; Cho, Y.K.; Madou, M.; Sharma, A. Improved graphitization and electrical conductivity of suspended carbon nanofibers derived from carbon nanotube/polyacrylonitrile composites by directed electrospinning. Carbon 2012, 50, 1753–1761. [Google Scholar] [CrossRef]
- Su, Z.Q.; Ding, J.W.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef]
- Wang, S.X.; Yap, C.C.; He, J.T.; Chen, C.; Wong, S.Y.; Li, X. Electrospinning: A facile technique for fabricating functional nanofibers for environmental applications. Nanotechnol. Rev. 2016, 5, 51–73. [Google Scholar] [CrossRef]
- He, Z.X.; Li, M.M.; Li, Y.H.; Zhu, J.; Jiang, Y.Q.; Meng, W.; Zhou, H.Z.; Wang, L.; Dai, L. Flexible electrospun carbon nanofiber embedded with TiO2 as excellent negative electrode for vanadium redox flow battery. Electrochim. Acta 2018, 281, 601–610. [Google Scholar] [CrossRef]
- Chung, D.D.L. Carbon materials for structural self-sensing, electromagnetic shielding and thermal interfacing. Carbon 2012, 50, 3342–3353. [Google Scholar] [CrossRef]
- Matlock-Colangelo, L.; Baeumner, A.J. Recent progress in the design of nanofiber-based biosensing devices. Lab Chip 2012, 12, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Marin-Barroso, E.; Messina, G.A.; Bertolino, F.A.; Raba, J.; Pereira, S.V. Electrochemical immunosensor modified with carbon nanofibers coupled to a paper platform for the determination of gliadins in food samples. Anal. Methods 2019, 11, 2170–2178. [Google Scholar] [CrossRef]
- Yue, Y.; Hu, G.Z.; Zheng, M.B.; Guo, Y.; Cao, J.M.; Shao, S.J. A mesoporous carbon nanofiber-modified pyrolytic graphite electrode used for the simultaneous determination of dopamine, uric acid, and ascorbic acid. Carbon 2012, 50, 107–114. [Google Scholar] [CrossRef]
- Koehne, J.E.; Marsh, M.; Boakye, A.; Douglas, B.; Kim, I.Y.; Chang, S.Y.; Jang, D.P.; Bennet, K.E.; Kimble, C.; Andrews, R.; et al. Carbon nanofiber electrode array for electrochemical detection of dopamine using fast scan cyclic voltammetry. Analyst 2011, 136, 1802–1805. [Google Scholar] [CrossRef]
- Rand, E.; Periyakaruppan, A.; Tanaka, Z.; Zhang, D.A.; Marsh, M.P.; Andrews, R.J.; Lee, K.H.; Chen, B.; Meyyappan, M.; Koehne, J.E. A carbon nanofiber based biosensor for simultaneous detection of dopamine and serotonin in the presence of ascorbic acid. Biosens. Bioelectron. 2013, 42, 434–438. [Google Scholar] [CrossRef]
