Synergistic Effect and Characterization of Graphene/Carbon Nanotubes/Polyvinyl Alcohol/Sodium Alginate Nanofibrous Membranes Formed Using Continuous Needleless Dynamic Linear Electrospinning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
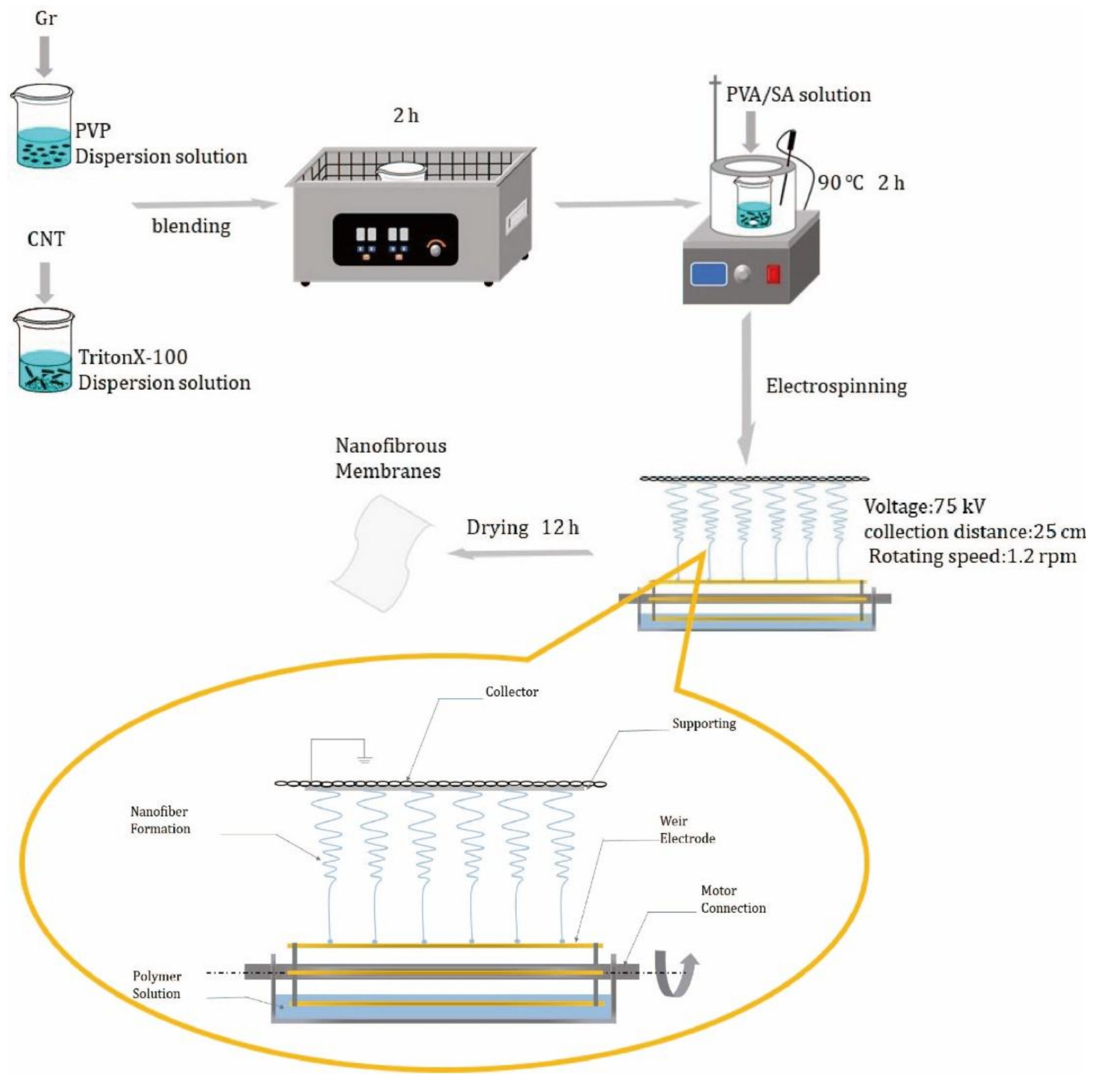
2.2. Preparation of Nanofibrous Membranes
2.3. Characterizations of Nanofibrous Membranes
3. Results and Discussion
3.1. Conductivity of Gr and CNT Solutions
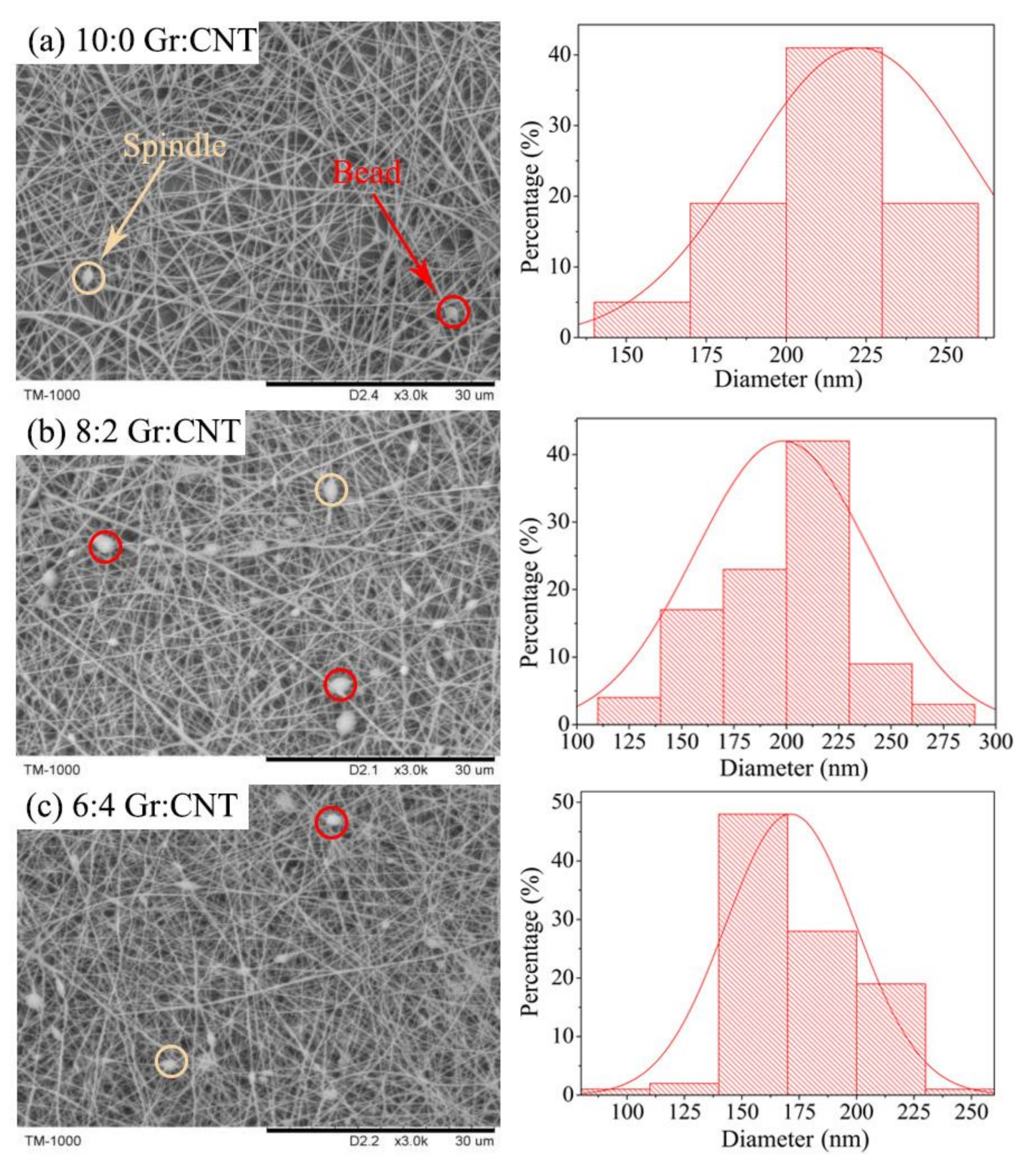
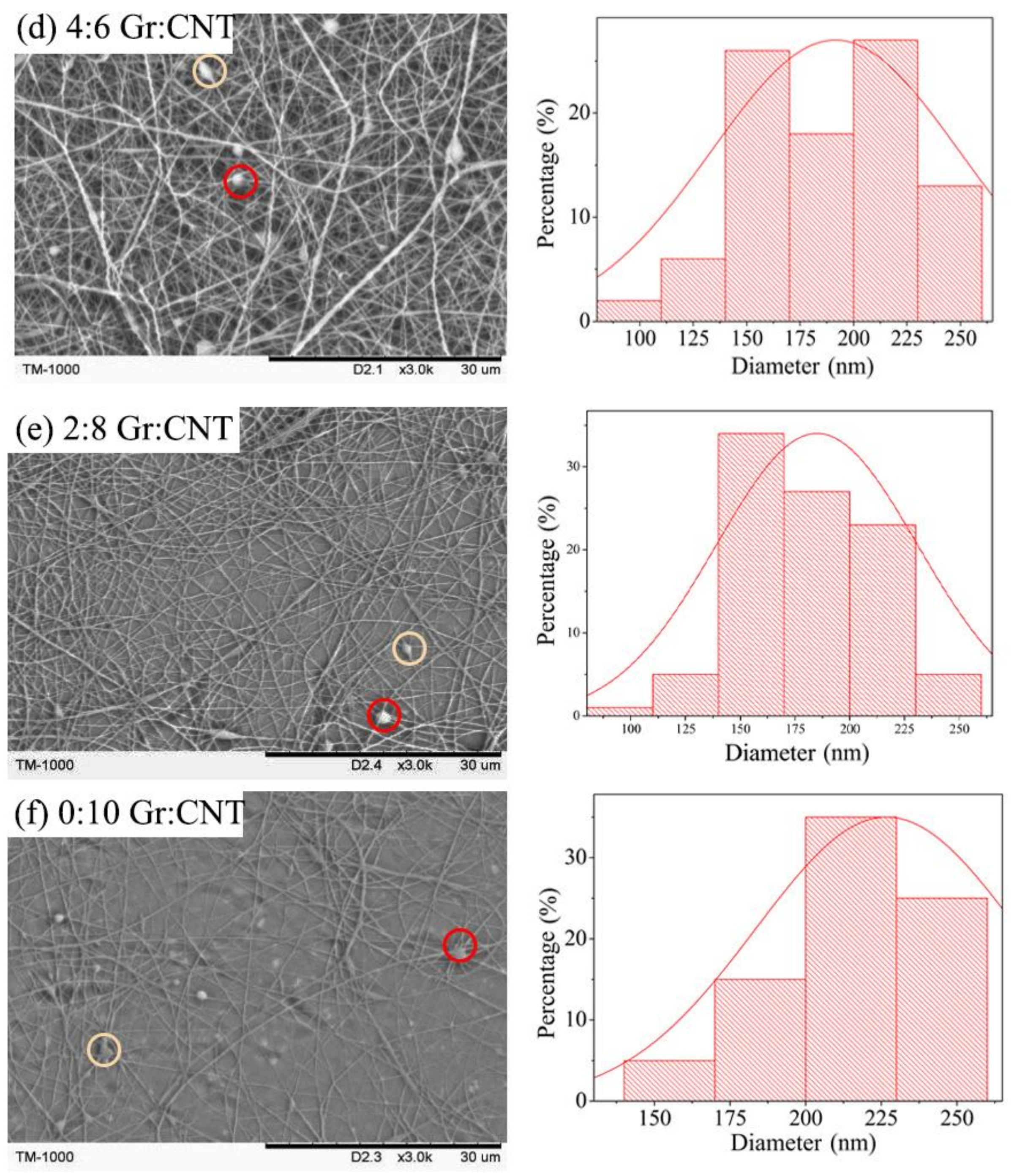
3.2. Morphology of Nanofibrous Membranes
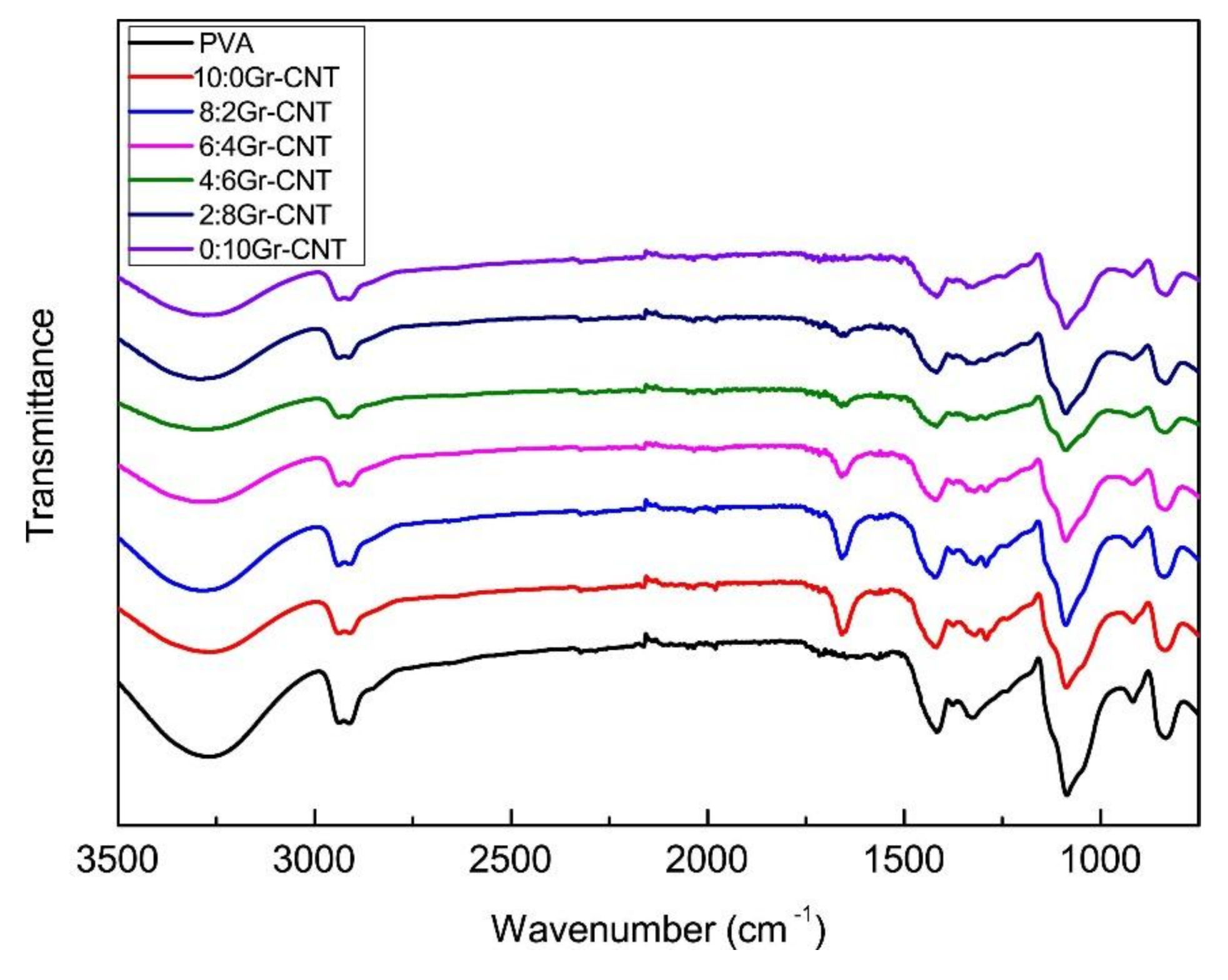
3.3. FTIR Spectrum Analyses
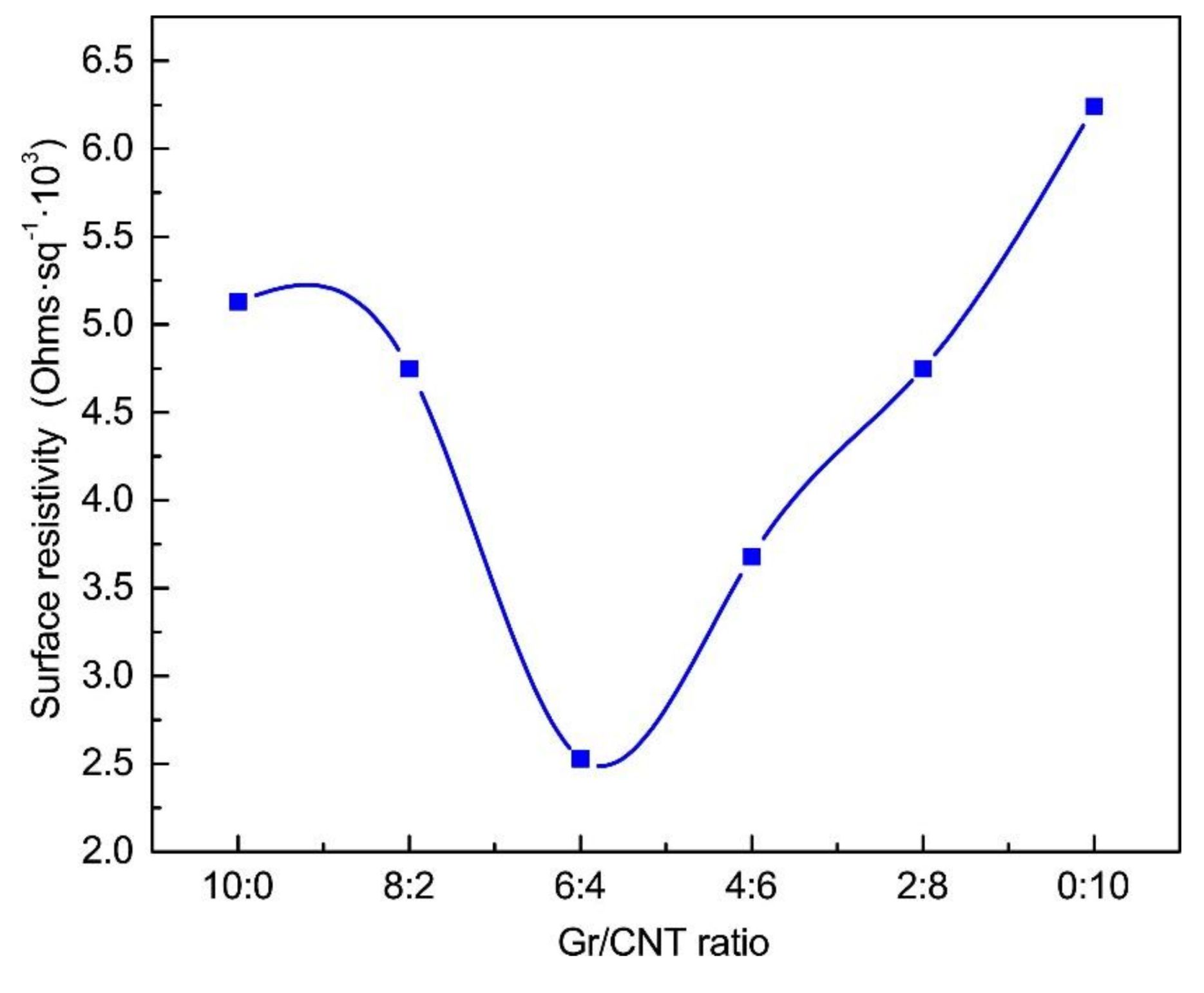
3.4. Surface Resistivity
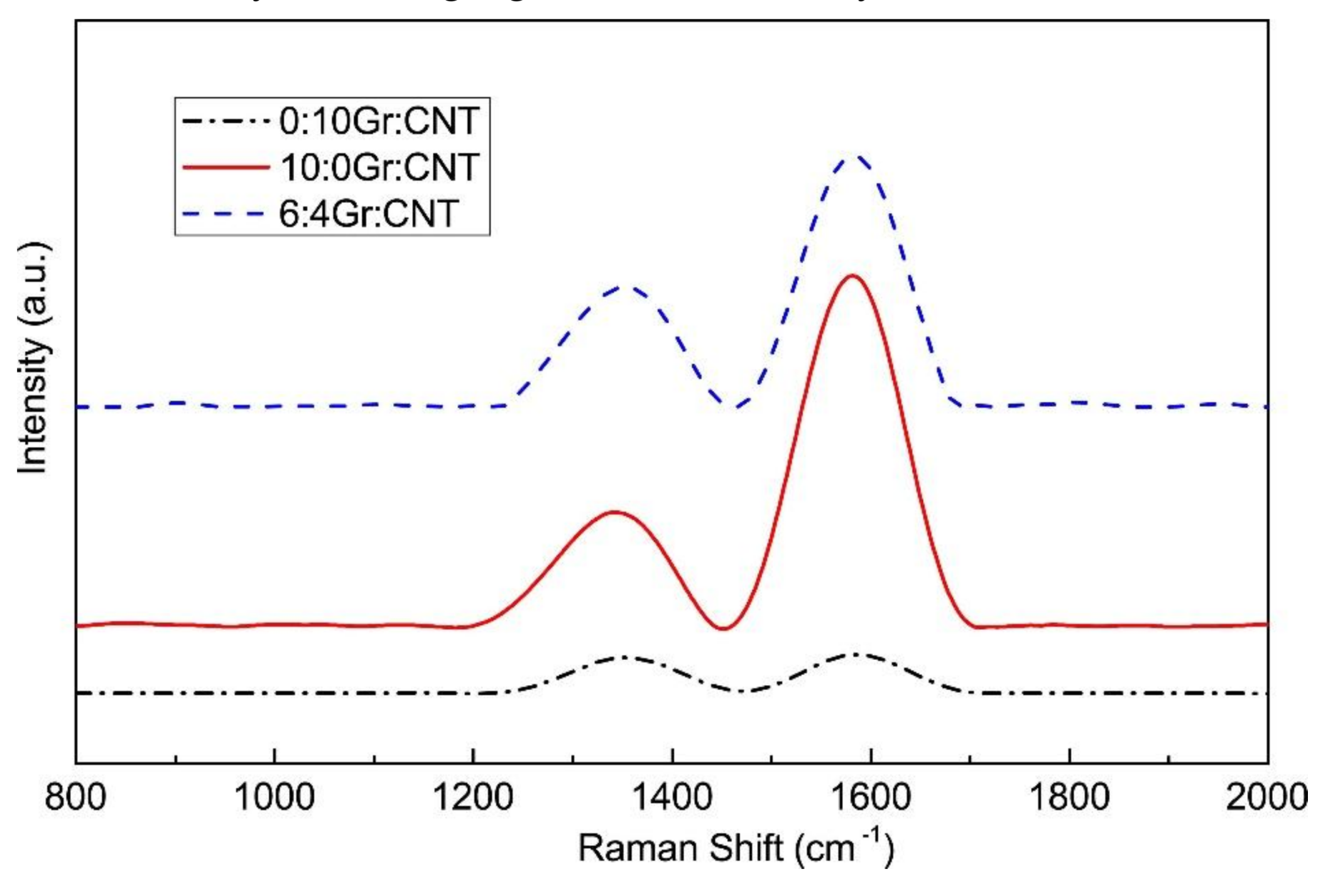
3.5. Raman Spectrometry
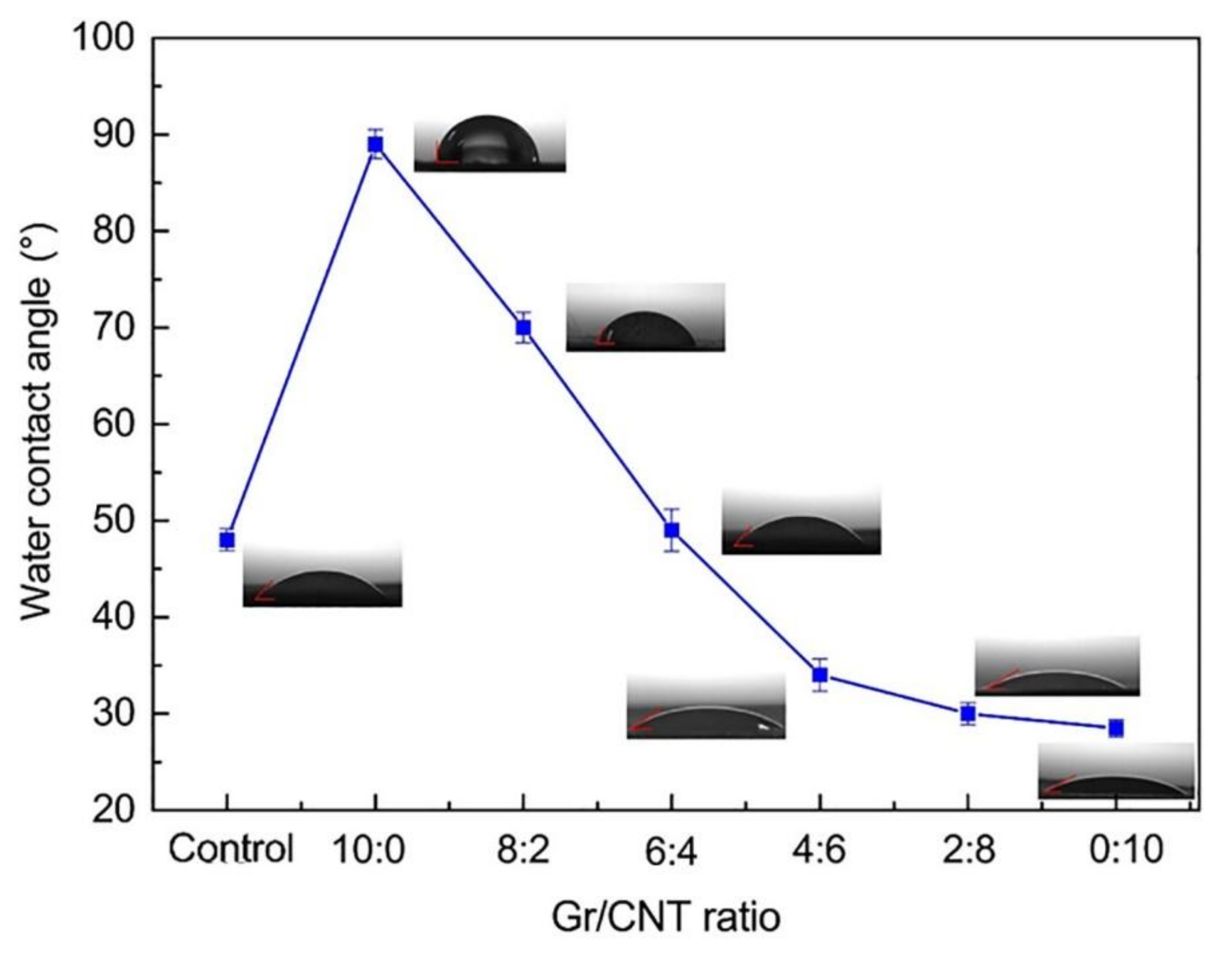
3.6. Water Contact Angle
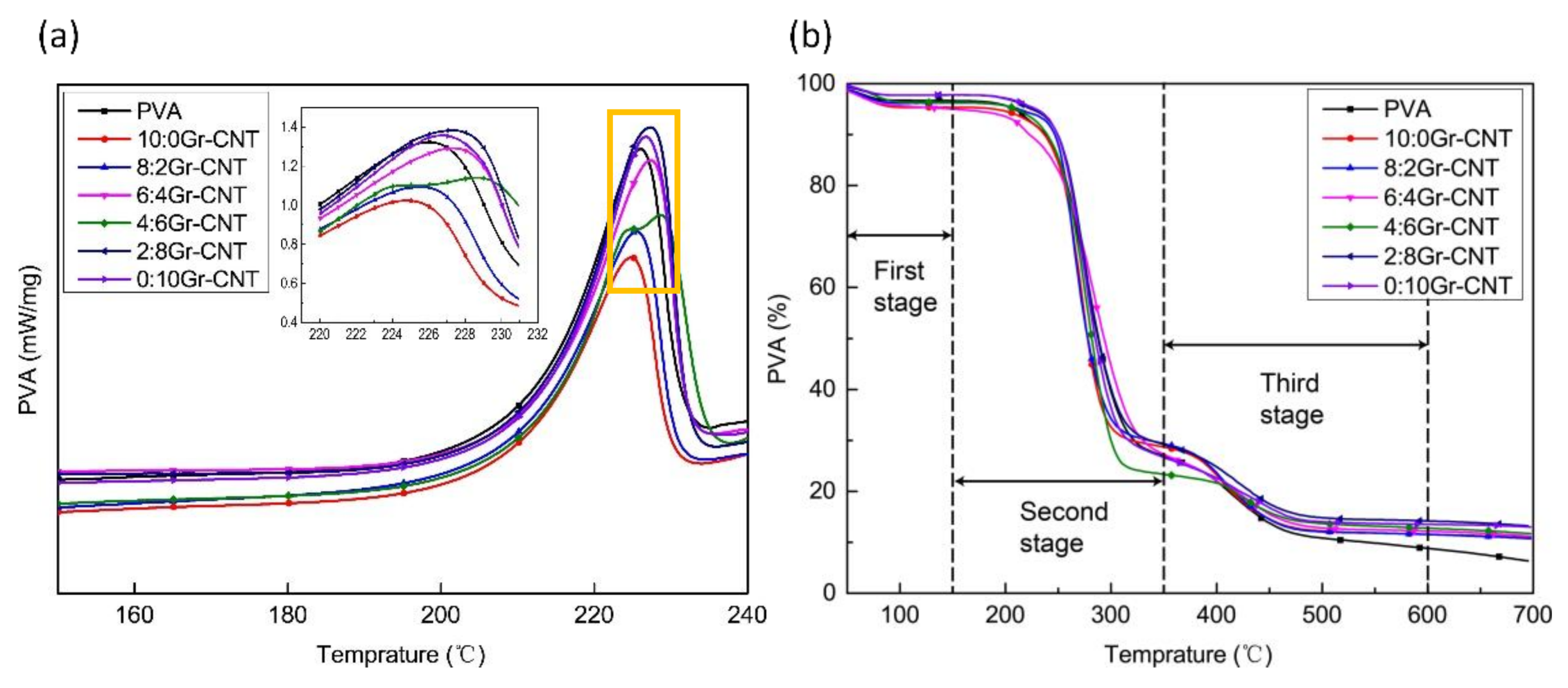
3.7. Thermal Property Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ficke, B.W.; Chaudhari, N.M. Nerve Injury; Eltorai, A., Eberson, C., Daniels, A., Eds.; Orthopedic Surgery Clerkship: New York, NY, USA, 2017; pp. 302–306. [Google Scholar]
- Chen, J.; Li, Y.; Zhang, Y.; Zhu, Y. Preparation and characterization of graphene oxide reinforced pva film with boric acid as crosslinker. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Jose, J.; Al-Harthi, M.A.; Alma’Adeed, A.A.; Bhadra Dakua, J.; De, S.K. Effect of graphene loading on thermomechanical properties of poly(vinyl alcohol)/starch blend. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Yang, J.M.; Yang, J.H.; Tsou, S.C.; Ding, C.H.; Wang, J.S. Cell proliferation on pva/sodium alginate and pva/poly(γ-glutamic acid) electrospun fiber. Mater. Sci. Eng. C 2016, 66, 170–177. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly(vinyl alcohol)/nano zno composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Merkle, V.M.; Tran, P.L.; Hutchinson, M.; Ammann, K.R.; DeCook, K.; Wu, X.; Slepian, M.J. Core–shell PVA/gelatin electrospun nanofibers promote human umbilical vein endothelial cell and smooth muscle cell proliferation and migration. Acta Biomater. 2015, 27, 77–87. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Das, T.K.; Prusty, S. Graphene: A revolution in nanobiotechnology. Propellants Explos. Pyrotech. 2012, 1, 19–30. [Google Scholar]
- Dong, L.X.; Chen, Q. Properties, synthesis, and characterization of graphene. Propellants Explos. Pyrotech. 2015, 24, 159–162. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ohnishi, K.; Kiyotani, T.; Sekine, T.; Ueda, H.; Nakamura, T.; Endo, K.; Shimizu, Y. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (pga)-collagen tube filled with laminin-coated collagen fibers: A histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000, 868, 315–328. [Google Scholar] [CrossRef]
