Effect of Varying the Ratio of Carbon Black to Vapor-Grown Carbon Fibers in the Separator on the Performance of Li–S Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the VGCF and Super-P Carbon Composite
2.3. Preparation of the Modified Separators
2.4. Preparation of the Sulfur Cathode and Cell Assembly
2.5. Electrochemical Testing
2.6. Characterization and Electrochemical Measurements
3. Results and Discussion
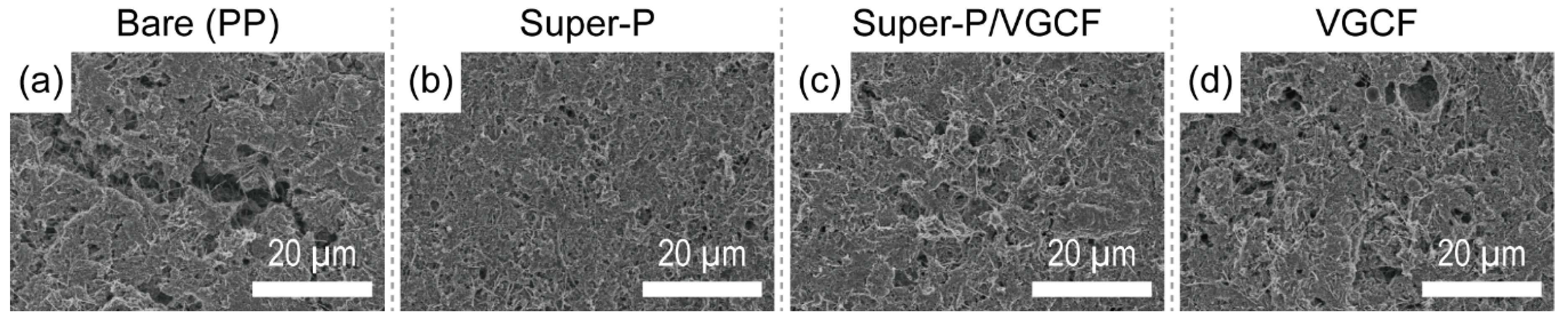
3.1. Morphology and Physical Characterization of the Carbon-Coated Separator
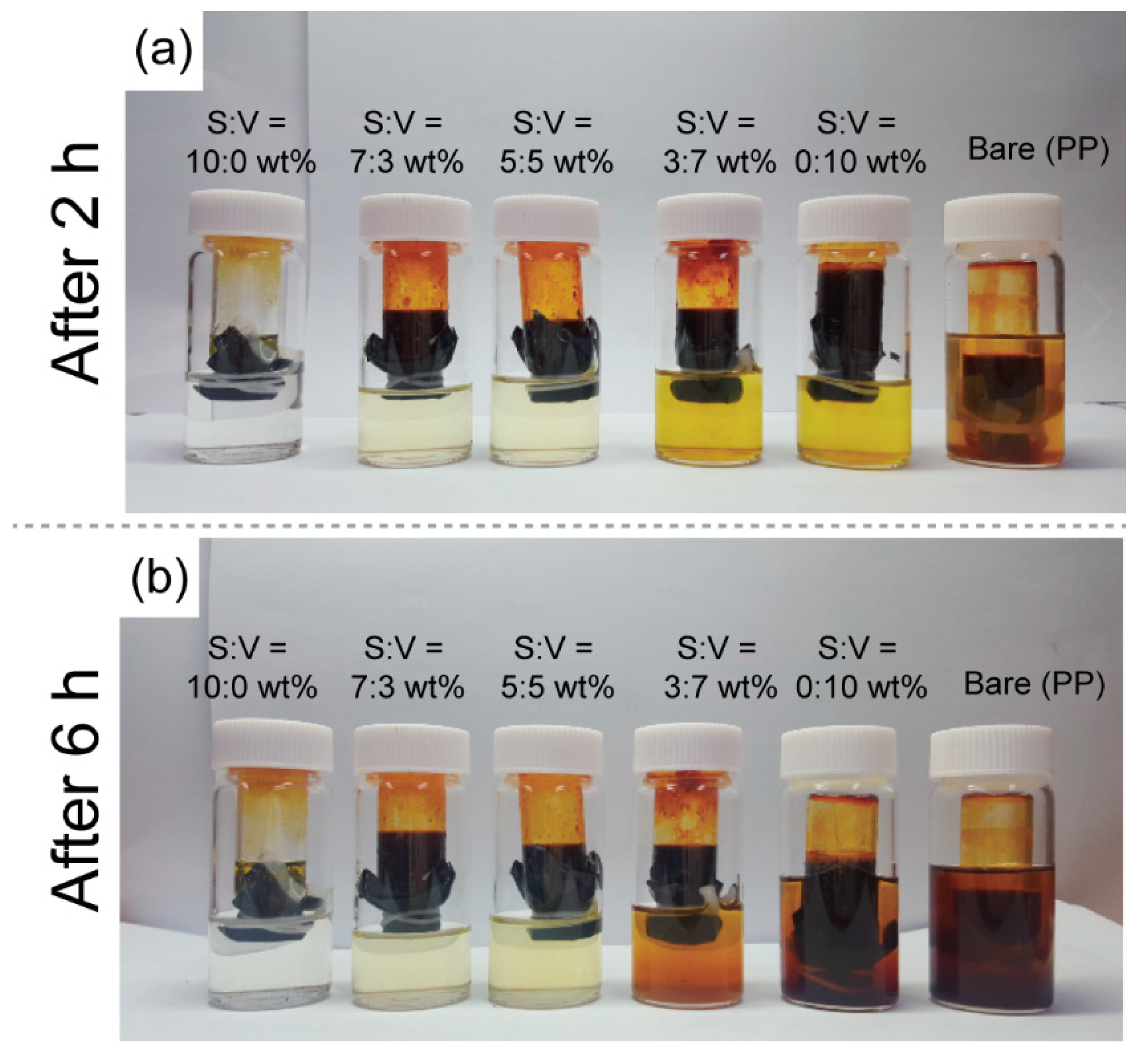
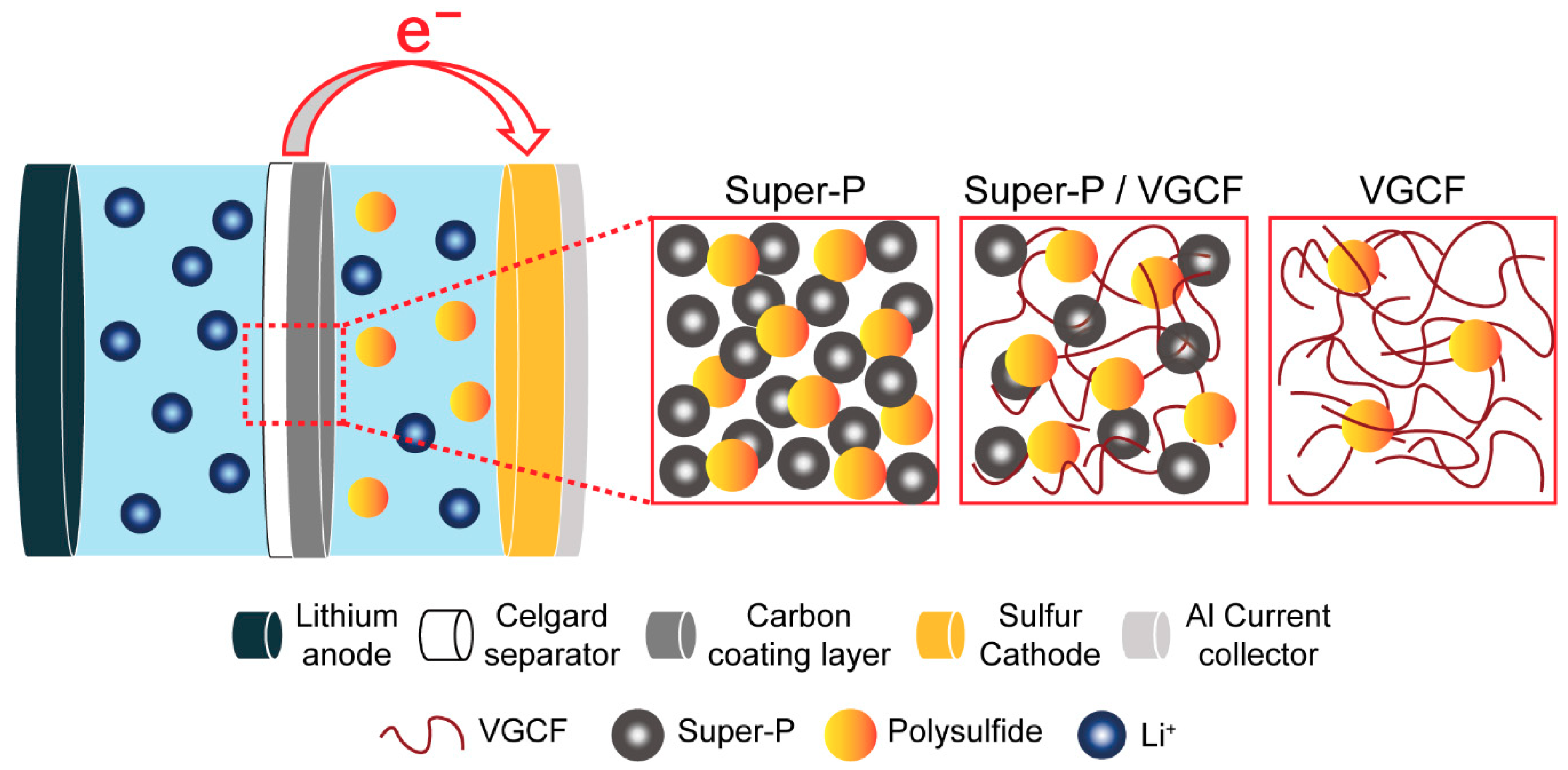
3.2. Polysulfide Suppression Behaviors of Carbon-Coated Separators
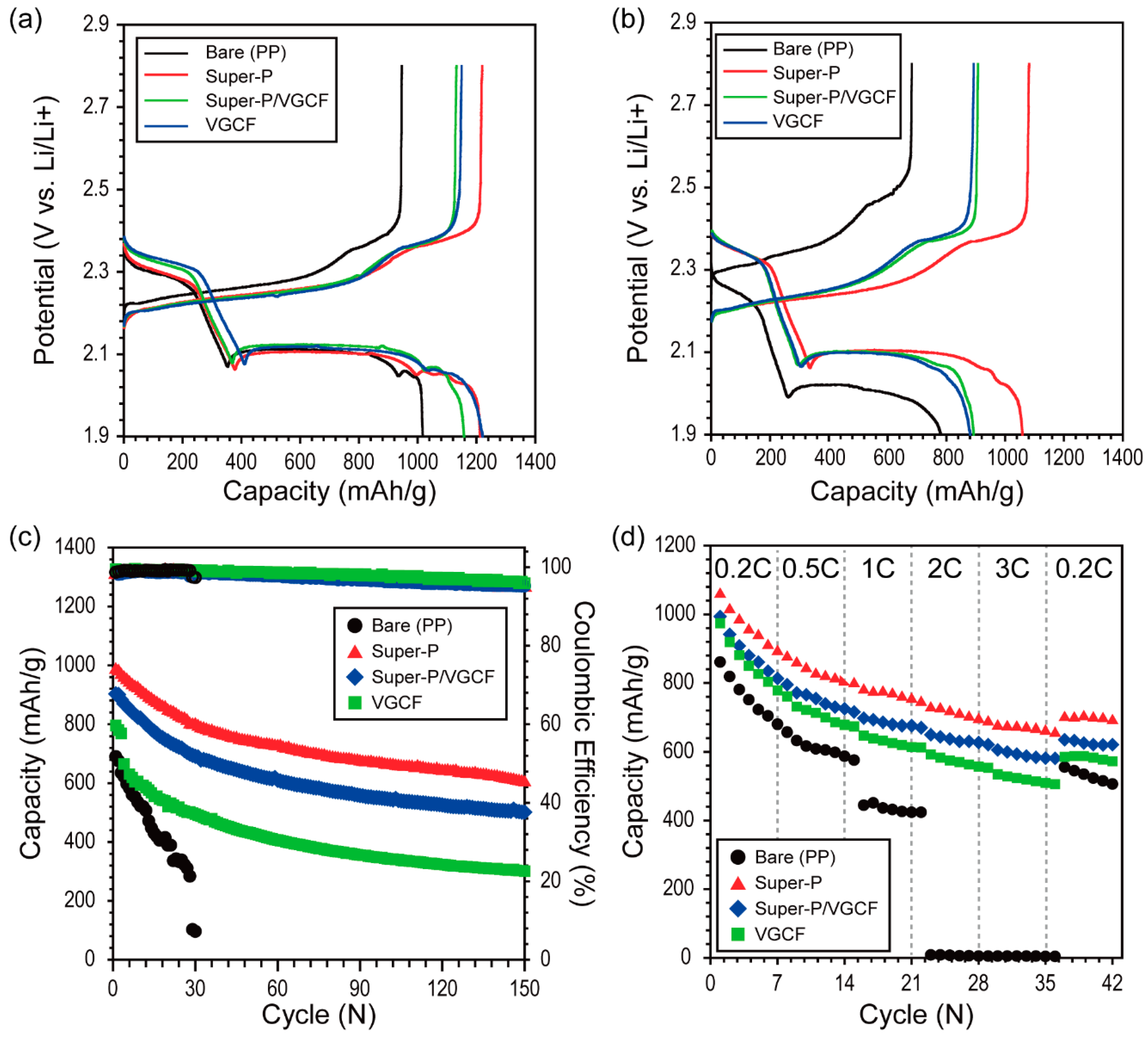
3.3. Electrochemical Performance
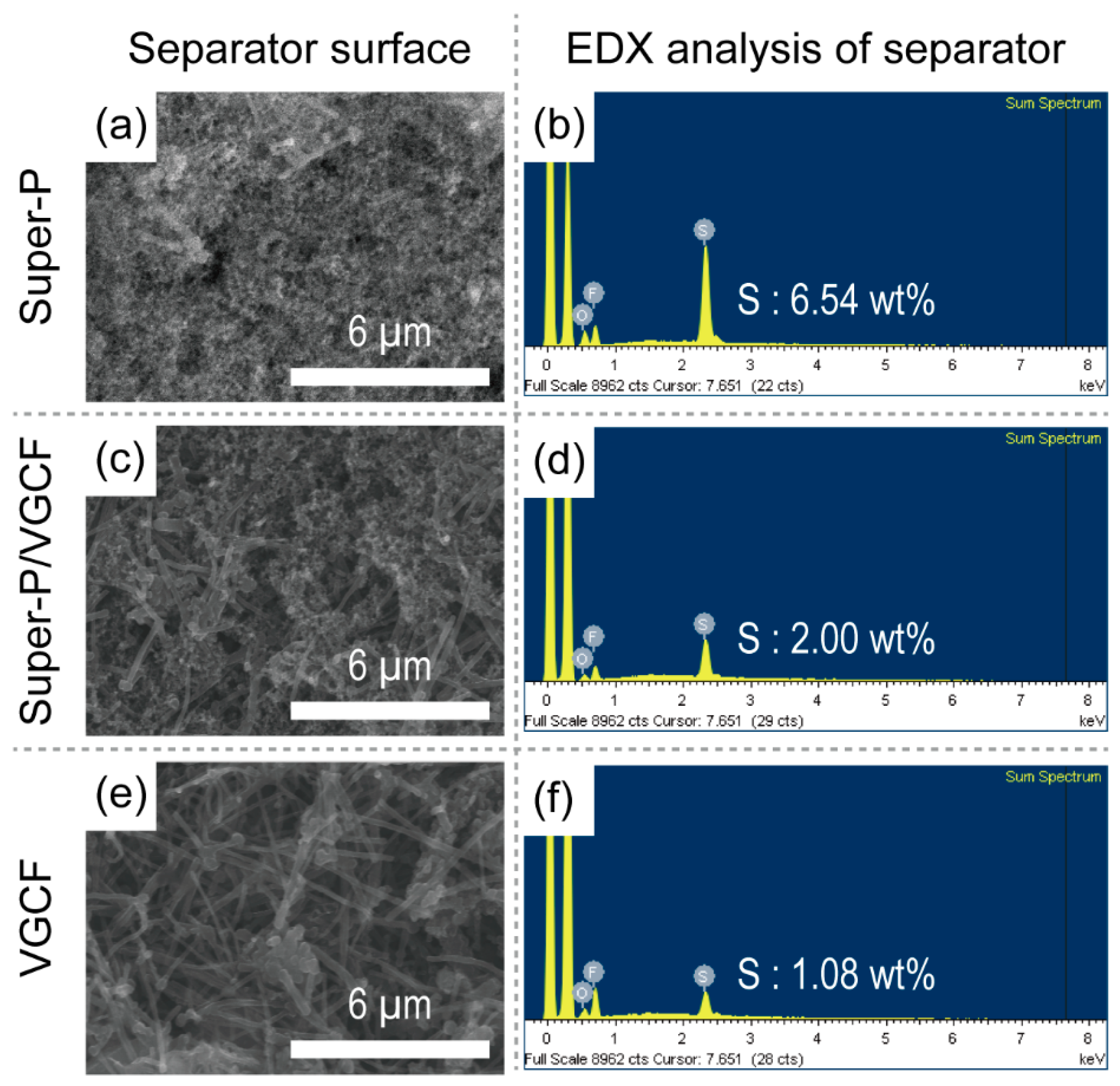
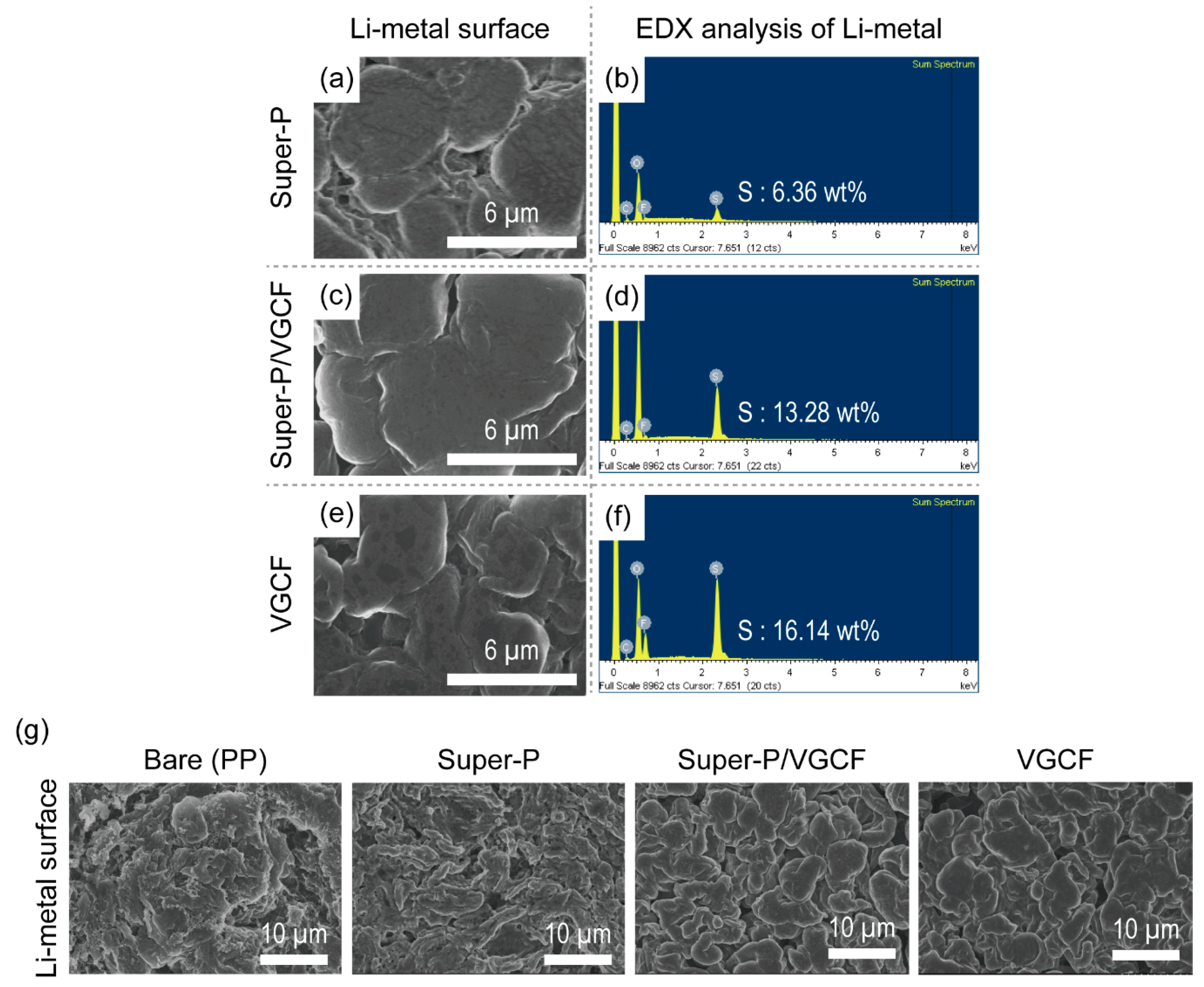
3.4. Post-Mortem Analysis of Li–S Batteries after Cycling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Li, Y.; Yao, Y.; Lin, Z.; Ji, L.; Xu, G.; Liang, Y.; Shi, Q.; Zhang, X. Electrospun Li4Ti5O12/C composites for lithium-ion batteries with high rate performance. Solid State Ionics 2011, 204, 61–65. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Nazar, L.F. Advances in Li–S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Lu, S.; Cheng, Y.; Wu, X.; Liu, J. Significantly improved long-cycle stability in high-rate Li–S batteries enabled by coaxial graphene wrapping over sulfur-coated carbon nanofibers. Nano Lett. 2013, 13, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fu, K.; Lu, Y.; Zhu, J.; Xue, L.; Hu, Y.; Zhang, X. Use of a tin antimony alloy-filled porous carbon nanofiber composite as an anode in sodium-ion batteries. RSC Adv. 2015, 5, 30793–30800. [Google Scholar] [CrossRef]
- Ge, Y.; Jiang, H.; Zhu, J.; Lu, Y.; Chen, C.; Hu, Y.; Qiu, Y.; Zhang, X. High cyclability of carbon-coated TiO2 nanoparticles as anode for sodium-ion batteries. Electrochim. Acta 2015, 157, 142–148. [Google Scholar] [CrossRef]
- Peng, H.-J.; Liang, J.; Zhu, L.; Huang, J.-Q.; Cheng, X.-B.; Guo, X.; Ding, W.; Zhu, W.; Zhang, Q. Catalytic self-limited assembly at hard templates: A mesoscale approach to graphene nanoshells for lithium–sulfur batteries. ACS Nano 2014, 8, 11280–11289. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, W.; Zhao, W.; Li, G.; Hou, Y.; Liu, M.; Zhou, L.; Ye, F.; Li, H.; Wei, Z. High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Lett. 2014, 14, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-L.; Xu, Y.-F.; Fang, J.-C.; Peng, X.-X.; Fu, F.; Huang, L.; Li, J.-T.; Sun, S.-G. Porous graphitic carbon loading ultra high sulfur as high-performance cathode of rechargeable lithium–sulfur batteries. ACS Appl. Mater. Interface 2013, 5, 10782–10793. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Pyun, J.; Char, K. Recent approaches for the direct use of elemental sulfur in the synthesis and processing of advanced materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef] [PubMed]
- Qie, L.; Manthiram, A. A facile layer-by-layer approach for high-areal-capacity sulfur cathodes. Adv. Mater. 2015, 27, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Shi, L.; Mu, D.; Xu, H.; Wu, B. A hierarchical carbon fiber/sulfur composite as cathode material for Li–S batteries. Carbon 2015, 86, 146–155. [Google Scholar] [CrossRef]
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Rui, K.; Wu, X.; Wu, M.; Zhang, J. Enhanced performance of lithium sulfur battery with polypyrrole warped mesoporous carbon/sulfur composite. J. Power Sources 2014, 254, 353–359. [Google Scholar] [CrossRef]
- Li, G.; Cai, W.; Liu, B.; Li, Z. A multi functional binder with lithium ion conductive polymer and polysulfide absorbents to improve cycleability of lithium–sulfur batteries. J. Power Sources 2015, 294, 187–192. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Z.; Monroe, C.W.; Yang, J.; Nuli, Y. Carbonyl-β-cyclodextrin as a novel binder for sulfur composite cathodes in rechargeable lithium batteries. Adv. Funct. Mater. 2013, 23, 1194–1201. [Google Scholar] [CrossRef]
- Li, M.-T.; Sun, Y.; Zhao, K.-S.; Wang, Z.; Wang, X.-L.; Su, Z.-M.; Xie, H.-M. Metal–organic framework with aromatic rings tentacles: High sulfur storage in Li–S batteries and efficient benzene homologues distinction. ACS Appl. Mater. Interface 2016, 8, 33183–33188. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, J.; Wu, D.; Gu, M.; Xu, W.; Wang, C.; Gao, F.; Engelhard, M.H.; Zhang, J.