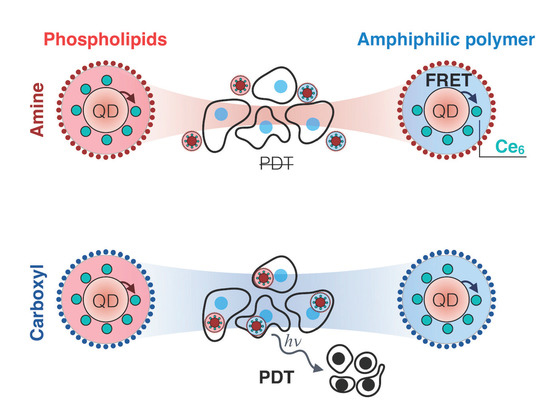
Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spectral Studies
2.3. Cell Culturing and Labeling
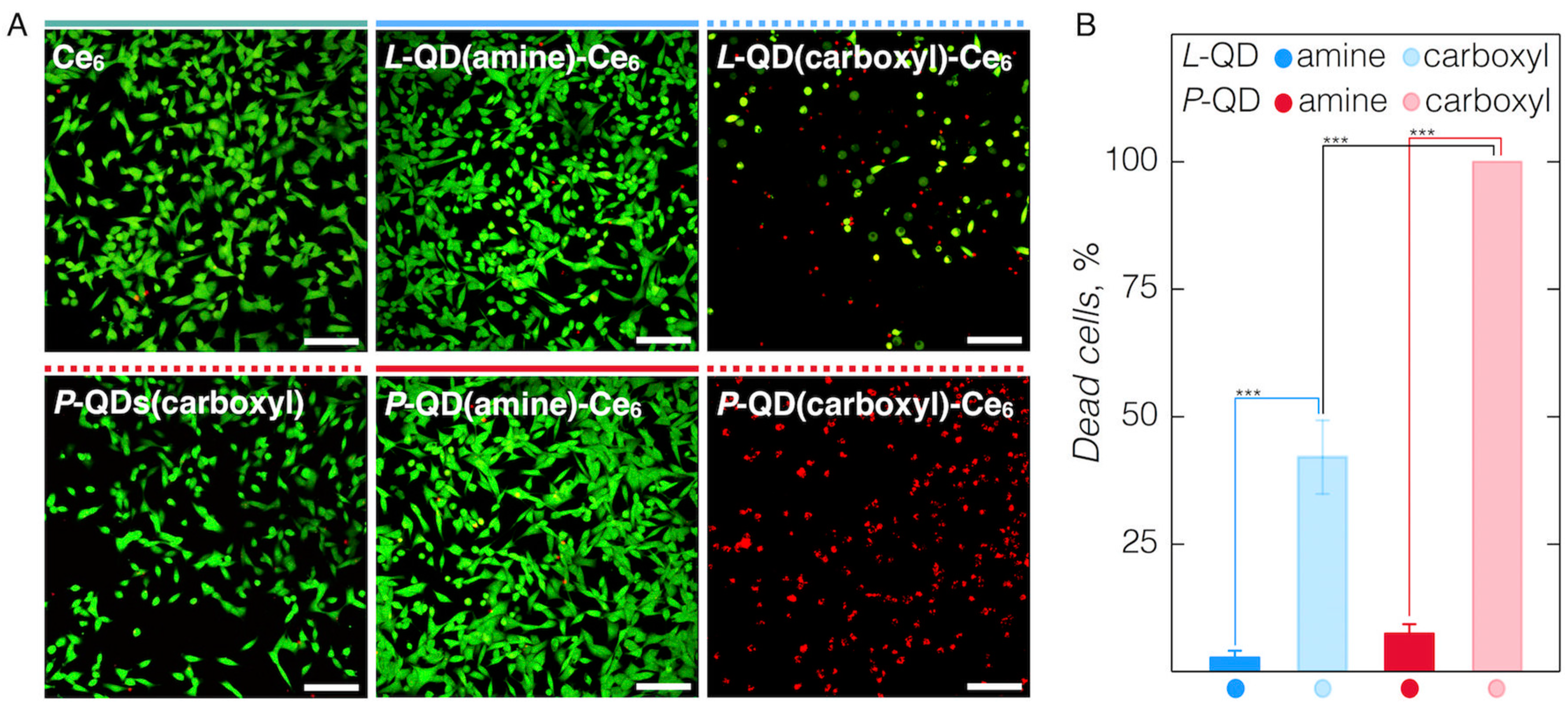
2.4. PDT Studies
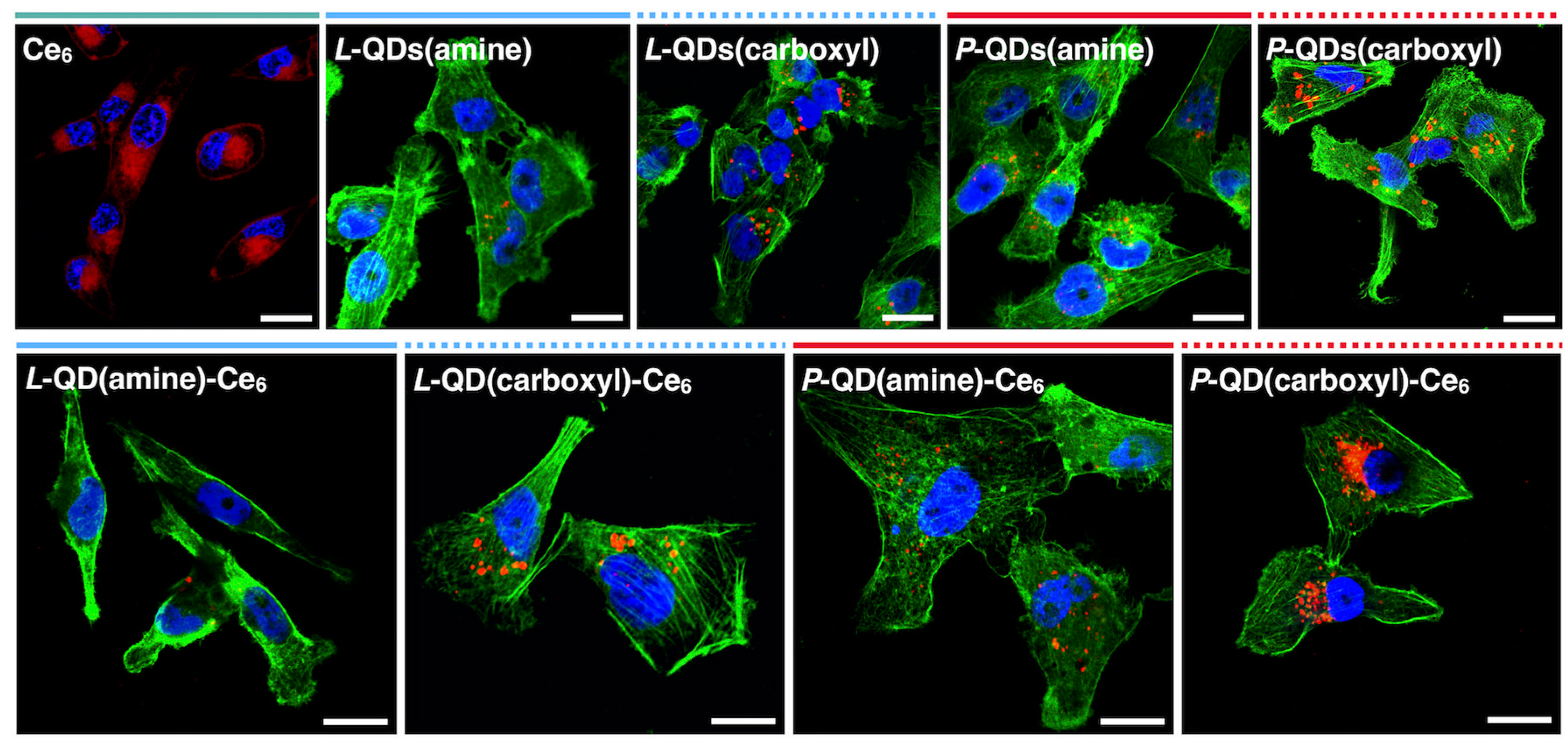
2.5. Cellular Microscopy
3. Results and Discussion
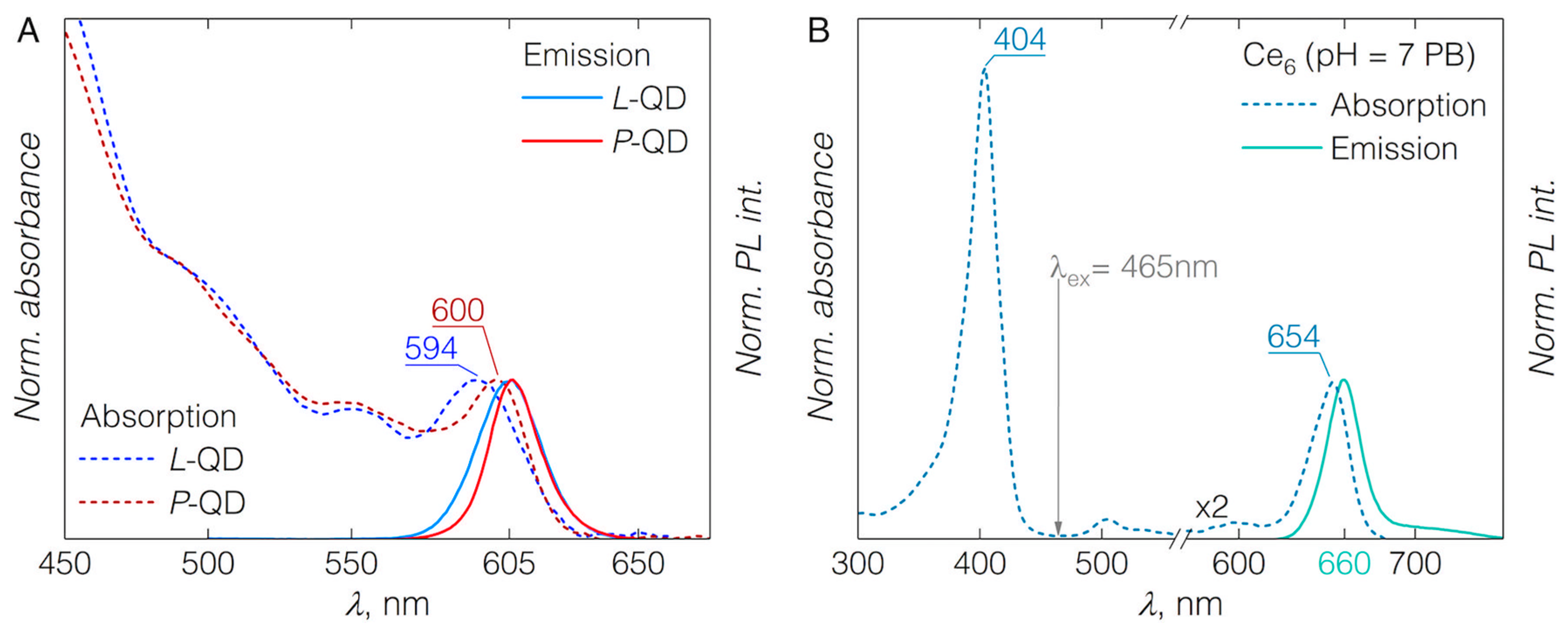
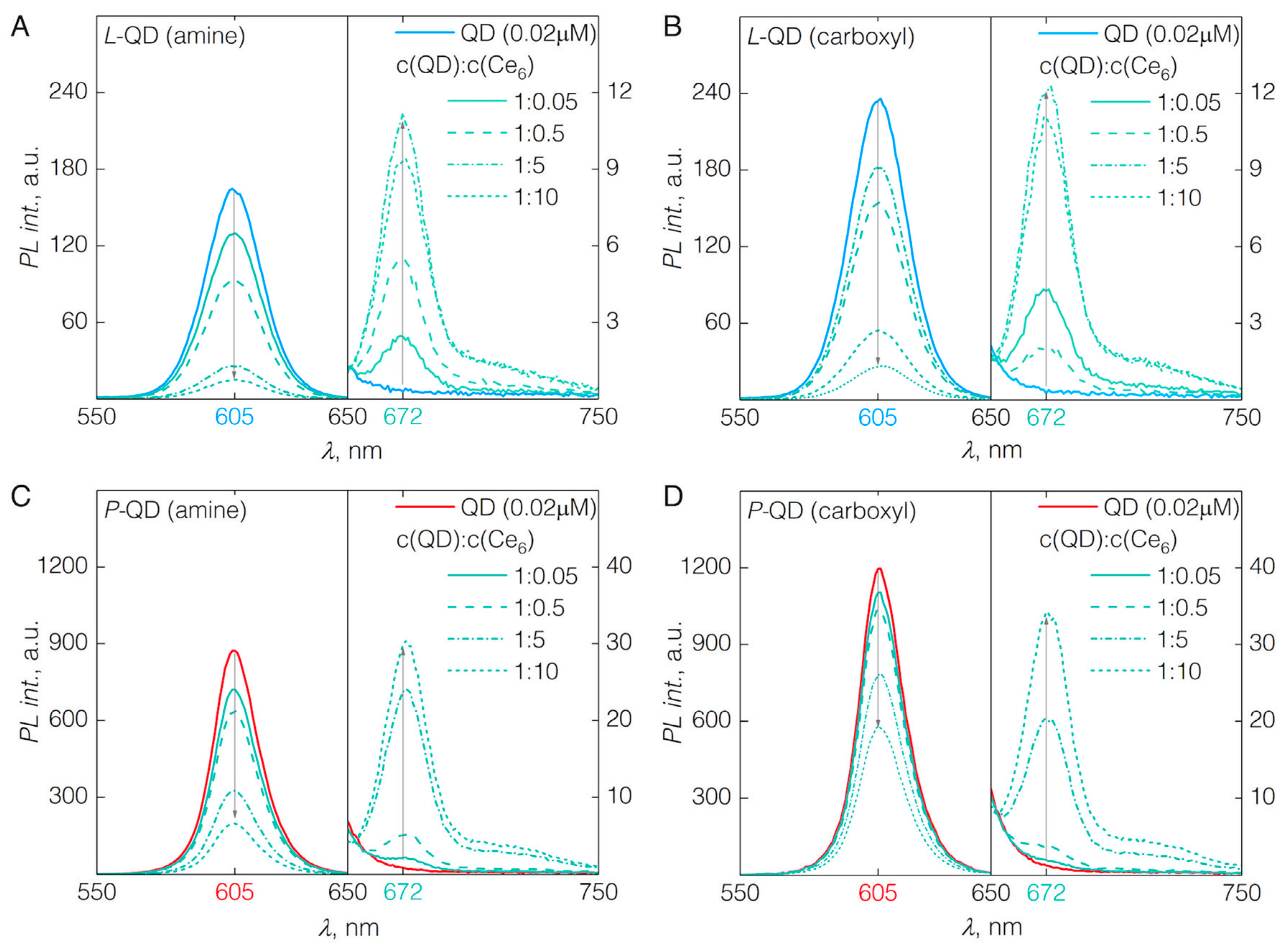
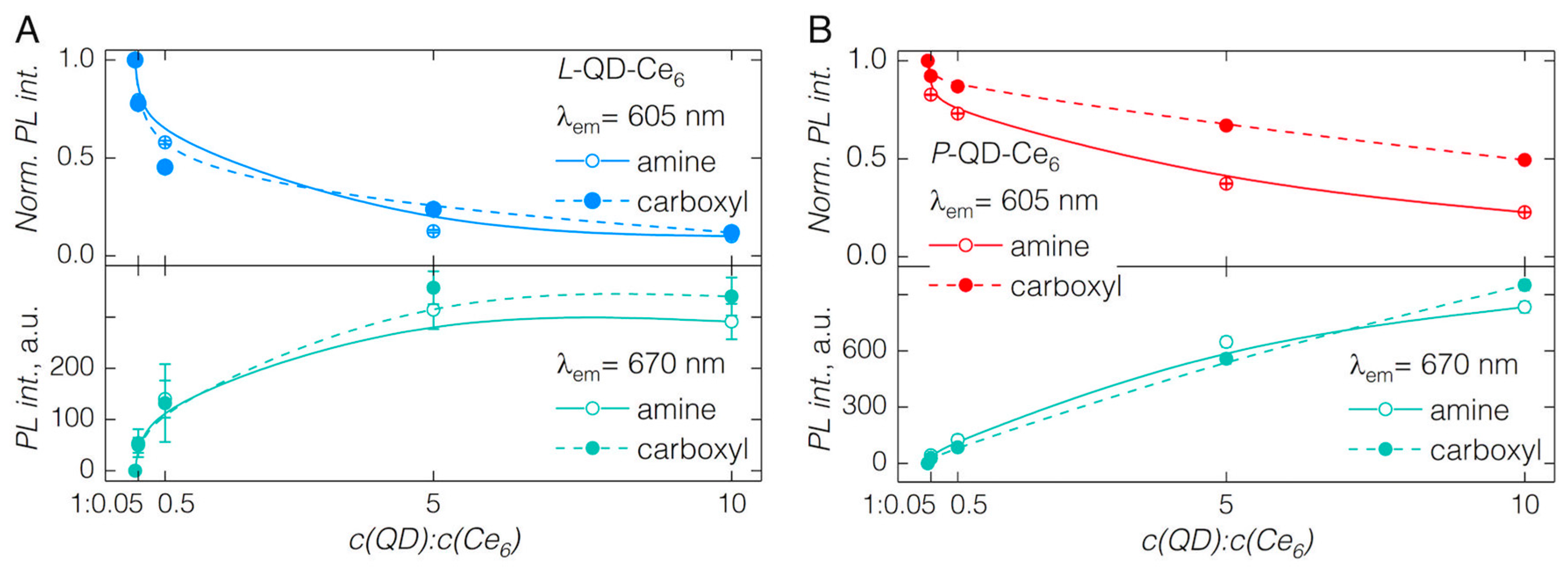
3.1. QD-Ce6 Complex Formation
3.2. FRET from QDs to Ce6
3.3. Cellular Accumulation of QD-Ce6 in Serum-Free Environment and PDT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Samia, A.C.; Chen, X.; Burda, C. Semiconductor Quantum Dots for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, N.; Spillmann, C.M.; Algar, W.R.; Pons, T.; Stewart, M.H.; Oh, E.; Susumu, K.; Diaz, S.A.; Delehanty, J.B.; Medintz, I.L. Energy Transfer with Semiconductor Quantum Dot Bioconjugates: A Versatile Platform for Biosensing, Energy Harvesting, and Other Developing Applications. Chem. Rev. 2016, 117, 536–711. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hernandez, B.; Selke, M. Singlet Oxygen Generation from Water-Soluble Quantum Dot-Organic Dye Nanocomposites. J. Am. Chem. Soc. 2006, 128, 6278–6279. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-M.; Sun, X.-J.; Wang, L.-L.; Fei, M.-Y.; Yan, Z.-Y. Singlet Oxygen-Generating from Fluorescence Probes Based on Denatured Bovine Serum Albumin-Conjugated Cdte Quantum Dots and Photosensitizer Chlorin E6. J. Nanopart. Res. 2014, 16, 2701. [Google Scholar] [CrossRef]
