Biocompatible Polymer Materials with Antimicrobial Properties for Preparation of Stents
Abstract
1. Introduction
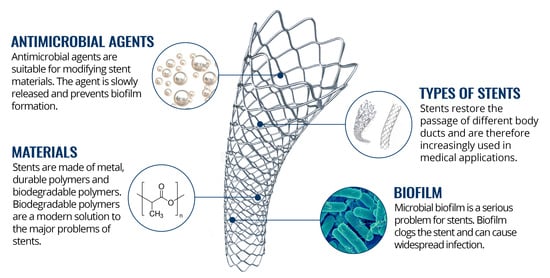
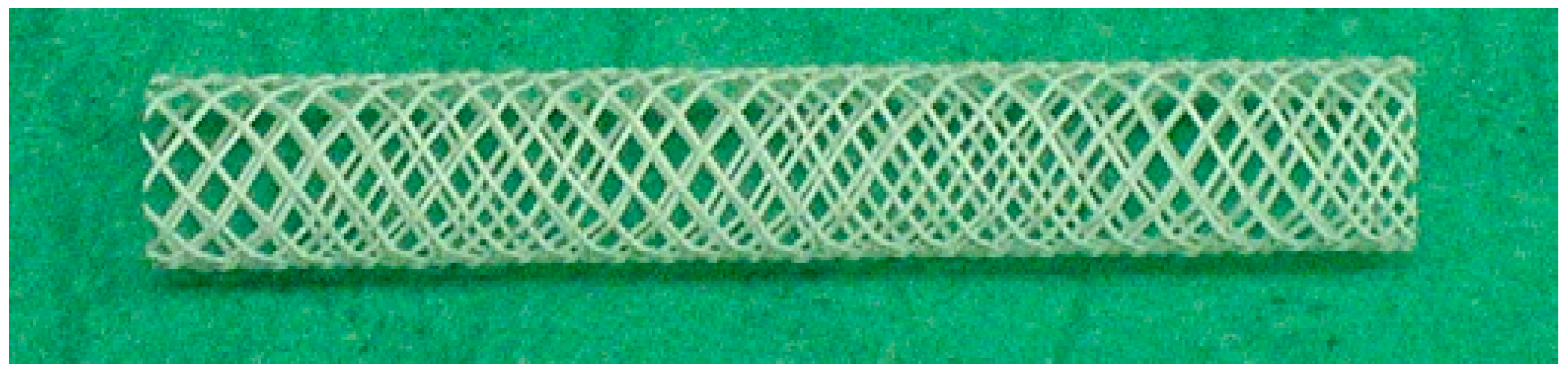
2. Stents
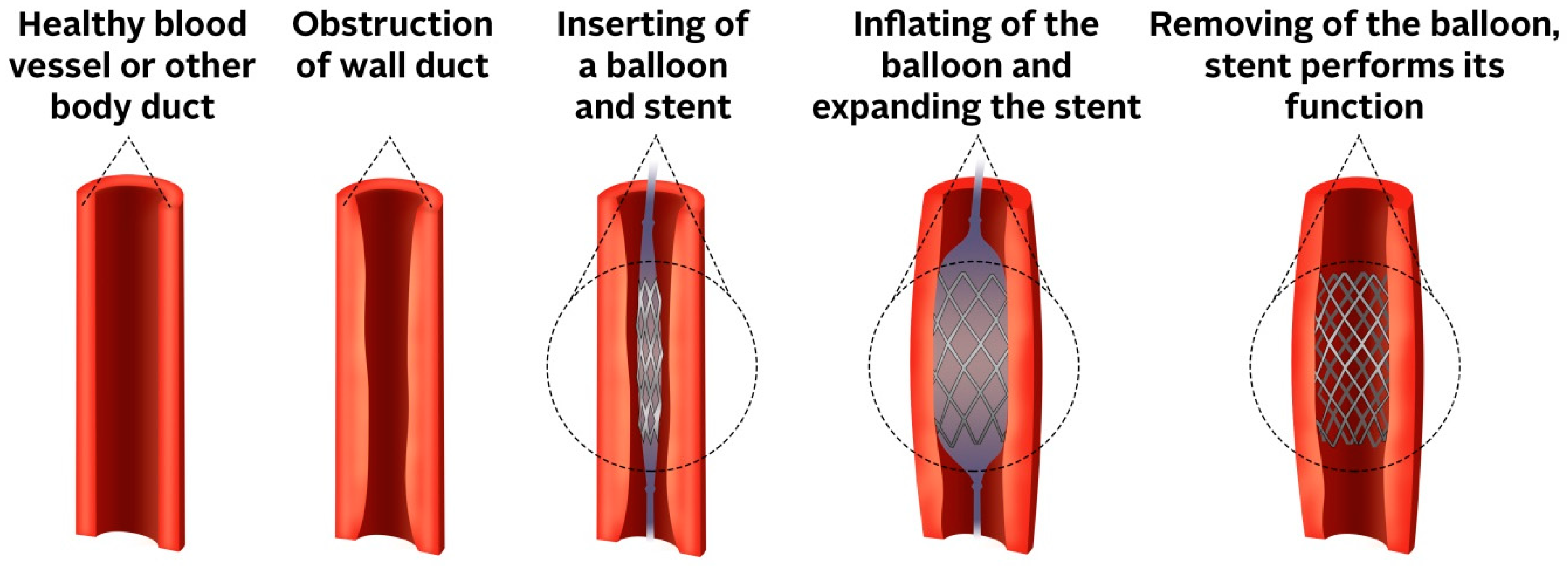
2.1. The Principle of Stents
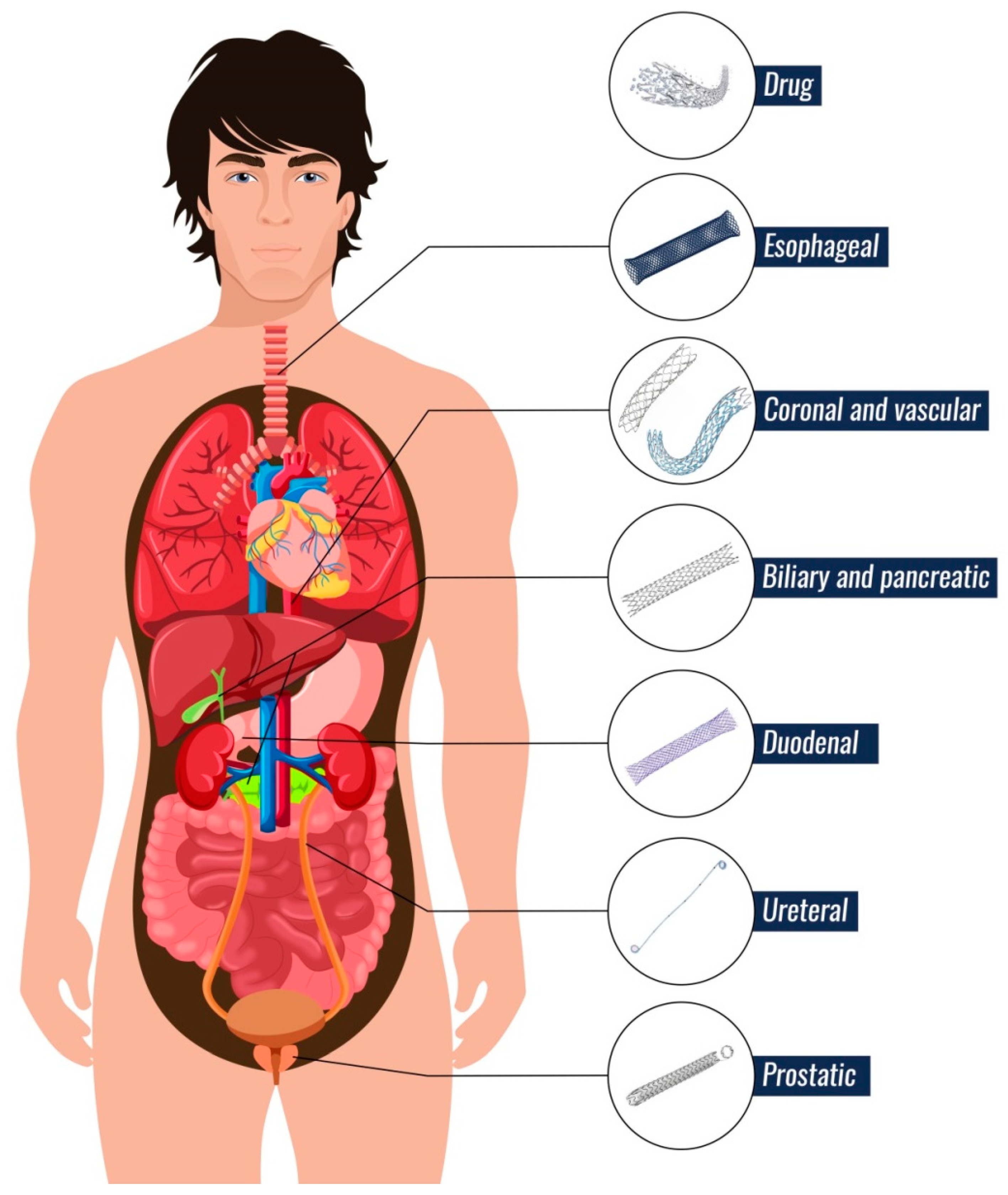
2.2. Types of Stents
2.2.1. Vascular and Coronary Arteries
2.2.2. Esophageal Ducts
2.2.3. Duodenal and Pancreatic Ducts
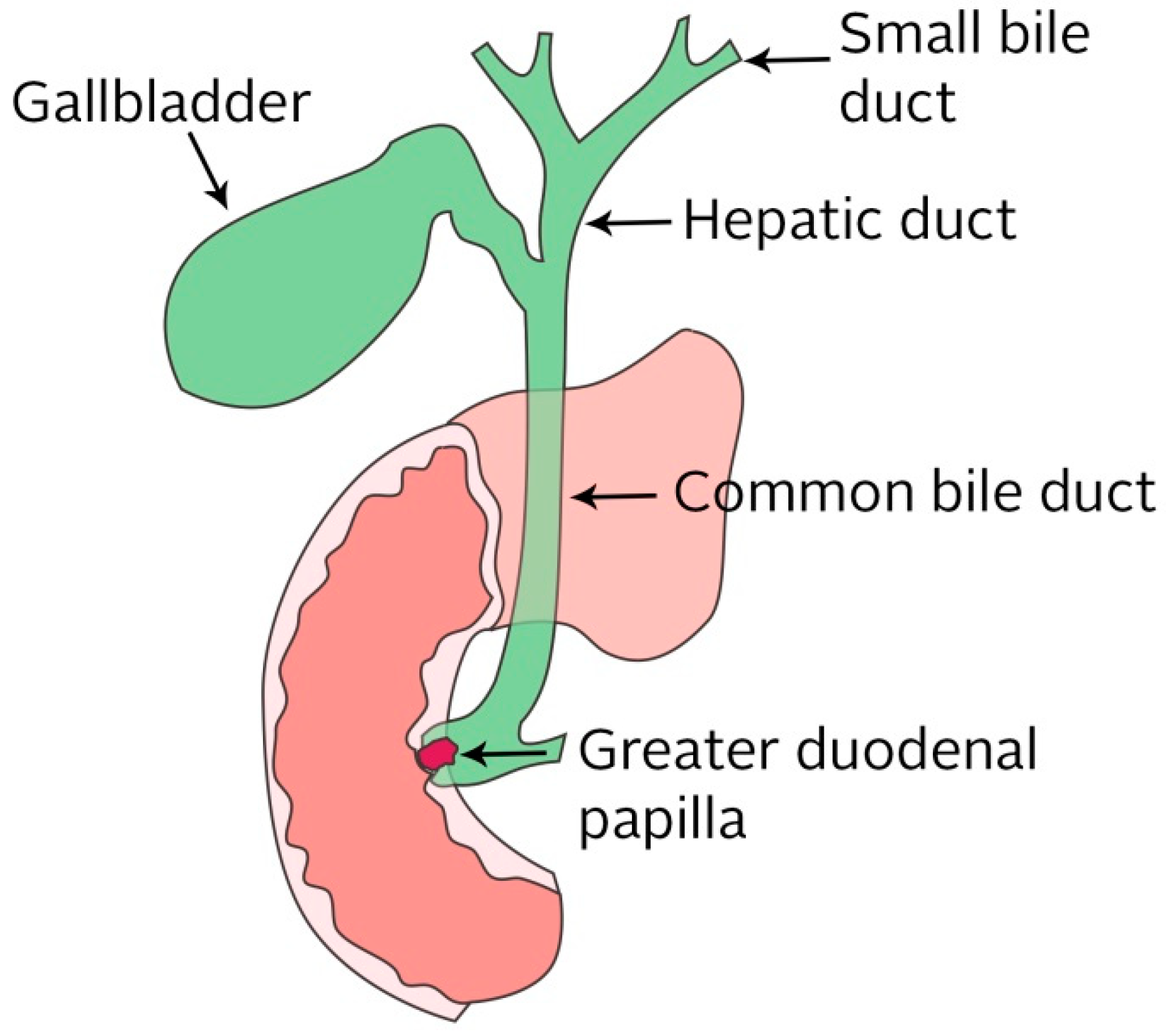
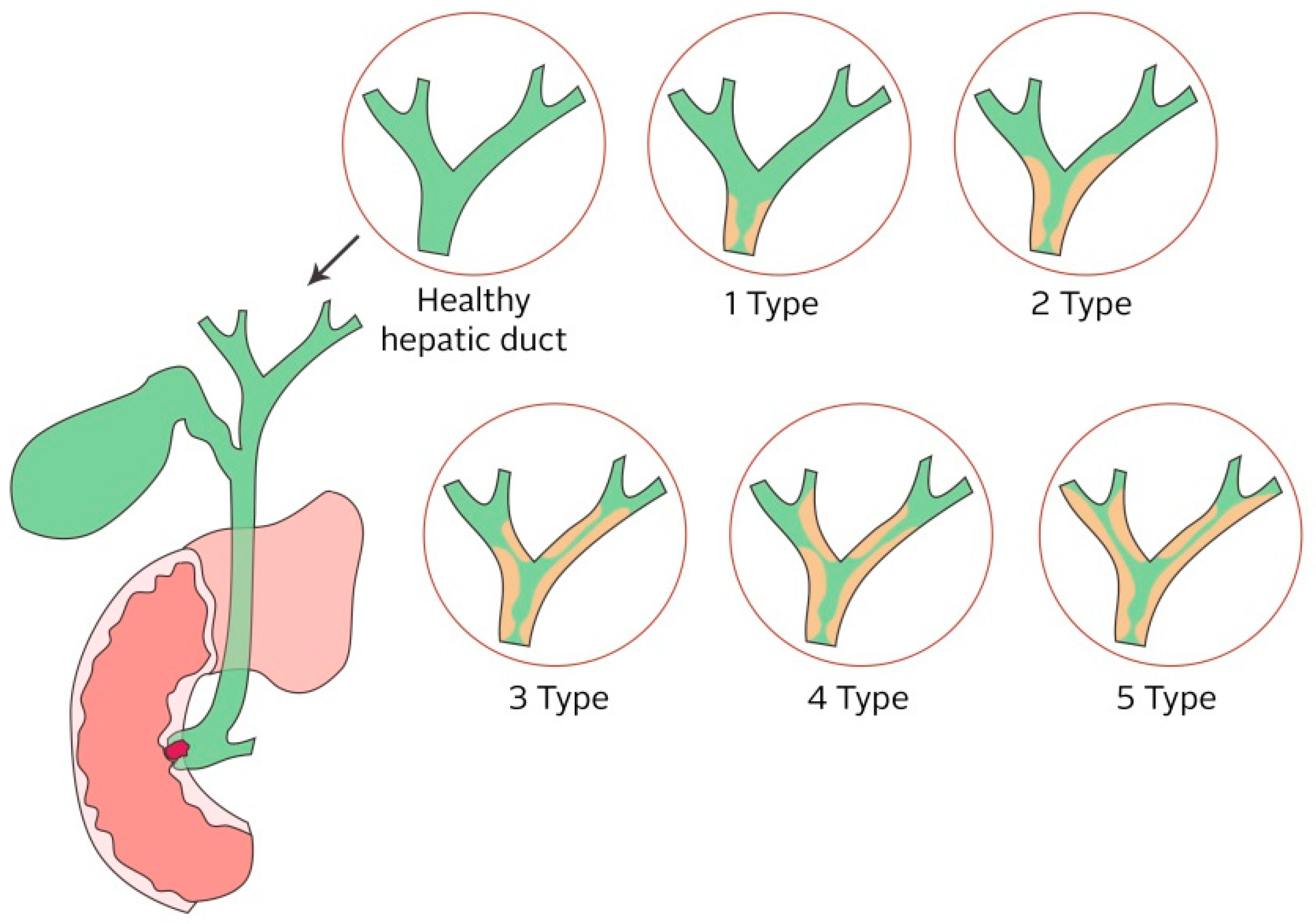
2.2.4. Biliary Ducts
2.2.5. Ureteral Ducts
2.2.6. Prostatic Ducts
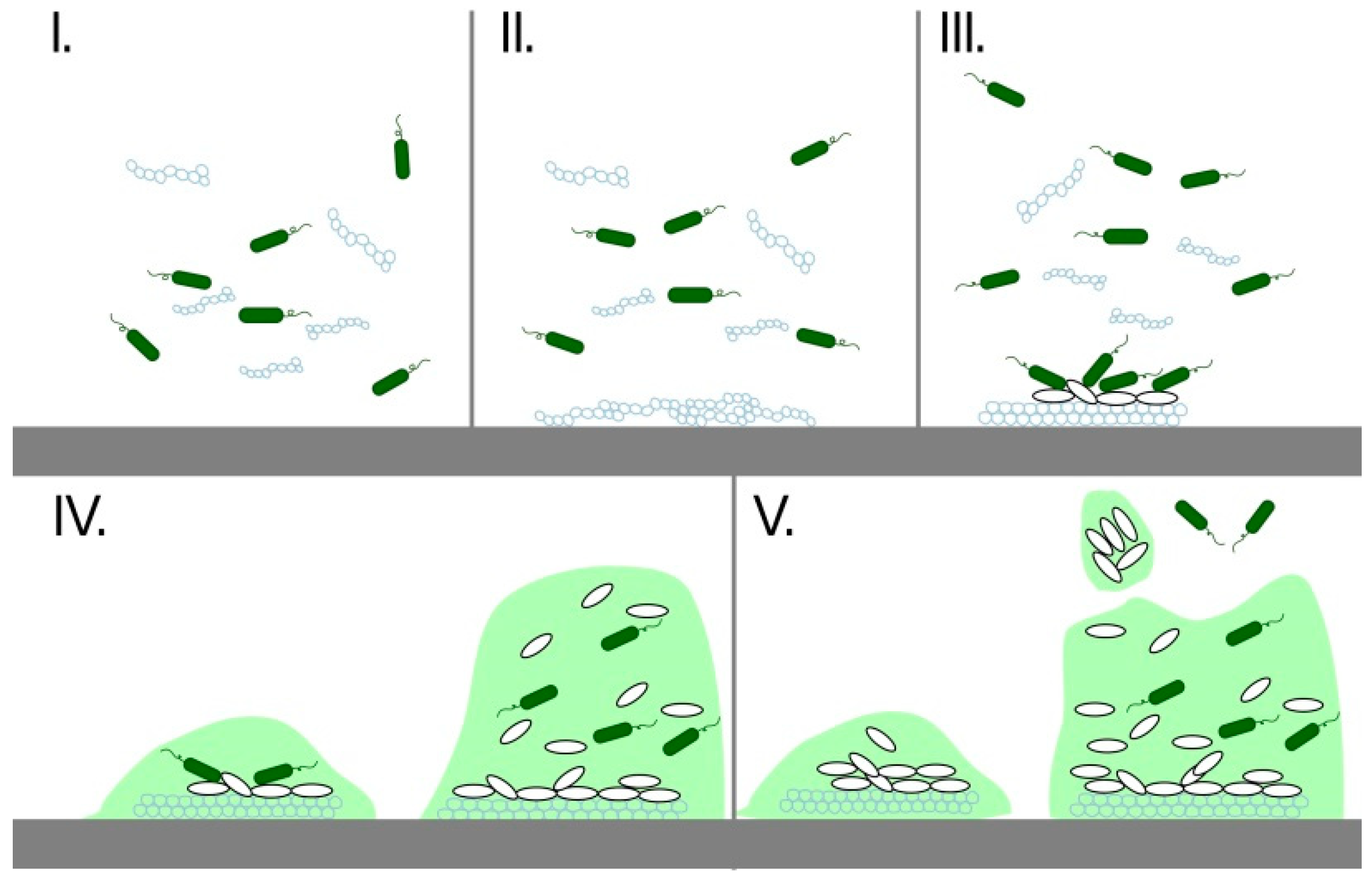
3. Biofilms
4. Biliary Stent
4.1. Biliary Obstruction
4.2. Types of Biliary Stents
4.2.1. Metal Stents
4.2.2. Permanent Polymer Stents
Polyethylene and Polyurethane Stents
Teflon Stents
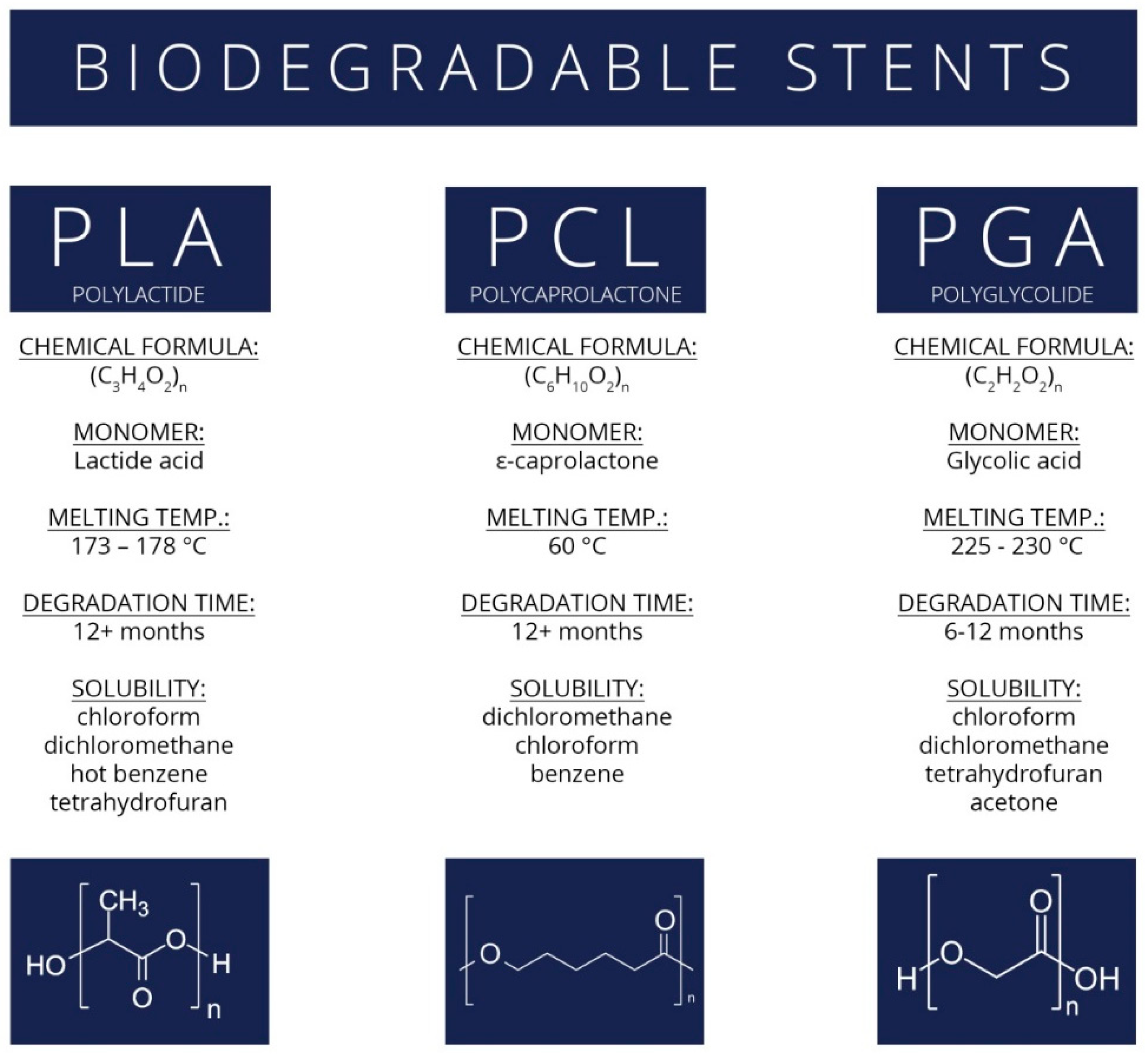
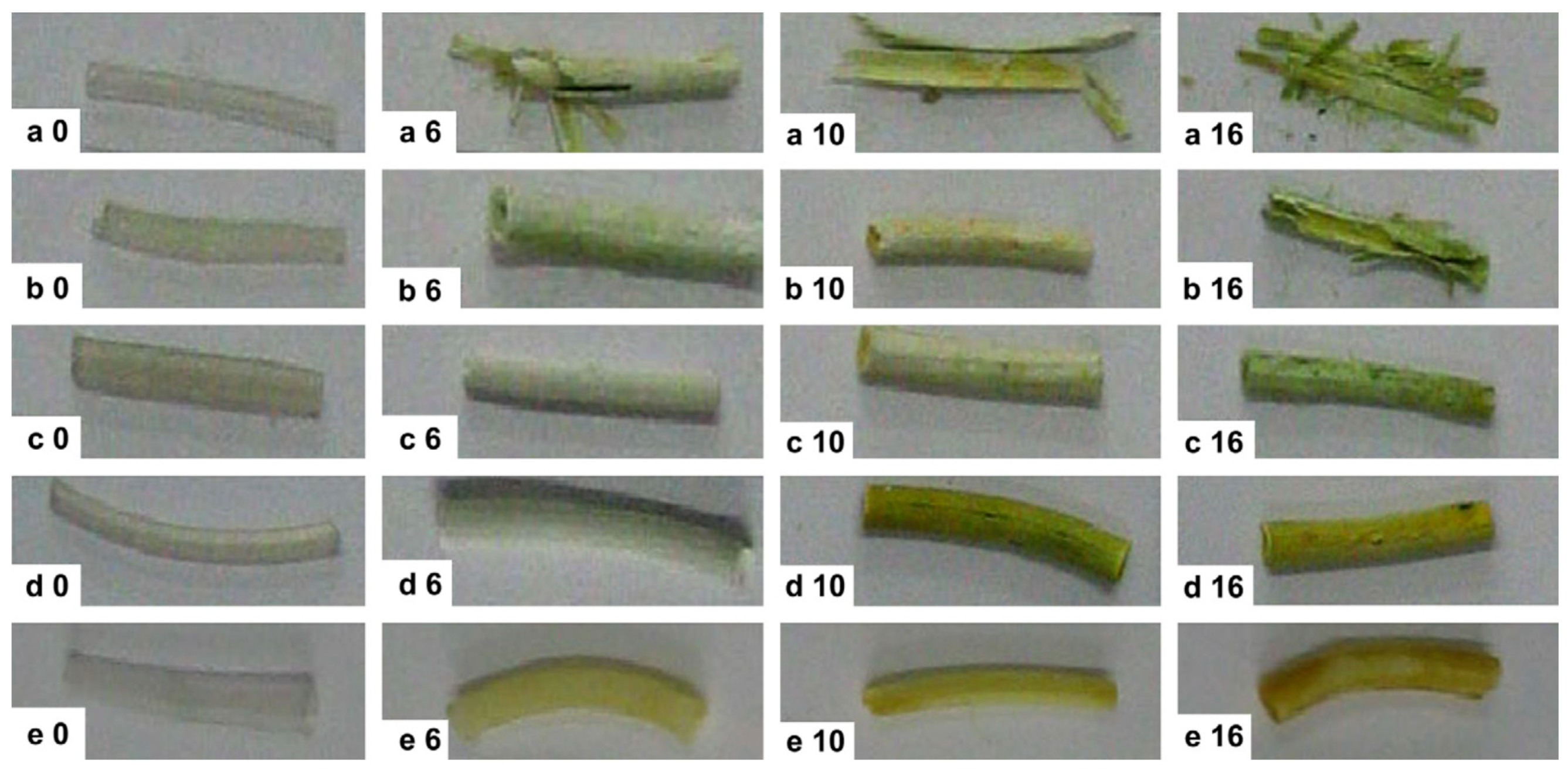
Biodegradable Stents
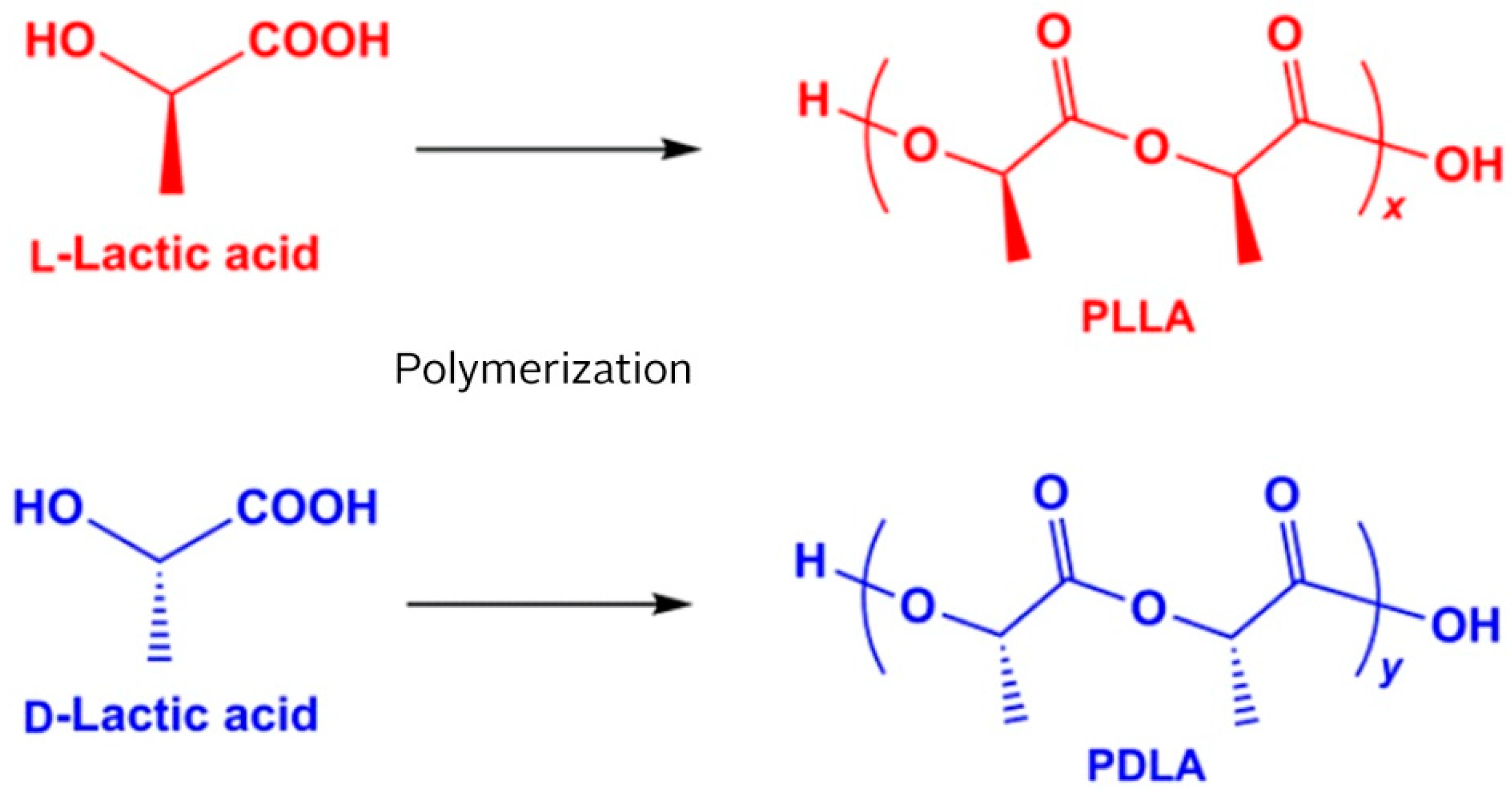
Polylactide Stents for Biliary Duct
Polycaprolactone, Polyglycolide, and Polydioxanone Stents
5. Challenges for New Materials with Antimicrobial Properties
5.1. Antimicrobial Agents
5.1.1. Silver
5.1.2. Copper
5.1.3. Zinc Oxide
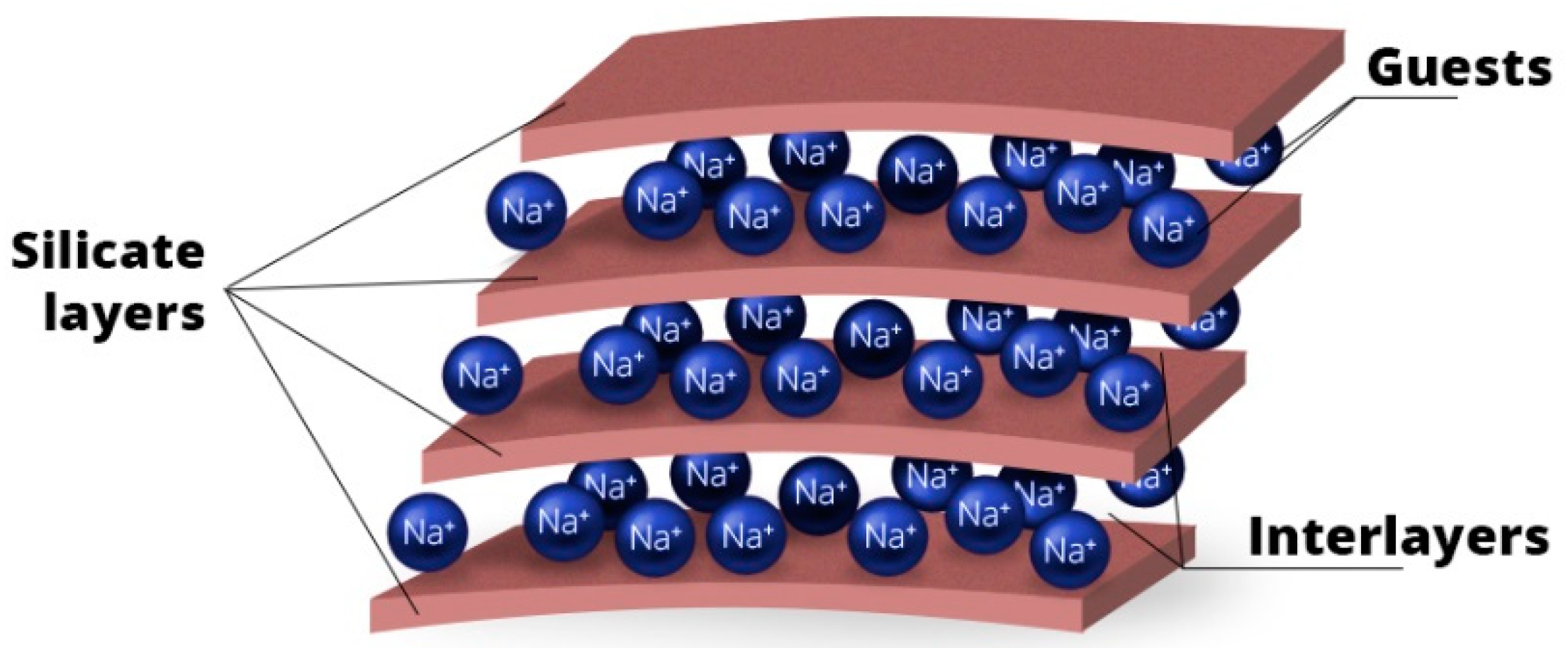
5.1.4. Clay Minerals
5.1.5. Hydroxyapatite
5.1.6. Carbon Nanomaterials
Graphene Oxide with Antimicrobial Agents
Carbon Nanotubes
5.1.7. Antimicrobial Polymeric Materials
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Góra, A.; Pliszka, D.; Mukherjee, S.; Ramakrishna, S. Tubular tissues and organs of human body—Challenges in regenerative medicine. J. Nanosci. Nanotechnol. 2016, 16, 19–39. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fu, W.; Zheng, J. Cell sources for trachea tissue engineering: Past, present and future. Regen. Med. 2012, 7, 851–863. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Bertollo, N.; Cearbhaill, E.D.O.; Salber, J.; Pierucci, L.; Duffy, P.; Dürig, T.; Bi, V.; Wang, W. Bio-resorbable polymer stents: A review of material progress and prospects. Prog. Polym. Sci. 2018, 83, 79–96. [Google Scholar] [CrossRef]
- Carey, F.A.; Sheppard, M.N. Diseases of blood vessels. Surgery 2018, 36, 259–264. [Google Scholar] [CrossRef]
- Welch, T.R.; Nugent, A.W.; Veeram Reddy, S.R. Biodegradable stents for congenital heart disease. Interv. Cardiol. Clin. 2019, 8, 81–94. [Google Scholar] [CrossRef]
- Meraj, P.M.; Jauhar, R.; Singh, A. Bare metal stents versus drug eluting stents: Where do we stand in 2015? Curr. Treat. Options Cardiovasc. Med. 2015, 17, 39. [Google Scholar] [CrossRef]
- Iqbal, J.; Gunn, J.; Serruys, P.W. Coronary stents: Historical development, current status and future directions. Br. Med. Bull. 2013, 106, 193–211. [Google Scholar] [CrossRef]
- Holm, A.N.; De la Mora Levy, J.G.; Gostout, C.J.; Topazian, M.D.; Baron, T.H. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointest. Endosc. 2008, 67, 20–25. [Google Scholar] [CrossRef]
- Testoni, P.A.; Testoni, P.A.; Gastroenterology, D. Endoscopic pancreatic duct stent placement for inflammatory pancreatic diseases. World J. Gastroenterol. 2007, 13, 5971–5978. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ueno, M.; Kameda, R.; Moriya, S.; Irie, K.; Goda, Y.; Tezuka, S.; Yanagida, N.; Ohkawa, S.; Aoyama, T.; et al. Duodenal stenting followed by systemic chemotherapy for patients with pancreatic cancer and gastric outlet obstruction. Pancreatology 2016, 16, 1085–1091. [Google Scholar] [CrossRef]
- Gerber, T.C.; Fasseas, P.; Lennon, R.J.; Valeti, V.U.; Wood, C.P.; Breen, J.F.; Berger, P.B. Clinical safety of magnetic resonanceimaging early after coronary artery stent placement. J. Am. Coll. Cardiol. 2003, 42, 1295–1298. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-K.; Park, S.A.; Lee, D.-W. Biodegradable polymer material based smart stent: Wireless pressure sensor and 3D printed stent. Microelectron. Eng. 2019, 206, 1–5. [Google Scholar] [CrossRef]
- Hermawan, H.; Dubé, D.; Mantovani, D. Developments in metallic biodegradable stents. Acta Biomater. 2010, 6, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, S.J.; Lim, K.S.; Bae, I.H.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Park, J.K. In vivo evaluation and characterization of a bio-absorbable drug-coated stent fabricated using a 3D-printing system. Mater. Lett. 2015, 141, 355–358. [Google Scholar] [CrossRef]
- Died 2017-Institute of Health Information and Statistics of the Czech Republic, 2017. Available online: https://www.uzis.cz/system/files/demozem2017.pdf (accessed on 30 August 2019).
- Cassar, A.; Holmes, D.R.; Rihal, C.S.; Gersh, B.J. Chronic coronary artery disease: Diagnosis and management. Mayo Clin. Proc. 2009, 84, 1130–1146. [Google Scholar] [CrossRef]
- Károly, D.; Charalambous, D.; Pogácsás, B.; Micsik, T.; Barile, C.; Casavola, K. Preparation of explanted coronary stents for investigation of material properties. Mater. Today Proc. 2016, 3, 997–1002. [Google Scholar] [CrossRef]
- Tomberli, B.; Mattesini, A.; Baldereschi, G.I.; Di Mario, C. Breve historia de los stents coronarios. Rev. Española Cardiol. 2018, 71, 312–319. [Google Scholar] [CrossRef]
- Rebagay, G.; Bangalore, S. Biodegradable polymers and stents: The next generation? Curr. Cardiovasc. Risk Rep. 2019, 13, 2–7. [Google Scholar] [CrossRef]
- Zanchin, C.; Ueki, Y.; Zanchin, T.; Häner, J.; Otsuka, T.; Stortecky, S.; Koskinas, K.C.; Siontis, G.C.M.; Praz, F.; Moschovitis, A.; et al. Everolimus-eluting biodegradable polymer versus everolimus-eluting durable polymer stent for coronary revascularization in routine clinical practice. JACC Cardiovasc. Interv. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Buiten, R.A.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; Stoel, M.G.; Hartmann, M.; Tjon, R.M.; et al. Thin, very thin, or ultrathin strut biodegradable- or durable-polymer- coated drug-eluting stents. JACC Cardiovasc. Interv. 2019, 12, 1650–1660. [Google Scholar] [CrossRef]