-G.; Liu, J. Lewis acid–base interactions between polysulfides and metal organic framework in lithium sulfur batteries. Nano Lett. 2014, 14, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Ohtomo, T.; Mizuno, F.; Tadanaga, K.; Tatsumisago, M. All-solid-state Li/S batteries with highly conductive glass–ceramic electrolytes. Electrochem. Commun. 2003, 5, 701–705. [Google Scholar] [CrossRef]
- Tao, X.; Liu, Y.; Liu, W.; Zhou, G.; Zhao, J.; Lin, D.; Zu, C.; Sheng, O.; Zhang, W.; Lee, H.-W. Solid-state lithium–sulfur batteries operated at 37C with composites of nanostructured Li7La3Zr2O12/carbon foam and polymer. Nano Lett. 2017, 17, 2967–2972. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-L.; Zong, J.; Xu, H.; Wang, W.; Liu, X.-J. Effects of LAGP electrolyte on suppressing polysulfide shuttling in Li–S cells. RSC Adv. 2016, 6, 57346–57356. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.; Fu, W.; Dudney, N.J.; Liang, C. Phosphorous pentasulfide as a novel additive for high-performance lithium-sulfur batteries. Adv. Funct. Mater. 2013, 23, 1064–1069. [Google Scholar] [CrossRef]