- Charron, G.; Stuchinskaya, T.; Edwards, D.R.; Russell, D.A.; Nann, T. Insights into the Mechanism of Quantum Dot-Sensitized Singlet Oxygen Production for Photodynamic Therapy. J. Phys. Chem. C 2012, 116, 9334–9342. [Google Scholar] [CrossRef]
- Tsay, J.M.; Trzoss, M.; Shi, L.; Kong, X.; Selke, M.; Jung, M.E.; Weiss, S. Singlet Oxygen Production by Peptide-Coated Quantum Dot-Photosensitizer Conjugates. J. Am. Chem. Soc. 2007, 129, 6865–6871. [Google Scholar] [CrossRef]
- Yaghini, E.; Pirker, K.F.; Kay, C.W.; Seifalian, A.M.; MacRobert, A.J. Quantification of Reactive Oxygen Species Generation by Photoexcitation of Pegylated Quantum Dots. Small 2014, 10, 5106–5115. [Google Scholar] [CrossRef]
- Dayal, S.; Burda, C. Semiconductor Quantum Dots as Two-Photon Sensitizers. J. Am. Chem. Soc. 2008, 130, 2890–2891. [Google Scholar] [CrossRef]
- Wen, Y.-N.; Song, W.-S.; An, L.-M.; Liu, Y.-Q.; Wang, Y.-H.; Yang, Y.-Q. Activation of Porphyrin Photosensitizers by Semiconductor Quantum Dots Via Two-Photon Excitation. Appl. Phys. Lett. 2009, 95, 143702. [Google Scholar] [CrossRef]
- Skripka, A.; Valanciunaite, J.; Dauderis, G.; Poderys, V.; Kubiliute, R.; Rotomskis, R. Two-Photon Excited Quantum Dots as Energy Donors for Photosensitizer Chlorin E6. J. Biomed. Opt. 2013, 18, 078002. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.-C.; Yang, M.