Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
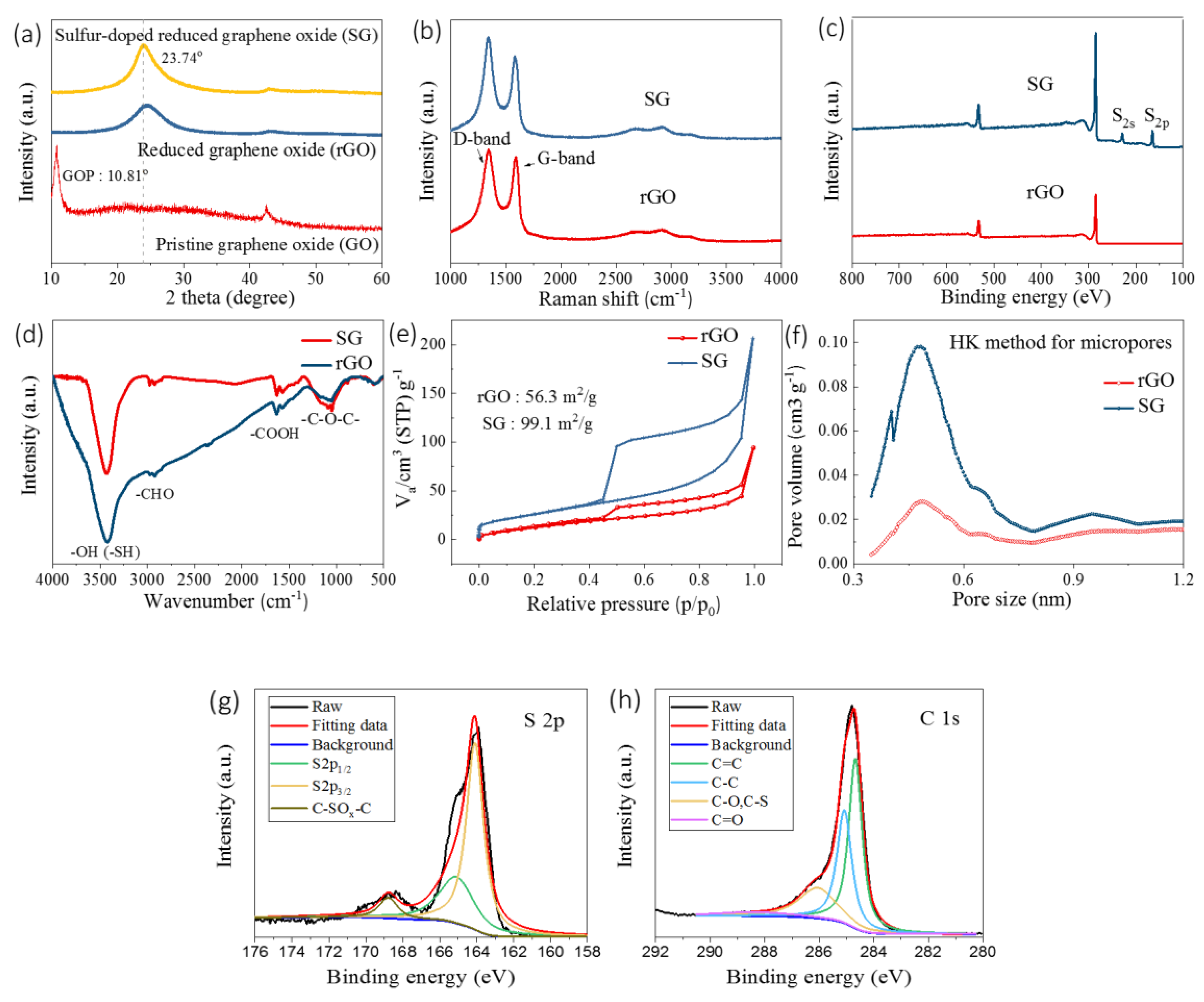
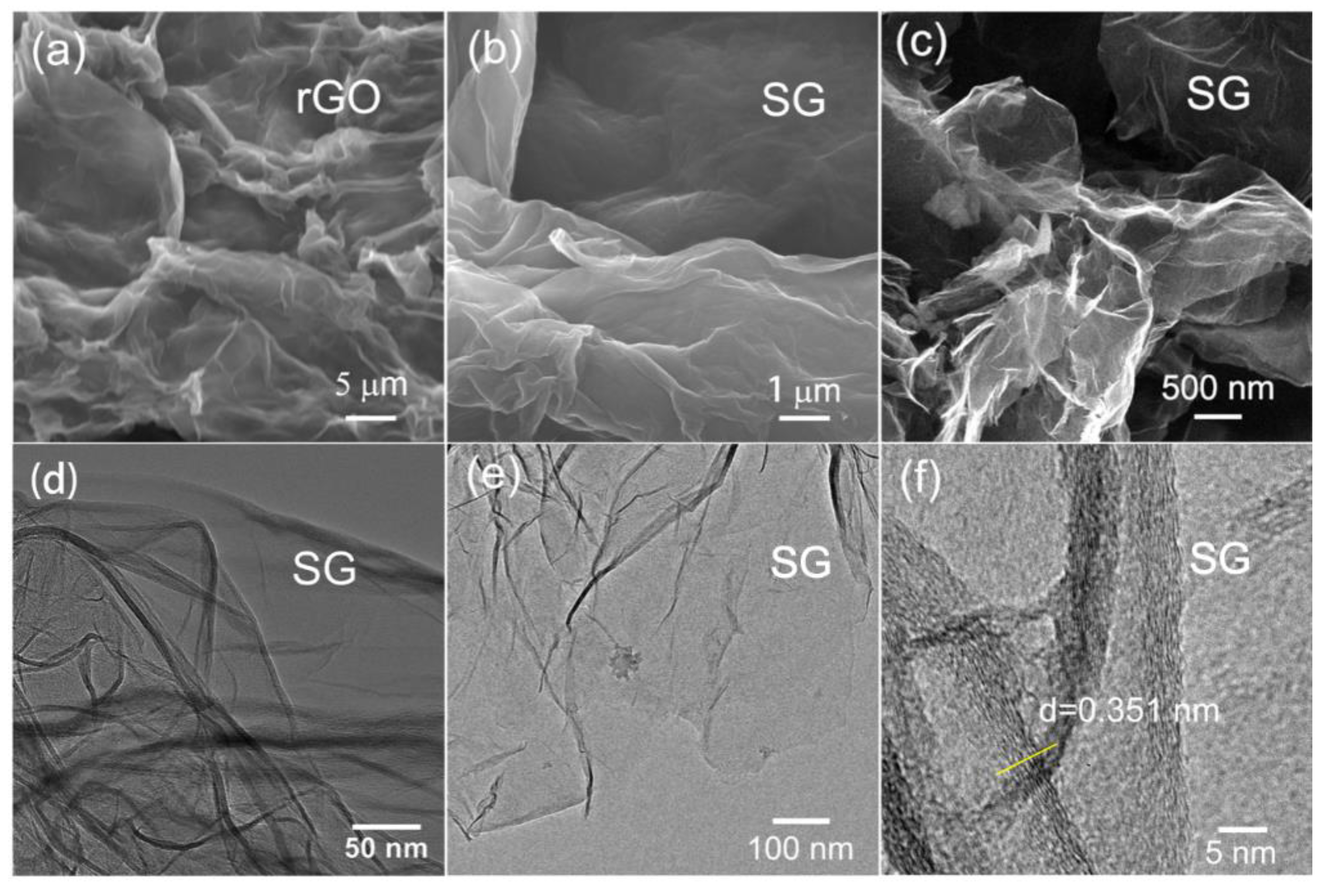
3.1. Morphology and Structural Characterization
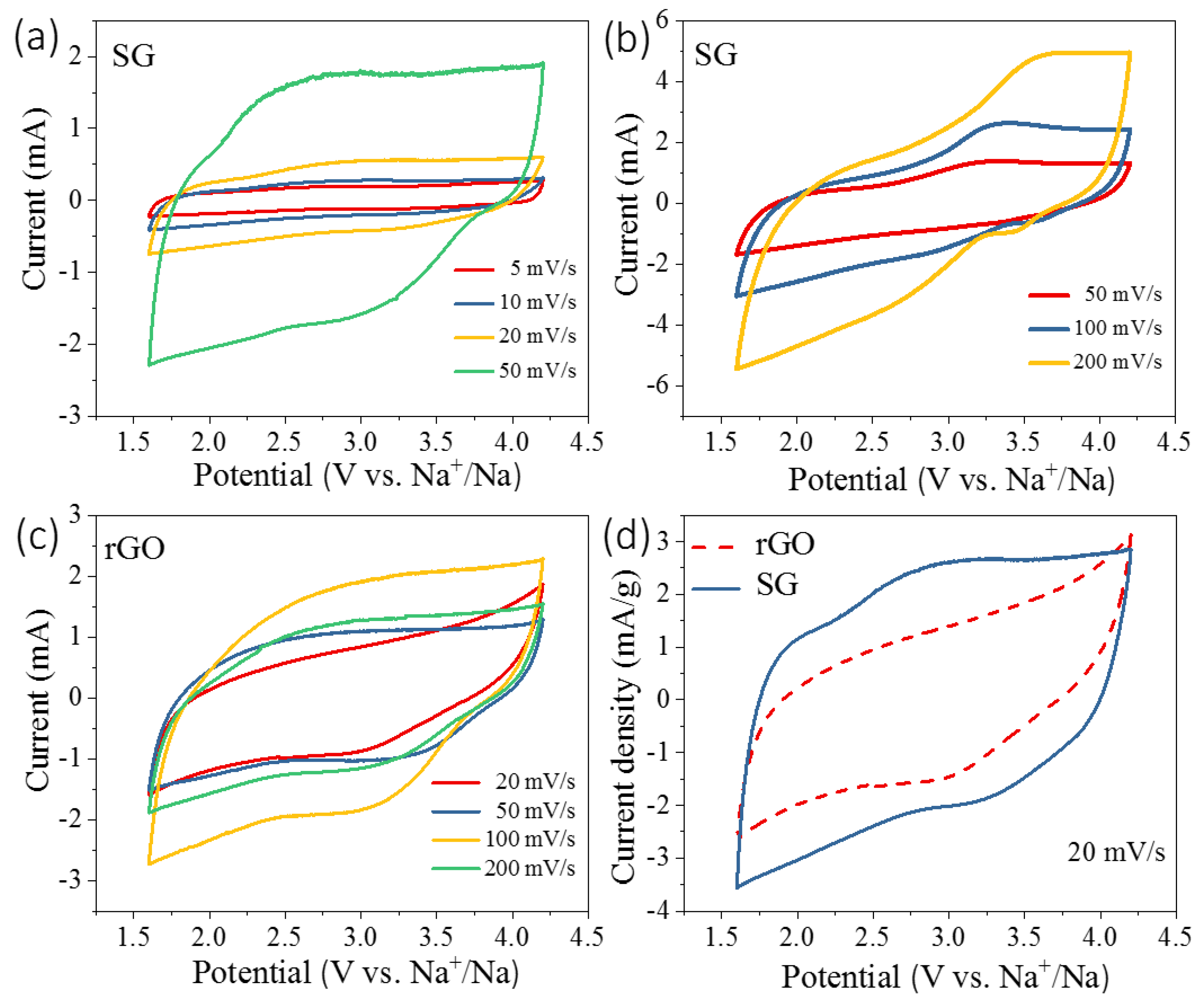
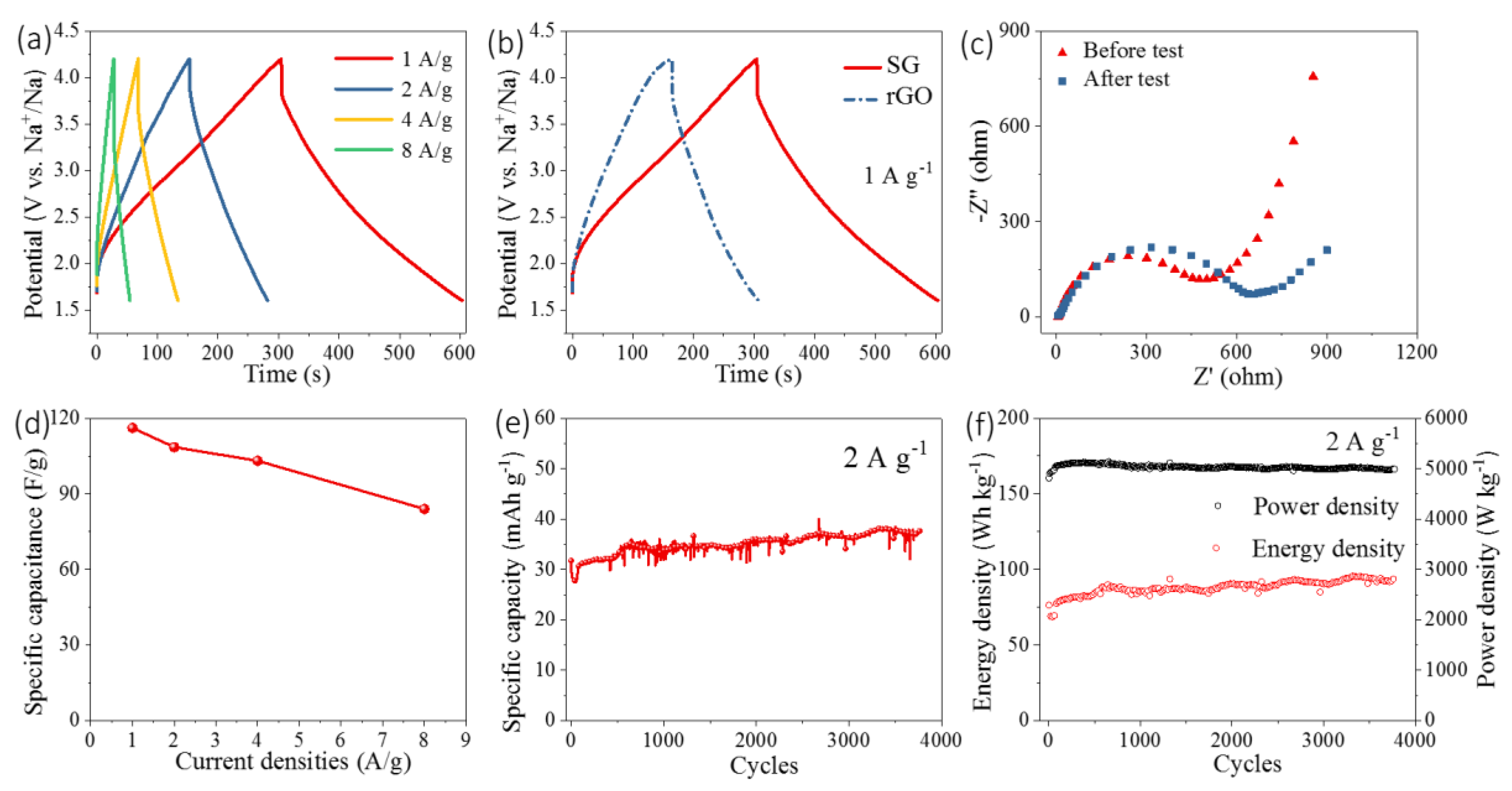
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hou, P.Y.; Chu, G.; Gao, J.; Zhang, Y.T.; Zhang, L.Q. Li-ion batteries: Phase transition. Chin. Phys. B 2016, 25, 11. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Wang, Q.; Zhu, X.T.; Jiang, F.Y. Three-Dimensional SnS Decorated Carbon Nano-Networks as Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2018, 8, 9. [Google Scholar]
- Zhou, Y.L.; Zhu, Q.; Tian, J.; Jiang, F.Y. TiO2 Nanobelt@ Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Muñoz-García, A.B.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Unveiling the controversial mechanism of reversible Na storage in TiO2 nanotube arrays: Amorphous versus anatase TiO2. Nano Res. 2017, 10, 2891–2903. [Google Scholar] [CrossRef]
- Bella, F.; Muñoz-García, A.B.; Coloò, F.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Combined Structural, Chemometric, and Electrochemical Investigation of Vertically Aligned TiO2 Nanotubes for Na-ion Batteries. ACS Omega 2018, 3, 8440–8450. [Google Scholar] [CrossRef]
- Liu, F.S.; Sun, X.X.; Liu, Y.T.; Song, X.Y.; Gao, J.; Qin, G.H. TiO2 nanorods confined in porous V2O5 nanobelts and interconnected carbon channels for sodium ion batteries. Appl. Surf. Sci. 2019, 473, 873–884. [Google Scholar] [CrossRef]