- Periyakaruppan, A.; Gandhiraman, R.P.; Meyyappan, M.; Koehne, J.E. Label-free detection of cardiac troponin-I using carbon nanofiber based nanoelectrode arrays. Anal. Chem. 2013, 85, 3858–3863. [Google Scholar] [CrossRef]
- Tang, X.F.; Liu, Y.; Hou, H.Q.; You, T.Y. A nonenzymatic sensor for xanthine based on electrospun carbon nanofibers modified electrode. Talanta 2011, 83, 1410–1414. [Google Scholar] [CrossRef]
- Tang, X.F.; Liu, Y.; Hou, H.Q.; You, T.Y. Electrochemical determination of L-tryptophan, L-tyrosine and L-cysteine using electrospun carbon nanofibers modified electrode. Talanta 2010, 80, 2182–2186. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.H.; Huang, J.S.; Chen, P.Q.; Liu, Y.; Hou, H.Q.; You, T.Y. Simultaneous determination of catechol and hydroquinone using electrospun carbon nanofibers modified electrode. Sens. Actuators B Chem. 2012, 163, 179–185. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.W.; Xu, L.; Hou, H.Q.; You, T.Y. A novel and simple route to prepare a pt nanoparticle-loaded carbon nanofiber electrode for hydrogen peroxide sensing. Biosens. Bioelectron. 2011, 26, 4585–4590. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.E.; Hu, G.Z.; Nitze, F.; Barzegar, H.R.; Sharifi, T.; Tai, C.W.; Wagberg, T. Synthesis of palladium/helical carbon nanofiber hybrid nanostructures and their application for hydrogen peroxide and glucose detection. ACS Appl. Mater. Interfaces 2013, 5, 12017–12022. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.J.; Loaiza, O.A.; Anorga, L.; Jubete, E.; Borghei, M.; Ruiz, V.; Ochoteco, E.; Cabanero, G.; Grande, H.J. Disposable amperometric biosensor based on lactate oxidase immobilised on platinum nanoparticle-decorated carbon nanofiber and poly(diallyldimethylammonium chloride) films. Biosens. Bioelectron. 2014, 56, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Luo, L.; Pang, Z.Y.; Ding, L.; Wang, Q.Q.; Ke, H.Z.; Huang, F.L.; Wei, Q.F. Novel phenolic biosensor based on a magnetic polydopamine-laccase-nickel nanoparticle loaded carbon nanofiber composite. ACS Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Wang, D.W.; Hou, H.Q.; You, T.Y. Electrospun palladium nanoparticle-loaded carbon nanofibers and their electrocatalytic activities towards hydrogen peroxide and nadh. Adv. Funct. Mater. 2008, 18, 441–448. [Google Scholar] [CrossRef]
- Huang, J.S.; Liu, Y.; Hou, H.Q.; You, T.Y. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.S.; Wang, D.W.; Hou, H.Q.; You, T.Y. Electrochemical determination of oxalic acid using palladium nanoparticle-loaded carbon nanofiber modified electrode. Anal. Methods 2010, 2, 855–859. [Google Scholar] [CrossRef]