-J.; Hsu, C.-C.; Lai, C.-W.; Hsieh, C.-C.; Lin, S.H.; Cheng, Y.-M.; Chou, P.-T. The Empirical Correlation between Size and Two-Photon Absorption Cross Section of Cdse and Cdte Quantum Dots. Small 2006, 2, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.O.; Martynenko, I.V.; Maslov, V.G.; Fedorov, A.V.; Gun’ko, Y.K.; Baranov, A.V. Investigation of Complexes of Cdte Quantum Dots with the Aloh-Sulphophthalocyanine Molecules in Aqueous Media. J. Phys. Chem. C 2013, 117, 23425–23431. [Google Scholar] [CrossRef]
- Zenkevich, E.; Cichos, F.; Shulga, A.; Petrov, E.P.; Blaudeck, T.; von Borczyskowski, C. Nanoassemblies Designed from Semiconductor Quantum Dots and Molecular Arrays. J. Phys. Chem. B 2005, 109, 8679–8692. [Google Scholar] [CrossRef] [PubMed]
- Valanciunaite, J.; Klymchenko, A.S.; Skripka, A.; Richert, L.; Steponkiene, S.; Streckyte, G.; Mely, Y.; Rotomskis, R. A Non-Covalent Complex of Quantum Dots and Chlorin E6: Efficient Energy Transfer and Remarkable Stability in Living Cells Revealed by Flim. RSC Adv. 2014, 4, 52270–52278. [Google Scholar] [CrossRef]
- Valanciunaite, J.; Skripka, A.; Streckyte, G.; Rotomskis, R. Complex of Water-Soluble Cdse/Zns Quantum Dots and Chlorin E6: Interaction and Fret. Laser Appl. Life Sci. 2010, 7376, 737607. [Google Scholar] [CrossRef]
- Steponkiene, S.; Valanciunaite, J.; Skripka, A.; Rotomskis, R. Cellular Uptake and Photosensitizing Properties of Quantum Dot-Chlorin E6 Complex: In Vitro Study. J. Biomed. Nanotechnol. 2014, 10, 679–686. [Google Scholar] [CrossRef]