- Luo, J.; Zheng, J.; Nai, J.; Jin, C.; Yuan, H.; Sheng, O.; Liu, Y.; Fang, R.; Zhang, W.; Huang, H.; et al. Atomic Sulfur Covalently Engineered Interlayers of Ti3C2 MXene for Ultra-Fast Sodium-Ion Storage by Enhanced Pseudocapacitance. Adv. Funct. Mater. 2019, 29, 10. [Google Scholar] [CrossRef]
- Zheng, G.X.; Chen, M.H.; Yin, J.H.; Zhang, H.R.; Liang, X.Q.; Zhang, J.W. Metal Organic Frameworks Derived Nano Materials for Energy Storage Application. Int. J. Electrochem. Sci. 2019, 14, 2345–2362. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy-power rivals lithium ion capacitors. Energy Environ. Sci. 2015, 8, 941–955. [Google Scholar] [CrossRef]
- Que, L.F.; Yu, F.D.; He, K.W.; Wang, Z.B.; Gu, D.M. Robust and Conductive Na2Ti2O5-x Nanowire Arrays for High Performance Flexible Sodium-Ion Capacitor. Chem. Mater. 2017, 29, 9133–9141. [Google Scholar] [CrossRef]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.S.; Madhavi, S. Insertion-Type Electrodes for Nonaqueous Li-Ion Capacitors. Chem Rev. 2014, 114, 11619–11635. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.D.; Hu, W.B.; Qiao, J.L.; Zhang, L.; Zhang, J.J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Two-Dimensional Vanadium Carbide (MXene) as Positive Electrode for Sodium-Ion Capacitors. J. Phys. Chem. Lett. 2015, 6, 2305–2309. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.S.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide (vol 4, pg 4806, 2010). ACS Nano 2018, 12, 2078. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Ren, W.C.; Xu, L.; Li, F.; Cheng, H.M. Doped Graphene Sheets As Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.A.