- Windecker, S.; Serruys, P.W.; Wandel, S.; Buszman, P.; Trznadel, S.; Linke, A.; Lenk, K.; Ischinger, T.; Klauss, V.; Eberli, F.; et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): A randomised non-inferiority trial. Lancet 2008, 372, 1163–1173. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Meredith, I.T.; Windecker, S.; Lee Jobe, R.; Mehta, S.R.; Sarembock, I.J.; Feldman, R.L.; Stein, B.; Dubois, C.; Grady, T.; et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. Circ. Cardiovasc. Interv. 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Staehr, P. ABSORB bioresorbable vascular scaffold system—The 4th revolution in interventional cardiology. In Proceedings of the 17th Asian Harmonization Working Party Annual Conference, Taipei, Taiwan, 2–6 November 2012. [Google Scholar]
- Gogas, B.D. Coronary interventions bioresorbable scaffolds for percutaneous coronary interventions. Glob. Cardiol. Sci. Pr. 2014, 2014, 409–427. [Google Scholar]
- Regazzoli, D.; Leone, P.P.; Colombo, A.; Latib, A. New generation bioresorbable scaffold technologies: An update on novel devices and clinical results. J. Thorac. Dis. 2017, 9, 979–985. [Google Scholar] [CrossRef]
- Capodanno, D. Bioresorbable scaffolds in coronary intervention: Unmet needs and evolution. Korean Circ. J. 2018, 48, 24–35. [Google Scholar] [CrossRef]
- Verheye, S.; Ormiston, J.A.; Stewart, J.; Webster, M.; Sanidas, E.; Costa, R.; Costa, J.R.; Chamie, D.; Abizaid, A.S.; Pinto, I.; et al. A next-generation bioresorbable coronary scaffold system: From bench to first clinical evaluation. JACC Cardiovasc. Interv. 2014, 7, 89–99. [Google Scholar] [CrossRef]
- Nef, H.M.; Wiebe, J.; Foin, N.; Blachutzik, F.; Dörr, O.; Toyloy, S.; Hamm, C.W. A new novolimus-eluting bioresorbable coronary scaffold: Present status and future clinical perspectives. Int. J. Cardiol. 2017, 227, 127–133. [Google Scholar] [CrossRef]
- Serruys, P.W.; Regar, E.; Carter, A.J. Rapamycin eluting stent: The onset of a new era in interventional cardiology. BMJ 2002, 305–308. [Google Scholar] [CrossRef]
- Iqbal, J.; Onuma, Y.; Ormiston, J.; Abizaid, A.; Waksman, R.; Serruys, P. Bioresorbable scaffolds: Rationale, current status, challenges, and future. Eur. Heart J. 2014, 35, 765–776. [Google Scholar] [CrossRef]
- Verheye, S.; Webster, M.; Stewart, J.; Abizaid, A.; Costa, R.; Costa, J.; Yan, J.; Bhat, V.; Morrison, L.; Toyloy, S.; et al. TCT-563 multi-center, first-in-man evaluation of the myolimus-eluting bioresorbable coronary scaffold: 6-month clinical and imaging results. J. Am. Coll. Cardiol. 2012, 60, B163. [Google Scholar] [CrossRef][Green Version]
- Mattesini, A.; Bartolini, S.; Dini, C.S.; Valente, S.; Parodi, G.; Meucci, F.; Mario, C. Di The DESolve novolimus bioresorbable Scaffold: From bench to bedside. J. Thorac. Disease 2017, 9, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Sreenivas, A. Interventional cardiology indigenous stents: Examining the clinical data on new technologies. Interv. Cardiol. 2014, 6, 319–333. [Google Scholar]
- Zhang, Y.; Li, M.; Wei, L.; Zhu, L.; Hu, S.; Wu, S.; Ma, S.; Gao, Y. Differential protein expression in perfusates from metastasized rat livers. Proteome Sci. 2013, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Makaroun, M.S.; Marone, L.K.; Cho, J.S.; Rhee, R.; Chaer, R.A. Long-term outcomes of internal carotid artery dissection. J. Vasc. Surg. 2011, 54, 370–375. [Google Scholar] [CrossRef]
- Fiorani, P.; Speziale, F.; Calisti, A.; Misuraca, M.; Zaccagnini, D.; Rizzo, L.; Giannoni, M.F. Endovascular graft infection: Preliminary results of an international enquiry. J. Endovasc. Ther. 2004, 10, 919–927. [Google Scholar] [CrossRef]
- Vögeling, H.; Pinnapireddy, S.R.; Seitz, B.; Bakowsky, U. Indocyanine green loaded PLGA film coated coronary stents for photo-triggered in situ biofilm eradication. Colloids Interface Sci. Commun. 2018, 27, 35–39. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshida, K.; Suetsugu, T.; Imai, T.; Matsuhashi, N.; Yamaguchi, K. Recent advancements in esophageal cancer treatment in Japan. Ann. Gastroenterol. Surg. 2018, 2, 253–265. [Google Scholar] [CrossRef]
- Fuccio, L.; Scagliarini, M.; Frazzoni, L.; Battaglia, G. Development of a prediction model of adverse events after stent placement for esophageal cancer. Gastrointest. Endosc. 2016, 83, 746–752. [Google Scholar] [CrossRef]
- Repici, A.; Conio, M.; De Angelis, C.; Battaglia, E.; Musso, A.; Pellicano, R.; Goss, M.; Venezia, G.; Rizzetto, M.; Saracco, G. Temporary placement of an expandable polyester silicone-covered stent for treatment of refractory benign esophageal strictures. Gastrointest. Endosc. 2004, 60, 513–519. [Google Scholar] [CrossRef]
- Langer, F.B.; Wenzl, E.; Prager, G.; Salat, A.; Miholic, J.; Mang, T.; Zacherl, J. Management of postoperative esophageal leaks with the polyflex self-expanding covered plastic stent. Ann. Thorac. Surg. 2005, 79, 398–403. [Google Scholar] [CrossRef]
- Lin, M.; Firoozi, N.; Tsai, C.-T.; Wallace, M.B.; Kang, Y. 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies. Acta Biomater. 2019, 83, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J. Gastroenterol. 2007, 13, 3977. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, M.; Nitta, N.; Furukawa, A.; Andoh, A.; Saito, Y.; Fujiyama, Y.; Murata, K. Newly developed biodegradable stents for benign gastrointestinal tract stenoses: A preliminary clinical trial. Digestion 2006, 74, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Gregory, C.J.; Pursnani, K.G.; Ward, J.B.; Stockwell, R.C. The use of biodegradable (SX-ELLA) oesophageal stents to treat dysphagia due to benign and malignant oesophageal disease. Surg. Endosc. 2012, 26, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Imaz-Iglesia, I.; García-Pérez, S.; Nachtnebel, A.; Martín-Águeda, B.; Sánchez-Piedra, C.; Karadayi, B.; Demirbaş, A.R. Biodegradable stents for the treatment of refractory or recurrent benign esophageal stenosis. Expert Rev. Med. Devices 2016, 13, 583–599. [Google Scholar] [CrossRef] [PubMed]
