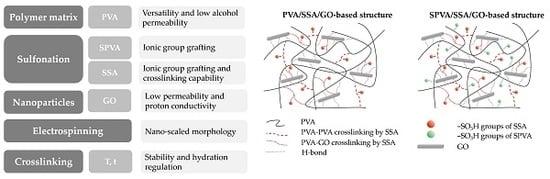
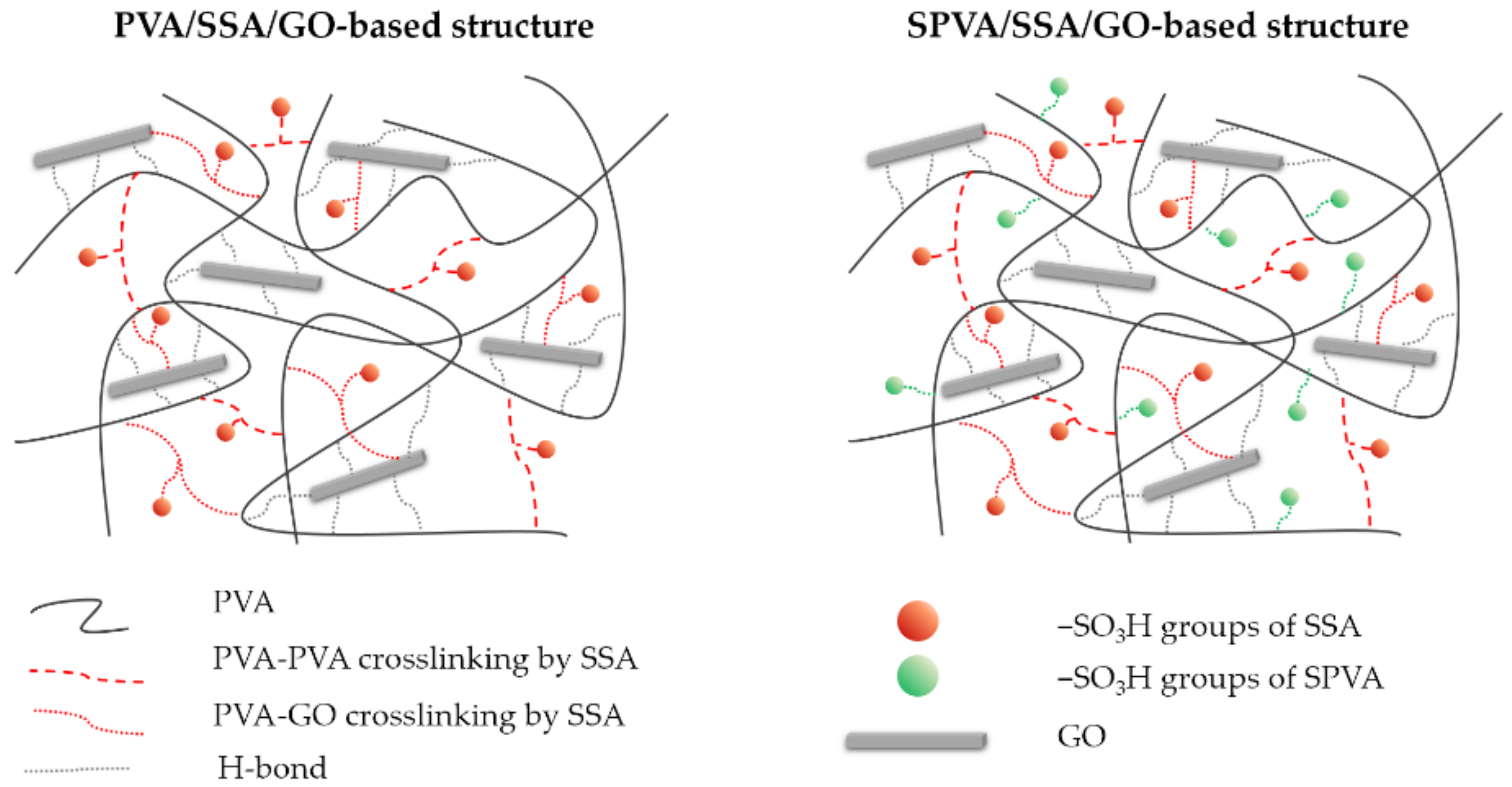
Crosslinked Sulfonated Poly(vinyl alcohol)/Graphene Oxide Electrospun Nanofibers as Polyelectrolytes
Abstract
1. Introduction
2. Materials and Methods
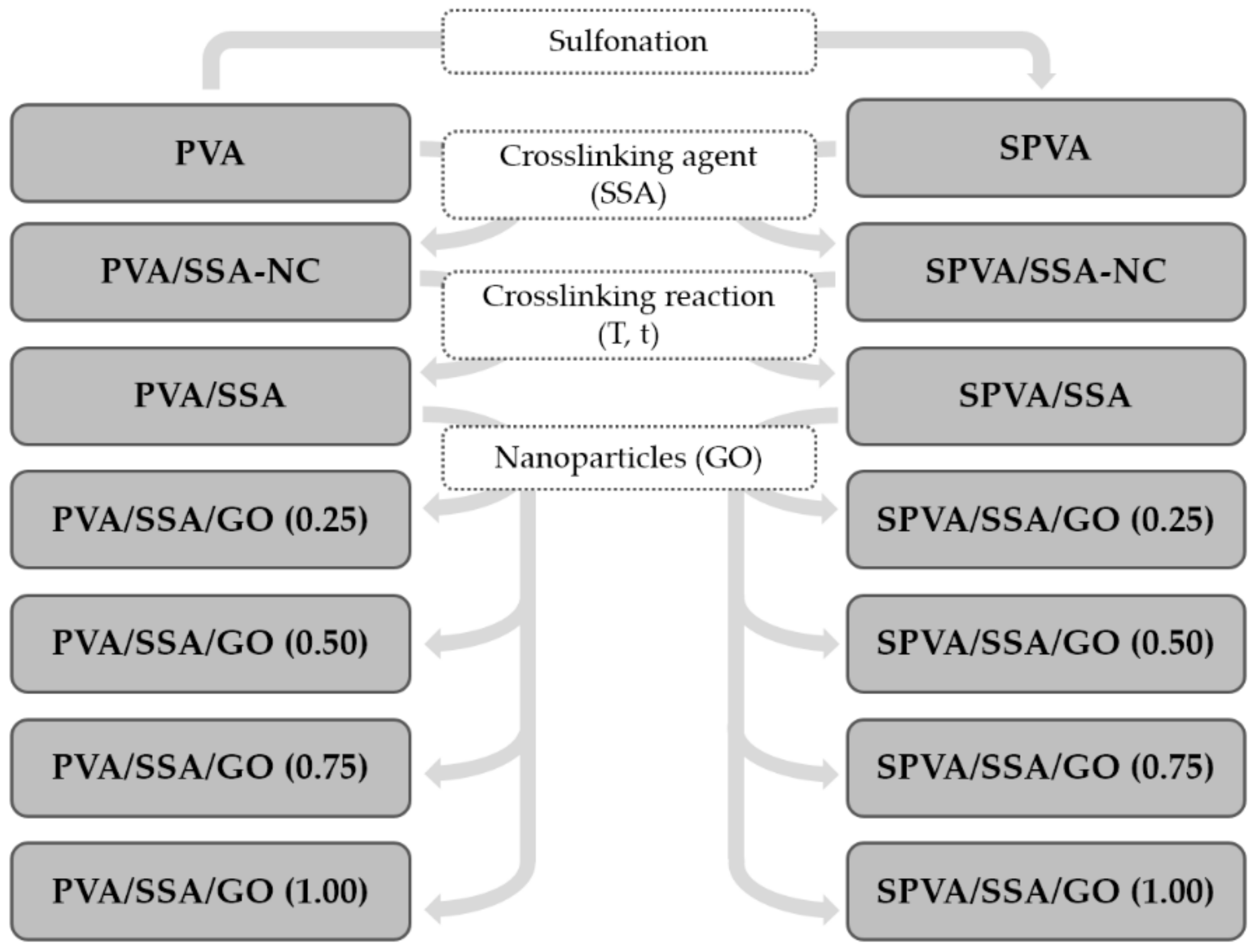
2.1. Obtaining of Nanofibers
2.1.1. Materials
2.1.2. Solution Preparation
2.1.3. Electrospinning
2.1.4. Nanofiber Crosslinking
2.2. Analytical Assessment
2.2.1. Field-Emission Scanning Electron Microscopy (FE-SEM)
2.2.2. Fourier-Transformed Infrared Spectroscopy (FT-IR)
2.2.3. Thermogravimetry (TGA)
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Electrical and Proton Conductometry
2.2.6. Immersion in Simulated Service Conditions
3. Results and Discussion
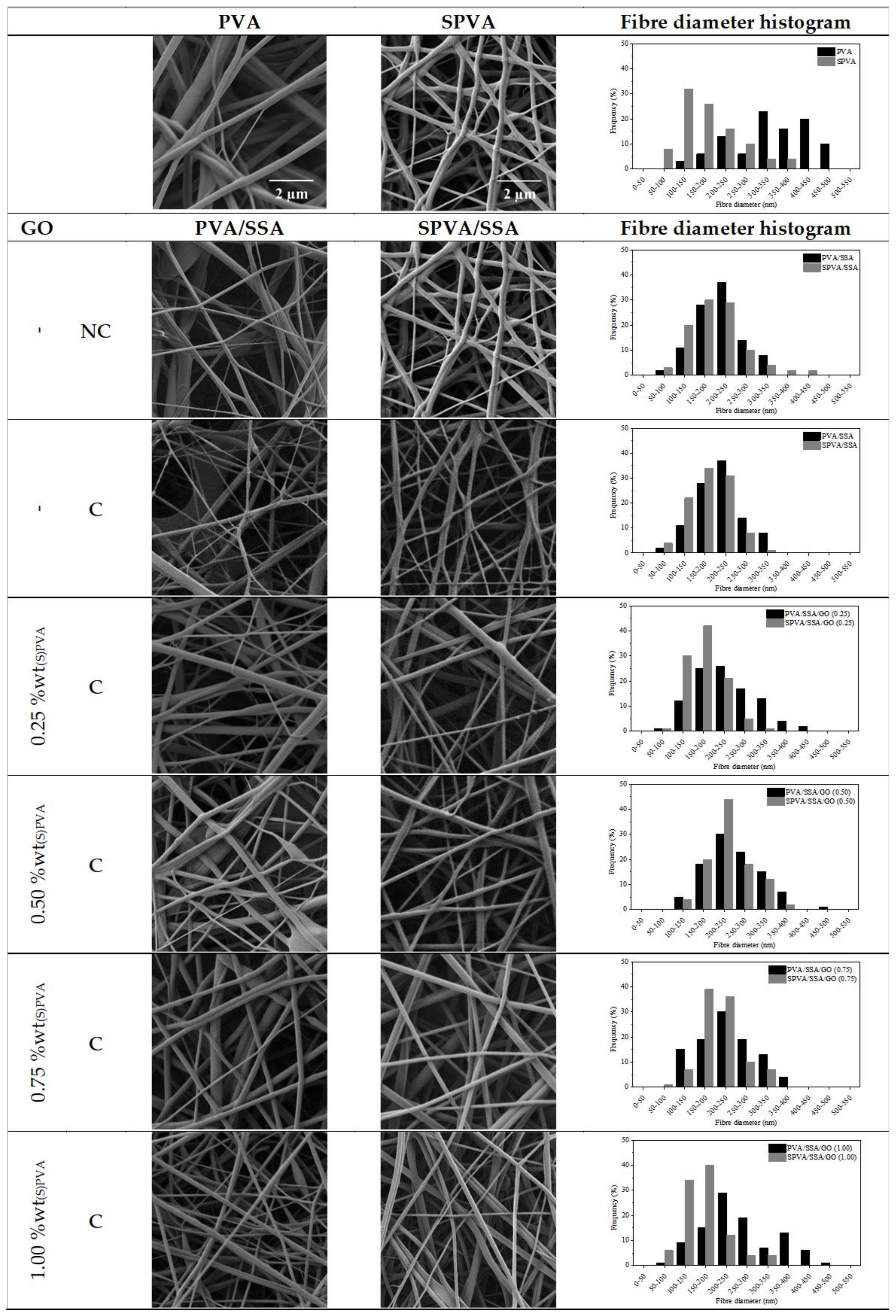
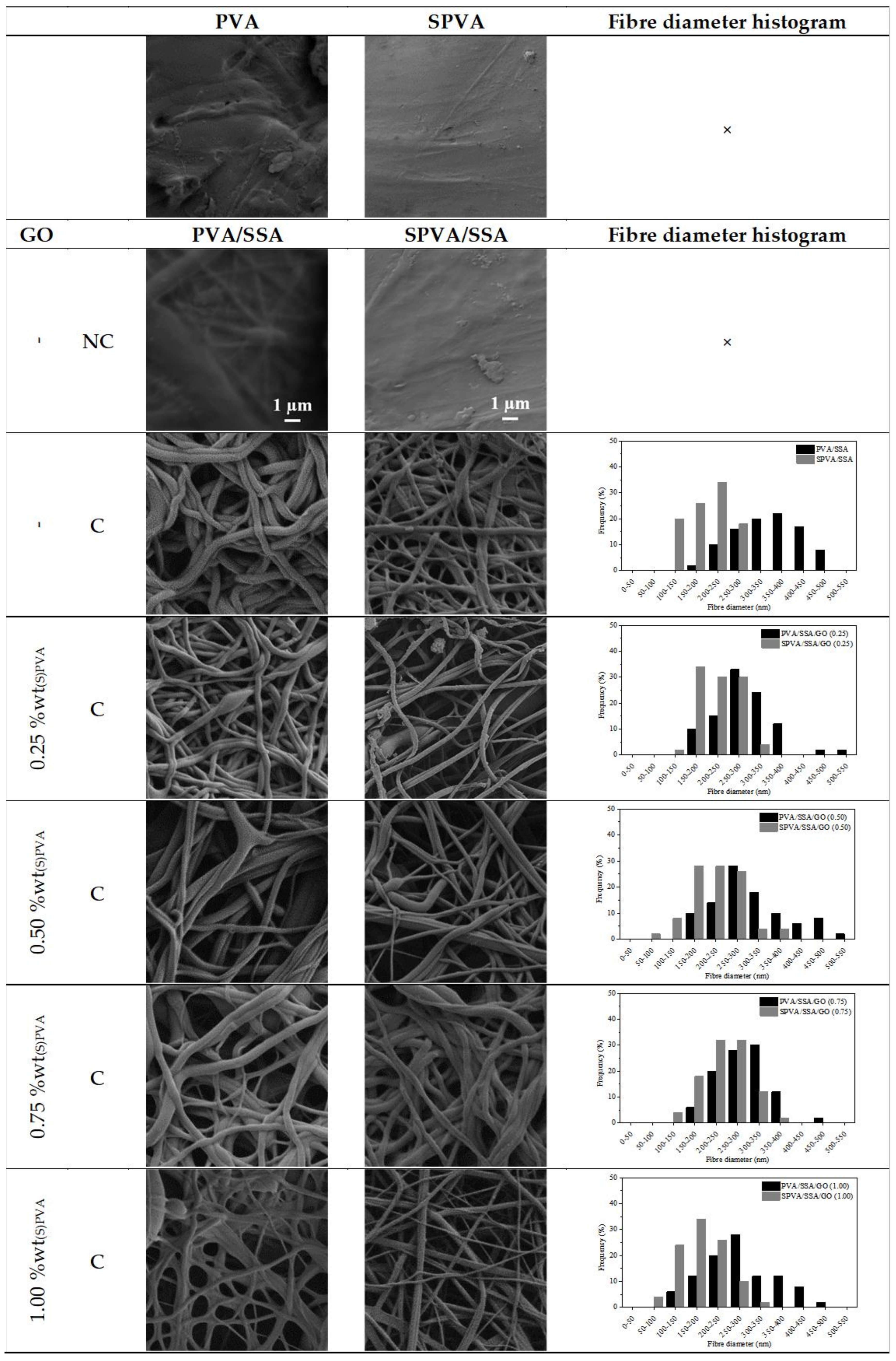
3.1. Surface Morphology
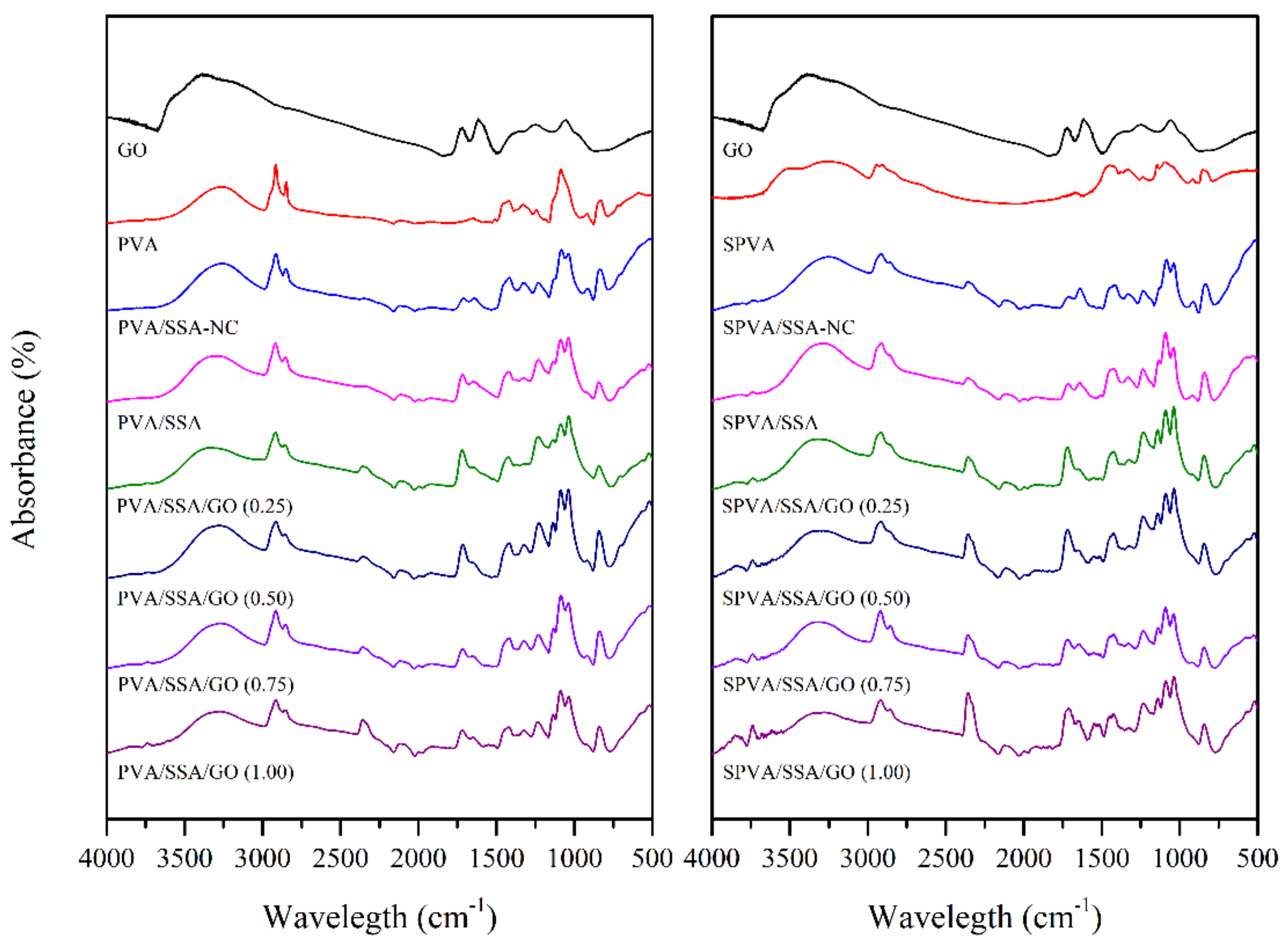
3.2. Chemical Composition
3.3. Thermal Properties
3.4. Thermo-Oxidative Stability
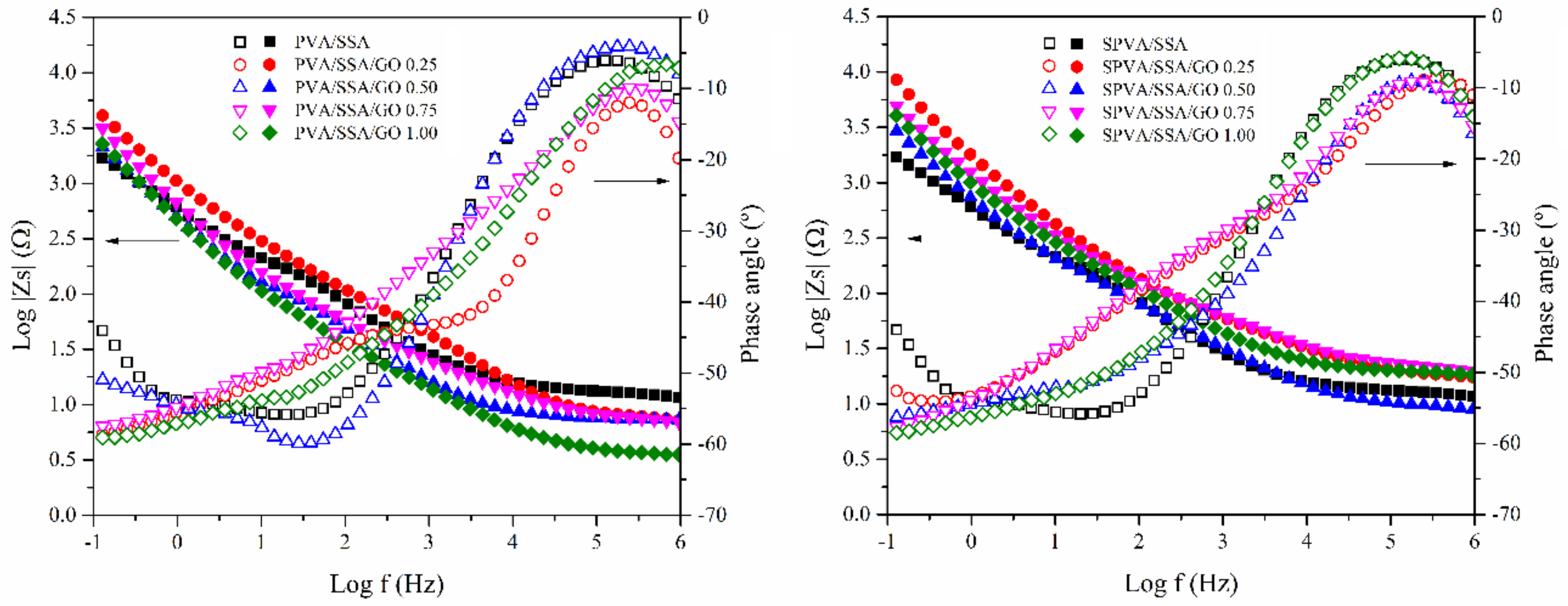
3.5. Electrical Conductivity
3.6. Proton Conductivity
3.7. Hydrothermal Stability in Simulated Service Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shabani, I.; Hasani-Sadrabadi, M.M.; Haddadi-Asl, V.; Soleimani, M. Nanofiber-based polyelectrolytes as novel membranes for fuel cell applications. J. Memb. Sci. 2011, 368, 233–240. [Google Scholar] [CrossRef]
- Sood, R.; Cavaliere, S.; Jones, D.J.; Rozière, J. Electrospun nanofibre composite polymer electrolyte fuel cell and electrolysis membranes. Nano Energy 2016, 26, 729–745. [Google Scholar] [CrossRef]
- Ding, B.; Kikuchi, M.; Li, C.; Shiratori, S. Electrospun nanofibrous polyelectrolytes membranes as high sensitive coatings for QCM-based gas sensors. In Nanotechnology at the Leading Edge; Dirote, E.V., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2006; pp. 1–28. [Google Scholar]
- Rajesh, S.; Zhao, Y.; Fong, H.; Menkhaus, T.J. Polyacrylonitrile nanofiber membranes modified with ionically crosslinked polyelectrolyte multilayers for the separation of ionic impurities. Nanoscale 2016, 8, 18376–18389. [Google Scholar] [CrossRef] [PubMed]
- Junoh, H.; Jaafar, J.; Mohd Norddin, M.N.A.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Wan Salleh, W.N.; Ilbeygi, H. A Review on the Fabrication of Electrospun Polymer Electrolyte Membrane for Direct Methanol Fuel Cell. J. Nanomater. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Effect of the dissolution time into an acid hydrolytic solvent to tailor electrospun nanofibrous polycaprolactone scaffolds. Eur. Polym. J. 2017, 87, 174–187. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.D.; Ribes-Greus, A. Tailored electrospun nanofibrous polycaprolactone/gelatin scaffolds into an acid hydrolytic solvent system. Eur. Polym. J. 2018, 101, 273–281. [Google Scholar] [CrossRef]