- Stevens, M.M. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Surudžić, R.; Janković, A.; Mitrić, M.; Matić, I.; Juranić, Z.D.; Živković, L.; Mišković-Stanković, V.B.; Rhee, K.Y.; Park, S.J.; Hui, D. The effect of graphene loading on mechanical, thermal, and biological properties of poly(vinyl alcohol)/graphene nanocomposites. J. Ind. Eng. Chem. 2015, 34, 250–257. [Google Scholar] [CrossRef]
- Huang, C.L.; Peng, S.Y.; Wang, Y.J.; Chen, W.C.; Lin, J.H. Microstructure and characterization of electrospun poly(vinyl alcohol) nanofiber scaffolds filled with graphene nanosheets. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Song, W.; Markel, D.C.; Wang, S.; Shi, T.; Ren, W. Electrospun polyvinyl alcohol-collagen-hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, X.; Cai, C.; Rytting, E.; Steele, T.; Kissel, T. Biodegradable branched polyesters poly(vinyl sulfonate-covinyl alcohol)-graft poly(d,l-lactic-coglycolic acid) as a negatively charged polyelectrolyte platform for drug delivery: Synthesis and characterization. Macromolecules 2008, 41, 2791–2799. [Google Scholar] [CrossRef]
- Andrews, R.; Weisenberger, M.C. Carbon nanotube polymer composites. Curr. Opin. Solid State Mater. Sci. 2004, 8, 31–37. [Google Scholar] [CrossRef]
- Lu, X.; Dou, H.; Yang, S.; Hao, L.; Zhang, L.; Shen, L.; Zhang, F.; Zhang, X. Fabrication and electrochemical capacitance of hierarchical graphene/polyaniline/carbon nanotube ternary composite film. Electrochimica Acta 2011, 56, 9224–9232. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.N.; Huang, Y.L.; Tien, H.W.; Wang, J.Y.; Ma, C.C.; Li, S.M.; Wang, Y.S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Yu, D.; Dai, L. Self-assembled graphene/carbon nanotube hybrid films for supercapacitors. J. Phys. Chem. Lett. 2010, 1, 467–470. [Google Scholar] [CrossRef]
