Optimization of ZnO Nanorod-Based Surface Enhanced Raman Scattering Substrates for Bio-Applications
Abstract
1. Introduction
2. Experimental Methods
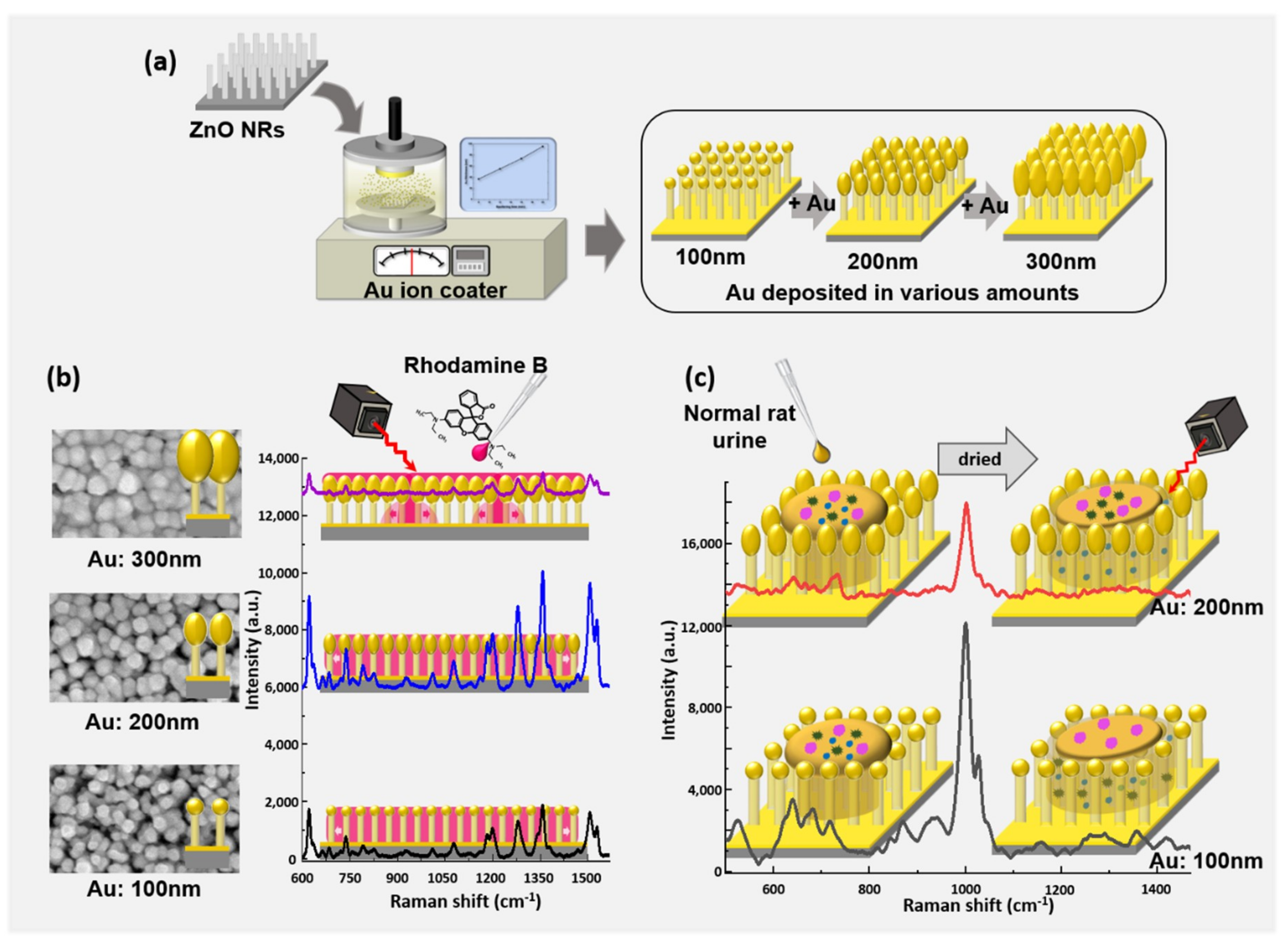
2.1. SERS Substrate Manufacture
2.1.1. ZnO Nanorod Growth
2.1.2. Au Thickness Control
2.2. SERS Measurement
2.2.1. Analytic Substances
2.2.2. Raman Measurement Method and Analysis
3. Results and Discussion
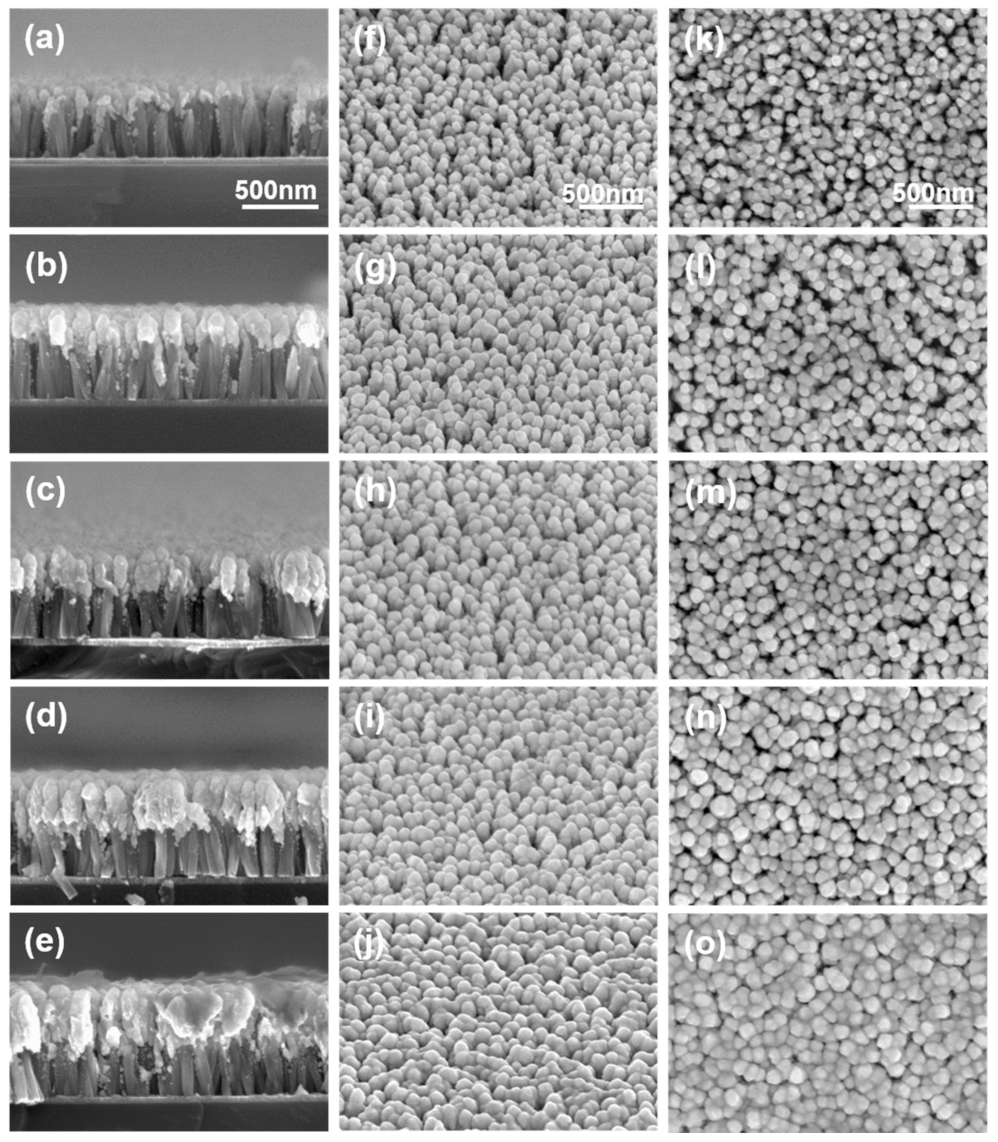
3.1. Morphology of SERS Substrate
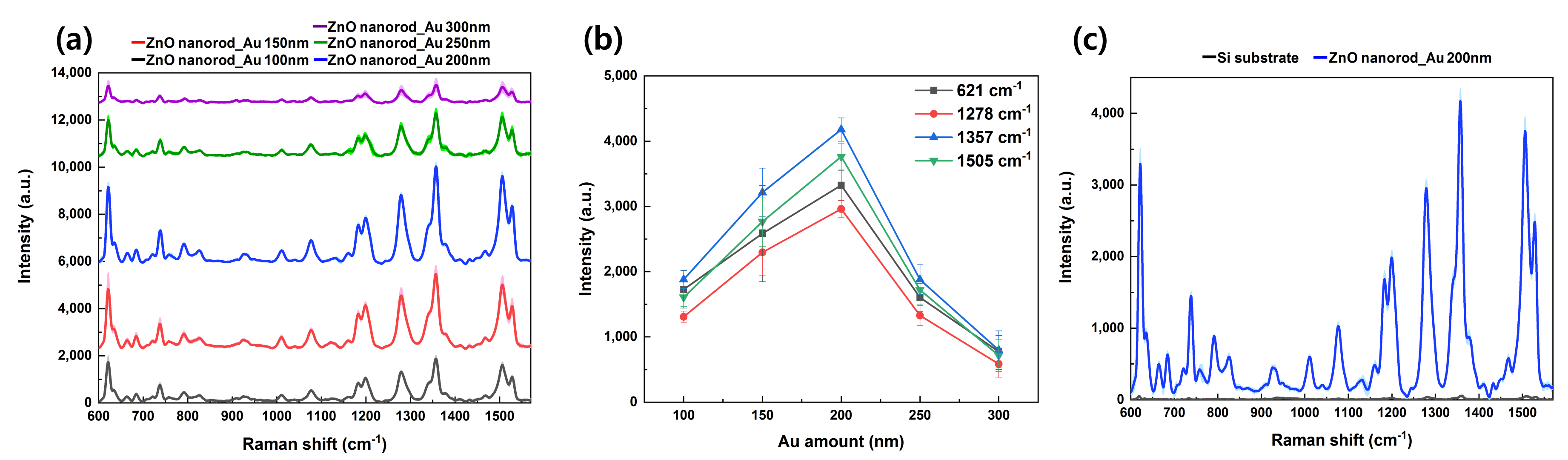
3.2. Raman Measurement of Rhodamine B
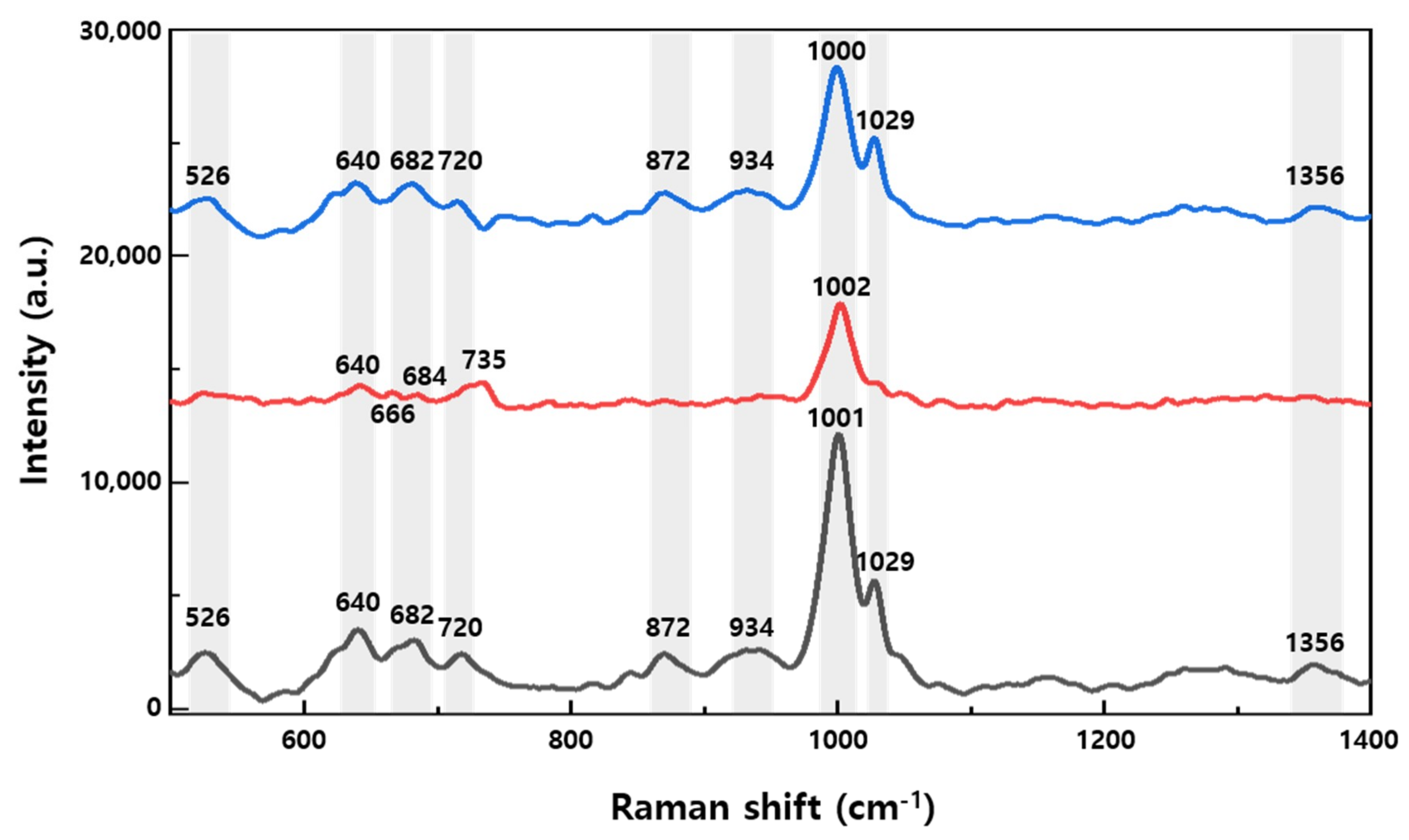
3.3. Raman Measurement of Healthy Rat Urine
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Liao, P.F.; Bergman, J.G.; Chemla, D.S.; Wokaun, A.; Melngailis, J.; Hawryluk, A.M.; Economou, N.P. Surface-enhanced raman scattering from microlithographic silver particle surfaces. Chem. Phys. Lett. 1981, 82, 355–359. [Google Scholar] [CrossRef]
- Howard, R.E.; Liao, P.F.; Skocpol, W.J.; Jackel, L.D.; Craighead, H.G. Microfabrication as a Scientific Tool. Science 1983, 221, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Etchegoin, P.G.; Le Ru, E.C. A perspective on single molecule SERS: Current status and future challenges. Phys. Chem. Chem. Phys. 2008, 10, 6079–6089. [Google Scholar] [CrossRef] [PubMed]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Sun, X.; Li, Y. Surface enhanced Raman scattering effects of silver colloids with different shapes. J. Phys. Chem. B 2005, 109, 12544–12548. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Jeon, K.S.; Hwang, J.H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef]
