Environmentally-Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their Anti-Fungal, Ovicidal, and Larvicidal Properties
Abstract
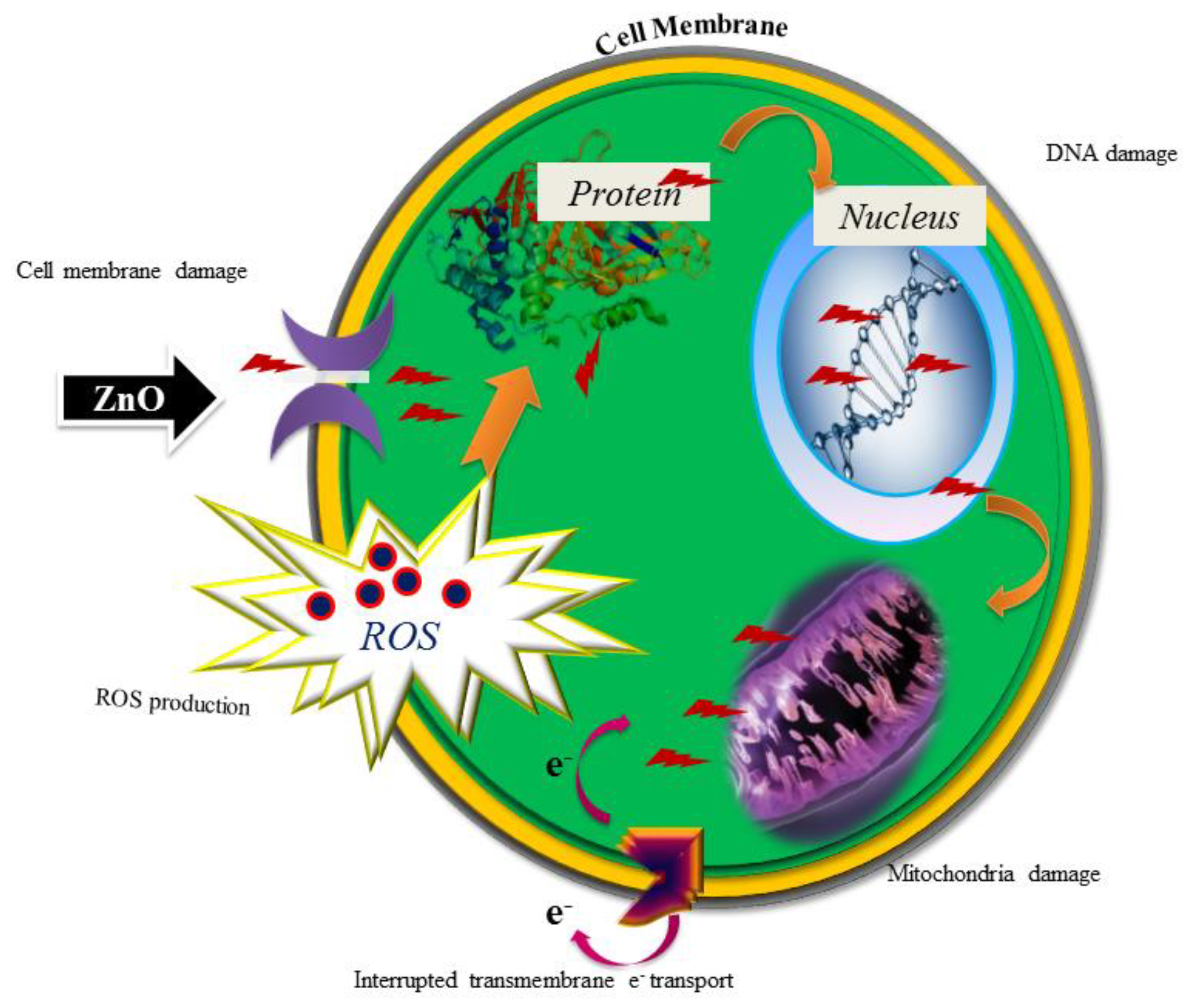
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of the Scadoxus multiflorus Leaf Powder Aqueous Extract Sample
2.3. Production of Zinc Oxide Nanoparticles
2.4. Analytical Techniques
2.5. Larvicidal and Ovicidal Properties of Synthesized Zinc Oxide Nanoparticles
2.6. Antifungal Activity of Zinc Oxide Nanoparticles
3. Results and Discussion
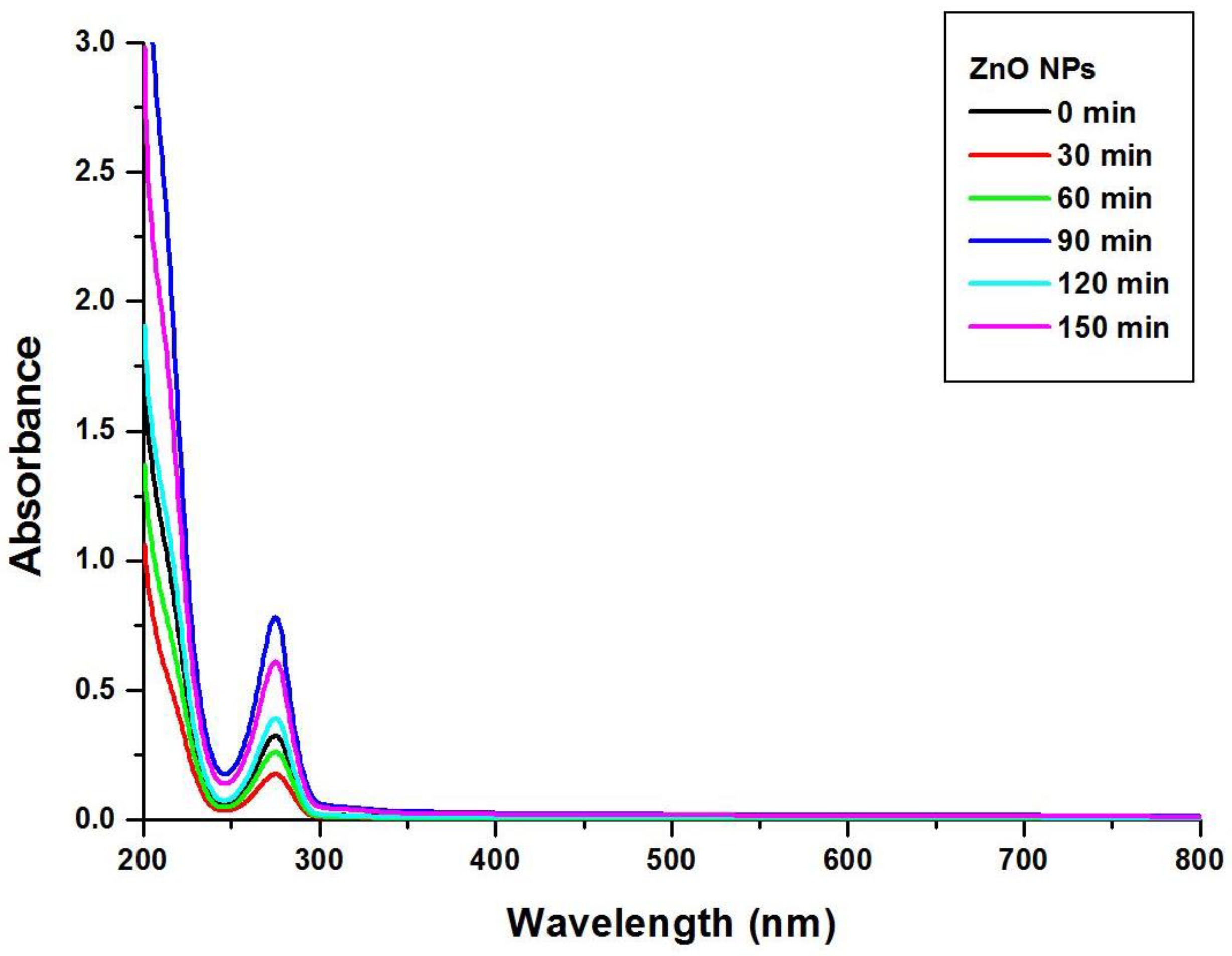
3.1. UV–Visible Spectroscopy
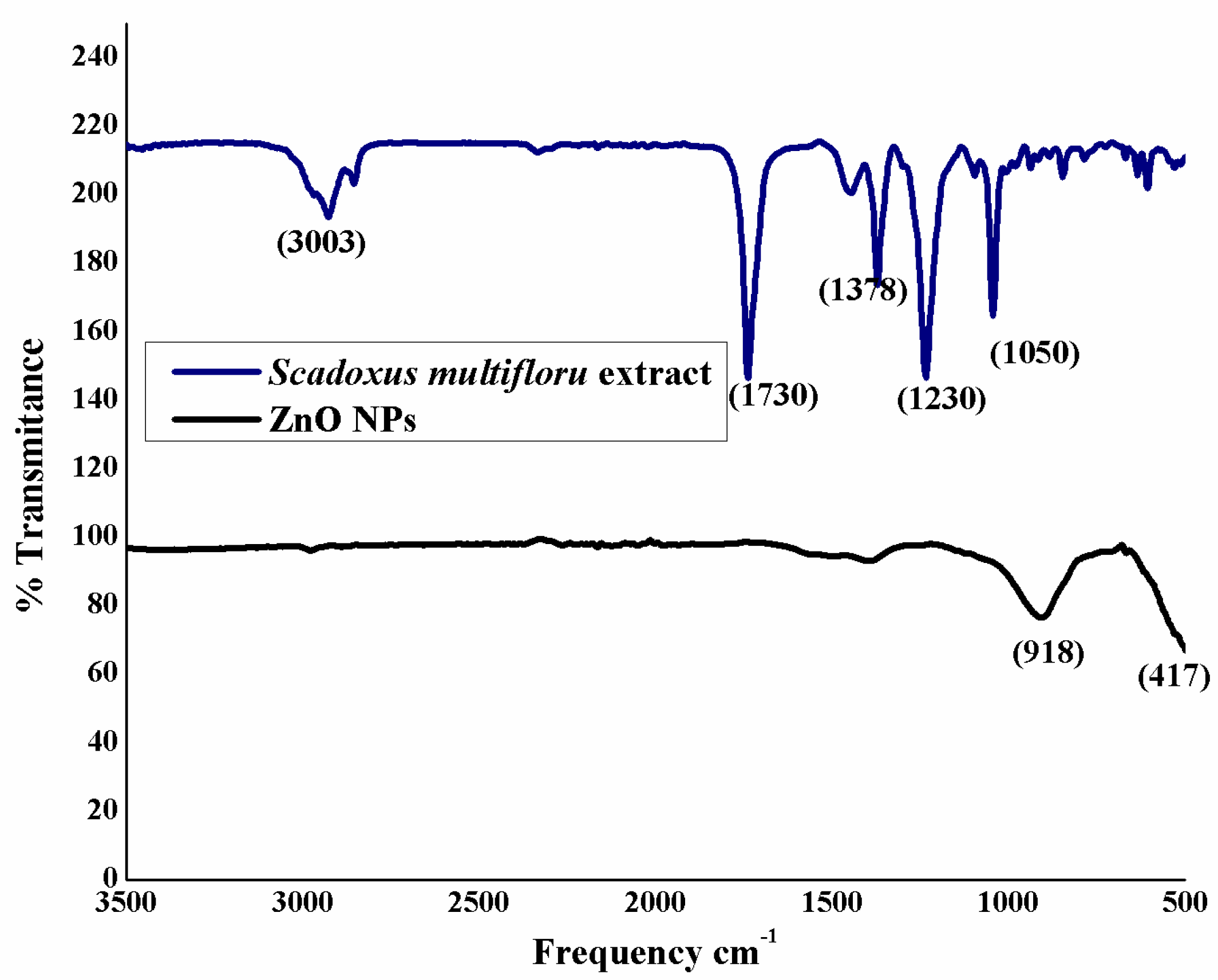
3.2. FTIR Analysis of Zinc Oxide Nanoparticles
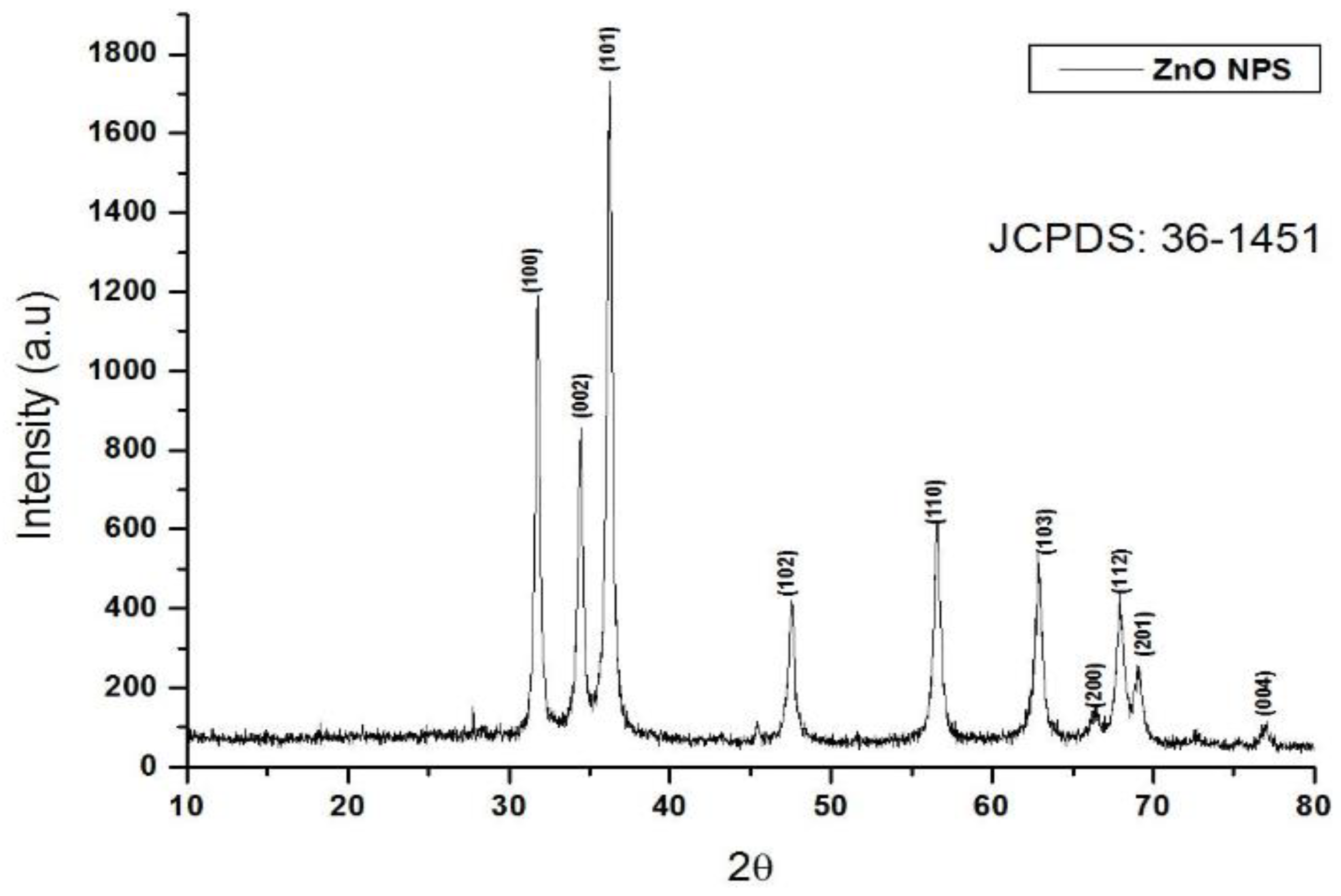
3.3. XRD Analysis of Zinc Oxide Nanoparticles
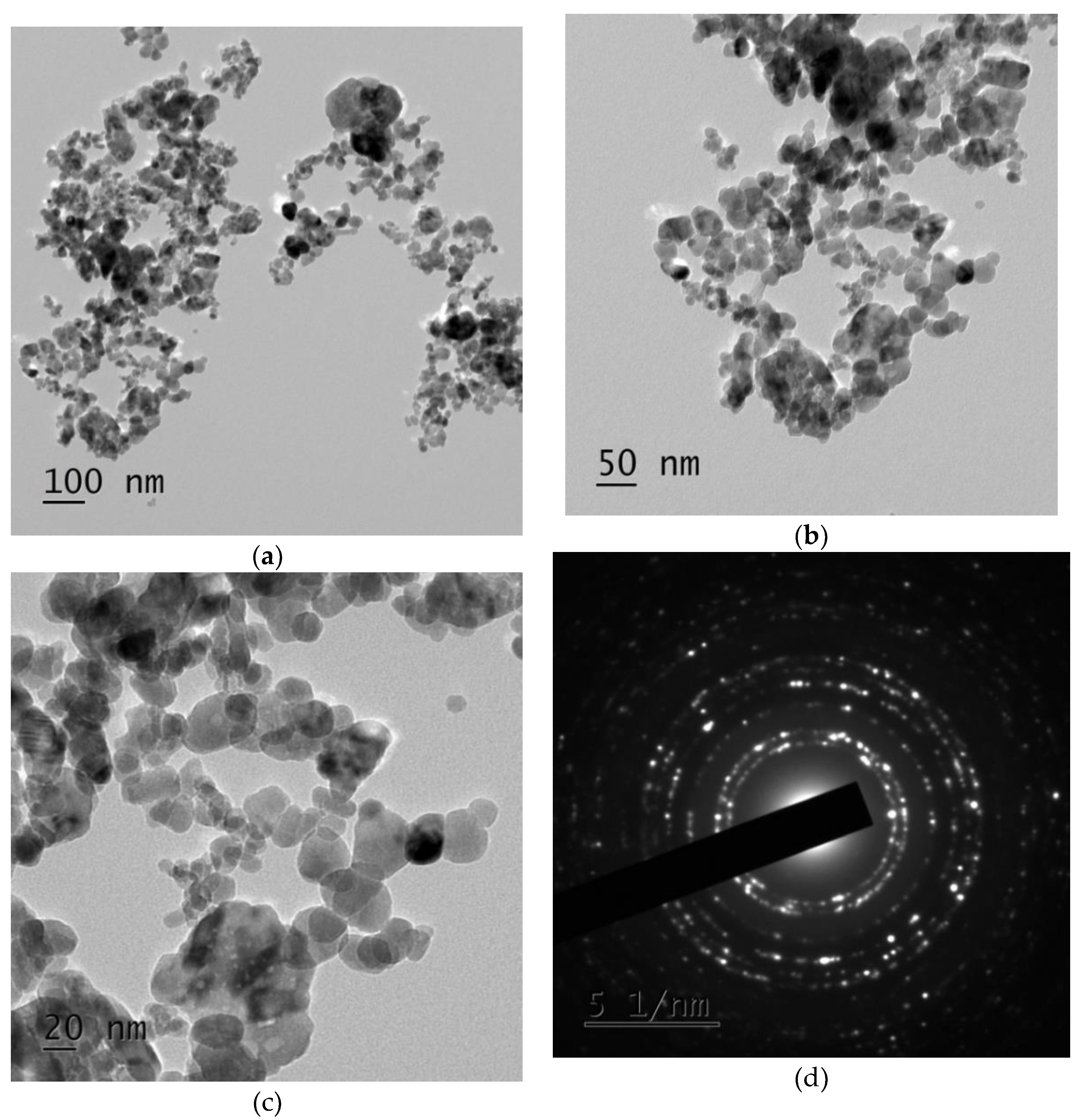
3.4. Zinc Oxide Nanoparticles Morphological Studies
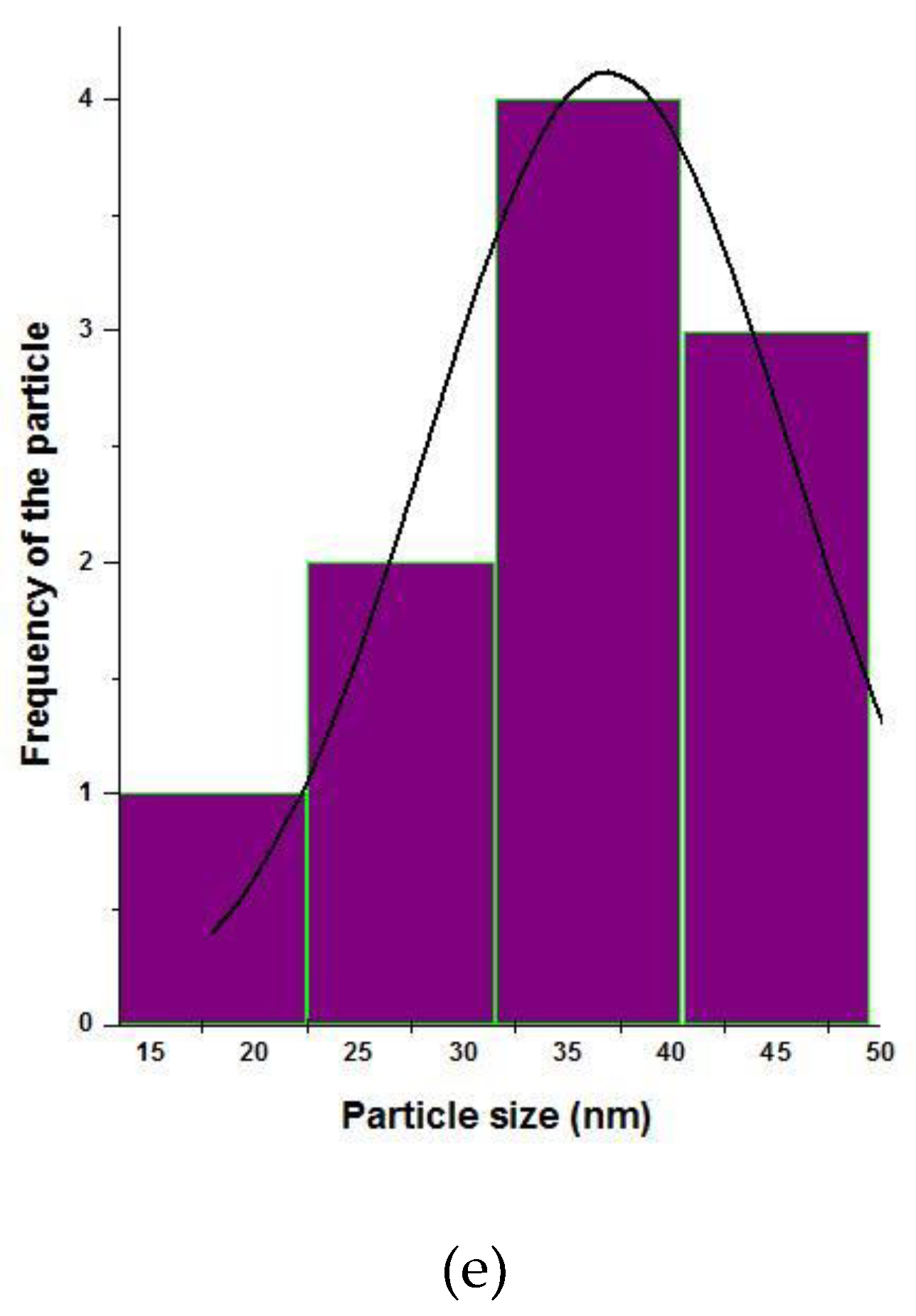
3.5. Particle Size Histogram Analysis of the Zinc Oxide Nanoparticles
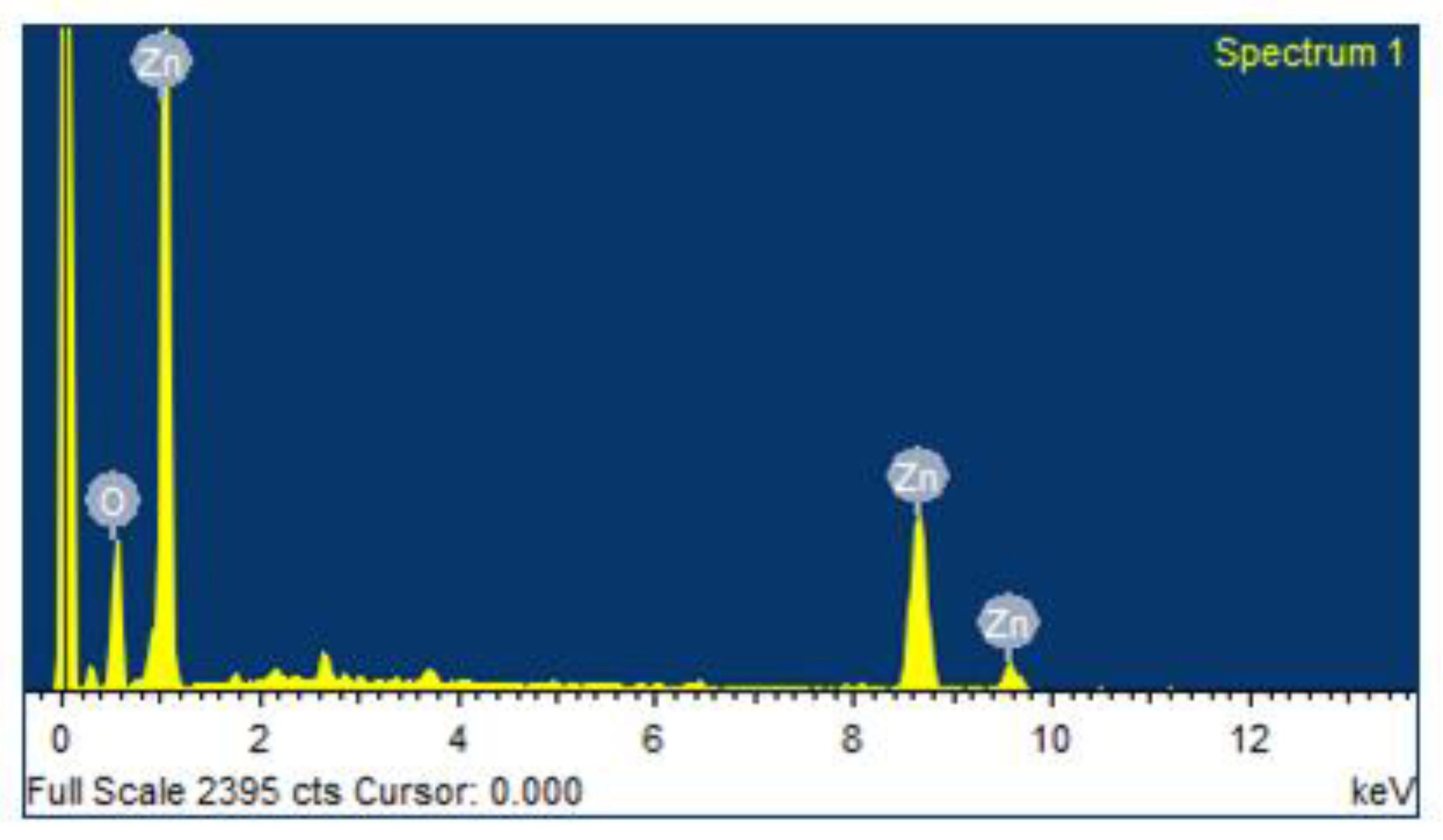
3.6. Energy Dispersive X-ray Analysis (EDAX) Spectrum of Zinc Oxide Nanoparticles
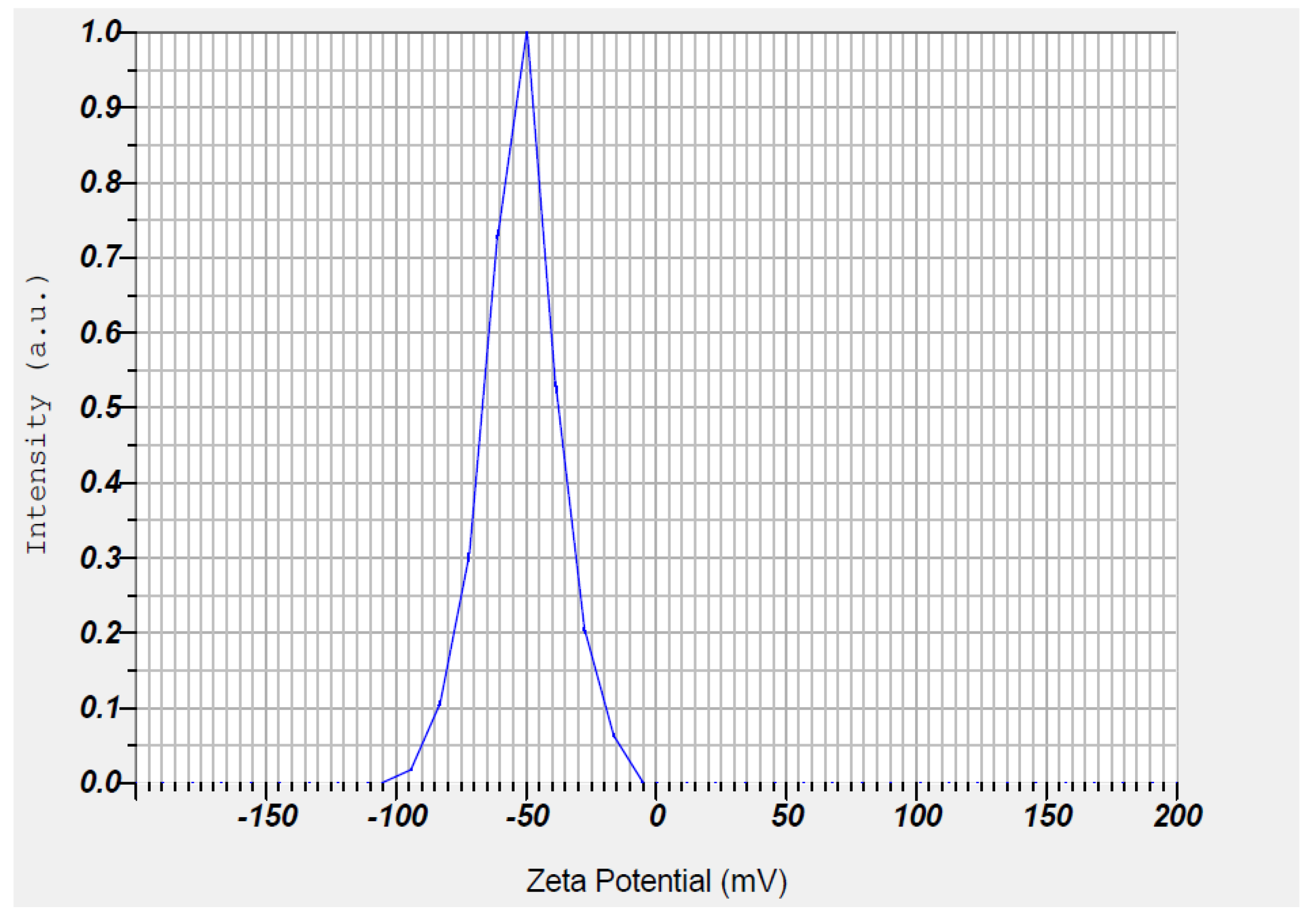
3.7. Stability of Synthesized Zinc Oxide Nanoparticles
3.8. Atomic Absorption Spectroscopy
3.9. Larvicidal Activity of Zinc Oxide Nanoparticles
3.10. Ovicidal Activity of Zinc Oxide Nanoparticles
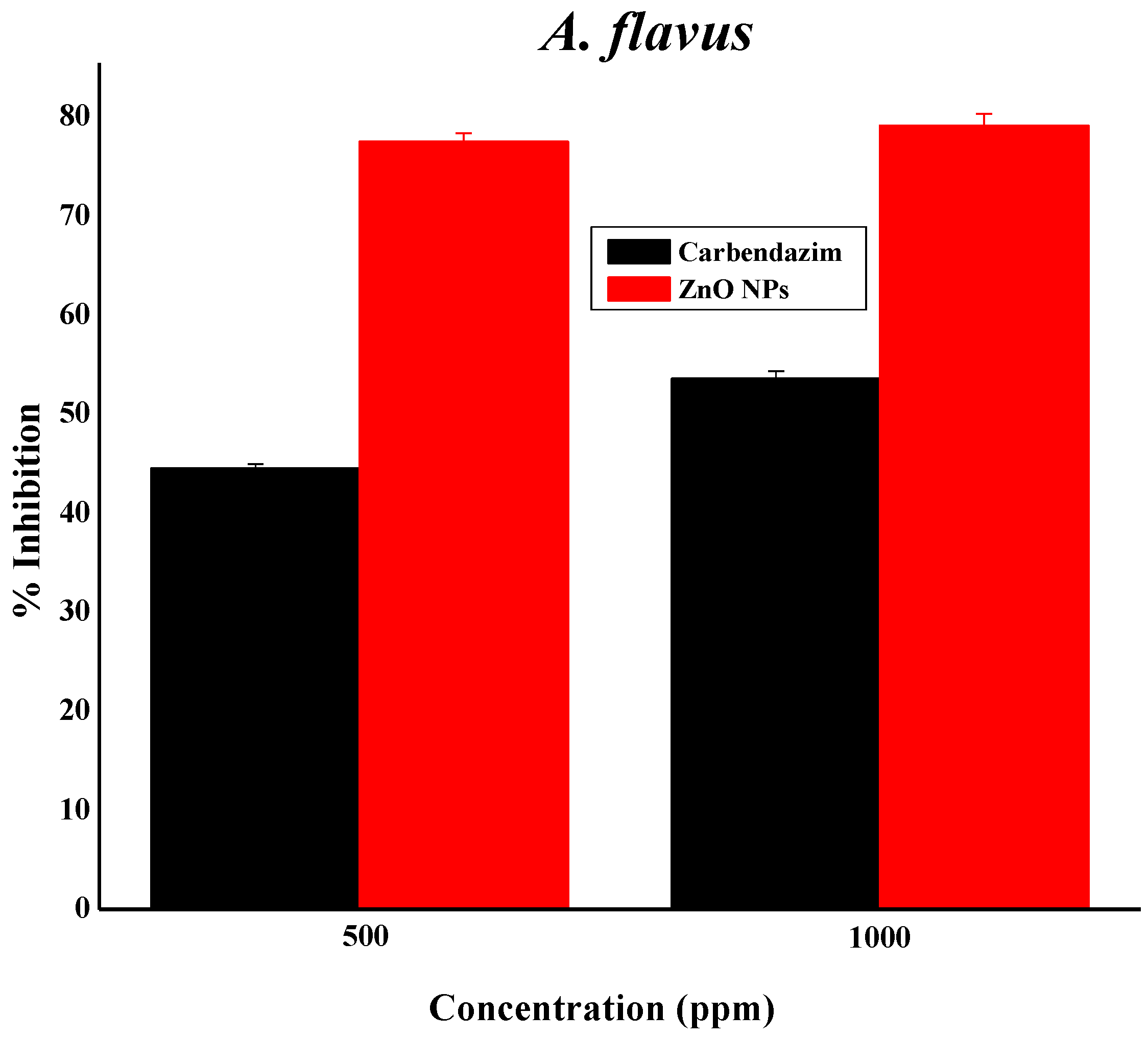
3.11. Zinc Oxide Nanoparticles as Fungicides