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y.L. Synthesis of Phosphorus-Doped Graphene and its Multifunctional Applications for Oxygen Reduction Reaction and Lithium Ion Batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Cha, H.A.; Jeong, H.M.; Kang, J.K. Nitrogen-doped open pore channeled graphene facilitating electrochemical performance of TiO2 nanoparticles as an anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 5182–5186. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene Double-Layer Capacitor with ac Line-Filtering Performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Long, J.; He, J.; Zhang, J.; Zheng, H.; Gou, X. Nitrogen and Phosphorus Dual-doped Porous Carbon Nanosheets for Efficient Oxygen Reduction in Both Alkaline and Acidic Media. ChemCatChem 2018, 10, 4038–4046. [Google Scholar] [CrossRef]
- Tuček, J.; Błoński, P.; Sofer, Z.; Šimek, P.; Petr, M.; Pumera, M.; Otyepka, M.; Zbořil, R. Sulfur Doping Induces Strong Ferromagnetic Ordering in Graphene: Effect of Concentration and Substitution Mechanism. Adv. Mater. 2016, 28, 5045–5053. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, Y.Y.; Matson, D.W.; Li, J.H.; Lin, Y.H. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, X.Y.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K.; Li, M.; Fu, H. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis Of Nitrogen-Doped Graphene Films For Lithium Battery Application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef]
- Su, Z.; Wei, Z.X.; Lai, C.; Deng, H.Q.; Liu, Z.X.; Ma, J.M. Robust pseudo-capacitive Li-I-2 battery enabled by catalytic, adsorptive N-doped graphene interlayer. Energy Storage Mater. 2018, 14, 129–135. [Google Scholar] [CrossRef]