- Tanaka, M. Development of ion conductive nanofibers for polymer electrolyte fuel cells. Polym. J. 2016, 48, 51–58. [Google Scholar] [CrossRef]
- Li, H.-Y.; Liu, Y.-L. Nafion-functionalized electrospun poly(vinylidene fluoride) (PVDF) nanofibers for high performance proton exchange membranes in fuel cells. J. Mater. Chem. A 2014, 2, 3783–3793. [Google Scholar] [CrossRef]
- Pedroza, O.J.O.; Dutra Filho, J.C.; Picciani, P.H.S.; Dias, M.L. Morphology and proton conductivity of composite membranes based on poly(styrene sulfonic acid–maleic anhydride) nanofibers prepared by electrospinning. Ionics 2015, 21, 755–764. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.M.; Wycisk, R.; Pintauro, P.N.; Mather, P.T. Sulfonated Polysulfone/POSS Nanofiber Composite Membranes for PEM Fuel Cells. J. Electrochem. Soc. 2010, 157, B914. [Google Scholar] [CrossRef]
- Reyes-Rodriguez, J.L.; Solorza-Feria, O.; García-Bernabé, A.; Giménez, E.; Sahuquillo, O.; Compañ, V. Conductivity of composite membrane-based poly(ether-ether-ketone) sulfonated (SPEEK) nanofiber mats of varying thickness. RSC Adv. 2016, 6, 56986–56999. [Google Scholar] [CrossRef]
- Baştürk, E.; Çakmakçi, E.; Madakbaş, S.; Kahraman, M.V. Surface and proton conductivity properties of electrospun poly(vinyl butyral)/polyaniline nanofibers. Adv. Polym. Technol. 2017, 37, 1774–1781. [Google Scholar] [CrossRef]
- Ito, G.; Tanaka, M.; Kawakami, H. Sulfonated polyimide nanofiber framework: Evaluation of intrinsic proton conductivity and application to composite membranes for fuel cells. Solid State Ionics 2018, 317, 244–255. [Google Scholar] [CrossRef]
- Supaphol, P.; Chuangchote, S. On the electrospinning of poly(vinyl alcohol) nanofiber mats: A revisit. Appl. Polym. 2008, 108, 969–978. [Google Scholar] [CrossRef]
- Liao, G.-M.; Li, P.-C.; Lin, J.-S.; Ma, W.-T.; Yu, B.-C.; Li, H.-Y.; Liu, Y.-L.; Yang, C.-C.; Shih, C.-M.; Lue, S.J. Highly conductive quasi-coaxial electrospun quaternized polyvinyl alcohol nanofibers and composite as high-performance solid electrolytes. J. Power Sources 2016, 304, 136–145. [Google Scholar] [CrossRef]
- Barzegar, F.; Bello, A.; Fabiane, M.; Khamlich, S.; Momodu, D.; Taghizadeh, F.; Dangbegnon, J.; Manyala, N. Preparation and characterization of poly(vinyl alcohol)/graphene nanofibers synthesized by electrospinning. J. Phys. Chem. Solids 2015, 77, 139–145. [Google Scholar] [CrossRef]