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madhumitha, G.; Elango, G.; Roopan, S.M. Bio-functionalized doped silver nanoparticles and its antimicrobial studies. J. Sol-Gel Sci. Technol. 2014, 73, 476–483. [Google Scholar] [CrossRef]
- Fatimah, I. Biosynthesis and characterization of ZnO nanoparticles using rice bran extract as low-cost templating agent. J. Eng. Sci. Technol. 2018, 13, 409–420. [Google Scholar]
- Roopan, S.M.; Rohit; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crop. Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Madhumitha, G.; Rajakumar, G.; Roopan, S.M.; Rahuman, A.A.; Priya, K.M.; Saral, A.M.; Khan, F.R.N.; Khanna, V.G.; Velayutham, K.; Jeyaseelan, C.; et al. Acaricidal, insecticidal, and larvicidal efficacy of fruit peel aqueous extract of Annona squamosa and its compounds against blood-feeding parasites. Parasitol. Res. 2012, 111, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Roopan, S.M.; Bharathi, A.; Kumar, R.; Khanna, V.G.; Prabhakarn, A. Acaricidal, insecticidal, and larvicidal efficacy of aqueous extract of Annona squamosa L. peel as biomaterial for the reduction of palladium salts into nanoparticles. Colloids Surf. B 2012, 92, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Roopan, S.M.; Prabhakarn, A.; Khanna, V.G.; Chakroborty, S. Agricultural waste Annona squamosa peel extract: Biosynthesis of silver nanoparticles. Spectrochim. Acta A 2012, 90, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.A.; Palanichamy, V.; Roopan, S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta A 2014, 127, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta A 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Ahmaruzzaman, M.; Adhikari, P.P. Ecofriendly bio-synthetic route to synthesize ZnO nanoparticles using Eryngium foetidum L. and their activity against pathogenic bacteria. Mat. Lett. 2018, 228, 37–41. [Google Scholar] [CrossRef]
- Elango, G.; Roopan, S.M.; Al-Dhabi, N.A.; Arasu, M.V.; Dhamodharan, K.I.; Elumalai, K. Coir mediated instant synthesis of Ni-Pd nanoparticles and its significance over larvicidal, pesticidal and ovicidal activities. J. Mol. Liq. 2016, 223, 1249–1255. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Rahuman, A.A.; Roopan, S.M.; Krithi, A.V.; Venkatesan, J.; Kim, S.K.; Iyappan, M.; Siva, C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta A 2013, 107, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Anzabi, Y. Biosynthesis of ZnO nanoparticles using barberry (Berberis vulgaris) extract and assessment of their physico-chemical properties and antibacterial activities. Green Process. Syn. 2018, 7, 114–121. [Google Scholar] [CrossRef]
- Velayutham, K.; Rahuman, A.A.; Rajakumar, G.; Roopan, S.M.; Elango, G.; Kamaraj, C.; Marimuthu, S.; SanthoshKumar, T.; Iyappan, M.; Siva, C. Larvicidal activity of green synthesized silver nanoparticles using bark aqueous extract of Ficus racemosa against Culex quinquefasciatus and Culex gelidus. Asian Pac. J. Trop. Med. 2013, 6, 95–101. [Google Scholar] [CrossRef]