- Ioniță, M.; Crică, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Lovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, H.; Wang, X.; Lin, T. Needleless electrospinning of uniform nanofibers using spiral coil spinnerets. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Li, T.T.; Yan, M.; Xu, W.; Shiu, B.C.; Lou, C.W.; Lin, J.H. Mass-Production and Characterizations of Polyvinyl Alcohol/Sodium Alginate/Graphene Porous Nanofiber Membranes Using Needleless Dynamic Linear Electrospinning. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Luo, H.; Xie, J.; Xiong, L.; Zhu, Y.; Yang, Z.; Wan, Y. Fabrication of flexible, ultra-strong, and highly conductive bacterial cellulose-based paper by engineering dispersion of graphene nanosheets. Compos. Part B Eng. 2019, 162, 484–490. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.; Shen, M.; Qi, R.; Fang, Y.; Guo, R.; Cai, H.; Cao, X.; Tomásd, H.; Zhu, M.; et al. Carbon nanotube-incorporated multilayered cellulose acetate nanofibers for tissue engineering applications. Carbohydr. Polym. 2013, 91, 419–427. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Schexnailder, P.; Kaul, V.; Akkus, O.; Zakharov, D.; Seifert, S.; Schmidt, G. Highly extensible bio-nanocomposite films with direction-dependent properties. Adv. Funct. Mater. 