- DiMagno, E.P.; Reber, H.A.; Tempero, M.A. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. Gastroenterology 1999, 117, 1464–1484. [Google Scholar] [CrossRef]
- Adler, D. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: Experience in 36 patients. Am. J. Gastroenterol. 2002, 97, 72–78. [Google Scholar] [CrossRef]
- Kozarek, R.A.; Ball, T.J.; Patterson, D.J. Metallic self-expanding stent application in the upper gastrointestinal tract: Caveats and concerns. Gastrointest. Endosc. 1992, 38, 1–6. [Google Scholar] [CrossRef]
- Tokar, J.L.; Banerjee, S.; Barth, B.A.; Desilets, D.J.; Kaul, V.; Kethi, S.R.; Pedrosa, M.C.; Pfau, P.R.; Pleskow, D.K.; Varadarajulu, S.; et al. Drug-eluting/biodegradable stents. Gastrointest. Endosc. 2011, 74, 954–958. [Google Scholar] [CrossRef]
- Wong, Y.T.; Brams, D.M.; Munson, L.; Sanders, L.; Heiss, F.; Chase, M.; Birkett, D.H. Gastric outlet obstruction secondary to pancreatic cancer. Surg. Endosc. Other Interv. Tech. 2002, 16, 310–312. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Li, R.; Li, Y.; Ruan, L. Biodegradable intestinal stents: A review. Prog. Nat. Sci. Mater. Int. 2014, 24, 423–432. [Google Scholar] [CrossRef]
- Calcagno, P.; Viti, M.; Cornelli, A.; Galli, D.; Urbano, C.D. Intestinal obstruction caused by endometriosis: Endoscopic stenting and expedited laparoscopic resection avoiding stoma. A case report and review of the literature. Int. J. Surg. Case Rep. 2018, 44, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.; Alvarez, C.; Robert, M.; Ashley, S.W.; Reber, H.A.; Lehman, G.A. Polyethylene pancreatic duct stent-induced changes in the normal dog pancreas. Gastrointest. Endosc. 1993, 39, 658–664. [Google Scholar] [CrossRef]
- Kassab, C.; Prat, F.; Liguory, C.; Meduri, B.; Ducot, B.; Fritsch, J.; Choury, A.D.; Pelletier, G. Endoscopic management of post-laparoscopic cholecystectomy biliary strictures. Gastroentérologie Clin. Biol. 2006, 30, 124–129. [Google Scholar] [CrossRef]
- Piñol, V.; Castells, A.; Bordas, J.M.; Real, M.I.; Llach, J.; Montañà, X.; Feu, F.; Navarro, S. Percutaneous self-expanding metal stents versus endoscopic polyethylene endoprostheses for treating malignant biliary obstruction: Randomized clinical trial. Radiology 2002, 225, 27–34. [Google Scholar] [CrossRef]
- Schneider, G.; Siveke, J.T.; Eckel, F.; Schmid, R.M. Pancreatic cancer: Basic and clinical aspects. Gastroenterology 2005, 128, 1606–1625. [Google Scholar] [CrossRef]
- Lubezky, N.; Konikoff, F.M.; Rosin, D.; Carmon, E.; Kluger, Y.; Ben-Haim, M. Endoscopic sphincterotomy and temporary internal stenting for bile leaks following complex hepatic trauma. Br. J. Surg. 2006, 93, 78–81. [Google Scholar] [CrossRef]
- Weber, A.; Zellner, S.; Wagenpfeil, S.; Schneider, J.; Gerngross, C.; Baur, D.M.; Neu, B.; Bajbouj, M.; von Delius, S.; Algül, H.; et al. Long-term follow-up after endoscopic stent therapy for benign biliary strictures. J. Clin. Gastroenterol. 2014, 48, 88–93. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahmed, O. Endoscopic management of pancreatic cancer. Surg. Oncol. Clin. N. Am. 2019, 28, 147–159. [Google Scholar] [CrossRef]
- Lee, T.H.; Jung, M.K.; Kim, T.-K.; Pack, C.G.; Park, Y.K.; Kim, S.-O.; Park, D.H. Safety and efficacy of a metal stent covered with a silicone membrane containing integrated silver particles in preventing biofilm and sludge formation in endoscopic drainage of malignant biliary obstruction: Phase II pilot study. Gastrointest. Endosc. 2019, 90, 663–672. [Google Scholar] [CrossRef]
- Shroff, S. Polymers as ureteral stents. J. Endourol. 2010, 24, 191–198. [Google Scholar]
- Forbes, C.; Scotland, K.B.; Lange, D.; Chew, B.H. Innovations in ureteral stent technology. Urol. Clin. North Am. 2019, 46, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Brierly, R.D.; Mostafid, A.H.; Kontothanassis, D.; Thomas, P.J.; Fletcher, M.S.; Harrison, N.W. Is transurethral resection of the prostate safe and effective in the over 80-year-old? Ann. R. Coll. Surg. Engl. 2001, 83, 50–53. [Google Scholar] [PubMed]
- Rutman, M.P. Prostatic Stents. In A Comprehensive Guide to the Prostate: Eastern and Western Approaches for Management of BPH; Academic Press: Cambridge, MA, USA, 2018; Chapter 19; ISBN 9780128114643. [Google Scholar]
- Nordling, J.; Ovesen, H.; Poulsen, A.L. The intraprostatic spiral: Clinical results in 150 consecutive patients. J. Urol. 1992, 147, 645–647. [Google Scholar] [CrossRef]
- Pétas, A.; Vuopio-Varkila, J.; Siitonen, A.; Välimaa, T.; Talja, M.; Taari, K. Bacterial adherence to self-reinforced polyglycolic acid and self-reinforced poly-lactic acid 96 urological spiral stents in vitro. Biomaterials 1998, 19, 677–681. [Google Scholar] [CrossRef]
- Madersbacher, S. Stents for prostatic diseases: Any progress after 25 years? Eur. Urol. 2006, 49, 212–214. [Google Scholar] [CrossRef]
- Davey, M.E.; O’toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survivalmechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M. Bacterial adhesion: Molecular and ecological diversity. In Wiley Series in Ecological and Applied Microbiology; John Wiley & Sons: Hoboken, NJ, USA, 1996; p. 361. ISBN 0471021857. [Google Scholar]
- Szczotka-Flynn, L.B.; Pearlman, E.; Ghannoum, M. Microbial contamination of contact lenses, lens care solutions, and their accessories: A literature review. Eye Contact Lens Sci. Clin. Pract. 2010, 36, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Archibald, L.K.; Gaynes, R.P. Hospital-acquired infections in the United States. Infect. Dis. Clin. North Am. 1997, 11, 245–255. [Google Scholar] [CrossRef]
- Maria-Litrán, T.; Allison, D.G.; Gilbert, P. An evaluation of the potential of the multiple antibiotic resistance operon (mar) and the multidrug efflux pump acrAB to moderate. J. Antimicrob. Chemother. 2000, 45, 789–795. [Google Scholar] [CrossRef]