- Claramunt, S.; Monereo, O.; Boix, M.; Leghrib, R.; Prades, J.D.; Cornet, A.; Merino, P.; Merino, C.; Cirera, A. Flexible gas sensor array with an embedded heater based on metal decorated carbon nanofibres. Sens. Actuators B Chem. 2013, 187, 401–406. [Google Scholar] [CrossRef]
- Hu, G.Z.; Zhou, Z.P.; Guo, Y.; Hou, H.Q.; Shao, S.J. Electrospun rhodium nanoparticle-loaded carbon nanofibers for highly selective amperometric sensing of hydrazine. Electrochem. Commun. 2010, 12, 422–426. [Google Scholar] [CrossRef]
- Fu, J.P.; Qiao, H.; Li, D.W.; Luo, L.; Chen, K.; Wei, Q.F. Laccase biosensor based on electrospun copper/carbon composite nanofibers for catechol detection. Sensors 2014, 14, 3543–3556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Teng, H.; Hou, H.Q.; You, T.Y. Nonenzymatic glucose sensor based on renewable electrospun Ni nanoparticle-loaded carbon nanofiber paste electrode. Biosens. Bioelectron. 2009, 24, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Rathod, D.; Dickinson, C.; Egan, D.; Dempsey, E. Platinum nanoparticle decoration of carbon materials with applications in non-enzymatic glucose sensing. Sens. Actuators B Chem. 2010, 143, 547–554. [Google Scholar] [CrossRef]
- Hu, N.; Itoi, T.; Akagi, T.; Kojima, T.; Xue, J.M.; Yan, C.; Atobe, S.; Fukunaga, H.; Yuan, W.F.; Ning, H.M.; et al. Ultrasensitive strain sensors made from metal-coated carbon nanofiller/epoxy composites. Carbon 2013, 51, 202–212. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, O.S.; Park, S.J.; Park, E.Y.; You, S.A.; Yoon, H.; Jang, J. Fabrication of ultrafine metal-oxide-decorated carbon nanofibers for dmmp sensor application. ACS Nano 2011, 5, 7992–8001. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kwon, O.S.; Shin, D.H.; Jang, J. Wo3 nanonodule-decorated hybrid carbon nanofibers for NO2 gas sensor application. J. Mater. Chem. A 2013, 1, 9099–9106. [Google Scholar] [CrossRef]
- Hu, A.; Curran, C.; Tran, C.; Kapllani, A.; Kalra, V. Fabrication of transition metal oxide-carbon nanofibers with novel hierarchical architectures. J. Nanosci. Nanotechnol. 2014, 14, 5501–5507. [Google Scholar] [CrossRef]
- Xia, G.L.; Zhang, L.J.; Fang, F.; Sun, D.L.; Guo, Z.P.; Liu, H.K.; Yu, X.B. General synthesis of transition metal oxide ultrafine nanoparticles embedded in hierarchically porous carbon nanofibers as advanced electrodes for lithium storage. Adv. Funct. Mater. 2016, 26, 6188–6196. [Google Scholar] [CrossRef]