- Singh, S.; Krishna, T.H.A.; Kamalraj, S.; Kuriakose, G.C.; Valayil, J.M.; Jayabaskaran, C. Phytomedicinal importance of Saracaasoca (Ashoka): An exciting past, an emerging present and a promising future. Curr. Sci. 2015, 109, 1790–1801. [Google Scholar] [CrossRef]
- Raut, S.; Thorat, P.V.; Thakra, R. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Ocimum tenuiflorum Leaves. Int. J. Sci. Res. 2015, 4, 7–13. [Google Scholar]
- Ramesh, P.; Rajendran, A.; Subramanian, A. Synthesis of zinc oxide nanoparticle from fruit of Citrus aurantifolia by chemical and green method. Asian J. Phytom. Clin. Res. 2014, 2, 189–195. [Google Scholar]
- Harrington, L.C.; Scott, T.W.; Lerdthusnee, K.; Coleman, R.C.; Costero, A.; Clark, G.G.; Jones, J.J.; Kitthawee, S.; Kittayapong, P.; Sithiprasana, R.; et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 2005, 72, 209–220. [Google Scholar] [PubMed]
- Nimmannitya, S.; Halstead, S.B.; Cohen, S.N. Dengue and Chikungunya virus infection in man in Thailand, 1962–1964. I. Observations on hospitalized patients with haemorrhagic fever. Am. J. Trop. Med. Hyg. 1969, 18, 954–971. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Baskar, G.; Chandhuru, J.; Fahad, K.S.; Praveen, A.S. Mycological Synthesis, Characterization and Antifungal Activity of Zinc Oxide Nanoparticles. Asian J. Pharm. Technol. 2013, 4, 142–146. [Google Scholar]