- Costerton, J.W. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Kuhn, D.M. Comparison of biofilms formed by candidaalbicans and candidaparapsilosis on bioprosthetic surfaces. Infect. Immun. 2002, 70, 878–888. [Google Scholar] [CrossRef]
- Stein, P.D.; Harken, D.E.; Dexter, L. The nature and prevention of prosthetic valve endocarditis. Am. Heart J. 1966, 71, 393–407. [Google Scholar] [CrossRef]
- Shah, H.; Bosch, W.; Thompson, K.M.; Hellinger, W.C. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013, 3, 144–151. [Google Scholar] [CrossRef]
- Nickel, J.C.; Costerton, J.W. Bacterial biofilms and catheters: A key to understanding bacterial strategies in catheter-associated urinary tract infection. Can. J. Infect. Dis. 1992, 3, 261–267. [Google Scholar] [CrossRef]
- Song, Z.; Borgwardt, L.; Høiby, N.; Wu, H.; Sørensen, T.S.; Borgwardt, A. Prosthesis infections after orthopedic joint replacement: The possible role of bacterial biofilms. Orthop. Rev. (Pavia) 2013, 5, 14. [Google Scholar] [CrossRef]
- Gibbs, K.; Holzman, I.R. Endotracheal tube: Friend or foe? Bacteria, the endotracheal tube, and the impact of colonization and infection. Semin. Perinatol. 2012, 36, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Molinari, G.; Pugliese, V.; Schito, G.C.; Guzmán, C.A. Bacteria involved in the blockage of biliary stents and their susceptibility to antibacterial agents. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, M.O.; Asch, M.R.; Vellahottam, A.; Puri, G.; Andrews, K.; Myers, A. Safety and efficacy of gastrointestinal stents in cancer patients at a community hospital. Can. J. Surg. 2008, 51, 130-4. [Google Scholar] [PubMed]
- Kim, J.H.; Song, H.-Y.; Shin, J.H.; Choi, E.; Kim, T.W.; Lee, S.K.; Kim, B.S. Stent Collapse as a delayed complication of placement of a covered gastroduodenal stent. Am. J. Roentgenol. 2007, 188, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, M.R.; Adams, D.B.; Simpson, J.P.; Cunningham, J.T. Management of biliary strictures due to laparoscopic cholecystectomy. J. Surg. Res. 1995, 58, 86–89. [Google Scholar] [CrossRef]
- Christoforidis, E.; Vasiliadis, K.; Goulimaris, I.; Tsalis, K.; Kanellos, I.; Papachilea, T.; Tsorlini, E.; Betsis, D. A single center experience in minimally invasive treatment of postcholecystectomy bile leak, complicated with biloma formation. J. Surg. Res. 2007, 141, 171–175. [Google Scholar] [CrossRef]
- Baillie, J. Clinical trial report: Endoscopic treatment of postoperative bile duct strictures using multiple stents: Long-term results. Curr. Gastroenterol. Rep. 2011, 13, 114–116. [Google Scholar] [CrossRef]
- Warshaw, A.L.; Castillo, C.F. Pancreatic carcinoma. N. Engl. J. Med. 1992, 326, 455–465. [Google Scholar] [CrossRef]
- Ballinger, A.B.; McHugh, M.; Catnach, S.M.; Alstead, E.M.; Clark, M.L. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut 1994, 35, 467–470. [Google Scholar] [CrossRef]
- Cubiella, J.; Castells, A.; Fondevila, C.; Sans, M.; Sabater, L.; Navarro, S.; Fernández-Cruz, L. Prognostic factors in nonresectable pancreatic adenocarcinoma: A rationale to design therapeutic trials. Am. J. Gastroenterol. 1999, 94, 1271. [Google Scholar] [CrossRef]
- Salgado, S.M.; Gaidhane, M.; Kahaleh, M. Endoscopic palliation of malignant biliary strictures. World J. Gastrointest. Oncol. 2016, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Donelli, G.; Guaglianone, E.; Di Rosa, R.; Fiocca, F.; Basoli, A. Plastic biliary stent occlusion: Factors involved and possible preventive approaches. Clin. Med. Res. 2007, 5, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rejchrt, S.; Kopacova, M.; Brozik, J.; Bures, J. Biodegradable stents for the treatment of benign stenoses of the small and large intestines. Endoscopy 2011, 43, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Zuber-Jerger, I.; Hempel, U.; Rockmann, F.; Klebl, F. Temporary stent placement in 2 cases of aortoesophageal fistula. Gastrointest. Endosc. 2008, 68, 599–602. [Google Scholar] [CrossRef]
- Pfau, P.R.; Pleskow, D.K.; Banerjee, S.; Barth, B.A.; Bhat, Y.M.; Desilets, D.J.; Gottlieb, K.T.; Maple, J.T.; Siddiqui, U.D.; Tokar, J.L.; et al. Pancreatic and biliary stents. Gastrointest. Endosc. 2013, 77, 319–327. [Google Scholar] [CrossRef]
- Ruys, A.T.; Rauws, E.A.; Busch, O.R.C.; Lameris, J.S.; Gouma, D.J.; van Gulik, T.M. Preoperative biliary drainage. In Hilar Cholangiocarcinoma; Springer: Dordrecht, The Netherlands, 2013; pp. 139–146. ISBN 9789400764736. [Google Scholar]
- Weber, A.; Prinz, C.; Gerngro, C.; Ludwig, L.; Huber, W.; Neu, B.; Ebert, M.P.; Meining, A.; Weidenbach, H.; Schmid, R.M.; et al. Long-term outcome of endoscopic and/or percutaneous transhepatic therapy in patients with biliary stricture after orthotopic liver transplantation. J. Gastroenterol. 2009, 44, 1195–1202. [Google Scholar] [CrossRef]
- Hermann, R.E. Shackelford’s surgery of the alimentary tract. JAMA J. Am. Med. Assoc. 1991, 266, 1576. [Google Scholar] [CrossRef]
- Laasch, H.-U.; Martin, D.F. Management of benign biliary strictures. Cardiovasc. Intervent. Radiol. 2002, 25, 457–466. [Google Scholar] [CrossRef]
- Padillo, F.J.; Cruz, A.; Briceño, J.; Martin-Malo, A.; Pera-Madrazo, C.; Sitges-Serra, A. Multivariate analysis of factors associated with renal dysfunction in patients with obstructive jaundice. Br. J. Surg. 2005, 92, 1388–1392. [Google Scholar] [CrossRef]
- Son, J.H.; Kim, J.; Lee, S.H.; Hwang, J.-H.; Ryu, J.K.; Kim, Y.-T.; Yoon, Y.B.; Jang, J.-Y.; Kim, S.-W.; Cho, J.Y.; et al. The optimal duration of preoperative biliary drainage for periampullary tumors that cause severe obstructive jaundice. Am. J. Surg. 2013, 206, 40–46. [Google Scholar] [CrossRef]