2010, 20, 429–436. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro- and Nanostructured Surface Morphology on Electrospun Polymer Fibers. Macromolecules 2002, 35, 456–466. [Google Scholar] [CrossRef]
- Noori, M.; Ravari, F.; Ehsani, M. Preparation of pva nanofibers reinforced with magnetic graphene by electrospinning method and investigation of their degradation kinetics using master plot analyses on solid state. J. Therm. Anal. Calorim. 2017, 132, 1–10. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and conduction. Rev. Mod. Phys. 1973, 45, 574–588. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, H.; Huang, C.; Du, Y. Mechanically- and electrically-enhanced cnt-collagen hydrogels as potential scaffolds for engineered cardiac constructs. ACS Biomater. Sci. Eng. 2017, 3, 3017–3021. [Google Scholar] [CrossRef]
- Djuzenova, C.S.; Zimmermann, U.; Frank, H.; Sukhorukov, V.L.; Richter, E.; Fuhr, G. Effect of medium conductivity and composition on the uptake of propidium iodide into electropermeabilized myeloma cells. Biochim. Biophys. Acta 1996, 1284. [Google Scholar] [CrossRef]
- Nikiel, L.; Jagodzinski, P.W. Raman spectroscopic characterization of graphites: A re-evaluation of spectra/structure correlation. Carbon 1993, 31, 1313–1317. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Nanda, S.S.; Kim, M.J.; Yeom, K.S.; An, S.S.A.; Ju, H.; Yi, D.K. Raman spectrum of graphene with its versatile future perspectives. TrAC Trends Anal. Chem. 2016, 80, 125–131. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Duzynska, A.; Swiniarski, M.; Wroblewska, A.; Lapinska, A.; Zeranska, K.; Judek, J.; Zdrojek, M. Phonon properties in different types of single-walled carbon nanotube thin films probed by raman spectroscopy. Carbon 2016, 105, 377–386. [Google Scholar] [CrossRef]
- Puech, P.; Flahaut, E.; Bassil, A.; Juffmann, T.; Beuneu, F.; Bacsa, W.S. Raman bands of double-wall carbon nanotubes: Comparison with single- and triple-wall carbon nanotubes, and influence of annealing and electron irradiation. J. Raman Spectrosc. 2007, 38, 714–720. [Google Scholar] [CrossRef]
- Talebi, M.; Abbasizadeh, S.; Keshtkar, A.R. Evaluation of single and simultaneous thorium and uranium sorption from water systems by an electrospun PVA/SA/PEO/HZSM5 nanofiber. Process Saf. Environ. Protect. 2017, 109, 340–356. [Google Scholar] [CrossRef]
- Xu, X. Modified wenzel and cassie equations for wetting on rough surfaces. SIAM J. Appl. Math. 2016, 76, 2353–2374. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.J.; Sun, G.H.; Wang, X.S. Generalized wenzel equation of spherical nanodroplets within a homogeneous and rough conical cavity. J. Comput. Theor. Nanosci. 2016, 13, 5322–5326. [Google Scholar] [CrossRef]