- Karabanovas, V.; Skripka, A.; Valanciunaite, J.; Kubiliute, R.; Poderys, V.; Rotomskis, R. Formation of Self-Assembled Quantum Dot–Chlorin E6 Complex: Influence of Nanoparticles Phospholipid Coating. J. Nanopart. Res. 2014, 16, 2508. [Google Scholar] [CrossRef]
- Valančiūnaitė, J.; Skripka, A.; Araminaitė, R.; Kalantojus, K.; Streckytė, G.; Rotomskis, R. Spectroscopic Study of Non-Covalent Complex Formation between Different Porphyrin Analogues and Quantum Dots with Lipidbased Coating. Chemija 2011, 22, 181–187. [Google Scholar]
- Skripka, A.; Marin, R.; Benayas, A.; Canton, P.; Hemmer, E.; Vetrone, F. Covering the Optical Spectrum through Collective Rare-Earth Doping of NaGdF4 Nanoparticles: 806 and 980 nm Excitation Routes. Phys. Chem. Chem. Phys. 2017, 19, 11825–11834. [Google Scholar] [CrossRef] [PubMed]
- Dapkute, D.; Steponkiene, S.; Bulotiene, D.; Saulite, L.; Riekstina, U.; Rotomskis, R. Skin-Derived Mesenchymal Stem Cells as Quantum Dot Vehicles to Tumors. Int. J. Nanomed. 2017, 12, 8129–8142. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.A.; Zenkevich, E.I.; Gurinovich, G.P.; Kochubeyev, G.A. Chlorin E6-Liposome Interaction. Investigation by the Methods of Fluorescence Spectroscopy and Inductive Resonance Energy Transfer. J. Photochem. Photobiol. B 1990, 7, 43–56. [Google Scholar] [CrossRef]