- Fraire, J.C.; Pérez, L.A.; Coronado, E.A. Cluster Size Effects in the Surface-Enhanced Raman Scattering Response of Ag and Au Nanoparticle Aggregates: Experimental and Theoretical Insight. J. Phys. Chem. C 2013, 117, 23090–23107. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Tang, Y.; Yao, K.F. Highly uniform and reproducible surface enhanced Raman scattering on air-stable metallic glassy nanowire array. Sci. Rep. 2014, 4, 5835. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.H.; Paulson, B.; Lee, J.C.; Kim, J.K. Enhancement of local surface plasmon resonance (LSPR) effect by biocompatible metal clustering based on ZnO nanorods in Raman measurements. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Cialla, D.; Marz, A.; Bohme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef]
- Laing, S.; Gracie, K.; Faulds, K. Multiplex in vitro detection using SERS. Chem. Soc. Rev. 2016, 45, 1901–1918. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Chen, S.; Zhang, K. Effect of toxicity of Ag nanoparticles on SERS spectral variance of bacteria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1061–1066. [Google Scholar] [CrossRef]
- Manekkathodi, A.; Lu, M.Y.; Wang, C.W.; Chen, L.J. Direct growth of aligned zinc oxide nanorods on paper substrates for low-cost flexible electronics. Adv. Mater. 2010, 22, 4059–4063. [Google Scholar] [CrossRef] [PubMed]
- Kurda, A.H.; Hassan, Y.M.; Ahmed, N.M. Controlling Diameter, Length and Characterization of ZnO Nanorods by Simple Hydrothermal Method for Solar Cells. World J. Nano Sci. Eng. 2015, 5, 34–40. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Eriksson, M.; AlSalhi, M.; Atif, M.; Ansari, A.; Willander, M. Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles. Materials 2013, 6, 3584–3597. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Lee, T.K.; Park, J.; Ha, M.; Kwak, S.K.; Ko, H. Particle-on-Film Gap Plasmons on Antireflective ZnO Nanocone Arrays for Molecular-Level Surface-Enhanced Raman Scattering Sensors. ACS Appl. Mater. Interfaces 2015, 7, 26421–26429. [Google Scholar] [CrossRef]
- Sinha, G.; Depero, L.E.; Alessandri, I. Recyclable SERS substrates based on Au-coated ZnO nanorods. ACS Appl. Mater. Interfaces 2011, 3, 2557–2563. [Google Scholar] [CrossRef]
- Tang, H.; Meng, G.; Huang, Q.; Zhang, Z.; Huang, Z.; Zhu, C. Arrays of Cone-Shaped ZnO Nanorods Decorated with Ag Nanoparticles as 3D Surface-Enhanced Raman Scattering Substrates for Rapid Detection of Trace Polychlorinated Biphenyls. Adv. Funct. Mater. 2012, 22, 218–224. [Google Scholar] [CrossRef]