- Ding, B.; Yu, J. Electrospun Nanofibers for Energy and Environmental Applications; Ding, B., Yu, J., Eds.; Springer: Berlin, Germany, 2014; ISBN 978-3-642-54159-9. [Google Scholar]
- Maiti, J.; Kakati, N.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Where do poly(vinyl alcohol) based membranes stand in relation to Nafion® for direct methanol fuel cell applications? J. Power Sources 2012, 216, 48–66. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Ye, Y.S.; Kao, K.Y.; Joseph, J.; Shen, W.C.; Rick, J.; Hwang, B.J. Interpenetrating network-forming sulfonated poly(vinyl alcohol) proton exchange membranes for direct methanol fuel cell applications. Int. J. Hydrogen Energy 2011, 36, 11936–11945. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Moliner-Estopiñán, C.; Imrie, C.T.; Ribes-Greus, A. Characterization of crosslinked poly(vinyl alcohol)-based membranes with different hydrolysis degrees for their use as electrolytes in direct methanol fuel cells. Appl. Polym. 2012, 124, 1000–1011. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, S.-B.; Han, N.-W. Kinetics of crosslinking reaction of PVA membrane with glutaraldehyde. Korean J. Chem. Eng. 1994, 11, 41–47. [Google Scholar] [CrossRef]
- Matty, F.S.; Sultan, M.T.; Amine, A.K. Swelling Behavior of Cross-link PVA with Glutaraldehyde. Pure Appl. Sci. Ibn Al-Haitham J. Pure Appl. Sci 2015, 28, 136–146. [Google Scholar]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly(vinyl alcohol) films crosslinked by glutaraldehyde under mild conditions. J. Appl. Polym. Sci. 2009, 111, 3074–3080. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Al-qudah, Y.H.F.; Mahmoud, G.A.; Abdel Khalek, M.A. Radiation crosslinked poly (vinyl alcohol)/acrylic acid copolymer for removal of heavy metal ions from aqueous solutions. J. Radiat. Res. Appl. Sci. 2014, 7, 135–145. [Google Scholar] [CrossRef]
- Kumeta, K.; Nagashima, I.; Matsui, S.; Mizoguchi, K. Crosslinking reaction of poly(vinyl alcohol) with poly(acrylic acid) (PAA) by heat treatment: Effect of neutralization of PAA. J. Appl. Polym. Sci. 2003, 90, 2420–2427. [Google Scholar] [CrossRef]
- Kim, D.S.; Guiver, M.D.; Nam, S.Y.; Yun, T., II; Seo, M.Y.; Kim, S.J.; Hwang, H.S.; Rhim, J.W. Preparation of ion exchange membranes for fuel cell based on crosslinked poly(vinyl alcohol) with poly(styrene sulfonic acid-co-maleic acid). J. Memb. Sci. 2006, 281, 156–162. [Google Scholar] [CrossRef]