- Huang, Y.P.; Miao, Y.E.; Ji, S.S.; Tjiu, W.W.; Liu, T.X. Electrospun carbon nanofibers decorated with Ag-Pt bimetallic nanoparticles for selective detection of dopamine. ACS Appl. Mater. Interfaces 2014, 6, 12449–12456. [Google Scholar] [CrossRef]
- Guo, Q.H.; Liu, D.; Zhang, X.P.; Li, L.B.; Hou, H.Q.; Niwa, O.; You, T.Y. Pd-Ni alloy nanoparticle/carbon nanofiber composites: Preparation, structure, and superior electrocatalytic properties for sugar analysis. Anal. Chem. 2014, 86, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, L.B.; Xiong, Y.P.; Liu, X.T.; Nsabimana, A.; Bo, X.J.; Guo, L.P. Bimetallic MCo (M= Cu, Fe, Ni, and Mn) nanoparticles doped-carbon nanofibers synthetized by electrospinning for nonenzymatic glucose detection. Sens. Actuators B Chem. 2015, 207, 614–622. [Google Scholar] [CrossRef]
- Wu, S.Y.; Zhang, J.; Ladani, R.B.; Rayindran, A.R.; Mouritz, A.P.; Kinloch, A.J.; Wang, C.H. Novel electrically conductive porous PDMS/carbon nanofiber composites for deformable strain sensors and conductors. ACS Appl. Mater. Interfaces 2017, 9, 14207–14215. [Google Scholar] [CrossRef] [PubMed]
- Vamvakaki, V.; Hatzimarinaki, M.; Chaniotakis, N. Bionumetically synthesized silica-carbon nanofiber architectures for the development of highly stable electrochemical biosensor systems. Anal. Chem. 2008, 80, 5970–5975. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.B.; Zhou, J.H.; Lu, W.; Liu, Q.; Li, J.H. Carbon nanofiber-based composites for the construction of mediator-free biosensors. Biosens. Bioelectron. 2008, 23, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Wei, S.Y.; Ryu, J.; Guo, Z.H. Strain-sensing elastomer/carbon nanofiber “metacomposites”. J. Phys. Chem. C 2011, 115, 13215–13222. [Google Scholar] [CrossRef]
- Baeza, F.J.; Galao, O.; Zornoza, E.; Garces, P. Multifunctional cement composites strain and damage sensors applied on reinforced concrete (RC) structural elements. Materials 2013, 6, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Azhari, F.; Banthia, N. Cement-based sensors with carbon fibers and carbon nanotubes for piezoresistive sensing. Cem. Concr. Compos. 2012, 34, 866–873. [Google Scholar] [CrossRef]
- Tallman, T.N.; Gungor, S.; Wang, K.W.; Bakis, C.E. Tactile imaging and distributed strain sensing in highly flexible carbon nanofiber/polyurethane nanocomposites. Carbon 2015, 95, 485–493. [Google Scholar] [CrossRef]
