Removal of Tetracycline Pollutants by Adsorption and Magnetic Separation Using Reduced Graphene Oxide Decorated with α-Fe2O3 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of RGO and α-Fe2O3/RGO Hybrid Materials
2.3. Adsorption Experiments of Tetracycline on RGO and α-Fe2O3/RGO Hybrid Materials
2.4. Characterization α-Fe2O3/RGO Hybrid Materials
3. Results
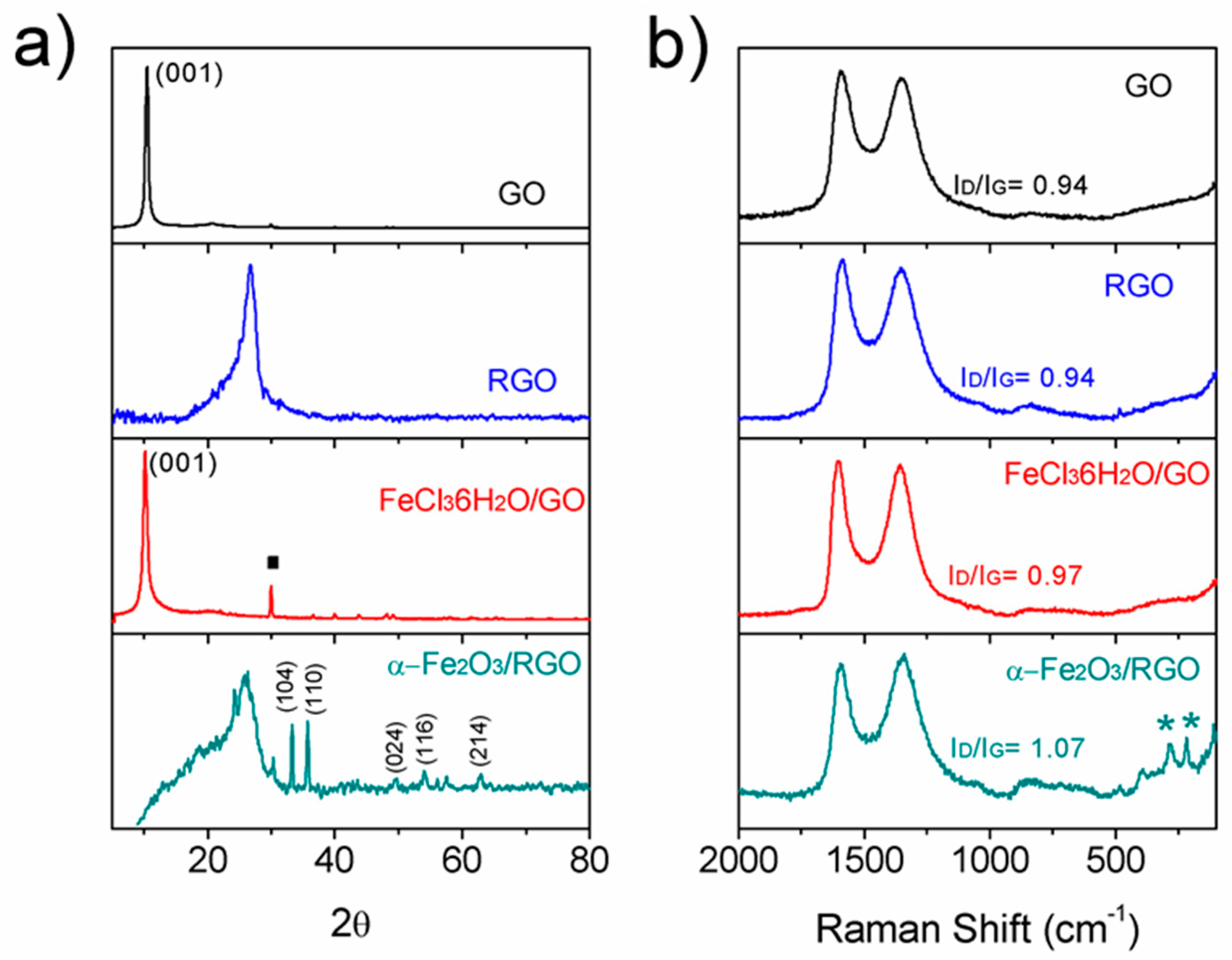
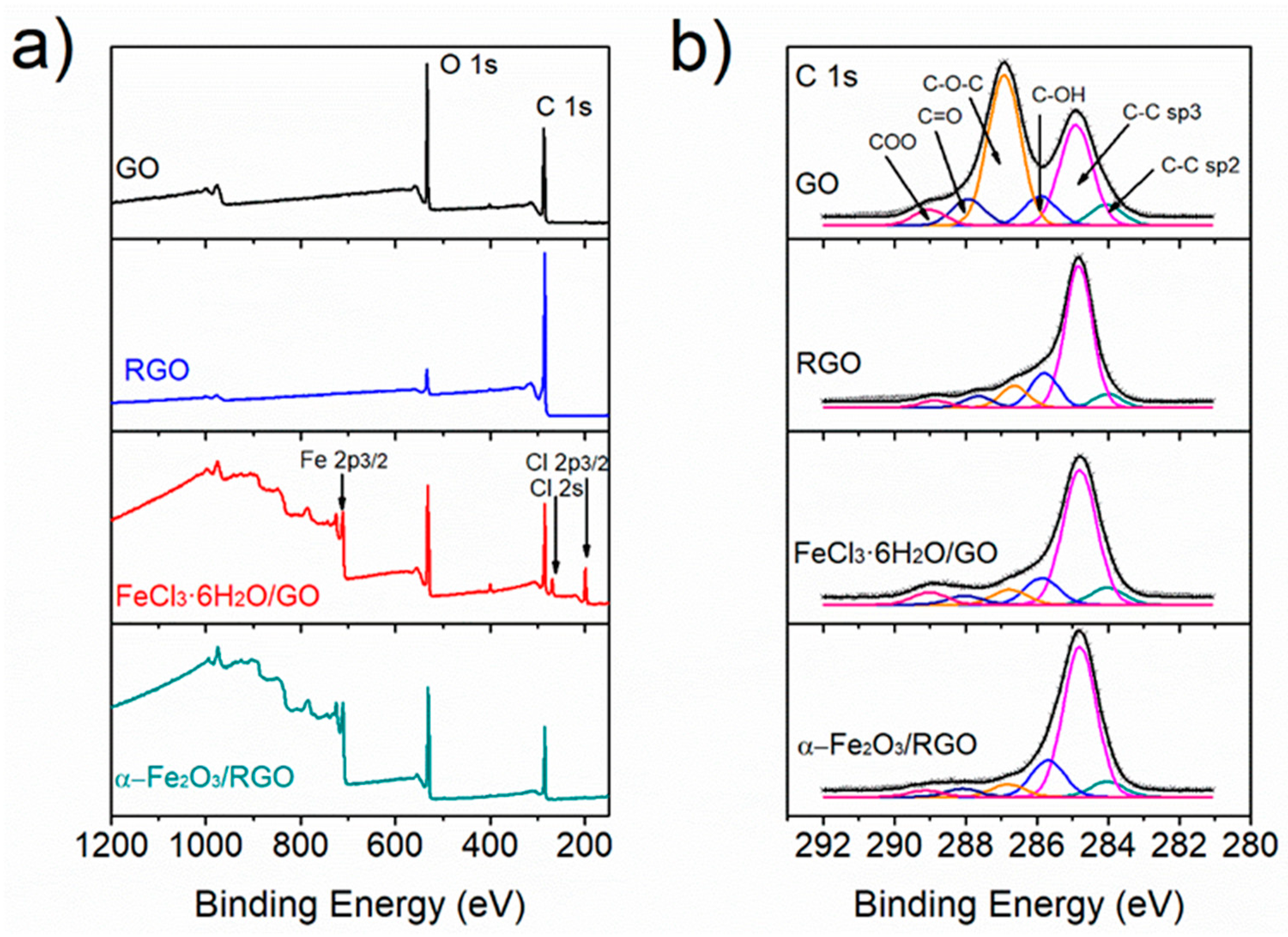
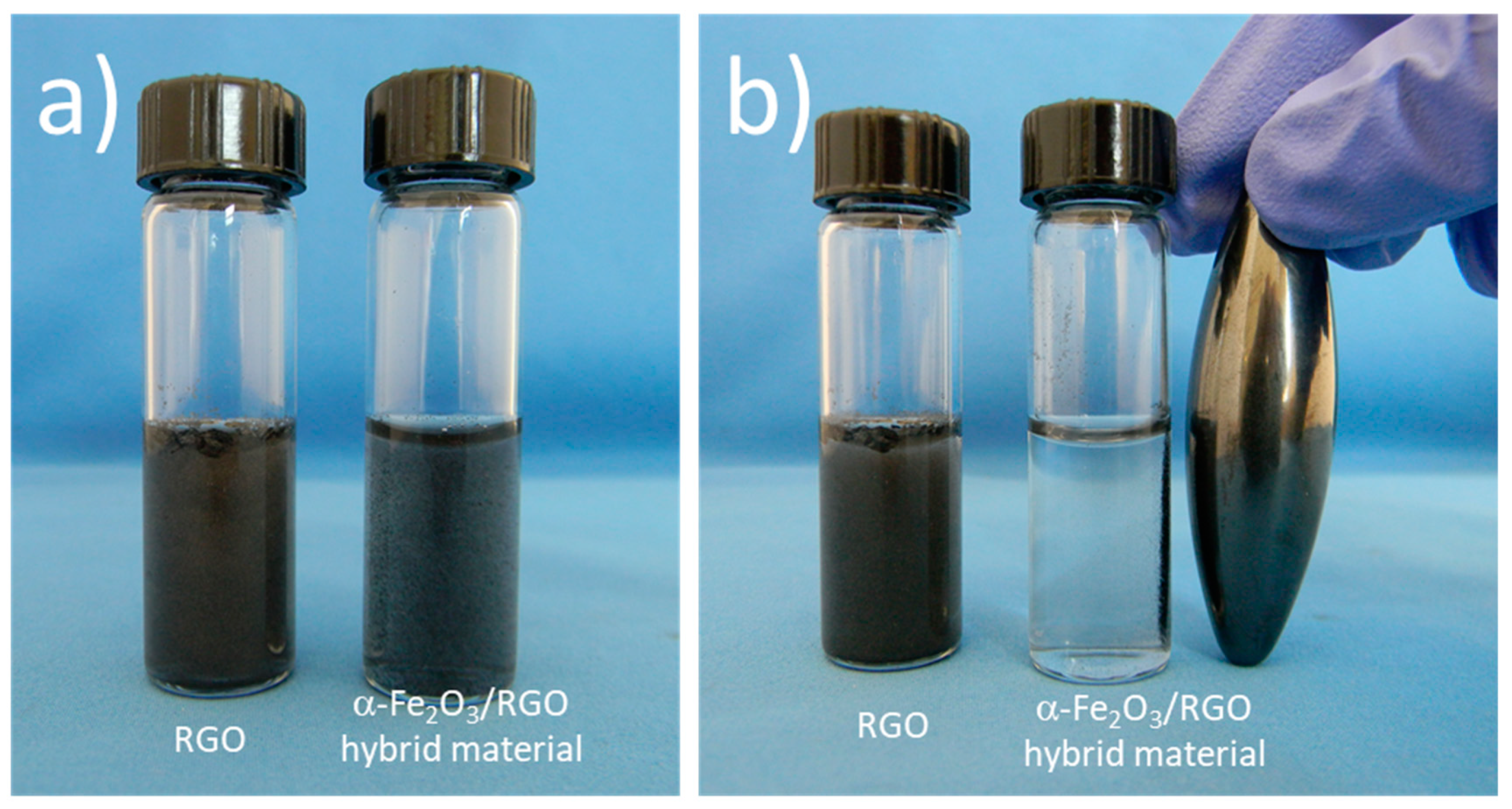
3.1. Synthesis of α-Fe2O3/RGO
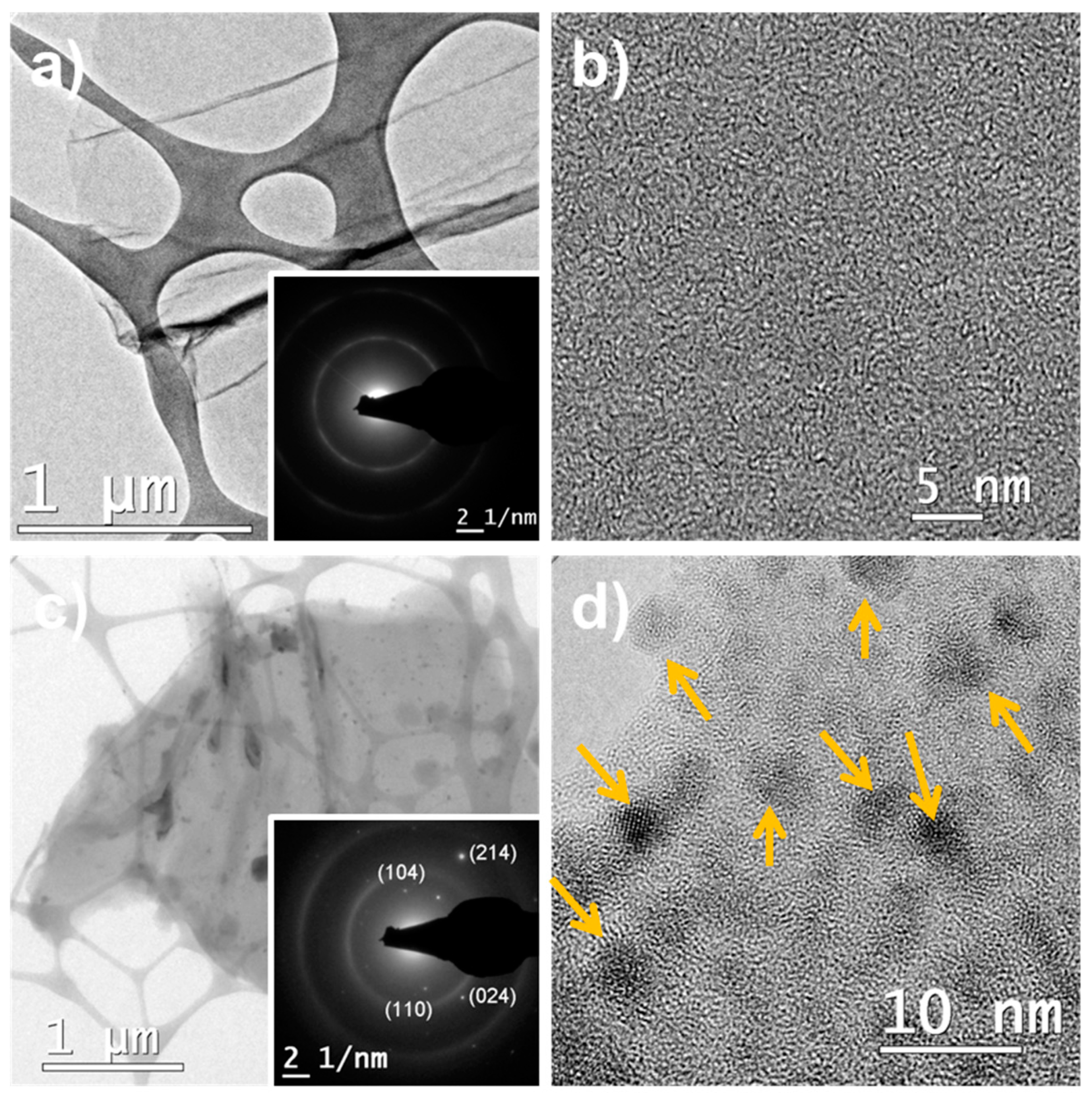
3.2. Microstructural Characterization of α-Fe2O3/RGO Hybrid Materials
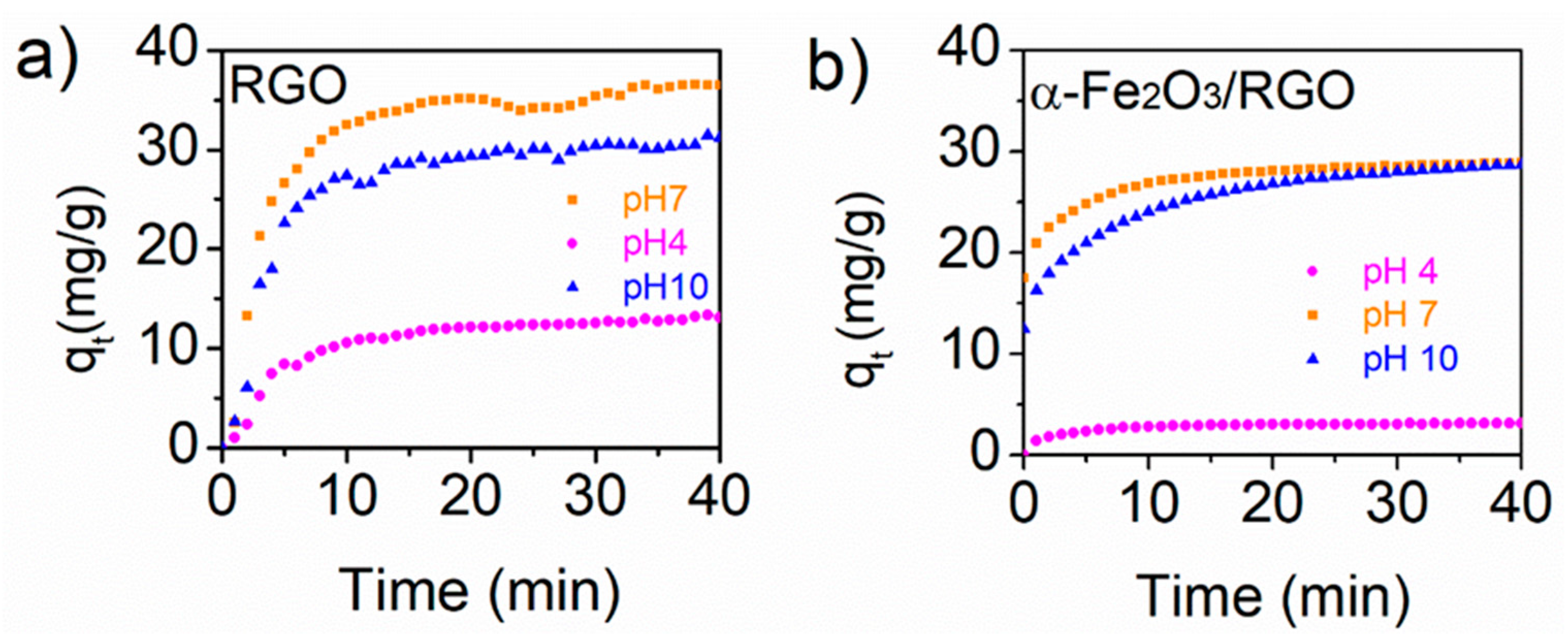
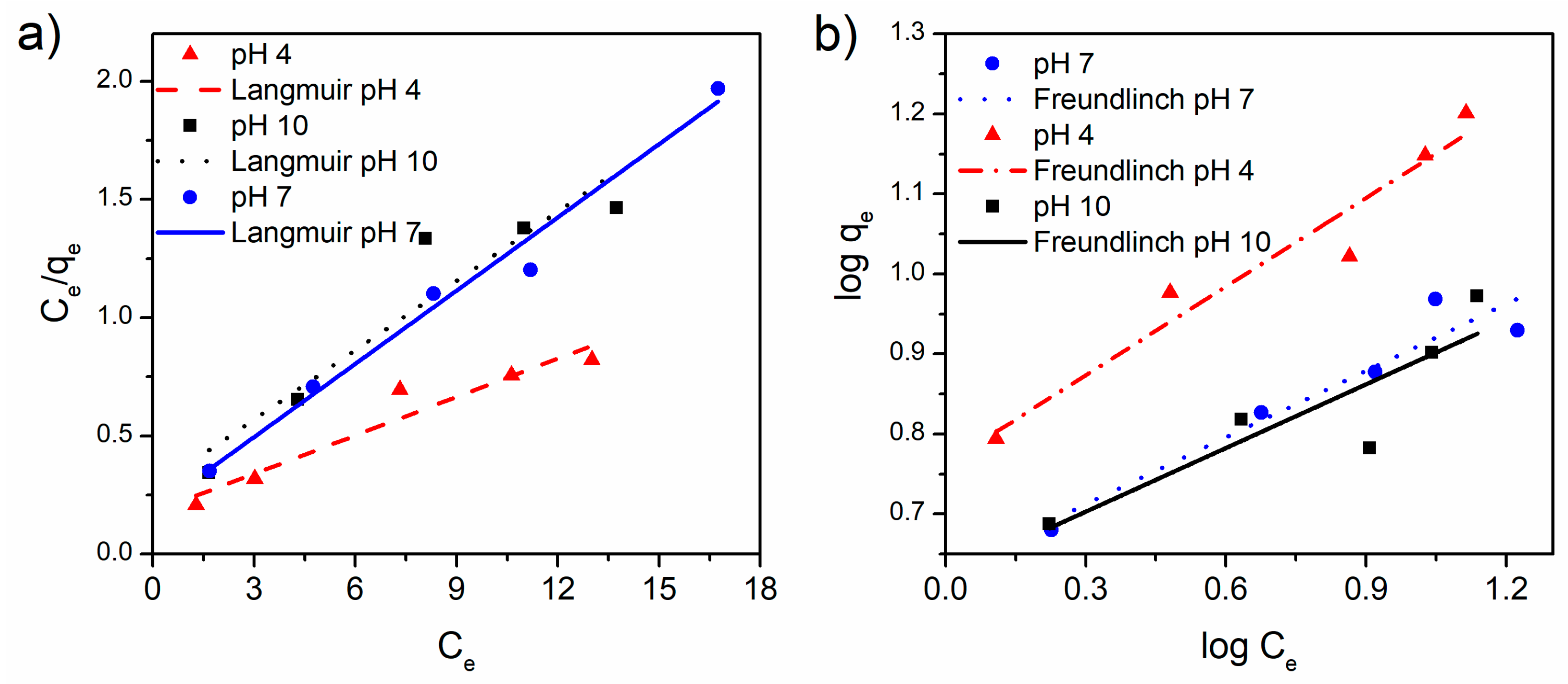
3.3. Tetracycline Adsorption Studies
3.4. Magnetic Characterization of α-Fe2O3/RGO Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aminov, R. History of antimicrobial drug discovery: Major classes and health impact. Biochem. Pharmacol. 2017, 133, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Rybak, M.J. Tigecycline: First of a New Class of Antimicrobial Agents. Pharmacotherapy 2006, 26, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A. Review. CLEAN-Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Zhang, T.; Fang, H.H.P. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef]
- Le-Minh, N.; Khan, S.J.; Drewes, J.E.; Stuetz, R.M. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010, 44, 4295–4323. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: Review. Environ. Toxicol. Pharmacol. 2017, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, X.; Liu, X.; Yang, L.; Pan, F.; Lv, J. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem. Eng. J. 2011, 178, 26–33. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interf. Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Ghadim, E.E.; Manouchehri, F.; Soleimani, G.; Hosseini, H.; Kimiagar, S.; Nafisi, S. Adsorption Properties of Tetracycline onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. PLOS ONE 2013, 8, e79254. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Lei, S.; Wang, M.-Z.; Yang, J.; Ge, X.-W. Fabrication of macroporous polystyrene/graphene oxide composite monolith and its adsorption property for tetracycline. Chin. Chem. Lett. 2016, 27, 511–517. [Google Scholar] [CrossRef]
- Feng, Y.; Huynh, K.A.; Xie, Z.; Liu, G.; Gao, S. Heteroaggregation and sedimentation of graphene oxide with hematite colloids: Influence of water constituents and impact on tetracycline adsorption. Sci. Total Environ. 2019, 647, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Ma, W.; Bakr, A.; Rahaman, M.S. pH-sensitive and magnetically separable Fe/Cu bimetallic nanoparticles supported by graphene oxide (GO) for high-efficiency removal of tetracyclines. J. Colloid Interf. Sci. 2019, 534, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, B.; Zhang, L.; Huang, J.; Chen, F.; Yang, Z.; Yao, J.; Zhang, Z. Controlled assembly of Fe3O4 magnetic nanoparticles on graphene oxide. Nanoscale 2011, 3, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. Visible light activated photocatalytic degradation of tetracycline by a magnetically separable composite photocatalyst: Graphene oxide/magnetite/cerium-doped titania. J. Colloid Interface Sci. 2016, 467, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Q.; Chen, Y.