- Vickery, J.L.; Patil, A.J.; Mann, S. Fabrication of graphene-polymer nanocomposites with higher-order three-dimensional architectures. Adv. Mater. 2009, 21, 2180–2184. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Li, J.; Li, Y.; Niu, S.; Li, N. Ultrasonic-microwave assisted synthesis of three-dimensional polyvinyl alcohol carbonate/graphene oxide sponge and studies of surface resistivity and thermal stability. Ultrason. Sonochem. 2018, 42, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Ma, Z.; Li, N.; Niu, S.; Li, Y. Preparation of polyvinyl alcohol graphene oxide phosphonate film and research of thermal stability and mechanical properties. Ultrason. Sonochem. 2018, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Gr: CNT | 0:0 | 10:0 | 8:2 | 6:4 | 4:6 | 2:8 | 0:10 |
| Viscosity (mPa. s) | 214 | 221 | 219 | 198 | 190 | 207 | 193 |
| Surface tension (mN /m) | 42.7 | 41.2 | 37.6 | 35.2 | 31.5 | 30.9 | 30.1 |
| Gr: CNT | 0:0 | 10:0 | 8:2 | 6:4 | 4:6 | 2:8 | 0:10 |
| Contact angle (°) | 48 ± 1.14 | 89 ± 1.52 | 70 ± 1.58 | 49 ± 2.17 | 34 ± 1.67 | 30 ± 1.14 | 28.5 ± 0.89 |
| Gr: CNT | T5% (°C) | TMAX (°C) | Loss in the First Stage (%) | Loss in the Second Stage (%) | Residual Mass (%) |
|---|---|---|---|---|---|
| 0:0 | 208.6 | 276.6 | 96.66 | 26.92 | 6.34 |
| 10:0 | 190.8 | 271.5 | 95.67 | 28.68 | 10.84 |
| 8:2 | 212.9 | 265.0 | 94.98 | 29.35 | 10.70 |
| 6:4 | 216.4 | 264.1 | 96.36 | 27.25 | 11.09 |
| 4:6 | 210.2 | 281.7 | 96.36 | 23.34 | 11.67 |
| 2:8 | 224.7 | 275.1 | 97.78 | 29.18 | 13.22 |