- Vijayakumar, S.; Mahadevan, S.; Arulmozhi, P.; Sriram, S.; Praseetha, P.K. Green synthesis of zinc oxide nanoparticles using Atalantia monophylla leaf extracts: Characterization and antimicrobial analysis. Mater. Sci. Semicond. Process. 2018, 82, 39–45. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Pfaller, M.; Messer, S.A.; Knapp, C.C.; Holliday, N.; Killian, S.B. Multicenter comparison of the sensititre yeast one colorimetric antifungal panel with the NCCLS M27-A2 reference methods for testing new antifungal agents against clinical isolates of Candida spp. J. Clin. Microbiol. 2004, 42, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Madhiyazhagan, P.; Murugan, K.; Kumar, A.K.; Nataraj, T.; Subramaniam, J.; Chandramohan, B.; Panmeerselvam, C.; Dinesh, D.; Suresh, U.; Nicoletti, M.; et al. One pot synthesis of silver nanocrystals using the seaweed Gracilaria edulis: Biophysical characterization and potential against the filariasis vector Culex quinquefasciatus and the midge Chironomus circumdatus. J. Appl. Phycol. 2016. [Google Scholar] [CrossRef]
- Volker, C.; Oetken, M.; Oehlmann, J. The biological effects and possible modes of action of nanosilver. Rev. Environ. Contam. Toxicol. 2013, 223, 81–106. [Google Scholar] [PubMed]