- Huang, J.S.; Liu, Y.; You, T.Y. Carbon nanofiber based electrochemical biosensors: A review. Anal. Methods 2010, 2, 202–211. [Google Scholar] [CrossRef]
- Adabi, M.; Saber, R.; Faridi-Majidi, R.; Faridbod, F. Performance of electrodes synthesized with polyacrylonitrile-based carbon nanofibers for application in electrochemical sensors and biosensors. Mater. Sci. Eng. C 2015, 48, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Perebikovsky, A.; Benice, O.; Holmberg, S.; Madou, M.; Ghazinejad, M. Rapid iodine sensing on mechanically treated carbon nanofibers. Sensors 2018, 18, 1486. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.S.; Wang, Q.; Yu, Y.; Chen, Z.; Cao, C.Y.; Song, W.G. Low-cost synthesis of graphitic carbon nanofibers as excellent room temperature sensors for explosive gases. J. Mater. Chem. 2012, 22, 15342–15347. [Google Scholar] [CrossRef]
- Zhang, J.T.; Zhu, Z.J.; Chen, C.M.; Chen, Z.; Cai, M.Q.; Qu, B.H.; Wang, T.H.; Zhang, M. ZnO-carbon nanofibers for stable, high response, and selective H2S sensors. Nanotechnology 2018, 29, 275501. [Google Scholar] [CrossRef] [PubMed]
- Petruci, J.F.D.; Fortes, P.R.; Kokoric, V.; Wilk, A.; Raimundo, I.M.; Cardoso, A.A.; Mizaikoff, B. Real-time monitoring of ozone in air using substrate-integrated hollow waveguide mid-infrared sensors. Sci. Rep. 2013, 3, 3174. [Google Scholar] [CrossRef] [PubMed]
- Kosterev, A.; Wysocki, G.; Bakhirkin, Y.; So, S.; Lewicki, R.; Fraser, M.; Tittel, F.; Curl, R.F. Application of quantum cascade lasers to trace gas analysis. Appl. Phys. B 2008, 90, 165–176. [Google Scholar] [CrossRef]
- Viciani, S.; de Cumis, M.S.; Borri, S.; Patimisco, P.; Sampaolo, A.; Scamarcio, G.; De Natale, P.; D’Amato, F.; Spagnolo, V. A quartz-enhanced photoacoustic sensor for H2S trace-gas detection at 2.6 μm. Appl. Phys. B 2015, 119, 21–27. [Google Scholar] [CrossRef]
- Klocke, J.L.; Mangold, M.; Allmendinger, P.; Hugi, A.; Geiser, M.; Jouy, P.; Faist, J.; Kottke, T. Single-shot sub-microsecond mid-infrared spectroscopy on protein reactions with quantum cascade laser frequency combs. Anal. Chem. 2018, 90, 10494–10500. [Google Scholar] [CrossRef]
- Brandstetter, M.; Lendl, B. Tunable mid-infrared lasers in physical chemosensors towards the detection of physiologically relevant parameters in biofluids. Sens. Actuators B Chem. 2012, 170, 189–195. [Google Scholar] [CrossRef]