- Kim, D.S.; Cho, H., II; Kim, D.H.; Lee, B.S.; Lee, B.S.; Yoon, S.W.; Kim, Y.S.; Moon, G.Y.; Byun, H.; Rhim, J.W. Surface fluorinated poly(vinyl alcohol)/poly(styrene sulfonic acid-co-maleic acid) membrane for polymer electrolyte membrane fuel cells. J. Memb. Sci. 2009, 342, 138–144. [Google Scholar] [CrossRef]
- Liu, Y.; Park, M.; Shin, H.K.; Pant, B.; Choi, J.; Park, Y.W.; Lee, J.Y.; Park, S.J.; Kim, H.Y.; Liu, Y.; et al. Facile preparation and characterization of poly(vinyl alcohol)/chitosan/graphene oxide biocomposite nanofibers. J. Ind. Eng. Chem. 2014, 20, 4415–4420. [Google Scholar] [CrossRef]
- Cao, S.G.; Liu, Z.F.; Hu, B.H.; Liu, H.Q. Stabilization of electrospun poly(Vinyl Alcohol) nanofibrous mats in aqueous solutions. Chin. J. Polym. Sci. 2010, 28, 781–788. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.Y.; Lee, S.C.; Shao, C.L.; Lee, D.R.; Park, S.J.; Kwag, G.B.; Choi, K.J. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. J. Polym. Sci. B-Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Zhong, S.; Cui, X.; Gao, Y.; Liu, W.; Dou, S. Fabrication and properties of poly(vinyl alcohol)-based polymer electrolyte membranes for direct methanol fuel cell applications. Int. J. Hydrogen Energ. 2014, 39, 17857–17864. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, H.B.; Rhim, J.W.; Lee, Y.M. Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J. Memb. Sci. 2004, 240, 37–48. [Google Scholar] [CrossRef]
- Ebenezer, D.; Deshpande, A.P.; Haridoss, P. Cross-linked poly (vinyl alcohol)/sulfosuccinic acid polymer as an electrolyte/electrode material for H2-O2 proton exchange membrane fuel cells. J. Power Sources 2016, 304, 282–292. [Google Scholar] [CrossRef]
- Morancho, J.M.; Salla, J.M.; Cadenato, A.; Fernández-Francos, X.; Colomer, P.; Calventus, Y.; Ramis, X.; Ruíz, R. Thermal analysis of enhanced poly(vinyl alcohol)-based proton-conducting membranes crosslinked with sulfonation agents for direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 124, E57–E65. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, G.; Shi, J. Poly (vinyl alcohol)/sulfosuccinic acid (PVA/SSA) as proton-conducting membranes for fuel cells: Effect of cross-linking and plasticizer addition. ECS Trans. 2013, 53, 29–34. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-eli, Y. A Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- González-Guisasola, C.; Ribes-Greus, A. Dielectric relaxations and conductivity of cross-linked PVA/SSA/GO composite membranes for fuel cells. Polym. Test. 2018, 67, 55–67. [Google Scholar] [CrossRef]