-L.; Jiang, H. Slow Pyrolysis Magnetization of Hydrochar for Effective and Highly Stable Removal of Tetracycline from Aqueous Solution. Ind. Eng. Chem. Res. 2017, 56, 3059–3066. [Google Scholar] [CrossRef]
- Hazell, G.; Hinojosa-Navarro, M.; McCoy, T.M.; Tabor, R.F.; Eastoe, J. Responsive materials based on magnetic polyelectrolytes and graphene oxide for water clean-up. J. Colloid Interface Sci. 2016, 464, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wu, J.; Wang, L.; Liu, X.; Meng, J.; Tang, X.; Tang, C.; Xu, J. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem. Eng. J. 2019, 358, 1399–1409. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C.; et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Deepa, J.R.; Nair, A.S. Fabrication of chemically modified graphene oxide/nano hydroxyapatite composite for adsorption and subsequent photocatalytic degradation of aureomycine hydrochloride. J. Ind. Eng. Chem. 2017, 47, 415–430. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Wang, H.; Tan, X.; Yang, Y.; Liu, Y. Efficient Removal of Tetracycline from Aqueous Media with a Fe3O4 Nanoparticles@graphene Oxide Nanosheets Assembly. Int. J. Environ. Res. Public Health 2017, 14, 1495. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Bai, Y.; Ming, Z.; Yang, H.; Chen, L.; Hu, X.; Feng, S.; Yang, S.-T. Adsorption behaviors of tetracycline on magnetic graphene oxide sponge. Mater. Chem. Phys. 2017, 198, 283–290. [Google Scholar] [CrossRef]
- Song, L.; Khoerunnisa, F.; Gao, W.; Dou, W.; Hayashi, T.; Kaneko, K.; Endo, M.; Ajayan, P.M. Effect of high-temperature thermal treatment on the structure and adsorption properties of reduced graphene oxide. Carbon 2013, 52, 608–612. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Ma, C.-C.M.; Chiang, J.-C.; Ho, K.-K.; Chou, T.-Y.; Xie, X.; Tsai, C.-H.; Chang, L.-H.; Hsieh, C.-K. Thermally conductive and electrically insulating epoxy nanocomposites with thermally reduced graphene oxide–silica hybrid nanosheets. Nanoscale 2013, 5, 5863–5871. [Google Scholar] [CrossRef] [PubMed]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaría, R.; Granda, M.; Gutiérrez, M.D.; Rodríguez-Reinoso, F.; Menéndez, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation–reduction of graphite oxide. Carbon 2013, 52, 476–485. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.G.; Sibley, S.D.; Pedersen, J.A. Complexation of the antibiotic tetracycline with humic acid. Chemosphere 2007, 66, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. In Zeitschrift für Physikalische Chemie; Oldenbourg Wissenschaftsverlag: Berlin, Germany, 1907; Volume 57, pp. 385–470. [Google Scholar]
- Raming, T.P.; Winnubst, A.J.A.; van Kats, C.M.; Philipse, A.P. The Synthesis and Magnetic Properties of Nanosized Hematite (α-Fe2O3) Particles. J. Colloid Interface Sci. 2002, 249, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Malaidurai, M.; Thangavel, R. Study of structural and magnetic properties of co-precipitated α-Fe2O3 nanocrystals. Superlattice. Microst. 2018, 120, 553–560. [Google Scholar] [CrossRef]

| Adsorbent | Qmax (mg g−1) 1 | Adsorption Conditions | Additional Functionality | Reference |
|---|---|---|---|---|
| GO | 322.43 | 298 K; pH 3.6 | [17] | |
| GO | 313.48 | 298 K; pH 3.6 | [16] | |
| CNT | 269.54 | 293 K; pH 4.5–7 | [15] | |