- Mojzisova, H.; Bonneau, S.; Vever-Bizet, C.; Brault, D. The Ph-Dependent Distribution of the Photosensitizer Chlorin E6 among Plasma Proteins and Membranes: A Physico-Chemical Approach. Biochim. Biophys. Acta 2007, 1768, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Sotgiu, G.; Ferroni, C.; Duchi, S.; Lucarelli, E.; Martini, C.; Posati, T.; Guerrini, A.; Ballestri, M.; Corticelli, F.; et al. Chlorin E6 Keratin Nanoparticles for Photodynamic Anticancer Therapy. RSC Adv. 2016, 6, 33910–33918. [Google Scholar] [CrossRef]
- Parak, W.J.; Pellegrino, T.; Plank, C. Labelling of Cells with Quantum Dots. Nanotechnology 2005, 16, R9–R25. [Google Scholar] [CrossRef]
- Pellegrino, T.; Manna, L.; Kudera, S.; Liedl, T.; Koktysh, D.; Rogach, A.L.; Keller, S.; Rädler, J.; Natile, G.; Parak, W.J. Hydrophobic Nanocrystals Coated with an Amphiphilic Polymer Shell: A General Route to Water Soluble Nanocrystals. Nano Lett. 2004, 4, 703–707. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Orlova, A.O.; Maslov, V.G.; Baranov, A.V.; Fedorov, A.V.; Artemyev, M. Energy Transfer in Complexes of Water-Soluble Quantum Dots and Chlorin E6 Molecules in Different Environments. Beilstein J. Nanotechnol. 2013, 4, 895–902. [Google Scholar] [CrossRef]
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent Dependence of the Fluorescence Lifetimes of Xanthene Dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (Cdse)Zns Core−Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and Properties of Biocompatible Water-Soluble Silica-Coated Cdse/Zns Semiconductor Quantum Dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; Volume 1. [Google Scholar]