- Bismuth, H.; Majno, P.E. Biliary strictures: Classification based on the principles of surgical treatment. World J. Surg. 2001, 25, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Perri, V.; Familiari, P.; Tringali, A.; Boskoski, I.; Costamagna, G. Plastic biliary stents for benign biliary diseases. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 405–433. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, G.; Pandolfi, M. Endoscopic stenting for biliary and pancreatic malignancies. J. Clin. Gastroenterol. 2004, 38, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, P.G.; Vleggaar, F.P.; Siersema, P.D. Plastic or metal stents for benign extrahepatic biliary strictures: A systematic review. BMC Gastroenterol. 2009, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.; Sturgess, R. Endoscopic therapy in the management of malignant biliary obstruction. Eur. J. Surg. Oncol. 2008, 34, 313–317. [Google Scholar] [CrossRef]
- Isayama, H.; Mukai, T.; Itoi, T.; Maetani, I.; Nakai, Y.; Kawakami, H.; Yasuda, I.; Maguchi, H.; Ryozawa, S.; Hanada, K.; et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: The WATCH study. Gastrointest. Endosc. 2012, 76, 84–92. [Google Scholar] [CrossRef]
- Davids, P.H.P.; Groen, A.K.; Rauws, E.A.J.; Tytgat, G.N.J.; Huibregtse, K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet 1992, 340, 1488–1492. [Google Scholar] [CrossRef]
- Yeoh, K.G.; Zimmerman, M.J.; Cunningham, J.T.; Cotton, P.B. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest. Endosc. 1999, 49, 466–471. [Google Scholar] [CrossRef]
- Prat, F.; Chapat, O.; Ducot, B.; Ponchon, T.; Pelletier, G.; Fritsch, J.; Choury, A.D.; Buffet, C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest. Endosc. 1998, 47, 1–7. [Google Scholar] [CrossRef]
- Parviainen, M.; Sand, J.; Harmoinen, A.; Kainulainen, H.; Välimaa, T.; Törmälä, P.; Nordback, I. A new biodegradable stent for the pancreaticojejunal anastomosis after pancreaticoduodenal resection: In vitro examination and pilot experiences in humans. Pancreas 2000, 21, 14–21. [Google Scholar] [CrossRef]
- Soehendra, N.; Reynders-Frederix, V. Palliative bile duct drainage—A new endoscopic method of introducing a transpapillary drain. Endoscopy 1980, 12, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut 2005, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.F.; Geenen, J.E.; Lehman, G.A.; Siegel, J.H.; Jacob, L.; McKinley, M.J.; Raijman, I.; Meier, P.; Jacobson, I.; Kozarek, R.; et al. “Tannenbaum” Teflon stents versus traditional polyethylene stents for treatment of malignant biliary stricture. Gastrointest. Endosc. 2002, 55, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Leung, J.W.C.; Shaffer, E.A.; Lam, K.; Costerton, J.W. Bacterial biofilm, brown pigment stone and blockage of biliary stents. J. Gastroenterol. Hepatol. 1993, 8, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, A.M.; Boland, C.; Redekop, W.K.; Bergman, J.J.G.H.M.; Groen, A.K.; Tytgat, G.N.J.; Huibregtse, K. A Prospective randomized trial of teflon versus polyethylene stents for distal malignant biliary obstruction. Endoscopy 1998, 30, 681–686. [Google Scholar] [CrossRef] [PubMed]
- England, R.E.; Martin, D.F.; Morris, J.; Sheridan, M.B.; Frost, R.; Freeman, A.; Lawrie, B.; Deakin, M.; Fraser, I.; Smith, K. A prospective randomised multicentre trial comparing 10 Fr Teflon Tannenbaum stents with 10 Fr polyethylene Cotton-Leung stents in patients with malignant common duct strictures. Gut 2000, 46, 395–400. [Google Scholar] [CrossRef][Green Version]
- Terruzzi, V.; Comin, U.; De Grazia, F.; Toti, G.L.; Zambelli, A.; Beretta, S.; Minoli, G. Prospective randomized trial comparing Tannenbaum Teflon and standard polyethylene stents in distal malignant biliary stenosis. Gastrointest. Endosc. 2000, 51, 23–27. [Google Scholar] [CrossRef]
- Speer, A.G.; Cotton, P.B.; MacRae, K.D. Endoscopic management of malignant biliary obstruction: Stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest. Endosc. 1988, 34, 412–417. [Google Scholar] [CrossRef]
- Pedersen, F.M. Endoscopic management of malignant biliary obstruction is stent size of 10 french gauge better than 7 french gauge? Scand. J. Gastroenterol. 1993, 28, 185–189. [Google Scholar] [CrossRef]
- Huibregtse, K.; Haverkamp, H.J.; Tytgat, G.N. Transpapillary positioning of a large 3.2 mm biliary endoprosthesis. Endoscopy 1981, 13, 217–219. [Google Scholar] [CrossRef]
- Weber, A.; Mittermeyer, T.; Wagenpfeil, S.; Schmid, R.M.; Prinz, C. Self-expanding metal stents versus polyethylene stents for palliative treatment in patients with advanced pancreatic cancer. Pancreas 2009, 38, e7–e12. [Google Scholar] [CrossRef] [PubMed]
- Leong, Q.W.; Shen, M.L.; Au, K.W.; Luo, D.; Lau, J.Y.; Wu, J.C.; Chan, F.K.; Sung, J.J. A prospective, randomized study of the patency period of the plastic antireflux biliary stent: An interim analysis. Gastrointest. Endosc. 2016, 83, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Torki, M.M.; Hassanajili, S.; Jalisi, M.M. Design optimizations of PLA stent structure by FEM and investigating its function in a simulated plaque artery. Math. Comput. Simul. 2019. [Google Scholar] [CrossRef]
- Petrtýl, J.; Brůha, R.; Horák, L.; Zádorová, Z.; Doseděl, J.; Laasch, H.-U. Management of benign intrahepatic bile duct strictures: Initial experience with polydioxanone biodegradable stents. Endoscopy 2010, 42, E89–E90. [Google Scholar] [CrossRef]
- Itoi, T.; Kasuya, K.; Abe, Y.; Isayama, H. Endoscopic placement of a new short-term biodegradable pancreatic and biliary stent in an animal model: A preliminary feasibility study (with videos). J. Hepatobiliary Pancreat. Sci. 2011, 18, 463–467. [Google Scholar] [CrossRef]
- Yaszemski, M. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef]
- Isotalo, T.; Alarakkola, E.; Talja, M.; Tammela, T.L.J.; Välimaa, T.; Törmälä, P. Biocompatibility testing of a new bioabsorbable X-ray positive sr-pla 96/4 urethral stent. J. Urol. 1999, 162, 1764–1767. [Google Scholar] [CrossRef]