- Nunes, S.P; Ruffmann, B.; Rikowski, E.; Vetter, S.; Richau, K. Inorganic modification of proton conductive polymer membranes for direct methanol fuel cells. J. Membr. Sci. 2002, 203, 215–225. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef]
- Moore, T.T.; Mahajan, R.; Vu, D.Q.; Koros, W.J. Hybrid membrane materials comprising organic polymers with rigid dispersed phases. AIChE J. 2004, 50, 311–321. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Tai, Z.X.; Sun, D.F.; Chen, J.T.; Ma, H.B.; Yan, X.B.; Liu, B.; Xue, Q.J. Fabrication and characterization of poly(vinyl alcohol)/graphene oxide nanofibrous biocomposite scaffolds. J. Appl. Polym. Sci. 2013, 127, 1885–1894. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Z.; Zhang, J.; Cao, J.; Wang, S.; Tian, Q.; Gao, M.; Xu, Q. Fabrication of PVA/graphene oxide/TiO2 composite nanofibers through electrospinning and interface sol-gel reaction: Effect of graphene oxide on PVA nanofibers and growth of TiO2. Colloid. Surf. A-Physicochem. Eng. Asp. 2014, 457, 318–325. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, L.; Lv, J. Layer- Structured Poly(vinyl alcohol)/Graphene Oxide Nanocomposites with Improved Thermal and Mechanical Properties. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar]
- Zubair, N.A.; Rahman, N.A.; Lim, H.N.; Zawawi, R.M.; Sulaiman, Y. Electrochemical properties of PVA–GO/PEDOT nanofibers prepared using electrospinning and electropolymerization techniques. RSC Adv. 2016, 6, 17720–17727. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Ding, G.; Xie, X.; Jiang, M. Preparation and characterization of graphene oxide/poly(vinyl alcohol) composite nanofibers via electrospinning. J. Appl. Polym. Sci. 2013, 127, 3026–3032. [Google Scholar] [CrossRef]
- Yun, S.; Im, H.; Heo, Y.; Kim, J. Crosslinked sulfonated poly(vinyl alcohol)/sulfonated multi-walled carbon nanotubes nanocomposite membranes for direct methanol fuel cells. J. Memb. Sci. 2011, 380, 208–215. [Google Scholar] [CrossRef]
- Sánchez Ballester, S.C.; Ribes Greus, A.; Soria Sanchis, V. Synthesis and characterization of new polymer electrolytes to use in fuel cells fed with bio-alcohols. PhD Thesis, Universitat Politècnica de València, Valencia, Spain, 2017. [Google Scholar]
- Jia, L.; Qin, X. The effect of different surfactants on the electrospinning poly(vinyl alcohol) (PVA) nanofibers. J. Therm. Anal. Calorim. 2013, 112, 595–605. [Google Scholar] [CrossRef]