- Howland, M.C.; Szmodis, A.W.; Sanii, B.; Parikh, A.N. Characterization of Physical Properties of Supported Phospholipid Membranes Using Imaging Ellipsometry at Optical Wavelengths. Biophys. J. 2007, 92, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Lü, C.; Yang, B. High Refractive Index Organic–Inorganic Nanocomposites: Design, Synthesis and Application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Jennings, T.L.; Becker-Catania, S.G.; Triulzi, R.C.; Tao, G.; Scott, B.; Sapsford, K.E.; Spindel, S.; Oh, E.; Jain, V.; Delehanty, J.B.; et al. Reactive Semiconductor Nanocrystals for Chemoselective Biolabeling and Multiplexed Analysis. ACS Nano 2011, 5, 5579–5593. [Google Scholar] [CrossRef] [PubMed]
- Mercatali, L.; La Manna, F.; Groenewoud, A.; Casadei, R.; Recine, F.; Miserocchi, G.; Pieri, F.; Liverani, C.; Bongiovanni, A.; Spadazzi, C.; et al. Development of a Patient-Derived Xenograft (PDX) of Breast Cancer Bone Metastasis in a Zebrafish Model. Int. J. Mol. Sci. 2016, 17, 1375. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Stewart, R.L.; Chen, J.; Gao, T.; Scott, T.L.; Samayoa, L.M.; O’Connor, K.; Lane, A.N.; Xu, R. Collagen Prolyl 4-Hydroxylase 1 Is Essential for Hif-1α Stabilization and Tnbc Chemoresistance. Nat. Commun. 