- Ban, F.Y.; Jayabal, S.; Lim, H.N.; Lee, H.W.; Huang, N.M. Synthesis of nitrogen-doped reduced graphene oxide-multiwalled carbon nanotube composite on nickel foam as electrode for high-performance supercapacitor. Ceram. Int. 2017, 43, 20–27. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Chen, L.; Sun, Q.; Zhu, M.; Zhang, Y.; Liu, Y.; Liang, Z.; Si, P.; Lou, J.; et al. Potassium gluconate-derived N/S Co-doped carbon nanosheets as superior electrode materials for supercapacitors and sodium-ion batteries. J. Power Sources 2019, 414, 308–316. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, H.; Gan, X.; Yang, L.; Huang, Z.H.; Wang, D.W.; Kang, F.; Lv, R. Ultrahigh rate sodium ion storage with nitrogen-doped expanded graphite oxide in ether-based electrolyte. J. Mater. Chem. A 2018, 6, 1582–1589. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Wang, B.; Huang, C.C.; Wang, L.Z.; Hulicova-Jurcakova, D. Synthesis of Phosphorus-Doped Graphene and its Wide Potential Window in Aqueous Supercapacitors. Chem. Eur. J. 2015, 21, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.B.; Miwa, R.H.; da Silva, A.J.R.; Fazzio, A. Electronic and transport properties of boron-doped graphene nanoribbons. Phys. Rev. Lett. 2007, 98, 4. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Gao, H.L.; Bao, W.J.; Wang, F.B.; Xia, X.H. Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells. J. Mater. Chem. 2012, 22, 390–395. [Google Scholar] [CrossRef]
- Cui, D.; Li, H.; Li, M.; Li, C.; Qian, L.; Zhou, B.; Yang, B. Boron-Doped Graphene Directly Grown on Boron-Doped Diamond for High-Voltage Aqueous Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 1526–1536. [Google Scholar] [CrossRef]