- Benelli, G. Mode of action of nanoparticles against insects. Environ. Sci. Pollut. Res. 2018, 25, 12329–12341. [Google Scholar] [CrossRef] [PubMed]
- Surendra, T.V.; Roopan, S.M.; Al-dhabi, N.A.; Arasu, M.V.; Sarkar, G.; Suthindhiran, K. Vegetable Peel Waste for the Production of ZnO Nanoparticles and its Toxicological Efficiency, Antifungal, Hemolytic, and Antibacterial Activities. Nanoscale Res. Lett. 2016, 11, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Inakhunbi Chanu, T.; Samanta, D.; Chatterjee, S. A review on bio-synthesized zinc oxide nanoparticles using plant extracts as reductants and stabilizing agents. J. Photochem. Photobiol. B 2018, 183, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Elango, G.; Roopan, S.M.; Dhamodaran, K.I.; Elumalai, K.; Al-dhabi, N.A.; Arasu, M.V. Spectroscopic investigation of biosynthesized nickel nanoparticles and its larvicidal, pesticidal activities. J. Photochem. Photobiol. B 2016, 162, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Ashokan, A.P.; Paulpandi, M.; Dinesh, D.; Murugan, K.; Vadivalagan, C.; Benelli, G. Toxicity on Dengue Mosquito Vectors Through Myristica fragrans-Synthesized Zinc Oxide Nanorods, and Their Cytotoxic Effects on Liver Cancer Cells (HepG2). J. Clust. Sci. 2017, 28, 205–226. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Subbaiah, S.; Nazar, A.K.