- Birtalan, E.; Rudat, B.; Kolmel, D.K.; Fritz, D.; Vollrath, S.B.; Schepers, U.; Brase, S. Investigating rhodamine B-labeled peptoids: Scopes and limitations of its applications. Biopolymers 2011, 96, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Agarwal, A.; Buddharaju, K.D.; Khalid, N.M.; Salim, S.M.; Widjaja, E.; Garland, M.V.; Balasubramanian, N.; Kwong, D.L. DNA detection using nanostructured SERS substrates with Rhodamine B as Raman label. Biosens. Bioelectron. 2008, 24, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lim, C.; Chen, L.; Chon, H.; Choo, J.; Hong, J.; deMello, A.J. Surface-enhanced Raman scattering in nanoliter droplets: Towards high-sensitivity detection of mercury (II) ions. Anal. Bioanal. Chem. 2009, 394, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Jin, D.; Ma, P.; Wang, D.; Yang, Q.; Song, D.; Wang, X. Rapid Determination of Rhodamine B in Chili Powder by Surface-Enhanced Raman Spectroscopy. Anal. Lett. 2015, 48, 1918–1929. [Google Scholar] [CrossRef]
- Li, D.; Li, D.-W.; Li, Y.; Fossey, J.S.; Long, Y.-T. Cyclic electroplating and stripping of silver on Au@SiO2 core/shell nanoparticles for sensitive and recyclable substrate of surface-enhanced Raman scattering. J. Mater. Chem. 2010, 20, 3688–3693. [Google Scholar] [CrossRef]
- Sun, C.H.; Wang, M.L.; Feng, Q.; Liu, W.; Xu, C.X. Surface-enhanced Raman scattering (SERS) study on Rhodamine B adsorbed on different substrates. Russ. J. Phys. Chem. A 2014, 89, 291–296. [Google Scholar] [CrossRef]