2018, 9, 4456. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, C.C.; Payne, C.K. Nanoparticle Surface Charge Mediates the Cellular Receptors Used by Protein-Nanoparticle Complexes. J. Phys. Chem. B 2012, 116, 8901–8907. [Google Scholar] [CrossRef]
- Frohlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Karabanovas, V.; Zitkus, Z.; Kuciauskas, D.; Rotomskis, R.; Valius, M. Surface Properties of Quantum Dots Define Their Cellular Endocytic Routes, Mitogenic Stimulation and Suppression of Cell Migration. J. Biomed. Nanotechnol. 2014, 10, 775–786. [Google Scholar] [CrossRef]
- Damalakiene, L.; Karabanovas, V.; Bagdonas, S.; Rotomskis, R. Fluorescence-Lifetime Imaging Microscopy for Visualization of Quantum Dots’ Endocytic Pathway. Int. J. Mol. Sci. 2016, 17, 473. [Google Scholar] [CrossRef]
- Damalakiene, L.; Karabanovas, V.; Bagdonas, S.; Valius, M.; Rotomskis, R. Intracellular Distribution of Nontargeted Quantum Dots after Natural Uptake and Microinjection. Int. J. Nanomed. 2013, 8, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Variables Influencing Interactions of Untargeted Quantum Dot Nanoparticles with Skin Cells and Identification of Biochemical Modulators. Nano Lett. 2007, 7, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailander, V.; Landfester, K.; et al. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernandez, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
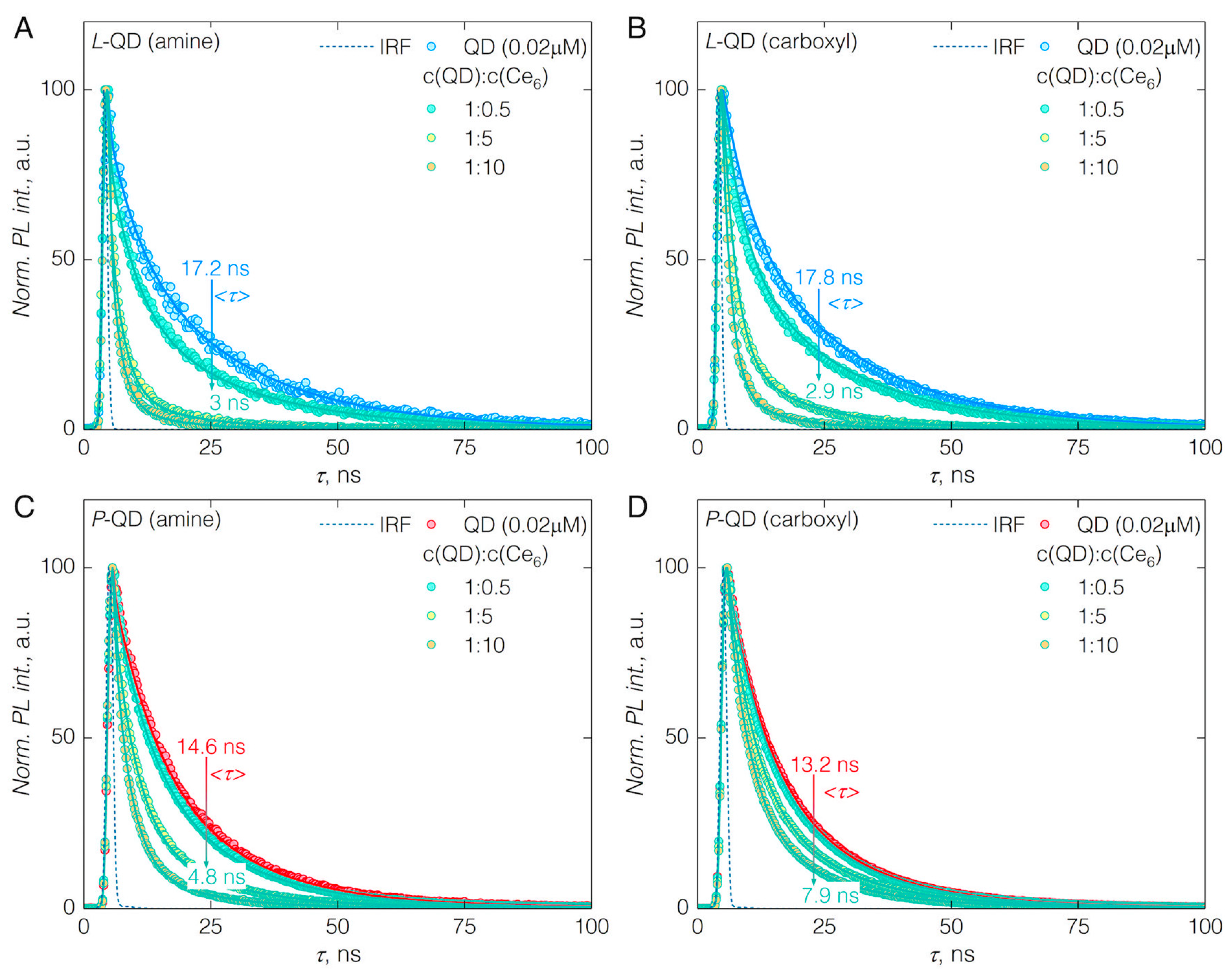