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-anbr, M.N. Sargassum wightii-synthesized ZnO nanoparticles—From antibacterial and insecticidal activity to immunostimulatory effects on the green tiger shrimp Penaeus semisulcatus. J. Photochem. Photobiol. B 2018, 183, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Veni, T.; Pushpanathan, P.; Mohanraj, J. Larvicidal and ovicidal activity of Terminalia chebula Retz. (Family: Combretaceae) medicinal plant extracts against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. J. Parasit. Dis. 2017, 41, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Usha Rani, P.; Desneux, N. Nanoparticles for pest control: current status and future perspectives. J. Pest. Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vaseeharan, B.; Vijayakumar, S.; Pandiselvi, K.; Kalanjiam, M.A.R.; Murugan, K.; Benelli, G. Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb. Pathogen. 2017, 104, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O. Influence of particle size on the antibacterial zinc oxide. Int. J. Inorg. Mater. 2013, 3, 643–646. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behavior of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Ramachandran, R.; Krishnaraj, C.; Stacey, L.H.; Soon-Il, Y.; Thangavel, P.K. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Indus. Crop. Prod. 2015, 70, 356–373. [Google Scholar]


| Concentration (ppm) | Mortality * (%) | LC50 (ppm) | 95% Confidence Limits (ppm) | LC90 (ppm) | 95% Confidence Limits (ppm) | χ2 Value | ||