- Synytsya, A.; Judexova, M.; Hoskovec, D.; Miskovicova, M.; Petruzelka, L. Raman spectroscopy at different excitation wavelengths (1064, 785 and 532 nm) as a tool for diagnosis of colon cancer. J. Raman Spectrosc. 2014, 45, 903–911. [Google Scholar] [CrossRef]
- Stone, N.; Stavroulaki, P.; Kendall, C.; Birchall, M.; Barr, H. Raman spectroscopy for early detection of laryngeal malignancy: Preliminary results. Laryngoscope 2000, 110, 1756–1763. [Google Scholar] [CrossRef]
- Premasiri, W.R.; Clarke, R.H.; Womble, M.E. Urine analysis by laser Raman spectroscopy. Lasers Surg. Med. 2001, 28, 330–334. [Google Scholar] [CrossRef]
- Del Mistro, G.; Cervo, S.; Mansutti, E.; Spizzo, R.; Colombatti, A.; Belmonte, P.; Zucconelli, R.; Steffan, A.; Sergo, V.; Bonifacio, A. Surface-enhanced Raman spectroscopy of urine for prostate cancer detection: A preliminary study. Anal. Bioanal. Chem. 2015, 407, 3271–3275. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Khan, A.; Maru, G.; Ingle, A.; Krishna, C.M. A preliminary Raman spectroscopic study of urine: Diagnosis of breast cancer in animal models. Analyst 2015, 140, 456–466. [Google Scholar] [CrossRef]
- Chan, J.W.; Taylor, D.S.; Zwerdling, T.; Lane, S.M.; Ihara, K.; Huser, T. Micro-Raman spectroscopy detects individual neoplastic and normal hematopoietic cells. Biophys. J. 2006, 90, 648–656. [Google Scholar] [CrossRef]
- Wang, H.; Huang, N.; Zhao, J.; Lui, H.; Korbelik, M.; Zeng, H. Depth-resolved in vivo micro-Raman spectroscopy of a murine skin tumor model reveals cancer-specific spectral biomarkers. J. Raman Spectrosc. 2011, 42, 160–166. [Google Scholar] [CrossRef]