- Balach, J.; Jaumann, T.; Klose, M.; Oswald, S.; Eckert, J.R.; Giebeler, L. Mesoporous carbon interlayers with tailored pore volume as polysulfide reservoir for high-energy lithium–sulfur batteries. J. Phys. Chem. C 2015, 119, 4580–4587. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. A hierarchical carbonized paper with controllable thickness as a modulable interlayer system for high performance Li–S batteries. Chem. Commun. 2014, 50, 4184–4187. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Manthiram, A. Bifunctional separator with a light-weight carbon-coating for dynamically and statically stable lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 5299–5306. [Google Scholar] [CrossRef]
- Yao, H.; Yan, K.; Li, W.; Zheng, G.; Kong, D.; Seh, Z.W.; Narasimhan, V.K.; Liang, Z.; Cui, Y. Improved lithium–sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode–separator interface. Energy Environ. Sci. 2014, 7, 3381–3390. [Google Scholar] [CrossRef]
- Yeon, D.; Lee, Y.; Ryou, M.-H.; Lee, Y.M. New flame-retardant composite separators based on metal hydroxides for lithium-ion batteries. Electrochim. Acta 2015, 157, 282–289. [Google Scholar] [CrossRef]
- Cho, I.; Choi, J.; Kim, K.; Ryou, M.-H.; Lee, Y.M. A comparative investigation of carbon black (Super-P) and vapor-grown carbon fibers (VGCFs) as conductive additives for lithium-ion battery cathodes. RSC Adv. 2015, 5, 95073–95078. [Google Scholar] [CrossRef]