- Qian, X.; Gu, N.; Cheng, Z.; Yang, X.; Wang, E.; Dong, S. Methods to study the ionic conductivity of polymeric electrolytes using a.c. impedance spectroscopy. J. Solid State Electrochem. 2001, 6, 8–15. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Kowsari, E. Synthesis and Characterization of Poly(vinyl alcohol)/Sulfonated Graphene Oxide Nanocomposite Membranes for Use in Proton Exchange Membrane Fuel Cells (PEMFCs). Ind. Eng. Chem. Res. 2014, 53, 16621–16632. [Google Scholar] [CrossRef]
- Gedde, U.W.; Ulf, W. The glassy amorphous state. In Polymer Physics; Chapman & Hall: Uxbridge, UK, 1995; p. 298. ISBN 9780412626401. [Google Scholar]
- Jobando, V.O.; Quarles, C.A. Effect of cross-linking on the free volume properties of natural rubber. Phys. Status Solidi C 2007, 4, 3759–3762. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Hu, Y.; Lamelas, F.J.; Wilkie, C.A.; Ivaska, A.; Sung, Y.; Ger, M.D.; Ma, C.C.M.; et al. Poly(vinyl alcohol) nanocomposites based on graphene and graphite oxide: a comparative investigation of property and mechanism. J. Mater. Chem. 2011, 21, 13942. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Das, G.; Lee, S.H.; Yoon, Y.S. An approach of balancing the ionic conductivity and mechanical properties of PVA based nanocomposite membrane for DMFC by various crosslinking agents with ionic liquid. Int. J. Hydrogen Energy 2015, 40, 7114–7123. [Google Scholar] [CrossRef]
- Mo, S.; Peng, L.; Yuan, C.; Zhao, C.; Tang, W.; Ma, C.; Shen, J.; Yang, W.; Yu, Y.; Min, Y.; et al. Enhanced properties of poly(vinyl alcohol) composite films with functionalized graphene. RSC Adv. 2015, 5, 97738–97745. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Teruel-Juanes, R.; Rodriguez, I.; Meseguer, F.; Ribes-Greus, A. Novel silicon microparticles to improve sunlight stability of raw polypropylene. Eur. Polym. J. 2015, 70, 247–261. [Google Scholar] [CrossRef]
- Rhim, J.W.; Park, H.B.; Lee, C.S.; Jun, J.H.; Kim, D.S.; Lee, Y.M. Crosslinked poly(vinyl alcohol) membranes containing sulfonic acid group: Proton and methanol transport through membranes. J. Memb. Sci. 2004, 238, 143–151. [Google Scholar] [CrossRef]
- Dong, B.; Gwee, L.; Salas-de la Cruz, D.; Winey, K.I.; Elabd, Y.A. Super Proton Conductive High-Purity Nafion Nanofibers. Nano Lett. 2010, 10, 3785–3790. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, J.; Kim, H. Preparation of poly(vinylidene fluoride)/phosphotungstic acid composite nanofiber membranes by electrospinning for proton conductivity. React. Funct. Polym. 2010, 70, 69–74. [Google Scholar] [CrossRef]
- Albu, A.M.; Maior, I.; Nicolae, C.A.; Bocăneală, F.L. Novel Pva Proton Conducting Membranes Doped with Polyaniline Generated by in-Situ Polymerization. Electrochim. Acta 2016, 211, 911–917. [Google Scholar] [CrossRef]