| MWCNT | 364.37 | 298 K | [27] | |
| Hydroxyapatite/GO | 16.16 | 293 K; pH 5–6 | Photocatalytic oxidation | [28] |
| Fe3O4/GO | 1272.45 | 313 K; pH 4 | Magnetic removal | [29] |
| Fe/Cu/GO | 302.5 | pH 5–7 | Magnetic removal | [20] |
| Fe3O4/GO sponge | 473 | 308 K; pH 3 | Magnetic removal | [30] |
| Adsorbent | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| pH | Qmax (mg/g) | KL (L/mg) | r2 | n | KF (L/mg) | r2 | |
| α-Fe2O3/RGO | 4 | 18.48 | 0.30 | 0.965 | 2.71 | 5.79 | 0.971 |
| 7 | 9.69 | 0.56 | 0.991 | 3.60 | 4.26 | 0.958 | |
| 10 | 10.25 | 0.35 | 0.945 | 3.77 | 4.20 | 0.894 | |
| RGO | 4 | 15.82 | 0.05 | 0.921 | 1.44 | 0.99 | 0.993 |
| 7 | 44.23 | 0.09 | 0.975 | 1.57 | 4.65 | 0.999 | |
| 10 | 39.94 | 0.07 | 0.994 | 1.31 | 2.83 | 0.995 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huízar-Félix, A.M.; Aguilar-Flores, C.; Martínez-de-la Cruz, A.; Barandiarán, J.M.; Sepúlveda-Guzmán, S.; Cruz-Silva, R. Removal of Tetracycline Pollutants by Adsorption and Magnetic Separation Using Reduced Graphene Oxide Decorated with α-Fe2O3 Nanoparticles. Nanomaterials 2019, 9, 313. https://doi.org/10.3390/nano9030313