- Poole-Warren, L.A.; Patton, A.J. Introduction to biomedical polymers and biocompatibility. In Biosynthetic Polymers for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–31. ISBN 9781782421139. [Google Scholar]
- Baudis, S.; Behl, M.; Lendlein, A. Smart polymers for biomedical applications. Macromol. Chem. Phys. 2014, 215, 2399–2402. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Abdelhamid, D. Biodegradable and bioerodible polymers for medical applications. In Biosynthetic Polymers for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 63–83. ISBN 9781782421139. [Google Scholar]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Liu, H.; Song, W.; Chen, F.; Guo, L.; Zhang, J. Interaction of microstructure and interfacial adhesion on impact performance of polylactide (PLA) ternary blends. Macromolecules 2011, 44, 1513–1522. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Avérous, L. Polylactic Acid: Synthesis, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 433–450. ISBN 9780080453163. [Google Scholar]
- Laukkarinen, J.; Nordback, I.; Mikkonen, J.; Kärkkäinen, P.; Sand, J. A novel biodegradable biliary stent in the endoscopic treatment of cystic-duct leakage after cholecystectomy. Gastrointest. Endosc. 2007, 65, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Laukkarinen, J.; Sand, J.; Leppiniemi, J.; Kellomäki, M.; Nordback, I. A novel technique for hepaticojejunostomy for nondilated bile ducts: A purse-string anastomosis with an intra-anastomotic biodegradable biliary stent. Am. J. Surg. 2010, 200, 124–130. [Google Scholar] [CrossRef]
- Laukkarinen, J.M.; Sand, J.A.; Chow, P.; Juuti, H.; Kellomäki, M.; Kärkkäinen, P.; Isola, J.; Yu, S.; Somanesan, S.; Kee, I.; et al. A novel biodegradable biliary stent in the normal duct hepaticojejunal anastomosis: An 18-month follow-up in a large animal model. J. Gastrointest. Surg. 2007, 11, 750–757. [Google Scholar] [CrossRef]
- Lämsä, T.; Jin, H.; Mikkonen, J.; Laukkarinen, J.; Sand, J.; Nordback, I. Biocompatibility of a new bioabsorbable radiopaque stent material (BaSO4 containing poly-L,D-lactide) in the rat pancreas. Pancreatology 2006, 6, 301–305. [Google Scholar] [CrossRef]
- Xu, X.; Liu, T.; Zhang, K.; Liu, S.; Shen, Z.; Li, Y.; Jing, X. Biodegradation of poly(l-lactide-co-glycolide) tube stents in bile. Polym. Degrad. Stab. 2008, 93, 811–817. [Google Scholar] [CrossRef]
- Ginsberg, G.; Cope, C.; Shah, J.; Martin, T.; Carty, A.; Habecker, P.; Kaufmann, C.; Clerc, C.; Nuutinen, J.-P.; Törmälä, P. In vivo evaluation of a new bioabsorbable self-expanding biliary stent. Gastrointest. Endosc. 2003, 58, 777–784. [Google Scholar] [CrossRef]
- Xu, X.; Liu, T.; Liu, S.; Zhang, K.; Shen, Z.; Li, Y.; Jing, X. Feasibility of biodegradable PLGA common bile duct stents: An in vitro and in vivo study. J. Mater. Sci. Mater. Med. 2009, 20, 1167–1173. [Google Scholar] [CrossRef]
- Meng, B.; Wang, J.; Zhu, N.; Meng, Q.-Y.; Cui, F.-Z.; Xu, Y.-X. Study of biodegradable and self-expandable PLLA helical biliary stent in vivo and in vitro. J. Mater. Sci. Mater. Med. 2006, 17, 611–617. [Google Scholar] [CrossRef]
- van Natta, F.J.; Hill, J.W.; Carothers, W.H. Studies of polymerization and ring formation. XXIII. 1 ε-caprolactone and its polymers. J. Am. Chem. Soc. 1934, 56, 455–457. [Google Scholar] [CrossRef]
- Luciani, A.; Coccoli, V.; Orsi, S.; Ambrosio, L.; Netti, P.A. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials 2008, 29, 4800–4807. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, H.Y.; Khil, M.S.; Ra, Y.M.; Lee, D.R. Characterization of nano-structured poly(ε-caprolactone) nonwoven mats via electrospinning. Polymer (Guildf) 2003, 44, 1287–1294. [Google Scholar] [CrossRef]
- Marrazzo, C.; Di Maio, E.; Iannace, S. Conventional and nanometric nucleating agents in poly(ε-caprolactone) foaming: Crystals vs. bubbles nucleation. Polym. Eng. Sci. 2008, 48, 336–344. [Google Scholar] [CrossRef]
- Cama, G.; Mogosanu, D.E.; Houben, A.; Dubruel, P. Synthetic biodegradable medical polyesters. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–105. ISBN 9780081003725. [Google Scholar]
- Aikawa, M.; Miyazawa, M.; Okada, K.; Toshimitsu, Y.; Torii, T.; Otani, Y.; Koyama, I.; Ikada, Y. Regeneration of extrahepatic bile duct—Possibility to clinical application by recognition of the regenerative process. J. Smooth Muscle Res. 2007, 43, 211–218. [Google Scholar] [CrossRef]
- Grolich, T.; Crha, M.; Novotný, L.; Kala, Z.; Hep, A.; Nečas, A.; Hlavsa, J.; Mitáš, L.; Misík, J. Self-expandable biodegradable biliary stents in porcine model. J. Surg. Res. 2015, 193, 606–612. [Google Scholar] [CrossRef]
- Hellmann, M.; Mehta, S.D.; Bishai, D.M.; Mears, S.C.; Zenilman, J.M. The estimated magnitude and direct hospital costs of prosthetic joint infections in the United States, 1997 to 2004. J. Arthroplasty 2010, 25, 766–771. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Uçkay, I.; Hoffmeyer, P.; Lew, D.; Pittet, D. Prevention of surgical site infections in orthopaedic surgery and bone trauma: State-of-the-art update. J. Hosp. Infect. 2013, 84, 5–12. [Google Scholar] [CrossRef]
- Rojas, K.; Canales, D.; Amigo, N.; Montoille, L.; Cament, A.; Rivas, L.M.; Gil-Castell, O.; Reyes, P.; Ulloa, M.T.; Ribes-Greus, A.; et al. Effective antimicrobial materials based on low-density polyethylene (LDPE) with zinc oxide (ZnO) nanoparticles. Compos. Part B Eng. 2019, 172, 173–178. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Azlin-Hasim, S.; Cruz-Romero, M.; Cummins, E.; Kerry, J.P.; Morris, M.A. Natural Antimicrobial Materials for Use in Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128119822. [Google Scholar]
- Lv, W.; Luo, J.; Deng, Y.; Sun, Y. Biomaterials immobilized with chitosan for rechargeable antimicrobial drug delivery. J. Biomed. Mater. Res. Part A 2013, 101A, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef]