- Galao, O.; Baeza, F.J.; Zornoza, E.; Garces, P. Strain and damage sensing properties on multifunctional cement composites with CNF admixture. Cem. Concr. Compos. 2014, 46, 90–98. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, C.; Liang, H.W.; Chen, J.F.; Yu, S.H. Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.Y.; Wang, R.H.; Yang, H.; Lin, Y.H.; Chen, T.M.; Huang, S.J. Flexible strain sensors fabricated with carbon nano-tube and carbon nano-fiber composite thin films. Thin Solid Films 2010, 518, 7343–7347. [Google Scholar] [CrossRef]
- Yan, T.; Wang, Z.; Wang, Y.Q.; Pan, Z.J. Carbon/graphene composite nanofiber yarns for highly sensitive strain sensors. Mater. Design 2018, 143, 214–223. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, Y.S.; Wan, B.L.; Han, B.G.; Cai, G.C.; Li, Z.Z. Properties and mechanisms of self-sensing carbon nanofibers/epoxy composites for structural health monitoring. Compos. Struct. 2018, 200, 669–678. [Google Scholar] [CrossRef]
- Liu, L.J.; Wang, Z.H.; Yang, J.H.; Liu, G.L.; Li, J.J.; Guo, L.; Chen, S.L.; Guo, Q.H. NiCo2O4 nanoneedle-decorated electrospun carbon nanofiber nanohybrids for sensitive non-enzymatic glucose sensors. Sens. Actuators B Chem. 2018, 258, 920–928. [Google Scholar] [CrossRef]
- Mao, X.W.; Yang, X.Q.; Rutledge, G.C.; Hatton, T.A. Ultra-wide-range electrochemical sensing using continuous electrospun carbon nanofibers with high densities of states. ACS Appl. Mater. Interfaces 2014, 6, 3394–3405. [Google Scholar] [CrossRef]
- Mang, J.; Lei, J.P.; Liu, Y.Y.; Zhao, J.W.; Ju, H.X. Highly sensitive amperometric biosensors for phenols based on polyaniline-ionic liquid-carbon nanofiber composite. Biosens. Bioelectron. 2009, 24, 1858–1863. [Google Scholar]
- Huo, Z.H.; Zhou, Y.L.; Liu, Q.; He, X.L.; Liang, Y.; Xu, M.T. Sensitive simultaneous determination of catechol and hydroquinone using a gold electrode modified with carbon nanofibers and gold nanoparticles. Microchim. Acta 2011, 173, 119–125. [Google Scholar] [CrossRef]
- Ye, D.X.; Liang, G.H.; Li, H.X.; Luo, J.; Zhang, S.; Chen, H.; Kong, J.L. A novel nonenzymatic sensor based on CuO nanoneedle/graphene/carbon nanofiber modified electrode for probing glucose in saliva. Talanta 2013, 116, 223–230. [Google Scholar] [CrossRef]