- Azadeh, M.S.S.; Kokabi, A.; Hosseini, M.; Fardmanesh, M. Tunable bandgap opening in the proposed structure of silicon-doped graphene. Micro Nano Lett. 2011, 6, 582–585. [Google Scholar] [CrossRef]
- Houmad, M.; Zaari, H.; Benyoussef, A.; El Kenz, A.; Ez-Zahraouy, H. Optical conductivity enhancement and band gap opening with silicon doped graphene. Carbon 2015, 94, 1021–1027. [Google Scholar] [CrossRef]
- Goyenola, C.; Gueorguiev, G.K.; Stafstrom, S.; Hultman, L. Fullerene-like CSx: A first-principles study of synthetic growth. Chem. Phys. Lett. 2011, 506, 86–91. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar]
- Hu, M.X.; Zhang, H.W.; Yang, L.; Lv, R.T. Ultrahigh rate sodium-ion storage of SnS/SnS2 heterostructures anchored on S-doped reduced graphene oxide by ion-assisted growth. Carbon 2019, 143, 21–29. [Google Scholar] [CrossRef]
- Zhang, B.X.; Gao, H.; Li, X.L. Synthesis and optical properties of nitrogen and sulfur co-doped graphene quantum dots. New J. Chem. 2014, 38, 4615–4621. [Google Scholar] [CrossRef]
- Wu, Z.S.; Tan, Y.Z.; Zheng, S.; Wang, S.; Parvez, K.; Qin, J.; Shi, X.; Sun, C.; Bao, X.; Feng, X.; et al. Bottom-Up Fabrication of Sulfur-Doped Graphene Films Derived from Sulfur-Annulated Nanographene for Ultrahigh Volumetric Capacitance Micro-Supercapacitors. J. Am. Chem. Soc. 2017, 139, 4506–4512. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, H.; Choi, M.S.; Suh, D.H.; Nakhanivej, P.; Park, H.S. Straightforward and controllable synthesis of heteroatom-doped carbon dots and nanoporous carbons for surface-confined energy and chemical storage. Energy Storage Mater. 2018, 12, 331–340. [Google Scholar] [CrossRef]