- Fukuda, R.K. Antimicrobial Resistance Global Report on Surveillance; World Health Organization: Cham, Switzerland, 2014. [Google Scholar]
- Piddock, L.J. V The crisis of no new antibiotics-what is the way forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef]
- Hasan, J.; Chatterjee, K. Recent advances in engineering topography mediated antibacterial surfaces. Nanoscale 2015, 7, 15568–15575. [Google Scholar] [CrossRef]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles biosynthesized by fungi and yeast: A review of their preparation, properties, and medical applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Choi, Y.J.; Han, J.W.; Kim, E.; Park, J.H.; Gurunathan, S.; Kim, J.H. Differential nanoreprotoxicity of silver nanoparticles in male somatic cells and spermatogonial stem cells. Int. J. Nanomedicine 2015, 10, 1335–1357. [Google Scholar] [PubMed]
- Hill, R.T. Plasmonic biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Sharma, A.; Tummalapalli, M.; Joy, J.; Bhan, S.; Gupta, B. A novel route for the preparation of silver loaded polyvinyl alcohol nanogels for wound care systems. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 894–905. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Alzohairy, M.A. Anti-biofi lm effi cacy of silver nanoparticles against MRSA and MRSE isolated from wounds in a tertiary care hospital. Indian J. Med. Microbiol. 2015, 33, 101–109. [Google Scholar] [CrossRef]
- Gosheger, G. Silver-coated megaendoprostheses in a rabbit model—An analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556. [Google Scholar] [CrossRef]
- Maharubin, S.; Nayak, C.; Phatak, O.; Kurhade, A.; Singh, M.; Zhou, Y.; Tan, G. Polyvinylchloride coated with silver nanoparticles and zinc oxide nanowires for antimicrobial applications. Mater. Lett. 2019, 249, 108–111. [Google Scholar] [CrossRef]
- Dutta, P.; Wang, B. Zeolite-supported silver as antimicrobial agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Hou, A.; Xie, K. Efficient antimicrobial silk composites using synergistic effects of violacein and silver nanoparticles. Mater. Sci. Eng. C 2019, 103, 109821. [Google Scholar] [CrossRef] [PubMed]
- Thokala, N.; Kealey, D.C.; Kennedy, D.J.; Brady, D.D.B.; Farrell, D.J. Comparative activity of silver-based antimicrobial composites for urinary catheters. Int. J. Antimicrob. Agents 2018, 52, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Falconer, J.L.; Grainger, D.W. 1.4 Silver antimicrobial biomaterials. Compr. Biomater. II 2017, 1, 79–91. [Google Scholar]
- Baygar, T.; Sarac, N.; Ugur, A.; Karaca, I.R. Antimicrobial characteristics and biocompatibility of the surgical sutures coated with biosynthesized silver nanoparticles. Bioorg. Chem. 2019, 86, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ontong, J.C.; Paosen, S.; Shankar, S.; Voravuthikunchai, S.P. Eco-friendly synthesis of silver nanoparticles using Senna alata bark extract and its antimicrobial mechanism through enhancement of bacterial membrane degradation. J. Microbiol. Methods 2019, 165, 105692. [Google Scholar] [CrossRef]
- Song, W.-H.; Ryu, H.S.; Hong, S.-H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. Part A 2009, 88A, 246–254. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mehtar, S.; Wiid, I.; Todorov, S.D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51. [Google Scholar] [CrossRef]
- Li, K.; Xia, C.; Qiao, Y.; Liu, X. Dose-response relationships between copper and its biocompatibility/antibacterial activities. J. Trace Elem. Med. Biol. 2019, 55, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Prokhorov, E.; España-Sánchez, B.L.; Luna-Bárcenas, G.; Padilla-Vaca, F.; Cruz-Soto, M.E.; Vázquez-Lepe, M.O.; Kovalenko, Y.; Elizalde-Peña, E.A. Chitosan/copper nanocomposites: Correlation between electrical and antibacterial properties. Colloids Surfaces B Biointerfaces 2019, 180, 186–192. [Google Scholar] [CrossRef]
- Rauf, A.; Ye, J.; Zhang, S.; Shi, L.; Akram, M.A.; Ning, G. Synthesis, structure and antibacterial activity of a copper(II) coordination polymer based on thiophene-2,5-dicarboxylate ligand. Polyhedron 2019, 166, 130–136. [Google Scholar] [CrossRef]
- Mercer, J.F.B. The molecular basis of copper-transport diseases. Trends Mol. Med. 2001, 7, 64–69. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Thill, A.; Flank, A.M. Cytotoxicity of CeO 2 Nanoparticles physico-chemical insight of the cytotoxicity mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 10–13. [Google Scholar] [CrossRef]
- Ghaffari-Moghaddam, M.; Hadi-Dabanlou, R. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. J. Ind. Eng. Chem. 2014, 20, 739–744. [Google Scholar] [CrossRef]
- Holešová, S.; Hundáková, M.; Pazdziora, E. Antibacterial kaolinite based nanocomposites. Procedia Mater. Sci. 2016, 12, 124–129. [Google Scholar] [CrossRef]
- Plachá, D.; Rosenbergová, K.; Slabotínský, J.; Kutláková, K.M.; Študentová, S.; Martynková, G.S. Modified clay minerals efficiency against chemical and biological warfare agents for civil human protection. J. Hazard. Mater. 2014, 271, 65–72. [Google Scholar] [CrossRef]
- Shah, N.J.; Hong, J.; Hyder, M.N.; Hammond, P.T. Osteophilic multilayer coatings for accelerated bone tissue growth. Adv. Mater. 2012, 24, 1445–1450. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide-silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Li, N.; Zeng, C.; Qin, Q.; Zhang, B.; Chen, L.; Luo, Z. Powerful antibacterial activity of graphene/nanoflower-like nickelous hydroxide nanocomposites. Nanomedicine 2018, 13, 2901–2916. [Google Scholar] [CrossRef]
- Jaleel, J.A.; Sruthi, S.; Pramod, K. Reinforcing nanomedicine using graphene family nanomaterials. J. Control. Release 2017, 255, 218–230. [Google Scholar] [CrossRef]