- Mao, X.W.; Simeon, F.; Rutledge, G.C.; Hatton, T.A. Electrospun carbon nanofiber webs with controlled density of states for sensor applications. Adv. Mater. 2013, 25, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Liao, Y.; Zheng, M.B.; Shao, S.J. In-situ growth of Co3O4 nanoparticles on mesoporous carbon nanofibers: A new nanocomposite for nonenzymatic amperometric sensing of H2O2. Microchim. Acta 2017, 184, 3689–3695. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xu, H.X.; Xu, Z.Q.; Gu, Y.; Yan, X.Y.; Liu, H.; Lu, N.N.; Zhang, S.Y.; Zhang, Z.Q.; Yang, M. A bioinspired antifouling zwitterionic interface based on reduced graphene oxide carbon nanofibers: Electrochemical aptasensing of adenosine triphosphate. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef] [PubMed]
- Arkan, E.; Paimard, G.; Moradi, K. A novel electrochemical sensor based on electrospun TiO2 nanoparticles/carbon nanofibers for determination of idarubicin in biological samples. J. Electroanal. Chem. 2017, 801, 480–487. [Google Scholar] [CrossRef]
- Eissa, S.; Alshehri, N.; Abduljabbar, M.; Rahman, A.M.A.; Dasouki, M.; Nizami, I.Y.; Al-Muhaizea, M.A.; Zourob, M. Carbon nanofiber-based multiplexed immunosensor for the detection of survival motor neuron 1, cystic fibrosis transmembrane conductance regulator and duchenne muscular dystrophy proteins. Biosens. Bioelectron. 2018, 117, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Koh, D.; Booth, M.A.; Ahmed, M.U. Combining a gold nanoparticle-polyethylene glycol nanocomposite and carbon nanofiber electrodes to develop a highly sensitive salivary secretory immunoglobulin a immunosensor. Sens. Actuators B Chem. 2018, 255, 557–563. [Google Scholar] [CrossRef]
- Arumugam, P.U.; Chen, H.; Siddiqui, S.; Weinrich, J.A.P.; Jejelowo, A.; Li, J.; Meyyappan, M. Wafer-scale fabrication of patterned carbon nanofiber nanoelectrode arrays: A route for development of multiplexed, ultrasensitive disposable biosensors. Biosens. Bioelectron. 2009, 24, 2818–2824. [Google Scholar] [CrossRef]
- Gupta, R.K.; Periyakaruppan, A.; Meyyappan, M.; Koehne, J.E. Label-free detection of C-reactive protein using a carbon nanofiber based biosensor. Biosens. Bioelectron. 2014, 59, 112–119. [Google Scholar] [CrossRef]
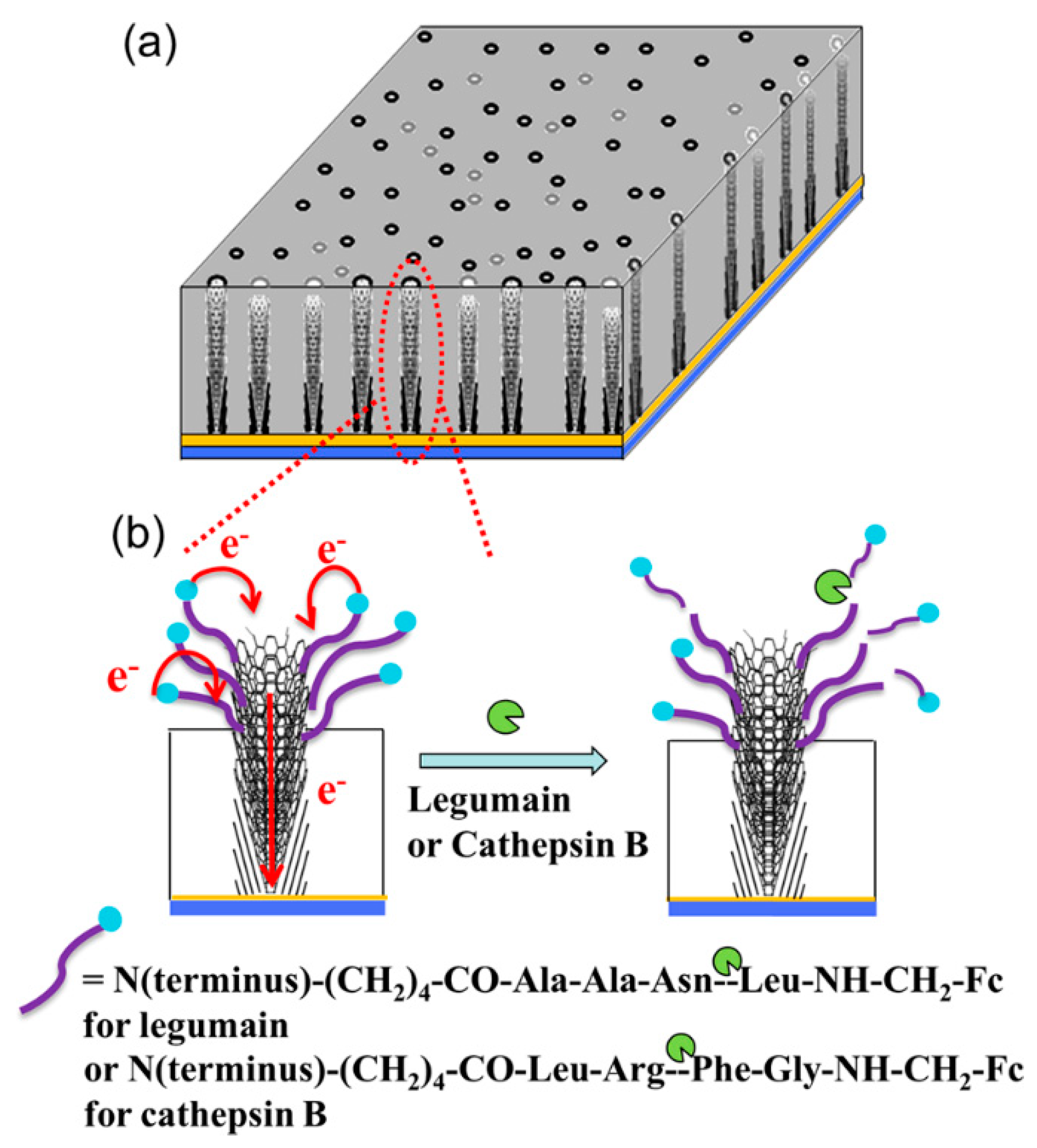
- Swisher, L.Z.; Syed, L.U.; Prior, A.M.; Madiyar, F.R.; Carlson, K.R.; Nguyen, T.A.; Hua, D.H.; Li, J. Electrochemical protease biosensor based on enhanced AC voltammetry using carbon nanofiber nanoelectrode arrays. J. Phys. Chem. C 2013, 117, 4268–4277. [Google Scholar] [CrossRef]