- Hao, J.; Meng, T.; Shu, D.; Song, X.; Cheng, H.; Li, B.; Zhou, X.; Zhang, F.; Li, Z.; He, C. Synthesis of three dimensional N&S co-doped rGO foam with high capacity and long cycling stability for supercapacitors. J. Colloid Interface Sci. 2019, 537, 57–65. [Google Scholar] [PubMed]
- Chen, X.A.; Chen, X.; Xu, X.; Yang, Z.; Liu, Z.; Zhang, L.; Xu, X.; Chen, Y.; Huang, S. Sulfur-doped porous reduced graphene oxide hollow nanosphere frameworks as metal-free electrocatalysts for oxygen reduction reaction and as supercapacitor electrode materials. Nanoscale 2014, 6, 13740–13747. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, M.; Ai, D.; Zhang, H.; Huang, Z.-H.; Lv, R.; Kang, F. Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance. Nanomaterials 2019, 9, 752. https://doi.org/10.3390/nano9050752
Wang Y, Hu M, Ai D, Zhang H, Huang Z-H, Lv R, Kang F. Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance. Nanomaterials. 2019; 9(5):752. https://doi.org/10.3390/nano9050752
Chicago/Turabian StyleWang, Yiting, Mingxiang Hu, Desheng Ai, Hongwei Zhang, Zheng-Hong Huang, Ruitao Lv, and Feiyu Kang. 2019. "Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance" Nanomaterials 9, no. 5: 752. https://doi.org/10.3390/nano9050752
APA StyleWang, Y., Hu, M., Ai, D., Zhang, H., Huang, Z.-H., Lv, R., & Kang, F. (2019). Sulfur-Doped Reduced Graphene Oxide for Enhanced Sodium Ion Pseudocapacitance. Nanomaterials, 9(5), 752. https://doi.org/10.3390/nano9050752