- Wang, Y.W.; Cao, A.N.; Jiang, Y.; Zhang, I.; Liu, J.H.; Liu, Y.F.; Wang, H.F. Superior antibacterial activity of zinc oxide/graphene oxide composites localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2790–2797. [Google Scholar]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Brunet, L.; Hinkal, G.W.; Wiesner, M.R.; Alvarez, P.J.J. Antibacterial activity of fullerene water suspensions (nC 60) is not due to ROS-mediated damage. Nano Lett. 2008, 8, 1539–1543. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Fernando, K.A.S.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.-P.; Bunker, C.E. Graphene oxide: A nonspecific enhancer of cellular growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, T.; Gopalan, A.I.; Lee, K.P.; Park, S.Y. Glucose sensing, photocatalytic and antibacterial properties of graphene-ZnO nanoparticle hybrids. Carbon NY 2012, 50, 2994–3000. [Google Scholar] [CrossRef]
- de Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surfaces B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.; Wang, Y.; Yan, X.; Sun, D.D. Facile synthesis of monodispersed silver nanoparticles on graphene oxide sheets with enhanced antibacterial activity. New J. Chem. 2011, 35, 1418. [Google Scholar] [CrossRef]
- Das, M.R.; Sarma, R.K.; Saikia, R.; Kale, V.S.; Shelke, M.V.; Sengupta, P. Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surfaces B Biointerfaces 2011, 83, 16–22. [Google Scholar] [CrossRef]
- Ali, Q.; Ahmed, W.; Lal, S.; Sen, T. Science direct novel multifunctional carbon nanotube containing silver and iron oxide nanoparticles for antimicrobial applications in water treatment. Mater. Today Proc. 2017, 4, 57–64. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Klodzinska, E. Zeta potential study of biodegradable antimicrobial polymers. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 483, 204–208. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-based polymers with antimicrobial properties towards sustainable development. Materials (Basel) 2019, 12, 641. [Google Scholar] [CrossRef]
- Paris, J.-B.; Seyer, D.; Jouenne, T.; Thébault, P. Various methods to combine hyaluronic acid and antimicrobial peptides coatings and evaluation of their antibacterial behaviour. Int. J. Biol. Macromol. 2019, 139, 468–474. [Google Scholar] [CrossRef]
- Sharma, D.; Choudhary, M.; Vashistt, J.; Shrivastava, R.; Bisht, G.S. Cationic antimicrobial peptide and its poly-N-substituted glycine congener: Antibacterial and antibiofilm potential against A. baumannii. Biochem. Biophys. Res. Commun. 2019, 518, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J.; Craik, D.J. The Vast Structural diversity of antimicrobial peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The amphibian antimicrobial peptide temporin b inhibits in vitro herpes simplex virus 1 infection. Antimicrob. Agents Chemother. 2018, 62, e02367-17. [Google Scholar] [CrossRef] [PubMed]
- Carter, V.; Underhill, A.; Baber, I.; Sylla, L.; Baby, M.; Larget-Thiery, I.; Zettor, A.; Bourgouin, C.; Langel, Ü.; Faye, I.; et al. Killer bee molecules: Antimicrobial peptides as effector molecules to target sporogonic stages of plasmodium. PLoS Pathog. 2013, 9, e1003790. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]

| Environment of the Biomaterial | Suspended Liquids | Main Components of the Host Products |
|---|---|---|
| Urethra | Urine, prostatic fluid | Mucopolysaccharides, Tamm Horsfall proteins, glycoproteins |
| Cardiovascular | Blood | Serum, albumin, fibrinogen, fibronectin |
| Ocular | Tears | Proteins (lysozymes), fibrin |
| The oral cavity | Sputum | Mucopolysaccharides, glycoproteins, serum albumin |
| Intestinal tract | Digested food and liquids | Mucopolysaccharides, glycoproteins, serum albumin, steroids |
| Biliary ducts | Bile | Steroids, bile salts, mucopolysaccharides |
| Respiratory passageways | Respiratory secretions | Mucopolysaccharides |
| Bones, joints | Synovial fluids, blood | Serum proteins, blood elements |
| Implant | Organisms | Associated Disease | References |
|---|---|---|---|
| Prosthetic valve | Staphylococcus aureus, Staphylococcus albus | Prosthetic valve endocarditis | [80] |
| Contact lenses | Pseudomonas aeruginosa, Staphylococcus epidermidis | Keratitis | [75] |
| Intravascular catheters | Staphylococcus epidermidis, Staphylococcus aureus | Septicaemia, endocarditis | [81] |
| Urinary catheters | Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis | Bacteraemia | [82] |
| Joint replacement | Pseudomonas aeruginosa, Staphylococcus epidermidis, Staphylococcus aureus | Septicaemia, device failure | [83] |
| Endotracheal tube | Pseudomonas aeruginosa, Escherichia coli, Ureaplasma urealyticum, Staphylococcus aureus | Pneumonia | [84] |
| Biliary stents | Enterobacteriaceae, Pseudomonas aeruginosa, Enterococcus, Candida albicans | Biliary obstruction, septicaemia | [85] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrlová, K.; Malachová, K.; Muñoz-Bonilla, A.; Měřinská, D.; Rybková, Z.; Fernández-García, M.; Plachá, D. Biocompatible Polymer Materials with Antimicrobial Properties for Preparation of Stents. Nanomaterials 2019, 9, 1548. https://doi.org/10.3390/nano9111548
Škrlová K, Malachová K, Muñoz-Bonilla A, Měřinská D, Rybková Z, Fernández-García M, Plachá D. Biocompatible Polymer Materials with Antimicrobial Properties for Preparation of Stents. Nanomaterials. 2019; 9(11):1548. https://doi.org/10.3390/nano9111548
Chicago/Turabian StyleŠkrlová, Kateřina, Kateřina Malachová, Alexandra Muñoz-Bonilla, Dagmar Měřinská, Zuzana Rybková, Marta Fernández-García, and Daniela Plachá. 2019. "Biocompatible Polymer Materials with Antimicrobial Properties for Preparation of Stents" Nanomaterials 9, no. 11: 1548. https://doi.org/10.3390/nano9111548
APA StyleŠkrlová, K., Malachová, K., Muñoz-Bonilla, A., Měřinská, D., Rybková, Z., Fernández-García, M., & Plachá, D. (2019). Biocompatible Polymer Materials with Antimicrobial Properties for Preparation of Stents. Nanomaterials, 9(11), 1548. https://doi.org/10.3390/nano9111548