- Huang, Z.; McWilliams, A.; Lui, H.; McLean, D.I.; Lam, S.; Zeng, H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jue, M.; Lee, S.; Paulson, B.; Namgoong, J.-M.; Yu, H.Y.; Kim, G.; Jeon, S.; Shin, D.-M.; Choo, M.-S.; Joo, J.; et al. Optimization of ZnO Nanorod-Based Surface Enhanced Raman Scattering Substrates for Bio-Applications. Nanomaterials 2019, 9, 447. https://doi.org/10.3390/nano9030447
Jue M, Lee S, Paulson B, Namgoong J-M, Yu HY, Kim G, Jeon S, Shin D-M, Choo M-S, Joo J, et al. Optimization of ZnO Nanorod-Based Surface Enhanced Raman Scattering Substrates for Bio-Applications. Nanomaterials. 2019; 9(3):447. https://doi.org/10.3390/nano9030447
Chicago/Turabian StyleJue, Miyeon, Sanghwa Lee, Bjorn Paulson, Jung-Man Namgoong, Hwan Yeul Yu, Gwanho Kim, Sangmin Jeon, Dong-Myung Shin, Myung-Soo Choo, Jinmyoung Joo, and et al. 2019. "Optimization of ZnO Nanorod-Based Surface Enhanced Raman Scattering Substrates for Bio-Applications" Nanomaterials 9, no. 3: 447. https://doi.org/10.3390/nano9030447
APA StyleJue, M., Lee, S., Paulson, B., Namgoong, J.-M., Yu, H. Y., Kim, G., Jeon, S., Shin, D.-M., Choo, M.-S., Joo, J., Moon, Y., Pack, C.-G., & Kim, J. K. (2019). Optimization of ZnO Nanorod-Based Surface Enhanced Raman Scattering Substrates for Bio-Applications. Nanomaterials, 9(3), 447. https://doi.org/10.3390/nano9030447