| Sensor | Target Molecule | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|
| CNFs | DA | 0.2–700,000 μM | 0.08 μM | [97] |
| Pd/CNFs | H2O2 and NADH | 0.2–20,000 μM (H2O2), 0.2–716.6 μM (NADH) | 0.2 μM(H2O2) | [57] |
| Pd/CNFs | DA, UA, AA | 0.5–160 μM (DA), 2–200 mΜ (UA), 0.05–4 mM (AA) | 0.2 μM (DA), 0.7 μM(UA), 15 μM(AA) | [57] |
| Ni/CNFs | Glucose | 2–2500 μM | 1 μM | [63] |
| PANI-IL-CNF | Phenol | 40–2100 nM | 0.1 nM | [98] |
| Pd/CNFs | OA | 0.2–45 mM | 0.2 mM | [59] |
| Pt/CNFs | Glucose | 2–20 mM | [64] | |
| Rh/CNFs | Hydrazine | 0.5–175 μM | 0.3 μM | [61] |
| CNFs | Xanthine | 0.03–21.19 μM | 20 nM | [50] |
| ZnO/CNFs | DMMP | 0.1–1000 ppb | 0.1 ppb | [66] |
| Pt/CNFs | H2O2 | 1–800 μM | 0.6 μM | [53] |
| GNPs/CNF/Au | CC and HQ | 5–350 μM (CC), 9–500 μM (HQ) | 0.36 μM (CC), 0.86 μM (HQ) | [99] |
| MCNF/PGE | DA, UA, AA | 0.05–30 μM (DA), 0.5–120 μΜ (UA), 0.1–10 mM (AA) | 0.02 μM (DA), 0.2 μM(UA), 50 μM(AA) | [46] |
| CNFs | Trp, Tyr, Cys | 0.1–119 μM (Trp), 0.2–107 μM (Tyr), 0.15–64 μM (Cys) | 0.1 μM | [51] |
| CNFs | CC, HQ | 1–200 μM | 0.2 μM (CC), 0.4 μM (HQ) | [52] |
| VACNFs | DA, 5-HT | 1–10 μM | 50 nM (DA), 250 nM (5-HT) | [48] |
| CuO/rGO/CNFs | Glucose | 1–5.3 mM | 0.1 μM | [100] |
| Pd-HCNF | H2O2, Glucose | 5–2100 μM (H2O2), 0.06–6 mM (glucose) | 3 μM(H2O2), 0.03 mM (glucose) | [54] |
| HRP-CNFs | H2O2 | 1–10 μM | 1.3 μM | [101] |
| PtNP-CNF | H2O2 | 25–1500 μM | 11 μM | [55] |
| Co3O4/CNFs | H2O2 | 1–2580 μM | 0.5 μM | [102] |
| Ag-Pt/pCNFs | DA | 10–500 μM | 0.11 μM | [70] |
| Cu/CNFs | Catechol | 9.95–9760 μM | 1.18 μM | [62] |
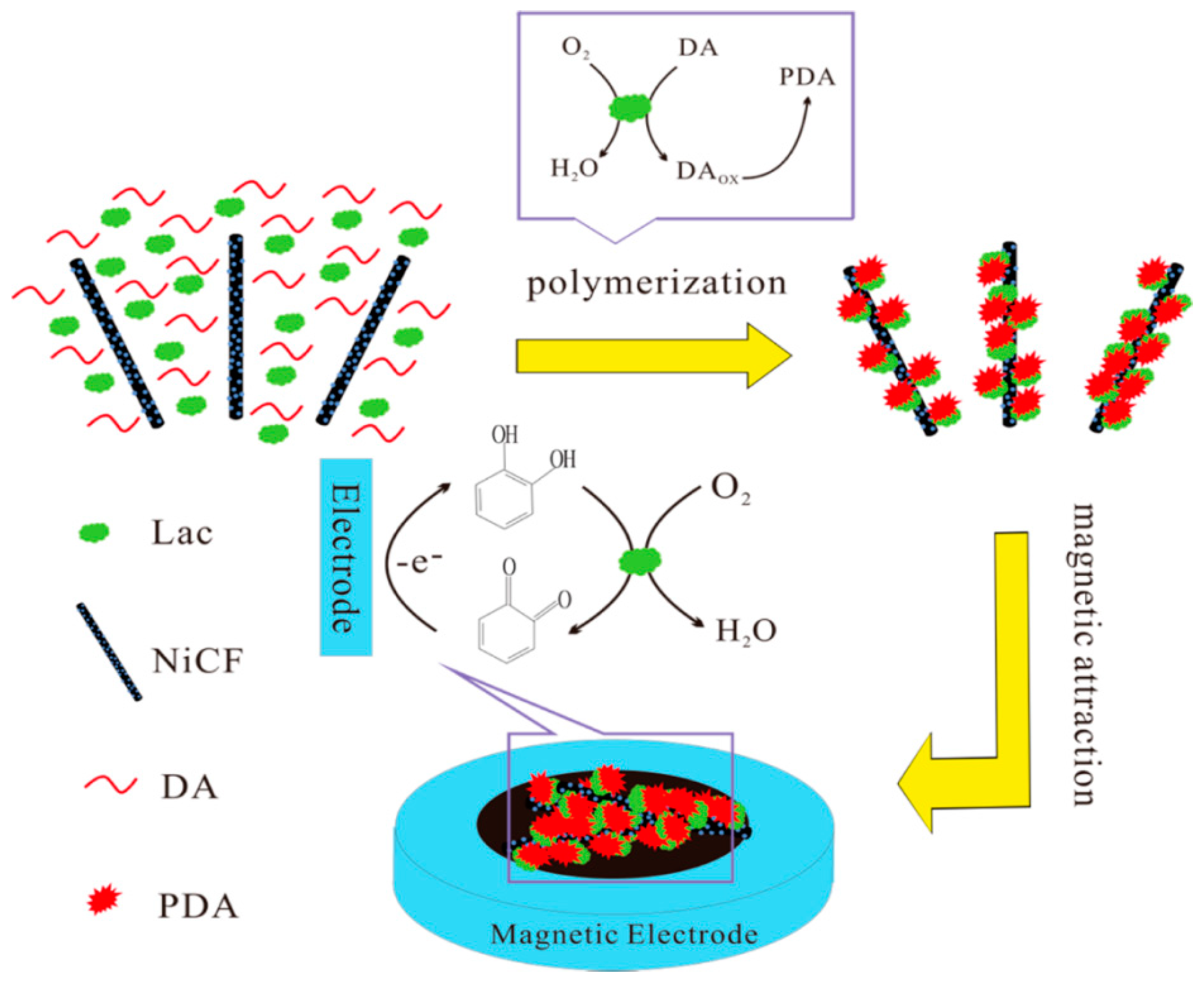
| PDA-Lac-NiCNFs | Catechol | 1–9100 μM | 0.69 μM | [56] |
| Pd-Ni/CNFs | Sugar | 0.03–800 μM | 7-20 nm | [71] |
| CuCo-CNFs | Glucose | 0.02–11 mM | 1 μM | [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. Nanomaterials 2019, 9, 1045. https://doi.org/10.3390/nano9071045
Wang Z, Wu S, Wang J, Yu A, Wei G. Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. Nanomaterials. 2019; 9(7):1045. https://doi.org/10.3390/nano9071045
Chicago/Turabian StyleWang, Zhuqing, Shasha Wu, Jian Wang, Along Yu, and Gang Wei. 2019. "Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications" Nanomaterials 9, no. 7: 1045. https://doi.org/10.3390/nano9071045
APA StyleWang, Z., Wu, S., Wang, J., Yu, A., & Wei, G. (2019). Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. Nanomaterials, 9(7), 1045. https://doi.org/10.3390/nano9071045