- Yang, M.; Hou, J. Membranes in lithium ion batteries. Membranes 2012, 2, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Freitag, A.; Stamm, M.; Ionov, L. Separator for lithium-sulfur battery based on polymer blend membrane. J. Power Sources 2017, 363, 384–391. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@sulfur composites for high-power lithium–sulfur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yuan, L.-X.; Zhang, W.-X.; Hu, X.-L.; Huang, Y.-H. Enhanced cyclability for sulfur cathode achieved by a water-soluble binder. J. Phys. Chem. C 2011, 115, 15703–15709. [Google Scholar] [CrossRef]
- Zhang, S. Understanding of sulfurized polyacrylonitrile for superior performance lithium/sulfur battery. Energies 2014, 7, 4588–4600. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.-C.; Cui, Y. Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar] [CrossRef] [PubMed]

| Celgard 2400 | Super-P | Super-P/VGCF | VGCF | |
|---|---|---|---|---|
| Thickness (µm) | 25 | 34 | 35 | 34 |
| Gurley number (s∙100 mL−1) | 546.4 | 633.2 | 583.2 | 570.6 |
| Surface resistance (mΩ∙cm) | N.A. | 286.1 | 165.2 | 84.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Oh, J.; Lee, Y.M.; Ryou, M.-H. Effect of Varying the Ratio of Carbon Black to Vapor-Grown Carbon Fibers in the Separator on the Performance of Li–S Batteries. Nanomaterials 2019, 9, 436. https://doi.org/10.3390/nano9030436
Jo H, Oh J, Lee YM, Ryou M-H. Effect of Varying the Ratio of Carbon Black to Vapor-Grown Carbon Fibers in the Separator on the Performance of Li–S Batteries. Nanomaterials. 2019; 9(3):436. https://doi.org/10.3390/nano9030436
Chicago/Turabian StyleJo, Hearin, Jeonghun Oh, Yong Min Lee, and Myung-Hyun Ryou. 2019. "Effect of Varying the Ratio of Carbon Black to Vapor-Grown Carbon Fibers in the Separator on the Performance of Li–S Batteries" Nanomaterials 9, no. 3: 436. https://doi.org/10.3390/nano9030436
APA StyleJo, H., Oh, J., Lee, Y. M., & Ryou, M.-H. (2019). Effect of Varying the Ratio of Carbon Black to Vapor-Grown Carbon Fibers in the Separator on the Performance of Li–S Batteries. Nanomaterials, 9(3), 436. https://doi.org/10.3390/nano9030436