- Badia, J.D.; Gil-Castell, O.; Ribes-Greus, A. Long-term properties and end-of-life of polymers from renewable resources. Polym. Degrad. Stab. 2017, 137, 35–57. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.D.; Kittikorn, T.; Strömberg, E.; Ek, M.; Karlsson, S.; Ribes-Greus, A.; Strömberg, E.; Ek, M.; Karlsson, S.; et al. Impact of hydrothermal ageing on the thermal stability, morphology and viscoelastic performance of PLA/sisal biocomposites. Polym. Degrad. Stab. 2016, 132, 87–96. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.D.; Kittikorn, T.; Strömberg, E.; Martínez-Felipe, A.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Hydrothermal ageing of polylactide/sisal biocomposites. Studies of water absorption behaviour and Physico-Chemical performance. Polym. Degrad. Stab. 2014, 108, 212–222. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Ontoria-Oviedo, I.; Castellano, D.; Marco, B.; Rabal, A.; Bou, J.J.; Serra, A.; Monreal, L.; Blanes, M.; et al. In vitro validation of biomedical polyester-based scaffolds: Poly(lactide-co-glycolide) as model-case. Polym. Test. 2018, 66, 256–267. [Google Scholar] [CrossRef]
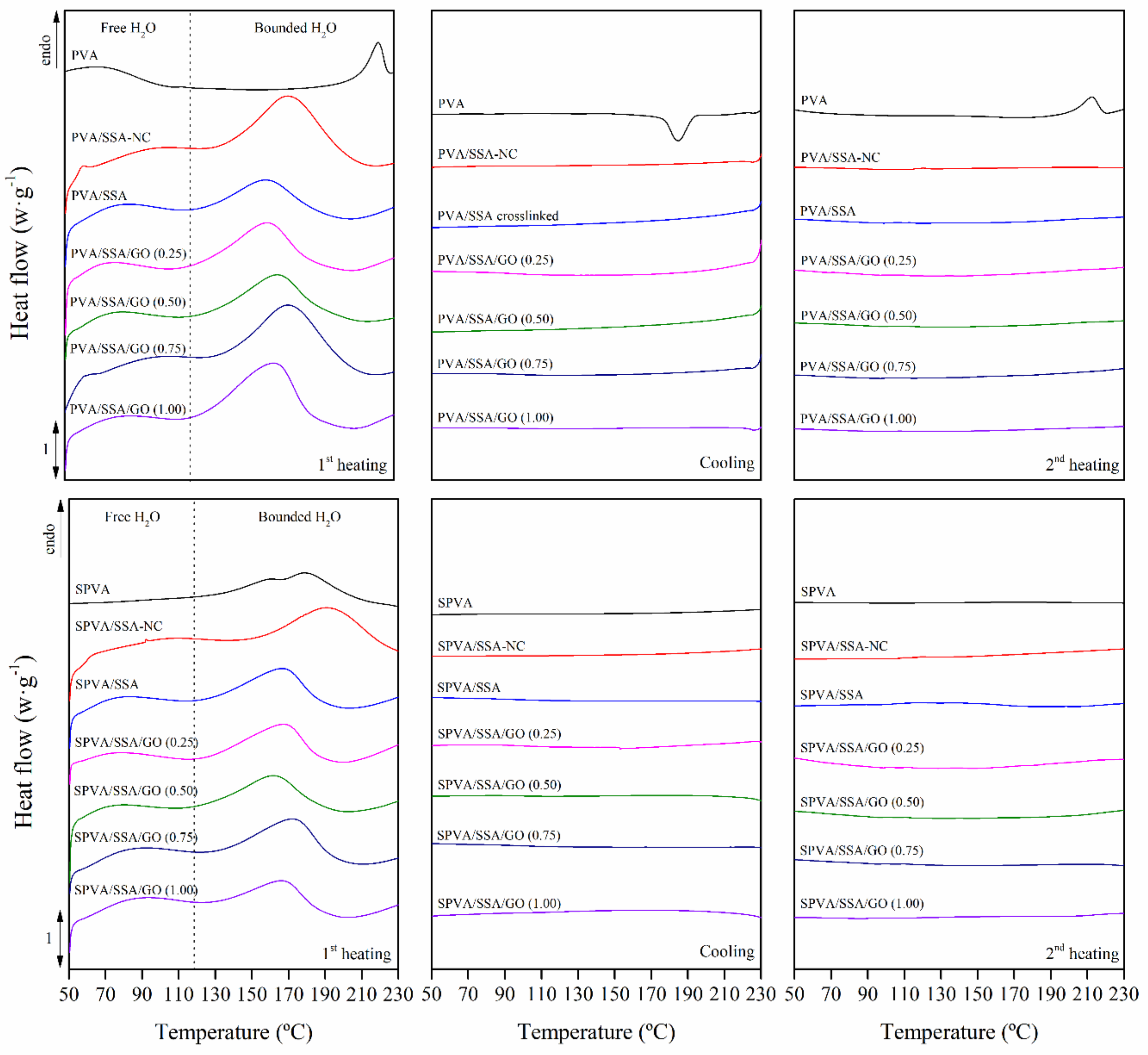
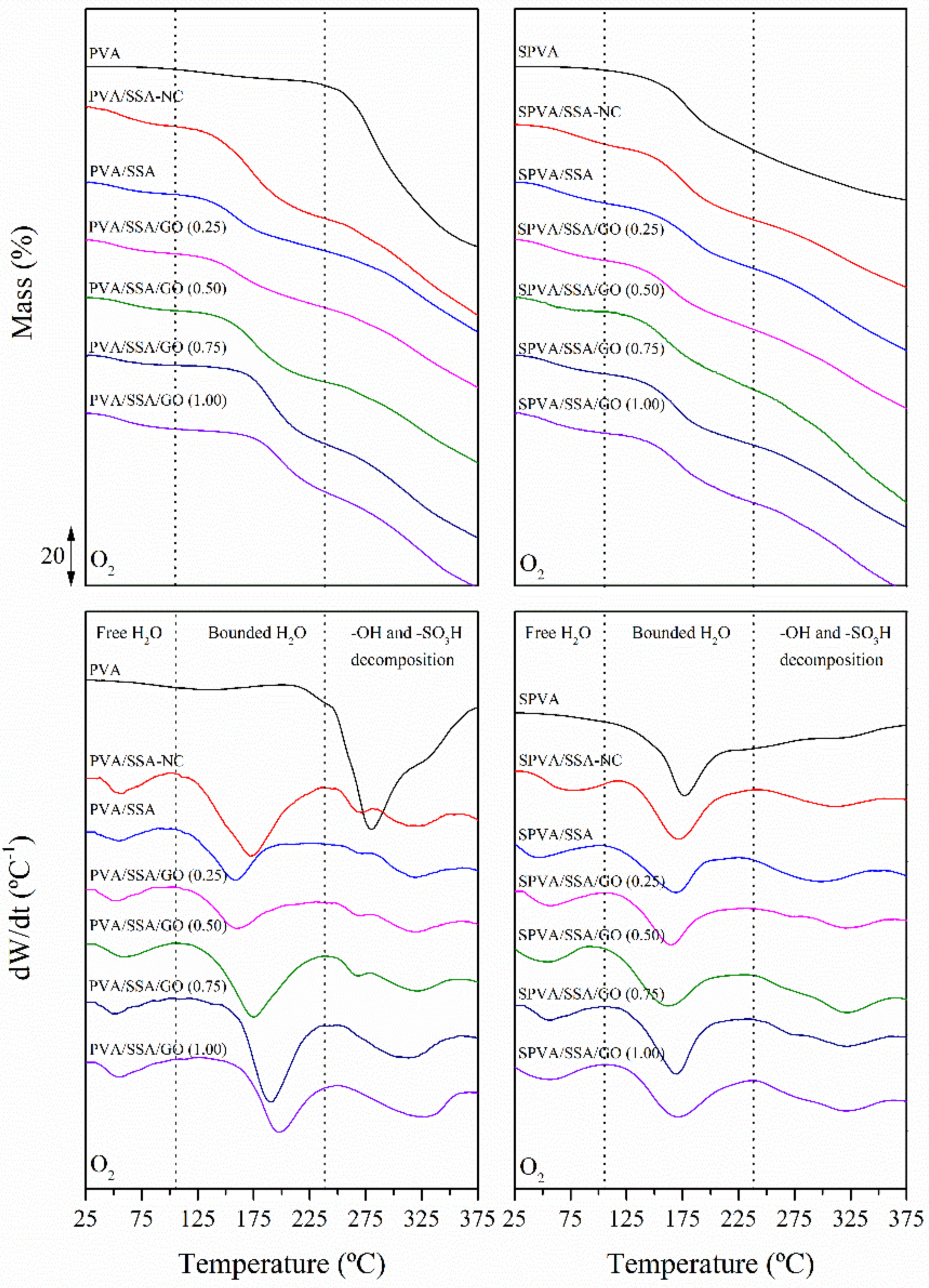
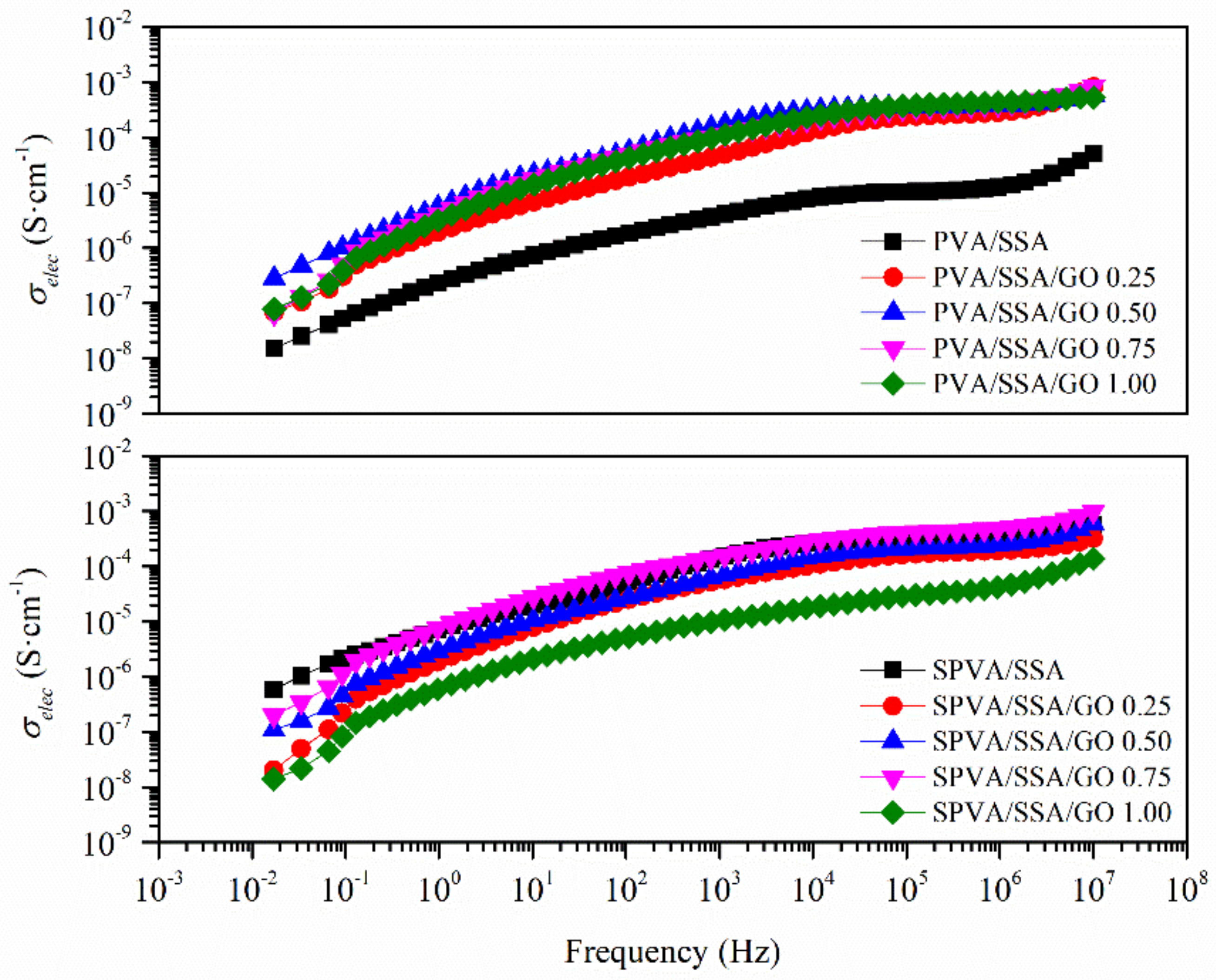
| Water Release | ||
|---|---|---|
| Tfree DSC (°C) | Tbound DSC (°C) | |
| PVA | 70.7 | - |
| PVA/SSA-NC | 87.0 | 176.1 |
| PVA/SSA | 70.8 | 162.8 |
| PVA/SSA/GO 0.25 | 70.5 | 169.4 |
| PVA/SSA/GO 0.50 | 69.9 | 171.1 |
| PVA/SSA/GO 0.75 | 63.3 | 170.9 |
| PVA/SSA/GO 1.00 | 62.8 | 170.4 |
| SPVA | 70.6 | 179.2 |
| SPVA/SSA-NC | 89.4 | 169.5 |
| SPVA/SSA | 62.9 | 166.1 |
| SPVA/SSA/GO 0.25 | 65.2 | 167.2 |
| SPVA/SSA/GO 0.50 | 71.2 | 167.7 |
| SPVA/SSA/GO 0.75 | 71.5 | 169.7 |
| SPVA/SSA/GO 1.00 | 72.2 | 170.0 |
| Water Release | Decomposition | |||||
|---|---|---|---|---|---|---|
| Tfree TGA | Tbound TGA | Mass-Loss | Td‒OH | Td‒SO3H | Mass-Loss | |
| (°C) | (°C) | (%) | (°C) | (°C) | (%) | |
| PVA | - | 123.3 | 5.1 | 280.1 | - | 60.9 |
| PVA/SSA-NC | 56.5 | 172.0 | 32.9 | 271.1 | 313.1 | 60.9 |
| PVA/SSA | 54.5 | 158.6 | 22.9 | 271.4 | 320.2 | 51.3 |
| PVA/SSA/GO 0.25 | 54.5 | 159.3 | 23.9 | 271.1 | 320.7 | 50.2 |
| PVA/SSA/GO 0.50 | 56.4 | 174.9 | 26.3 | 268.2 | 320.9 | 51.8 |
| PVA/SSA/GO 0.75 | 53.3 | 190.3 | 26.2 | - | 320.4 | 57.6 |
| PVA/SSA/GO 1.00 | 55.3 | 197.8 | 24.2 | - | 322.8 | 55.8 |
| SPVA | - | 177.8 | 24.7 | - | 300.4 | 25.3 |
| SPVA/SSA-NC | 65.6 | 172.4 | 34.6 | - | 312.9 | 60.7 |