|---|---|---|---|---|---|---|---|---|
| LCL | UCL | LCL | UCL | |||||
| Control | 1.6 ± 0.4 a | 34.04 | 14.82 | 50.32 | 78.06 | 58.75 | 143.75 | 3.189 |
| 15 | 28.6 ± 7.5 b | |||||||
| 30 | 42..4 ± 2.5 c | |||||||
| 60 | 82.2 ± 6.4 d | |||||||
| 120 | 98.4 ± 2.3 e | |||||||
| Concentrations (ppm) | % of Mortality |
|---|---|
| 15 | 35.5 ± 0.23 |
| 30 | 47.2 ± 1.21 |
| 60 | 63.7 ± 0.38 |
| 120 | 96.4 ± 0.24 |
| Neem azal (120) | 100 ± 0.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dhabi, N.A.; Valan Arasu, M. Environmentally-Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their Anti-Fungal, Ovicidal, and Larvicidal Properties. Nanomaterials 2018, 8, 500. https://doi.org/10.3390/nano8070500
Al-Dhabi NA, Valan Arasu M. Environmentally-Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their Anti-Fungal, Ovicidal, and Larvicidal Properties. Nanomaterials. 2018; 8(7):500. https://doi.org/10.3390/nano8070500
Chicago/Turabian StyleAl-Dhabi, Naif Abdullah, and Mariadhas Valan Arasu. 2018. "Environmentally-Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their Anti-Fungal, Ovicidal, and Larvicidal Properties" Nanomaterials 8, no. 7: 500. https://doi.org/10.3390/nano8070500
APA StyleAl-Dhabi, N. A., & Valan Arasu, M. (2018). Environmentally-Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their Anti-Fungal, Ovicidal, and Larvicidal Properties. Nanomaterials, 8(7), 500. https://doi.org/10.3390/nano8070500