| 0:10 | 229.3 | 275.5 | 97.79 | 26.65 | 12.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.-T.; Zhong, Y.; Yan, M.; Zhou, W.; Xu, W.; Huang, S.-Y.; Sun, F.; Lou, C.-W.; Lin, J.-H. Synergistic Effect and Characterization of Graphene/Carbon Nanotubes/Polyvinyl Alcohol/Sodium Alginate Nanofibrous Membranes Formed Using Continuous Needleless Dynamic Linear Electrospinning. Nanomaterials 2019, 9, 714. https://doi.org/10.3390/nano9050714
Li T-T, Zhong Y, Yan M, Zhou W, Xu W, Huang S-Y, Sun F, Lou C-W, Lin J-H. Synergistic Effect and Characterization of Graphene/Carbon Nanotubes/Polyvinyl Alcohol/Sodium Alginate Nanofibrous Membranes Formed Using Continuous Needleless Dynamic Linear Electrospinning. Nanomaterials. 2019; 9(5):714. https://doi.org/10.3390/nano9050714
Chicago/Turabian StyleLi, Ting-Ting, Yanqin Zhong, Mengxue Yan, Wei Zhou, Wenting Xu, Shih-Yu Huang, Fei Sun, Ching-Wen Lou, and Jia-Horng Lin. 2019. "Synergistic Effect and Characterization of Graphene/Carbon Nanotubes/Polyvinyl Alcohol/Sodium Alginate Nanofibrous Membranes Formed Using Continuous Needleless Dynamic Linear Electrospinning" Nanomaterials 9, no. 5: 714. https://doi.org/10.3390/nano9050714
APA StyleLi, T.-T., Zhong, Y., Yan, M., Zhou, W., Xu, W., Huang, S.-Y., Sun, F., Lou, C.-W., & Lin, J.-H. (2019). Synergistic Effect and Characterization of Graphene/Carbon Nanotubes/Polyvinyl Alcohol/Sodium Alginate Nanofibrous Membranes Formed Using Continuous Needleless Dynamic Linear Electrospinning. Nanomaterials, 9(5), 714. https://doi.org/10.3390/nano9050714