Huízar-Félix AM, Aguilar-Flores C, Martínez-de-la Cruz A, Barandiarán JM, Sepúlveda-Guzmán S, Cruz-Silva R. Removal of Tetracycline Pollutants by Adsorption and Magnetic Separation Using Reduced Graphene Oxide Decorated with α-Fe2O3 Nanoparticles. Nanomaterials. 2019; 9(3):313. https://doi.org/10.3390/nano9030313
Chicago/Turabian StyleHuízar-Félix, Adriana Magdalena, Celia Aguilar-Flores, Azael Martínez-de-la Cruz, José Manuel Barandiarán, Selene Sepúlveda-Guzmán, and Rodolfo Cruz-Silva. 2019. "Removal of Tetracycline Pollutants by Adsorption and Magnetic Separation Using Reduced Graphene Oxide Decorated with α-Fe2O3 Nanoparticles" Nanomaterials 9, no. 3: 313. https://doi.org/10.3390/nano9030313
APA StyleHuízar-Félix, A. M., Aguilar-Flores, C., Martínez-de-la Cruz, A., Barandiarán, J. M., Sepúlveda-Guzmán, S., & Cruz-Silva, R. (2019). Removal of Tetracycline Pollutants by Adsorption and Magnetic Separation Using Reduced Graphene Oxide Decorated with α-Fe2O3 Nanoparticles. Nanomaterials, 9(3), 313. https://doi.org/10.3390/nano9030313