| Quantity | L-QD(amine) | L-QD(carboxyl) | P-QD(amine) | P-QD(carboxyl) | ||||
|---|---|---|---|---|---|---|---|---|
| QY | 0.14 | 0.18 | 0.34 | 0.37 | ||||
| J, 10−13 M−1cm3 | 1.16 | 1.19 | 1.26 | 1.22 | ||||
| R0, Å | 38.0 | 39.8 | 44.7 | 45.0 | ||||
| m | E, % | r, Å | E, % | r, Å | E, % | r, Å | E, % | r, Å |
| 0.5 | 23.9 | 41.1 | 20.8 | 44.3 | 10.9 | 56.5 | 5.4 | 64.7 |
| 5 | 74.9 | 41.5 | 70.6 | 45.0 | 47.3 | 59.5 | 24.4 | 71.1 |
| 10 | 82.8 | 43.0 | 83.7 | 44.5 | 66.9 | 58.3 | 40.1 | 70.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skripka, A.; Dapkute, D.; Valanciunaite, J.; Karabanovas, V.; Rotomskis, R. Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy. Nanomaterials 2019, 9, 9. https://doi.org/10.3390/nano9010009
Skripka A, Dapkute D, Valanciunaite J, Karabanovas V, Rotomskis R. Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy. Nanomaterials. 2019; 9(1):9. https://doi.org/10.3390/nano9010009
Chicago/Turabian StyleSkripka, Artiom, Dominyka Dapkute, Jurga Valanciunaite, Vitalijus Karabanovas, and Ricardas Rotomskis. 2019. "Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy" Nanomaterials 9, no. 1: 9. https://doi.org/10.3390/nano9010009
APA StyleSkripka, A., Dapkute, D., Valanciunaite, J., Karabanovas, V., & Rotomskis, R. (2019). Impact of Quantum Dot Surface on Complex Formation with Chlorin e6 and Photodynamic Therapy. Nanomaterials, 9(1), 9. https://doi.org/10.3390/nano9010009