| SPVA/SSA | 51.3 | 167.6 | 30.2 | - | 315.2 | 60.2 |
| SPVA/SSA/GO 0.25 | 56.3 | 162.9 | 27.7 | - | 320.4 | 60.1 |
| SPVA/SSA/GO 0.50 | 54.7 | 161.5 | 26.9 | - | 320.5 | 61.1 |
| SPVA/SSA/GO 0.75 | 55.3 | 169.7 | 27.8 | - | 321.5 | 59.2 |
| SPVA/SSA/GO 1.00 | 53.1 | 171.2 | 26.5 | - | 320.2 | 60.4 |
| σprot (S·cm−1 × 10−4) | |
|---|---|
| PVA/SSA | 2.5 ± 0.1 |
| PVA/SSA/GO 0.25 | 2.7 ± 0.2 |
| PVA/SSA/GO 0.50 | 3.8 ± 0.3 |
| PVA/SSA/GO 0.75 | 3.9 ± 0.2 |
| PVA/SSA/GO 1.00 | 4.4 ± 0.2 |
| SPVA/SSA | 3.3 ± 0.3 |
| SPVA/SSA/GO 0.25 | 1.9 ± 0.2 |
| SPVA/SSA/GO 0.50 | 2.3 ± 0.1 |
| SPVA/SSA/GO 0.75 | 4.3 ± 0.2 |
| SPVA/SSA/GO 1.00 | 3.8 ± 0.1 |
| Average Fiber Diameter (nm) | Variation (%) | ||
|---|---|---|---|
| Initial | Final | ||
| PVA | 354 ± 120 | × | × |
| PVA/SSA-NC | 207 ± 60 | × | × |
| PVA/SSA | 198 ± 59 | 347 ± 69 | +75.3 |
| PVA/SSA/GO 0.25 | 229 ± 78 | 274 ± 53 | +19.6 |
| PVA/SSA/GO 0.50 | 241 ± 74 | 275 ± 60 | +14.1 |
| PVA/SSA/GO 0.75 | 249 ± 66 | 288 ± 58 | +15.7 |
| PVA/SSA/GO 1.00 | 238 ± 85 | 255 ± 86 | +7.1 |
| SPVA | 200 ± 66 | × | × |
| SPVA/SSA-NC | 189 ± 48 | × | × |
| SPVA/SSA | 182 ± 33 | 233 ± 88 | +28.0 |
| SPVA/SSA/GO 0.25 | 186 ± 64 | 220 ± 49 | +18.3 |
| SPVA/SSA/GO 0.50 | 212 ± 42 | 219 ± 55 | +3.3 |
| SPVA/SSA/GO 0.75 | 201 ± 50 | 217 ± 61 | +8.0 |
| SPVA/SSA/GO 1.00 | 183 ± 58 | 199 ± 52 | +8.7 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Castell, O.; Galindo-Alfaro, D.; Sánchez-Ballester, S.; Teruel-Juanes, R.; Badia, J.D.; Ribes-Greus, A. Crosslinked Sulfonated Poly(vinyl alcohol)/Graphene Oxide Electrospun Nanofibers as Polyelectrolytes. Nanomaterials 2019, 9, 397. https://doi.org/10.3390/nano9030397
Gil-Castell O, Galindo-Alfaro D, Sánchez-Ballester S, Teruel-Juanes R, Badia JD, Ribes-Greus A. Crosslinked Sulfonated Poly(vinyl alcohol)/Graphene Oxide Electrospun Nanofibers as Polyelectrolytes. Nanomaterials. 2019; 9(3):397. https://doi.org/10.3390/nano9030397
Chicago/Turabian StyleGil-Castell, Oscar, Diana Galindo-Alfaro, Soraya Sánchez-Ballester, Roberto Teruel-Juanes, José David Badia, and Amparo Ribes-Greus. 2019. "Crosslinked Sulfonated Poly(vinyl alcohol)/Graphene Oxide Electrospun Nanofibers as Polyelectrolytes" Nanomaterials 9, no. 3: 397. https://doi.org/10.3390/nano9030397
APA StyleGil-Castell, O., Galindo-Alfaro, D., Sánchez-Ballester, S., Teruel-Juanes, R., Badia, J. D., & Ribes-Greus, A. (2019). Crosslinked Sulfonated Poly(vinyl alcohol)/Graphene Oxide Electrospun Nanofibers as Polyelectrolytes. Nanomaterials, 9(3), 397. https://doi.org/10.3390/nano9030397